Abstract
We used an integrated morpho-physiological, biochemical, and genetic approach to investigate the salt responses of four lines (TN1.11, TN6.18, JA17, and A10) of Medicago truncatula. Results showed that TN1.11 exhibited a high tolerance to salinity, compared with the other lines, recording a salinity induced an increase in soluble sugars and soluble proteins, a slight decrease in malondialdehyde (MDA) accumulation, and less reduction in plant biomass. TN6.18 was the most susceptible to salinity as it showed less plant weight, had elevated levels of MDA, and lower levels of soluble sugars and soluble proteins under salt stress. As transcription factors of the APETALA2/ethylene responsive factor (AP2/ERF) family play important roles in plant growth, development, and responses to biotic and abiotic stresses, we performed a functional characterization of MtERF1 gene. Real-time PCR analysis revealed that MtERF1 is mainly expressed in roots and is inducible by NaCl and low temperature. Additionally, under salt stress, a greater increase in the expression of MtERF1 was found in TN1.11 plants than that in TN6.18. Therefore, the MtERF1 pattern of expression may provide a useful marker for discriminating among lines of M. truncatula and can be used as a tool in breeding programs aiming at obtaining Medicago lines with improved salt tolerance.
1. Introduction
Due to the unpredictability of environmental conditions and the inability of plants to move in order to avoid unfavorable conditions, a number of abiotic stress factors threaten plant productivity and sustainability [1,2] Salinity is one of the main environmental stressors limiting crop production globally [3], it causes oxidative damages, ion toxicity, and nutrition imbalance [2,4,5,6]. According to the United Nations (UN) Environment Program (UNEP), worldwide, approximately 50% of agricultural lands are now characterized as saline soils [6]. Additionally, this area increases every day due to inadequate irrigation practices and it aggravates the salinity problem [7].
Survival under this stress requires the integration of adaptive metabolic, physiological, and molecular responses. In response to salinity, plants employ different strategies and mechanisms to accumulate organic solutes in the tissue, and provide tissue tolerance to high salt concentrations [7,8,9,10] These organic solutes can protect plants against short term and high intensity salt stress [11]. Moreover, it is known that soluble protein content is an important indicator of physiological status of plants [12]. Thus, understanding the different adaption mechanisms to environmental stresses may lead to novel strategies for plant improvement.
According to the ability to grow on high salt medium, plants have been classified as glycophytes or halophytes, where glycophytes cannot grow well under salt stress condition whereas halophytes grow well under high salinity. Most crop species are glycophytes and cannot tolerate salt stress [13,14], so to cope with this stress, plants have evolved complex mechanisms and elaborated signaling network that perceives signals from their surroundings and appropriately responds to environmental changes by modulating the expression of responsive genes [15]. These genes encode two major groups of proteins: functional and regulatory proteins. The main regulators of abiotic stress mediated gene expression are transcription factors (TFs), which have the capacity to recognize and bind specifically to cis-elements in the promoters of stress-responsive genes, and regulate their transcription [15,16,17], TFs function as terminal transducers and directly modulate gene expression of an array of downstream genes [18,19,20,21] Due to this property, the manipulation of TFs is a very useful strategy for imparting multiple stress tolerance in plants [22,23,24]. The APETALA2/ethylene responsive factor (AP2/ERF) superfamily is one of the largest groups of transcription factors in plants [25]. This family is characterized by the presence of a common domain of about 60–70 amino acids residues known as the AP2 domain. A simple classification based on the copy number of AP2 domains yielded four families: AP2, ERF, RAV and soloist [26,27]. The AP2 family owns duplicated AP2/ERF domains, whereas the ERF family exhibits a single AP2/ERF domain, the RAV family has one B3 domain and one AP2/ERF domain and the Soloist family contains a small group of TFs with a highly divergent single AP2 domain (AP2-like domain) and gene structure.
The ERF family is further subdivided into the ERF and dehydration responsive element binding proteins (DREB) subfamilies on the basis of the similarities in amino acid residues of the AP2 domain [28]. The TFs from the ERF and DREB subfamilies, are closely associated with responses to environmental stress, such as pathogen and disease stimuli [28,29], salinity [30,31], drought [31], and freezing [32,33]. With more extensive plant genome sequences, AP2/ERF gene families have been identified in various plants, such as Arabidopsis [34], soybean [35], rice [36], potato [25], Medicago truncatula [37], barley [38], and Ammopiptanthus nanus [39], among others.
Moreover, legumes are the plants in family Fabaceae or Leguminosae, which are playing a crucial role in crop rotation due to their symbiotic nitrogen-fixing bacteria in structures [40,41]. Fabaceae are primarily grown for human consumption, for livestock forage and silage, and as soil-enhancing green manure [42,43]. Forage crops are the backbone of sustainable agriculture; they are often grown in less favorable areas and thus require sophisticated protective mechanisms to withstand severe environmental conditions [43]. The transcription factors play a crucial role in enhancing some legume species to adapt during abiotic stresses. Thus, we have been interested in characterizing certain transcription factor genes from M. truncatula, which is an omni-Mediterranean forage legume species and a model plant for legume biological studies in view of its small diploid genome, self-fertile nature, relatively short life cycle and high genetic transformation efficiency [42,44]. Due to these characteristics and to the fact that it is a close relative of alfalfa and clovers, M. truncatula has become the focus of intensive research around the globe, aimed at identifying and characterizing major stress responsive genes using modern tools, such as genomics as well as genetic transformation [44,45,46].
This study aims to (i) analyze the morpho-physiological and biochemical responses of four M. truncatula lines under salt stress, and (ii) to explore the expression of an ERF gene in M. truncatula under salt stress.
2. Results
2.1. Morpho-Physiological Responses
Results from ANOVA showed that the variation in measured traits were explained by the effects of line, treatment and the interaction of line × treatment (Table 1). The maximum effect was observed for the line factor. There were clear treatment and line effects on most of the traits. However, the variation of only six traits was explained by the interaction line × treatment. The traits include the length of stems, number of leaves, aerial fresh weight, aerial dry weight, root fresh weight, and root dry weight.

Table 1.
Effects of line, treatment and the interaction of line × treatment on measured traits for studied lines of M. truncatula under control treatment and 100 mM NaCl.
Salt stress significantly decreased the fresh and dry biomass of shoots and root organs of M. truncatula. Salinity stress was associated with 49%, 82%, 18%, and 86% decrease in shoot fresh weights while the shoot dry weight was reduced by 40%, 76%, 31%, and 68% in A10, JA17, TN1.11, and TN6.18 under salt stress, respectively. Similarly, compared to the control plants, the root fresh weight was reduced under salt stress by 61%, 66%, 29%, and 29% while the reductions in root dry weight were 93%, 76%, 30%, and 33% in A10, JA17, TN1.11, and TN6.18, respectively, in salinity treated plants (Table 2). In comparison to controls, the effect of salt stress on plant biomass was much more noticeable for TN6.18; which it had the least effect on root fresh weight and the biggest effect on aerial fresh weight.

Table 2.
Minimum, maximum, means, and broad-sense heritability (H2) of measured characters for the four lines of M. truncatula under control treatment and 100 mM NaCl.
Under salt stress, the length of stems was reduced by 36%, 46%, 14%, and 56% in the A10, JA17, TN1.11, and TN6.18 lines, respectively.
Salinity stress significantly (P ≤ 0.05) reduced the number of leaves in JA17 and TN6.18 lines (Figure 1).
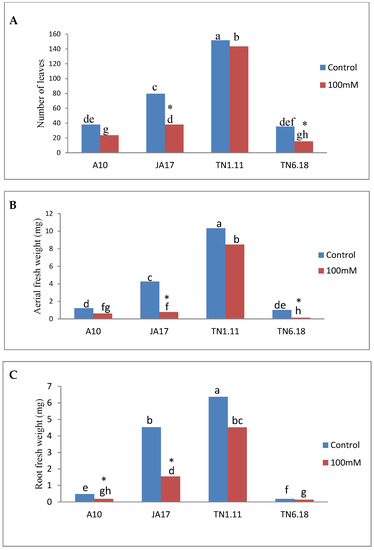
Figure 1.
Means of (A) leaves number, (B) aerial fresh weight, and (C) root fresh weight for the four lines of M. truncatula under control treatment and 100 mM NaCl. Different letters set in bold represent significant differences among treatments as tested using a Duncan’s test. Asterisks indicate tests are taken as significant at P ≤ 0.05.
All the measured traits for the four lines showed high heritability values (H2 > 0.4). The broad-sense heritability (H2) values of the traits ranged from 0.90 to 0.97 and from 0.91 to 0.99 under the control treatment and salt stress, respectively (Table 2).
Among the 30 possible correlations, 25 were significant and were positive (Table 3). Only the length of stems was not significantly correlated with any of the traits in the control treatment.

Table 3.
Matrices of correlations between measured traits for the four lines of M. truncatula under control treatment (down diagonal) and 100 mM NaCl (upper diagonal).
Principal Component Analysis and Clustering Analysis
The first three principal components with eigenvalues > 1 explained 100% of the total variation among the studied genotypes grown under salt stress. The first two axes explained 87% of the total phenotypic variation. The first axis was mainly correlated to the number of leaves, the aerial fresh weight and the aerial dry weight. The second axis was explained by the root dry weight. The distribution of the studied genotypes on the first two axes of principal component analysis (PCA) showed an important genetic variation.
The positive side of the PCA gathered the tolerant and the moderately tolerant lines (A10, TN1.11, and JA17), which were marked by high values of salt sensitivity index SSI (Figure 2B). However, the negative side of the PCA was associated with the sensitive line TN6.18.
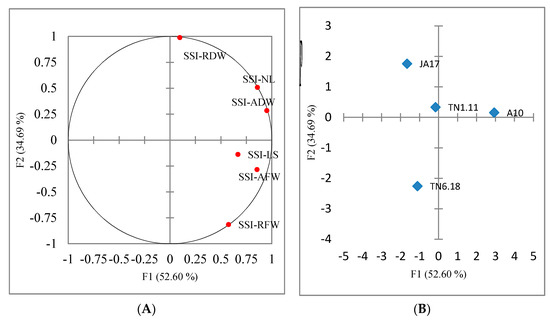
Figure 2.
Principal components analysis with traits recorded under 100 mM NaCl condition for 4 lines of M. truncatula. (A) Contribution of traits to the first two principal component analysis (PCA) axes. Length of stems (SSI-LS), number of leaves (SSI-NL), aerial fresh weight (SSI-AFW), aerial dry weight (SSI-ADW), root fresh weight (SSI-RFW), root dry weight (SSI-RDW). (B) Distribution of the four lines on the first two PCA axes.
The lines were clustered into three groups (Figure 3). The first group was formed by the tolerant line A10, the second group was constituted by the two moderately tolerant lines TN1.11 and JA17, and the third group was composed by the sensitive line TN6.18.
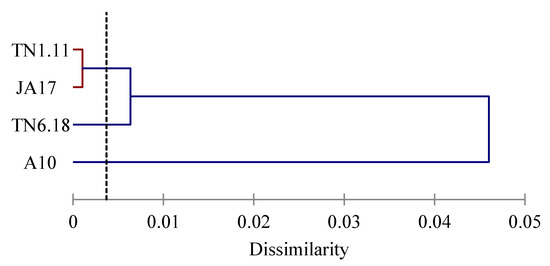
Figure 3.
Dendrogram of the lines of M. truncatula based on Euclidean distances of dissimilarity matrix using the Ward’s method.
2.2. Biochemical Responses
Salt stress influenced soluble sugars, proteins and malondialdehyde (MDA) content (Table 4). There were clear treatment and line effects on the soluble sugar. However, the effect of treatment was not significant for proteins and MDA content.

Table 4.
Effects of line, treatment and the interaction line × treatment on biochemical parameters for studied lines of M. truncatula under control treatment and 100 mM NaCl.
Under salt stress, there was a decrease of sugars and protein content in all the studied lines except for TN1.11 (Figure 4). This decrease was more pronounced for the soluble sugars (45%) and soluble proteins (30%) for the lines JA17 and A10, respectively. Compared with the control treatment, MDA content increased as a result of salt stress for all lines except TN1.11, indicating enhanced lipid peroxidation. However, the highest increase was observed in JA17 (54%) (Figure 4). Protein and soluble sugar content accumulate in TN1.11 subjected to salinity stress conditions to confer stress tolerance to this line.
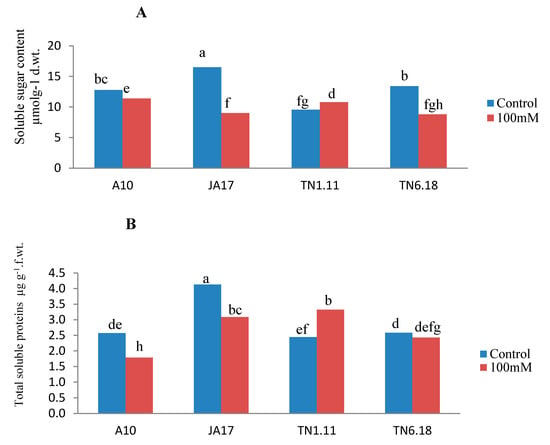
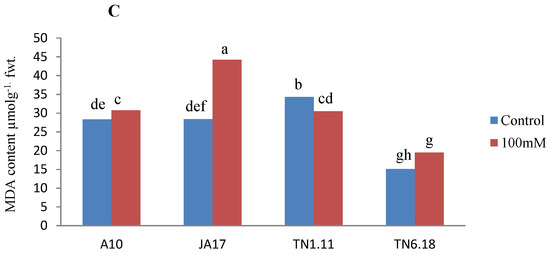
Figure 4.
Variability of soluble sugar (A), total proteins content (B), MDA content (C) in A10, JA17, TN1.11, and TN6.18 lines of M. truncatula under control treatment and 100 mM NaCl. Means with the same or common letters are not significantly different among studied lines as tested using a Duncan’s test.
2.3. Molecular Responses
2.3.1. Gene Expression Analysis
To study the role of MtERF1 in salt stress response, we examined the expression of MTERF1 in 21-day-old lines grown on a mixture of sand and compost (3:1. v/v) treated with 100 mM NaCl for 30 days. ANOVA showed that the variation in salt stress response was explained by the effects of line, tissue treatment, the interactions line × tissue, line × treatment, tissue × treatment, line × tissue × treatment. The maximum effect was observed for the treatment factor (Table 5).

Table 5.
Effects of line, tissue, treatment, the interaction line × tissue, the interaction line × treatment, the interaction tissue × treatment, and the interaction line × tissue × treatment on the expression of MtERF1 gene for studied lines of M. truncatula under control treatment and 100 mM NaCl.
The spatial expression pattern of MtERF1, was determined by analyzing the expression profiles of MtERF1 in three different organs: roots, stems, and leaves by qRT-PCR. Results showed that MtERF1 can be detected in all tissues of M. truncatula, but with different expression levels (Table 6). MtERF1 was mainly expressed in roots and the leaves. The highest induction was observed in roots under salt stress in all lines except in the sensitive one (TN.6.18) (Figure 5). In stems, only A10 showed a significant expression profile of MtERF1, and this may explain why only the length of stems was not correlated with any morphological traits.

Table 6.
Expression analysis of MtERF1 for the four lines of M. truncatula under control treatment and 100 mM NaCl.
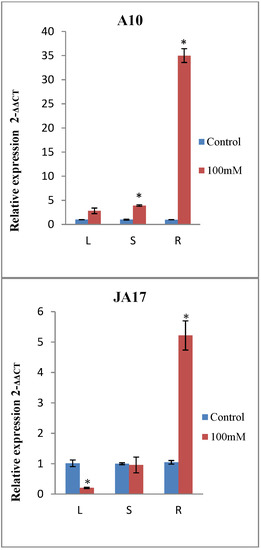
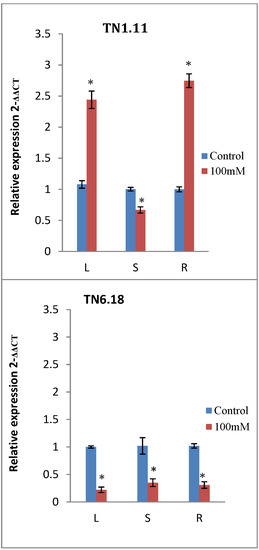
Figure 5.
Expression analysis of MtERF1 in different tissues of the four lines of M. truncatula under control treatment and 100 mM NaCl. Leaves (L), stems (S) and roots (R). Asterisks indicate significant differences between treatments as estimated by ANOVA (* P ≤ 0.05).
To determine the short-term response of MtERF1 to salt stress, we analyze the relative transcript levels after 6 and 24 h (Figure 6 and Figure 7). The abundance of MtERF1 increased about 14 times by salt treatment compared with the control after 6 h (Figure 7).
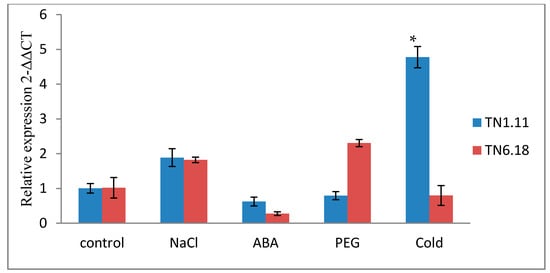
Figure 6.
Expression analysis of MtERF1 of two lines of M. truncatula TN1.11 and TN6.18 under different treatments: control, 200 mM NaCl, 10 µM ABA, 20% PEG, and cold 4 °C after 24 h. A representative example out of three biological replicates is shown, the error bars signify standard error and the asterisks as estimated by ANOVA (* P ≤ 0.05).
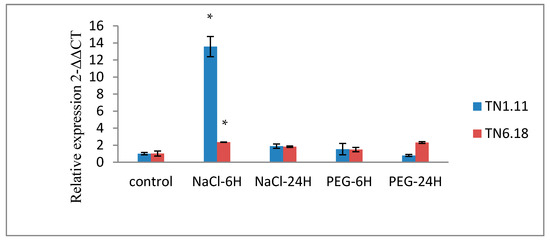
Figure 7.
Expression analysis of MtERF1 of two lines of M. truncatula TN1.11 and TN6.18 under different treatment: control, 200 mM NaCl and 20% PEG after 6 and 24 h. The error bars signify standard error in data from three independent experiments and the asterisks as estimated by ANOVA (* P ≤ 0.05).
For the line A10, MtERF1 gene expression showed a significant increase in stems and roots under NaCl treatment, but the highest level of expression was detected in roots (Figure 5).
As shown in Figure 5, salt treatment upregulated the expression of MtERF1 in leaves and roots for the line TN1.11, while a downregulation was observed in the stems.
In the case of JA17, MtERF1 gene expression decreased by the NaCl treatment in leaves, whereas a sharp increase was observed in roots.
For TN6.18, a strong reduction in the expression of MtERF1 in all examined tissues was observed by the application of salt treatment suggesting that TN6.18 was more sensitive to salt stress than the others lines (Figure 5).
2.3.2. Expression Analysis of MtERF1 under Abiotic Stresses and ABA Treatment
The expression profiles of MtERF1 by qRT-PCR showed a significant induction by cold and salt treatment (200 mM) for the tolerant line TN1.11 (Table 7) after 24 h for cold treatment (Figure 6) and after 6 h for salt treatment (Figure 7), which mean the implication of MtERF1 in early salt stress responses by providing initial protection and amplification of signals. However, under the 20% PEG treatment, the upregulation of MtERF1 was only detected for the line TN6.18, but it was not significant. Moreover, for the 10 µM ABA treatment, the expression levels were downregulated for both lines after 24 h (Figure 6).

Table 7.
ANOVA test for the analysis of MtERF1 of two lines of M. truncatula TN1.11 and TN6.18 under different treatment: control, 200 mM NaCl and 20% PEG (after 6 and 24 h), 10 µM ABA and 4 °C (after 24 h).
As shown in Figure 7, the amounts of the MtERF1 transcripts significantly increased after 6 h of 200 mM NaCl treatment in both lines. However, they increased to 14-fold higher than the control plants grown under normal conditions for the tolerant line TN1.11. Hence, the result indicates a rapid response of MtERF1 to salt stress because the accumulation decreases after 24 h of salt treatment.
Taken together, these results suggest that TN1.11 is a salt-tolerant line of Medicago truncatula and MtERF1 may play a role in regulating root growth and could be a marker gene for salt tolerance.
3. Discussion
3.1. Morphological and Photosynthetic Characteristics Variation
Reductions in the biomass under salt stress were indicative of severe growth limitations. Salinity had many effects not only on the biomass, but also on other morphological parameters, such as plant height, number of leaves, and root length salinity was reported to reduce shoot and root weights [47,48,49,50,51,52,53]. Similarly, in the present study, we recorded reduced growth during stress conditions. Our results revealed that the 100 mM NaCl stress treatment has caused reduction of the biomass in all lines, but more pronounced reduction was found for the sensitive line TN6.18.
The broad-sense heritability (H2) for the traits measured showed high values. This high heritability found for most analyzed traits may be explained by a large genetic variance rather than by a smaller environmental variance, as already was suggested by Barton and Turelli (1989) [54] and by Badri et al. (2016) [55]. Fitness components are generally less heritable than morphological or physiological characters [56].
3.2. Biochemical Characterization
At the physiological level, accumulation of osmolytes acting as osmoprotectants, such as proline, glycine betaine, soluble proteins, and soluble sugars, is a strategy to overcome osmotic stress provoked by salinity [57,58]. These osmoprotectants are essential to maintain cellular osmotic balance, detoxification of reactive oxygen species, maintenance of membrane integrity and stabilization of proteins [59].
In our study, two osmoprotectants (soluble proteins and soluble sugars) were measured in leaves. The line TN1.11 was found to be more salt tolerant than other lines. This was reflected by the increase in soluble proteins and soluble sugar. The increase in soluble protein content under stress may be the result of enhanced synthesis of specific stress-related proteins [59]. Furthermore, soluble sugars act as important osmolytes to maintain the cell homeostasis [60].
MDA is the principal product of polyunsaturated fatty acid peroxidation, which acts as toxic molecule and biological marker of oxidative stress [61]. The amount of MDA represents the degree of cell membrane damage under salt stress and is a common physiological indicator in evaluation of salt tolerance [61,62,63].
Previous studies have shown that the capacity to prevent membrane damage is correlated with the stress tolerance of plants [11,64]. The accumulation of MDA in salt treated M. truncatula implied clearly that the plants were suffering from stress. This high accumulation of MDA in M. truncatula made these plants more susceptible to oxidative damage under the conditions of abiotic stresses [65]. The low concentration of MDA and the stability of membrane integrity in TN1.11 suggest that this line was more protected against the oxidative stress than the other lines.
3.3. Expression Analysis of MtERF1 under Abiotic Stresses ABA Treatment
The AP2/ERF superfamily is one of the largest groups of the transcription factor family in plants which plays an important role in the regulation of plant development and tolerance to biotic and abiotic stresses [38]. It is considered as one of the most important families of gene regulators in plants [66].
ERFs, which contain an AP2 DNA-binding domain, form a plants specific superfamily of 123 transcriptional factors in M. truncatula [37], and play an important role in the transcriptional regulation of a variety of abiotic and biotic stress responses.
In previous studies, ERF subfamily gene expression levels were found to be higher than those of AP2 subfamily members, which may be related to the number of introns [67]. The gene expression rate is accelerated, leading to higher expression levels. when the number of introns is small [68]. Genes in the ERF subfamily are either devoid of introns or had only one to two introns [67]. In this study, a member of the AP2/ERF transcription factors, MtERF1, which is constituted of one exon, was studied under salt stress.
The MtERF1 gene was inducible by salt treatment and cold, indicating the involvement of this transcription factors in abiotic stress responses, demonstrated previously in several studies [68,69,70,71,72].
Moreover, ERF1 was rapidly induced by salt stress within few hours (6 h) in the model legume M. truncatula, which is in agreement with previous studies realized in several plant species, such as A. thaliana [73,74,75,76], tomato [77], L. japonicus [78], and moss [79], which support the strong implication of TFs in early salt stress responses. It has been speculated that the early responsive genes may provide initial protection and amplification of signals [80].
Accumulating evidence indicates that AP2/ERF genes have different expression patterns in different organs, and play roles in regulating plant growth and organs development. In the current study, MtERF1 expression was found to be most abundant in roots compared to leaves and stems, similarly to previous studies [36,79,81]. This organ/tissue-differentiated expression may be a consequence of differences in the existence/abundance of regulatory proteins that interact with cis-acting elements or other transcriptional/post-transcriptional regulators in the various organs/tissues.
3.4. MtERF1 Promoter Analysis of Cis-Acting Elements
Analysis of promoters provides useful information to understand the upstream transcription factors that govern the tightly controlled regulation of ERF genes [82]. In the past two decades, a large number of research studies on the regulation of ERFs have revealed a complex network of different transcription factors involved in their regulation, and the ERF promoters have been proposed to be the central hubs that integrate multiple environmental and internal developmental signals [83]. Our results showed that promoter regions of the MtERF1 contained a certain number of TATA motif, CAAT motif, MYB, and MYC elements. We did not find obvious differences after ABA treatment although the promoter contained multiple ABREs and a large number of MYB/MYC binding sites, which can be explained by the fact that MYB and MYC factors act in an ABA-dependent manner at a later stage of stress responses to high-salt and water stresses and we had only analyzed the expression at 24 h (Figure 8) [83,84,85,86].
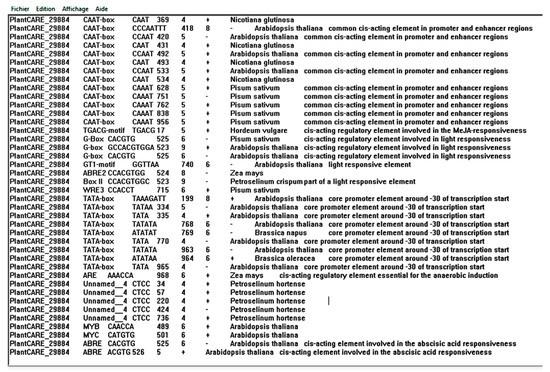
Figure 8.
Some cis-acting elements identified in the promotor using the PlantCARE search tool.
In brief, the large amount of stress-related cis-acting elements found in promoters of ERF subfamily genes supported their potential biological functions in regulating low-temperature stress and salt stress response in M. truncatula. In this study, qRT-PCR analysis of gene expression in response to ABA, PEG, cold and salt stresses showed that this gene did not respond significantly to ABA treatment, while it was induced by cold and salt stress and repressed by osmotic stress. These findings indicated that MtERF1 plays an active role in responses to cold and salt stress. Furthermore, Gruber et al. (2009) [87] reported that several TF are induced at much higher levels by salt stress than by other treatments, suggesting certain specificity of the salt response, which is similar to findings reported in this study [88].
4. Material and Methods
4.1. Plant Material and Culture Conditions
Four lines of M. truncatula were used. They include the two Tunisian lines TN1.11 and TN6.18, the Moroccan line A10, lines belonging to a collection of genotypes used in the M. truncatula HapMap project, and Jemalong A17 (JA17) from the Australian collection, which was used to obtain the reference genome for M. truncatula. Three biological replicates were used in all analysis.
Seeds were scarified using sandpaper q60, then were kept for 72 h in the dark at 4 °C and thereafter transferred at 21 °C during 24 h for germination. The germinated seeds were sown into black pots with a two liters capacity filled with a mixture of sand and compost (3:1. v/v). Plants were grown in a growth chamber under controlled conditions at a temperature of 24 °C/18 °C (day/night), a relative humidity of 60–80%, and a photoperiod of 16/8 h. Plants were irrigated with Fahräeus nutritive solution [89] once every 2 days, and after 21 days they were randomly divided into two groups one for the control treatment and the other for salt stress (100 mM NaCl).
4.2. Morphological and Photosynthetic Parameters
Fourteen parameters were measured for the studied lines including the length of stems (LS, cm), length of roots (LR, cm), number of leaves (NL), number of axes (NA), aerial fresh weight (AFW, g), aerial dry weight (ADW, g), root fresh weight (RFW, g), root dry weight (RDW, g), root dry weight and aerial dry weight ratio (RDW/ADW), relative water content (RWC, %), water content (WC), and the relative growth rate (RGR), chlorophyll a (Chla, mg.g−1 FW), and chlorophyll b (Chlb, mg.g−1 FW) content.
The relative water content (RWC) was calculated as follows: RWC (%) = 100 [(LFW−LDW)/(LTW−LDW)], where LFW is the leaf fresh weight, LDW is the leaf dry weight, and LTW is the leaf turgid weight. Briefly, the fresh weight of a young leaf is determined. The leaves were left floating on distilled water, in Petri dishes, for 24 h and the turgid weight is then recorded. After that, the leaf tissues were dried in an oven at 70 °C for 24 h and their dry weight was measured.
Relative growth rate (RGR) or the mean relative growth rate was determined as the rate of increase in total dry weight per unit of plant weight according to Hunt (1982) thus:
RGR = (In W2 − In W1/t2 − t1).
RGR in g.g−1 day−1,
where W is the total plant weight (g), t is the time (days), and the subscripts 1 and 2 are initial and final harvest of biomass yield.
4.3. MDA Determination
The extent of lipid peroxidation was estimated by determining the concentration of malondialdehyde [90]. Briefly, 150 mg of fresh leaves were homogenized in TCA (0.1%) and centrifuged (13,000× g, 20 min) at 4 °C. Thereafter, an aliquot of the supernatant was added to 0.5% (w/v) TBA in 20% (w/v) TCA and the mixture was incubated at 95 °C for 30 min. The reaction was stopped by transferring tubes to ice for 10 min followed by a centrifugation step at 10,000× g for 10 min. The absorbance of supernatant was measured at 532 nm and the value for non-specific absorption at 600 nm was subtracted. The concentration of MDA was determined from the extinction coefficient of 155 mmol.L−1cm−1. Three independent extractions were made for each sample.
4.4. Measurement of Soluble Sugars Content
Soluble sugar content was quantified the method described by Yemm and Willis (1954) [91]. Briefly, 25 mg of dry weight of leaves was homogenized with 5 mL of 80% ethanol for 30 min at 70 °C. The extract was separated by centrifugation at 6000× g for 15 min. A second extraction was carried out under the same conditions as above. Then, the soluble sugars content of the aqueous extract was determined using the sulfuric acid anthrone colorimetric method. The soluble sugar content was revealed through absorbance measurement at 640 nm. The standard curve was drawn using glucose (1 to 14 µg/mL).
4.5. Measurement of Soluble Proteins
Fresh leaf material (150 mg) was homogenized with 2 mL buffer (1 M Tris-HCl pH8, 0.5 M EDTA, 100 mM PMSF. The homogenate was then centrifuged for 30 min at 12,000× g at 4 °C. The protein concentration in the supernatant was measured by the protein assay using BSA as a standard [92].
4.6. Gene Expression Assay by Quantitative Real-Time (RT-qPCR)
The coding sequence of MtERF1 was identified from the National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/ (accessed on 15 August 2016)), its accession number is XM_003607935. Then this sequence was blasted against the M. truncatula genome with expected values ≤ (1 × 10−5). Finally, these proteins were submitted to the NCBI batch web CD-search tool (http://www.ncbi. nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi, (accessed on 20 August 2016)). Pfam (http://pfam.sanger.ac.uk/ (accessed on 3 March 2021)), and SMART (http://smart.embl-hei delberg.de/ (accessed on 3 March 2021)) to confirm the presence and completeness of the AP2 domain and the primers were used to amplify the CDS regions of MtERF1 and then it was sequenced to be sure if it is the right sequence.
To determine the expression pattern of MtERF1 in M. truncatula we analyzed its expression in three different organs: roots, stem, and leaves by real-time quantitative PCR (qRT-PCR) using MtERF1 specific primers. Total RNA was extracted from the different tissues of the four studied lines according to the procedures described by [93]. The concentration and purity of the total RNA were assessed with a NanoDrop-ND 1000 UV-Vis Spectrophotometer (NanoDrop Technologies, Auckland, New Zealand), using 1 μL of total RNA. RNA purity was estimated from the A260/A280 absorbance ratio. RNA samples were treated with recombinant RNase-free DNase I (Roche, Mannheim, Germany) for removing possible genomic DNA contamination. Then, 1 µg of each RNA extract was used to synthesize cDNA by using the iScript™. Reverse Transcription Supermix for RT-qPCR (Bio-Rad, Marnes-la-Coquette, France), according to the manufacturer’s protocol. Gene-specific primer pairs for RT-qPCR were designed with Primer 3 software (Whitehead Instutute for Biomedical Researech, Cambridge, MA, USA). Each gene was evaluated at least in two independent runs. Each sample was amplified in biological triplicate by quantitative qRT-PCR using a Roche 2.0 Real-Time PCR Detection System with SYBR Green (Roche, Mannheim, Germany). The cycle program used was as follows: Initial denaturation at 50 °C for 2 min. then 95 °C at 30 s. followed by 2 cycles for 1 min at 95 °C. then 10 min at 95 °C followed by 40 cycles at 95 °C (10 s) and 60 °C (30 s) followed by 65 °C for 15 s. The amplification of the Actin gene was used as the normalization control. The mRNA fold difference was relative to that of untreated samples used as calibrator. The relative quantification value for MtERF1 was calculated by the 2−ΔΔCT method.
4.7. Promoter Prediction and Analysis for MtERF1
In order to gain insight into the transcriptional regulation of MtERF1 gene, putative stress- or hormone-responsive cis-acting elements were identified in 1 kb lengths of M. truncatula DNA sequence located immediately 5′ to the gene coding sequence using the PlantCARE search tool (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 15 August 2019)).
4.8. Treatment with Various Abiotic Stress
We examined the expression profiles of MTERF1 genes by qRT-PCR as described above under ABA and abiotic stress treatment, including cold, NaCl, and PEG only for the two local lines TN1.11 and TN6.18.
The three additional abiotic elicitors which were used are: osmotic stress (PEG 20%), cold (4 °C) and ABA (10 µM). For the treatment with salt, PEG, and ABA, 21-day-old seedlings of M. truncatula were transferred to Fahräeus nutritive solution containing 200 mM NaCl or 20% PEG and incubated for 6 h and 24 h. For ABA treatment, the solution containing 10 µM ABA and incubated for 24 h. For cold treatment, 21-day-old seedlings of M. truncatula grown in soil were placed in a low temperature chamber at 4 °C for 24 h.
4.9. Statistical Analyses
A three-way analysis of variance was used to test for line, salt treatment differences, and line × treatment interaction effects. Only characters that showed a significant line × treatment interaction were used for further analysis. Means were compared using Duncan’s multiple range test at 5%. To find out whether or not traits are correlated to each other, Pearson coefficients were calculated. The significance level of associations between morphological traits and photosynthetic parameters was set to 0.05. All analyses were performed using SPSS software (SPSS Inc. Released 2007 SPSS for Windows. Version 16.0. Chicago, IL, USA).
Broad-sense heritability (H2) for each trait was estimated as described by Badri et al. (2016) [94].
where σ2 g is the genetic variance observed between the lines and σ2 e is the environmental variance corresponding to the residual error between the eight replicates of the same genotype (=line).
H2 = σ2 g/σ2 g+ σ2 e
To investigate the stratification of the lines, salt sensitivity index (SSI) for measured traits in lines of M. truncatula grown under salt stress were subjected to principal component analysis (PCA) and hierarchical cluster analysis (HCA). This analysis was carried out using XLSTAT software (Version 2014.5.03. Addinsoft, Paris, France).
5. Conclusions
Associations were discovered between exhibited phenotypes, lipid peroxidation, soluble proteins, and sugar accumulation, and gene expression. Salt tolerance seems to be related to lower MDA accumulation and a lower biomass reduction. Besides, accumulation of soluble proteins and soluble sugars was linked to salt-tolerance, being much higher for the tolerant genotype.
The expression pattern of MTERF1 supports the fact that this gene is involved in mechanisms associated with salt tolerance and could be considered as a marker to discriminate Medicago genotypes with differing tolerance to salinity. Our expression profiling analysis of the MtERF1 in various tissues and under different abiotic stresses should facilitate the identification of appropriate candidate genes for further functional characterization. The information obtained in this study showed that the MTERF1 gene is strongly induced by salt in root tissues. Thus. MTERF1 could be helpful in selecting candidate genotypes to be used by crop growers in salty areas or as progenitors in breeding programs for salt tolerance.
Author Contributions
Conceptualization, S.H., L.H., M.H., A.M., H.B.J., N.L., M.T.S.-B., C.A., and M.B.; data curation, S.H., M.H., and M.B.; writing—original draft preparation, S.H.; writing—review and editing, S.H., L.H., M.H., I.R., N.L., M.T.S.-B., and M.B.; supervision, C.A., and M.B.; project administration, M.B.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Tunisian–South African project (2016–2017) and the Tunisian Ministry of Higher Education and Scientific Research (CBBC02 LR15).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, W.; Jiang, W.; Liu, J.; Li, Y.; Gai, J. Genome-wide characterization of the aldehyde dehydrogenase gene superfamily in soybean and its potential role in drought stress response. BMC Genom. 2017, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Yin, X. Effects of salinity, growth condition, and soil type on shoot dry matter of Alfalfa (Medicago sativa)—A meta-analysis. Commun. Soil Sci. Plant Anal. 2021, 52, 201–211. [Google Scholar] [CrossRef]
- Mbarki, S.; Skalicky, M.; Vachova, P.; Hajihashemi, S.; Jouini, L.; Zivcak, M.; Tlustos, P.; Brestic, M.; Hejnak, V.; Khelil, A.Z. Comparing Salt Tolerance at Seedling and Germination Stages in Local Populations of Medicago ciliaris L. to Medicago intertexta L. and Medicago scutellata L. Plants 2020, 9, 526. [Google Scholar] [CrossRef]
- Yao, W.; Wang, L.; Zhou, B.; Wang, S.; Li, R.; Jiang, T. Over-expression of poplar transcription factor ERF76 gene confers salt tolerance in transgenic tobacco. J. Plant Physiol. 2016, 198, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, F.; Iqbal, M.; Ashraf, M.A.; Ali, M. Foliar applied fullerol differentially improves salt tolerance in wheat through ion compartmentalization, osmotic adjustments and regulation of enzymatic antioxidants. Physiol. Mol. Biol. Plants 2020, 26, 475–487. [Google Scholar] [CrossRef]
- Zafar, S.; Hasnain, Z.; Anwar, S.; Perveen, S.; Iqbal, N.; Noman, A.; Ali, M. Influence of melatonin on antioxidant defense system and yield of wheat (Triticum aestivum L.) genotypes under saline condition. Pak. J. Bot. 2019, 51, 1987–1994. [Google Scholar] [CrossRef]
- Sandhu, D.; Kaundal, A.; Acharya, B.R.; Forest, T.; Pudussery, M.V.; Liu, X.; Ferreira, J.F.S.; Suarez, D.L. Linking diverse salinity responses of 14 almond rootstocks with physiological, biochemical, and genetic determinants. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Sandhu, D.; Cornacchione, M.V.; Ferreira, J.F.S.; Suarez, D.L. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Understanding and Improving Salt Tolerance in Plants. Crop Sci. 2005, 45, 437–448. [Google Scholar]
- Doganlar, Z.B.; Demir, K.; Basak, H.; Gul, I. Effects of salt stress on pigment and total soluble protein contents of three different tomato cultivars. Afr. J. Agr. Res. 2010, 5, 2056–2065. [Google Scholar] [CrossRef]
- Sairam, R.K.; Tyagi, A. Physiology and Molecular Biology of Stress Tolerance in Plants; Springer: New York, NY, USA, 2006. [Google Scholar]
- Thouin, J.; Guo, M.Y.; Zribi, I.; Pauly, N.; Mouradi, M.; Ghoulam, C.; Sentenac, H.; Véry, A.A. The Medicago truncatula HKT family: Ion transport properties and regulation of expression upon abiotic stresses and symbiosis. bioRxiv 2019. [Google Scholar] [CrossRef]
- Niu, Y.; Hu, T.; Zhou, Y.; Hasi, A. Isolation and characterization of two Medicago falcate AP2/EREBP family transcription factor cDNA, MfDREB1 and MfDREB1s. Plant Physiol. Biochem. 2010, 48, 971–976. [Google Scholar] [CrossRef]
- Ohme-takagi, M.; Shinshi, H. Ethylene-inducible DNA binding proteins that interact with an ethylene responsive element. Plant Cell 1995, 7, 173–182. [Google Scholar] [PubMed]
- Ohta, M.; Ohme-Takagi, M.; Shinshi, H. Three ethylene-responsive transcription factors in tobacco with distinct transactivation functions. Plant J. 2000, 22, 29–38. [Google Scholar] [CrossRef]
- Živko, J.; Nemanja, S.; Aleksandar, M.; Svetlana, R.; Vesna, M. The expression of drought responsive element binding protein (DREB2A) related gene from pea (Pisum sativum L.) as affected by water stress. Aust. J. Crop Sci. 2013, 7, 1590–1596. [Google Scholar]
- Mitsuda, N.; Ohme-Takagi, M. Functional Analysis of Transcription Factors in Arabidopsis. Plant Cell Physiol. 2009, 50, 1232–1248. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Jha, B. Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol. Plant. 2010, 54, 201–212. [Google Scholar] [CrossRef]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014, 217–218, 109–119. [Google Scholar] [CrossRef]
- Erpen, L.; Devi, H.S.; Grosser, J.W.; Dutt, M. Potential use of the DREB/ERF, MYB, NAC and WRKY transcription factors to improve abiotic and biotic stress in transgenic plants. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 132, 1–25. [Google Scholar] [CrossRef]
- Pardo, J.M. Biotechnology of water and salinity stress tolerance. Curr. Opin. Biotechnol. 2010, 21, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Djemal, R.; Khoudi, H. Isolation and molecular characterization of a novel WIN1/SHN1 ethylene-responsive transcription factor TdSHN1 from durum wheat (Triticum turgidum L. subsp. durum). Protoplasma 2015, 252, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Charfeddine, M.; Saïdi, M.N.; Charfeddine, S.; Hammami, A.; Bouzid, R.G. Genome-Wide Analysis and Expression Profiling of the ERF Transcription Factor Family in Potato (Solanum tuberosum L.). Mol. Biotechnol. 2014, 57, 348–358. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-Wide Analysis of the ERF Gene Family. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef]
- Licausi, F.; Giorgi, F.M.; Zenoni, S.; Osti, F.; Pezzotti, M.; Perata, P. Genomic and transcriptomic analysis of the AP2/ERF superfamily in Vitis vinifera. BMC Genom. 2010, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, D.; Chen, A.; Tang, H.; Li, J.; Huang, S. RNA-seq for comparative transcript profiling of kenaf under salinity stress. J. Plant Res. 2016, 130, 365–372. [Google Scholar] [CrossRef]
- Zhao, X.J.; Lei, H.J.; Zhao, K.; Yuan, H.Z.; Li, T.H. Isolation and characterization of a dehydration responsive element binding factor MsDREBA5 in Malus sieversii Roem. Sci. Hortic. 2012, 142, 212–220. [Google Scholar] [CrossRef]
- Dong, L.; Cheng, Y.; Wu, J.; Cheng, Q.; Li, W.; Fan, S.; Jiang, L.; Xu, Z.; Kong, F.; Zhang, D.; et al. Overexpression of GmERF5, a new member of the soybean EAR motif-containing ERF transcription factor, enhances resistance to Phytophthora sojae in soybean. J. Exp. Bot. 2015, 66, 2635–2647. [Google Scholar] [CrossRef]
- Seo, Y.J.; Park, J.B.; Cho, Y.J.; Jung, C.; Seo, H.S.; Park, S.K.; Nahm, B.H.; Song, J.T. Overexpression of the ethylene-responsive factor gene BrERF4 from Brassica rapa increases tolerance to salt and drought in Arabidopsis plants. Mol. Cells 2010, 30, 271–277. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukuda, K.; Yang, Y.; Li, R.; Shi, F.; Fukuda, K.; Yang, Y. Effects of salt and alkali stresses on germination growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 2011, 56, 725–733. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 2010, 73, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K. Genome-Wide Identification and Testing of Superior Reference Genes for Transcript Normalization. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, X.; Stellwag, E.J. Identification of soybean microRNAs and their targets. Planta 2008, 229, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Narsai, R.; Ivanova, A.; Ng, S.; Whelan, J. Defining reference genes in Oryza sativa using organ, development, biotic and abiotic transcriptome datasets. BMC Plant Biol. 2010, 10, 56. [Google Scholar] [CrossRef]
- Eshu, Y.; Eliu, Y.; Ezhang, J.; Esong, L.; Eguo, C. Genome-Wide Analysis of the AP2/ERF Superfamily Genes and their Responses to Abiotic Stress in Medicago truncatula. Front. Plant Sci. 2016, 6, 1247. [Google Scholar] [CrossRef]
- Guo, B.; Wei, Y.; Xu, R.; Lin, S.; Luan, H.; Lv, C.; Zhang, X.; Song, X.; Xu, R. Genome-Wide Analysis of APETALA2/Ethylene-Responsive Factor (AP2/ERF) Gene Family in Barley (Hordeum vulgare L.). PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Li, X.; Gao, F.; Feng, J.; Zhou, Y. Characterization of the AP2/ERF Transcription Factor Family and Expression Profiling of DREB Subfamily under Cold and Osmotic Stresses in Ammopiptanthus nanus. Plants 2020, 9, 455. [Google Scholar] [CrossRef]
- Min, X.; Jin, X.; Liu, W.; Wei, X.; Zhang, Z.; Ndayambaza, B.; Wang, Y. Transcriptome-wide characterization and functional analysis of MATE transporters in response to aluminum toxicity in Medicago sativa L. PeerJ 2019, 7, 1–21. [Google Scholar] [CrossRef]
- Staniak, M.; Bojarszczuk, J.; Księżak, J. Changes in yield and gas exchange parameters in Festulolium and alfalfa grown in pure sowing and in mixture under drought stress. Acta Agric. Scand. Sect. B Plant Soil Sci. 2018, 68, 255–263. [Google Scholar] [CrossRef]
- Thomas, J.C.; Sepahi, M.; Arendall, B.; Bohnert, H.J. Enhancement of seed germination in high salinity by engineering mannitol expression in Arabidopsis thaliana. Plant Cell Environ. 1995, 18, 801–806. [Google Scholar] [CrossRef]
- Wang, Z.; Hopkins, A.; Mian, R. Forage and turf grass biotechnology. Crit. Rev. Plant Sci. 2001, 20, 573–619. [Google Scholar] [CrossRef]
- Young, N.D.; Debellé, F.; Oldroyd, G.E.D.; Geurts, R.; Cannon, S.B.; Udvardi, M.K.; Benedito, V.A.; Mayer, K.F.X.; Gouzy, J.; Schoof, H.; et al. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nat. Cell Biol. 2011, 480, 520–524. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Broeckling, C.D.; Blancaflor, E.B.; Sledge, M.K.; Sumner, L.W.; Wang, Z.Y. Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). Plant J. 2005, 42, 689–707. [Google Scholar] [CrossRef] [PubMed]
- Ané, J.M.; Zhu, H.; Frugoli, J. Recent Advances in Medicago truncatula Genomics. Int. J. Plant Genom. 2008, 2008, 1–11. [Google Scholar] [CrossRef]
- Yousef, A.N.; Sprent, J.I. Effects of NaCl on Growth, Nitrogen Incorporation and Chemical Composition of Inoculated and NH4NO3 Fertilized Viciafaba (L.) Plants. J. Exp. Bot. 1983, 34, 941–950. [Google Scholar] [CrossRef]
- Zahran, H.H.; Sprent, J.I. Effects of sodium chloride and polyethylene glycol on root-hair infection and nodulation of Vicitt faba L. plants by Rhizobium leguminosarum. Planta 1986, 3, 303–309. [Google Scholar] [CrossRef]
- Wignarajah, K. Growth response of phaseolus vulgaris to varying salinity regimes. Environ. Exp. Bot. 1990, 30, 141–147. [Google Scholar] [CrossRef]
- Aras, S.; Eşitken, A.; Karakurt, Y. Morphological and physiological responses and some WRKY genes expression in cherry rootstocks under salt stress. Span. J. Agric. Res. 2020, 17, e0806. [Google Scholar] [CrossRef]
- Farooq, M.; Rehman, A.; Al-Alawi, A.K.; Al-Busaidi, W.M.; Lee, D.J. Integrated use of seed priming and biochar improves salt tolerance in cowpea. Sci. Hortic. 2020, 272, 109507. [Google Scholar] [CrossRef]
- Kim, J.J.; Park, S.I.; Kim, Y.H.; Park, H.M.; Kim, Y.S.; Yoon, H.S. Overexpression of a proton pumping gene OVP1 enhances salt stress tolerance, root growth and biomass yield by regulating ion balance in rice (Oryza sativa L.). Environ. Exp. Bot. 2020, 175, 104033. [Google Scholar] [CrossRef]
- Qureshi, A.S.; Daba, A.W. Evaluating Growth and Yield Parameters of Five Quinoa (Chenopodium quinoa W.) Genotypes Under Different Salt Stress Conditions. J. Agric. Sci. 2020, 12, 128. [Google Scholar] [CrossRef]
- Barton, N.H.; Turelli, M. Evolutionary quantitave genetics: How little do we know? Rev. Genet. 1989, 23, 337–370. [Google Scholar] [CrossRef] [PubMed]
- Badri, M.; Mahfoudh, S.; Abdelly, C. Morph-physiological responses to water deficit in parental genotypes of Medicago truncatula recombinant inbred lines. Afr. J. Biotechnol. 2016, 15, 1339–1349. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Roff, D.A. Natural selection and the heritability of fitness components. Heredity 1987, 59, 181–197. [Google Scholar] [CrossRef]
- Khan, F.M.; Berentzen, I.; Berczik, P.; Just, A.; Mayer, L.; Nitadori, K.; Callegari, S. Formation and hardening of supermassive black hole binaries in minor mergers of disk galaxies. Astrophys. J. 2012, 756, 30. [Google Scholar] [CrossRef]
- Abdel, A.; Abdel, H.; He, L. Does Inoculation with Glomus mosseae Improve Salt Tolerance in Pepper Plants? J. Plant Growth Regul. 2014, 33, 644–653. [Google Scholar] [CrossRef]
- Yancey, P.H.; Clark, M.E.; Hand, S.C.; Bowlus, R.D.; Somero, G.N. Living with Water Stress: Evolution of Osmolyte Systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef]
- Gupta, A.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Boughanmi, N.; Michonneau, P.; Daghfous, D.; Fleurat-Lessard, P. Adaptation ofMedicago sativacv. Gabès to long-term NaCl stress. J. Plant Nutr. Soil Sci. 2005, 168, 262–268. [Google Scholar] [CrossRef]
- Peuthert, A.; Chakrabarti, S.; Pflugmacher, S. Uptake of Microcystins-LR and -LF (Cyanobacterial Toxins) in Seedlings of Several Important Agricultural Plant Species and the Correlation with Cellular Damage (Lipid Peroxidation). Environ. Toxicol. 2007, 22, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Song, Y.; Ji, D.; Li, S.; Wang, P.; Li, Q.; Xiang, F. The Dynamic Changes of DNA Methylation and Histone Modifications of Salt Responsive Transcription Factor Genes in Soybean. PLoS ONE 2012, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta Bioenerg. 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Xu, L.; Feng, G.; Yang, Z.; Xu, X.; Huang, L.; Yang, Q.; Zhang, X. Genome-wide AP2/ERF gene family analysis reveals the classification, structure, expression profiles and potential function in orchardgrass (Dactylis glomerata). Mol. Biol. Rep. 2020, 47, 5225–5241. [Google Scholar] [CrossRef]
- Krizek, B. AINTEGUMENTA and AINTEGUMENTA-LIKE6 Act Redundantly to Regulate Arabidopsis Floral Growth and Patterning. Plant Physiol. 2009, 150, 1916–1929. [Google Scholar] [CrossRef]
- Aharoni, A.; Dixit, S.; Jetter, R.; Thoenes, E.; Van Arkel, G.; Pereira, A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Plant Cell 2004, 16, 2463–2480. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, Y.S.; Kwon, C.W.; Park, H.K.; Jeong, J.S.; Kim, J.K. Overexpression of the Transcription Factor AP37 in Rice Improves Grain Yield under Drought Conditions. Plant Physiol. 2009, 150, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Ai, X.; Xu, F.; Quan, T.; Liu, S.; Xia, G. Isolation and characterization of a bread wheat salinity responsive ERF transcription factor. Gene 2012, 511, 38–45. [Google Scholar] [CrossRef]
- Rong, W.; Qi, L.; Wang, A.; Ye, X.; Du, L.; Liang, H.; Xin, Z.; Zhang, Z. The ERF transcription factor TaERF3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol. J. 2014, 12, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Kreps, J.A.; Wu, Y.; Chang, H.; Zhu, T.; Wang, X.; Harper, J.F. Transcriptome Changes for Arabidopsis in Response to Salt, Osmotic, and Cold Stress. Plant Physiol. 2002, 130, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Narusaka, M.; Ishida, J.; Nanjo, T.; Fujita, M.; Oono, Y.; Kamiya, A.; Enju, A.; Sakurai, T.; Satou, M. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses using full-length cDNA microarray. Plant Cell 2001, 13, 61–72. [Google Scholar] [CrossRef]
- Jiang, Y.; Deyholos, M.K. Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. Plant Biol. 2006, 20, 1–20. [Google Scholar] [CrossRef]
- Ouyang, S.; Park, G.; Atamian, H.S.; Han, C.S.; Stajich, J.E.; Kaloshian, I.; Borkovich, K.A. MicroRNAs Suppress NB Domain Genes in Tomato That Confer Resistance to Fusarium oxysporum. PLoS Pathog. 2014, 10, e1004464. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.H.; Lippold, F.; Redestig, H.; Hannah, M.A.; Erban, A.; Krämer, U.; Kopka, J.; Udvardi, M.K. Integrative functional genomics of salt acclimatization in the model legume Lotus japonicus. Plant J. 2007, 53, 973–987. [Google Scholar] [CrossRef]
- Cuming, A.C.; Cho, S.H.; Kamisugi, Y.; Graham, H.; Quatrano, R.S.; Cuming, A.C. Microarray analysis of transcriptional responses to abscisic acid and osmotic, salt and drought stress in the moss, Physcomitrella Patens. New Phytol. 1997, 176, 275–287. [Google Scholar] [CrossRef]
- Ramanjulu, S.; Bartels, D. Drought- and desiccation-induced modulation of gene. Plant Cell Environ. 2002, 25, 141–151. [Google Scholar] [CrossRef]
- Rashid, M.; Guangyuan, H.; Guangxiao, Y.; Hussain, J.; Xu, Y. AP2/ERF Transcription Factor in Rice: Genome-Wide Canvas and Syntenic Relationships between Monocots and Eudicots. Evol. Bioinform. 2012, 8, 321–325. [Google Scholar] [CrossRef]
- Singhal, P.; Jan, A.T.; Azam, M.; Haq, Q.M.R. Plant abiotic stress: A prospective strategy of exploiting promoters as alternative to overcome the escalating burden. Front. Life Sci. 2015, 9, 52–63. [Google Scholar] [CrossRef]
- Jakoby, M.; Vicente-carbajosa, J. bZIP transcription factors in Arabidopsis. Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef]
- Urao, T.; Yamaguchi-shinozaki, K.; Urao, C.S. An Arabidopsis myb Homolog 1 s lnduced by Dehydration Stress and Its Gene Product Binds to the Conserved MYB Recognition Sequence. Plant Cell. 1993, 5, 1529–1539. [Google Scholar]
- Abe, H.; Yamaguchi-shinozaki, K.; Urao, T.; Hosokawa, C.D. Role of Arabidopsis MYC and MYB Homologs in Drought- and Abscisic Acid-Regulated Gene Expression. Plant Cell. 1997, 9, 1859–1869. [Google Scholar]
- Min, M.K.; Kim, R.; Hong, W.J.; Jung, K.H.; Lee, J.Y.; Kim, B.G. OsPP2C09 Is a Bifunctional Regulator in Both ABA-Dependent and Independent Abiotic Stress Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 393. [Google Scholar] [CrossRef]
- Gruber, V.; Blanchet, S.; Diet, A.; Zahaf, O.; Boualem, A.; Kakar, K.; Alunni, B.; Udvardi, M.; Frugier, F.; Crespi, M. Identification of transcription factors involved in root apex responses to salt stress in Medicago truncatula. Mol. Genet. Genom. 2008, 281, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.P.; Prasanna, H.C.; Kumari, N.; Mishra, P.; Singh, B. Understanding salt tolerance mechanism using transcriptome profiling and de novo assembly of wild tomato Solanum chilense. Sci. Rep. 2020, 10, 1–20. [Google Scholar] [CrossRef]
- Gosta, B.Y. The Infection of Clover Root Hairs by Nodule Bacteria Studied by a Simple Glass Slide Technique. J. Gen. Microbiol. 1957, 2, 374–381. [Google Scholar]
- Draper, H.H.; Hadley, M. Isolation of a Guanine-Malondialdehyde Adduct from Rat and Human Urine. Lipids 1990, 2, 82–85. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 197. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, T. RNA isolation from highly viscous samples rich in polyphenols and polysaccharides. Plant Mol. Biol. Rep. 2002, 20, 417. [Google Scholar] [CrossRef]
- Badri, M.; Toumi, G.; Mahfoudh, S.; Hessini, K.; Abdelguerfi-Laouar, M.; Abdelguerfi, A.; Aouani, M.E.; Abdelly, C.; Djébali, N. Diversity of Response to Drought in a Collection of Lines of Medicago truncatula, M. ciliaris, and M. polymorpha. Crop. Sci. 2016, 56, 3125–3132. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).