Abstract
Drought is a severe and complex abiotic stress that negatively affects plant growth and crop yields. Numerous genes with various functions are induced in response to drought stress to acquire drought stress tolerance. The phytohormone abscisic acid (ABA) accumulates mainly in the leaves in response to drought stress and then activates subclass III SNF1-related protein kinases 2 (SnRK2s), which are key phosphoregulators of ABA signaling. ABA mediates a wide variety of gene expression processes through stress-responsive transcription factors, including ABA-RESPONSIVE ELEMENT BINDING PROTEINS (AREBs)/ABRE-BINDING FACTORS (ABFs) and several other transcription factors. Seed plants have another type of SnRK2s, ABA-unresponsive subclass I SnRK2s, that mediates the stability of gene expression through the mRNA decay pathway and plant growth under drought stress in an ABA-independent manner. Recent research has elucidated the upstream regulators of SnRK2s, RAF-like protein kinases, involved in early responses to drought stress. ABA-independent transcriptional regulatory systems and ABA-responsive regulation function in drought-responsive gene expression. DEHYDRATION RESPONSIVE ELEMENT (DRE) is an important cis-acting element in ABA-independent transcription, whereas ABA-RESPONSIVE ELEMENT (ABRE) cis-acting element functions in ABA-responsive transcription. In this review article, we summarize recent advances in research on cellular and molecular drought stress responses and focus on phosphorylation signaling and transcription networks in Arabidopsis and crops. We also highlight gene networks of transcriptional regulation through two major regulatory pathways, ABA-dependent and ABA-independent pathways, that ABA-responsive subclass III SnRK2s and ABA-unresponsive subclass I SnRK2s mediate, respectively. We also discuss crosstalk in these regulatory systems under drought stress.
1. Introduction
Drought stress is an environmental factor that results in reduced plant growth and yield productivity. Understanding the mechanisms that mediate drought stress responses and tolerance in plants can help improve crop productivity under drought stress [1,2,3,4]. When plants sense drought stress, rapid transcription of stress-related genes occurs through molecular signal transduction, such as protein phosphorylation, in the early cellular response to drought stress. The phytohormone abscisic acid (ABA) plays fundamental roles in plant responses to drought stress by regulating stomatal closure and gene expression [5,6]. In seed plants, there are three subclasses of SNF1-RELATED PROTEIN KINASES 2 (SnRK2s). Among them, subclass III SnRK2s act as key positive regulators of ABA signaling downstream of ABA receptors [7]. Subclass III SnRK2s phosphorylate and activate several transcription factors, such as ABRE-BINDING PROTEINS (AREBs)/ABRE-BINDING FACTORS (ABFs), to induce the expression of stress-responsive genes in an ABA-dependent manner [8]. In addition to these ABA-activated subclass III SnRK2s, seed plants have another type of ABA-unresponsive subclass I SnRK2s [9]. Subclass I SnRK2s regulate functional proteins, including mRNA decay factors, to adjust transcriptional stability and mediate plant growth under drought and salinity stress in an ABA-independent pathway [9,10,11,12]. DEHYDRATION-RESPONSIVE ELEMENT (DRE)-BINDING PROTEIN 2A (DREB2A) mediates transcriptional changes to acquire stress resistance in ABA-independent pathways [3,13].
In this review, we summarize recent knowledge of how plants regulate intracellular signal and gene expression through two different phosphorylation signaling pathways, ABA-dependent or ABA-independent pathways, in drought stress responses. We also discuss crosstalk of these regulatory systems in drought stress responses.
2. Importance of ABA-Dependent Responses under Drought Stress
The phosphorylation process was shown to play important roles in ABA signaling because ABA INSENSITIVE 1 (ABI1) was identified as a major protein phosphatase 2C in ABA signaling. In 2002, Arabidopsis SNF1-RELATED PROTEIN KINASES 2 (SnRK2s) was shown to play important roles in ABA-responsive stomatal closure and ABA-dependent gene expression [14,15,16]. One of the SnRK2s, SRK2E/SnRK2.6/OST1, is expressed in guard cells and is important for ABA-induced stomatal closure in Arabidopsis. SnRK2s are classified into 3 subclasses, I, II and III, and have been shown to function in not only ABA-responsive regulation but also ABA-independent regulation of drought stress responses [17,18]. Subclass III SnRK2s play key roles in ABA-dependent signaling pathways. Subclass II SnRK2s are also involved in ABA-dependent pathways [19]. However, subclass I SnRK2s are not activated by ABA. Here, we focus on the key roles of subclass III SnRK2s and their upstream and downstream factors in ABA-dependent cellular signaling.
2.1. ABA-Dependent Phosphorylation Signaling in the Dehydration Stress Response: Important Roles of Subclass III SnRK2 Protein Kinases
In Arabidopsis, three subclass III SnRK2s, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, are mainly involved in ABA-dependent drought stress signaling. The srk2dei (snrk2.2/snrk2.6/snrk2.3) triple mutant lacking all subclass III SnRK2s shows severe ABA-insensitive viviparity, ABA-insensitive germination and a drought-sensitive phenotype [20,21,22,23]. These observations show the key roles of subclass III SnRK2s in ABA responses in diverse developmental regulatory processes, such as germination, seed maturation and drought tolerance. The PYRABACTIN RESISTANCE1/PYR1-LIKE/REGULATORY COMPONENTS OF ABA RECEPTOR (PYR/PYL/RCAR) family contains important ABA receptors that bind to ABA to regulate ABA responses [20,23,24,25,26]. Their role in ABA perception is established as shown below (Figure 1). In the absence of ABA, the kinase activity of subclass III SnRK2s is inhibited by group A PROTEIN PHOSPHATASES 2C (PP2Cs) [17]. When plants are subjected to drought stress, ABA accumulates in plant cells in large amounts, and then the ABA receptors PYR/PYL/RCAR bind to ABA. ABA-PYR/PYL/RCAR complexes competitively interact with PP2C and release SnRK2s [20,23,24,25,26]. The released SnRK2s are activated by autophosphorylation or phosphorylation by other protein kinases [17,27] (Figure 1). These three components, PYR/PYL/RCAR, PP2C and SnRK2, are now well known as core components in ABA sensing and signaling.
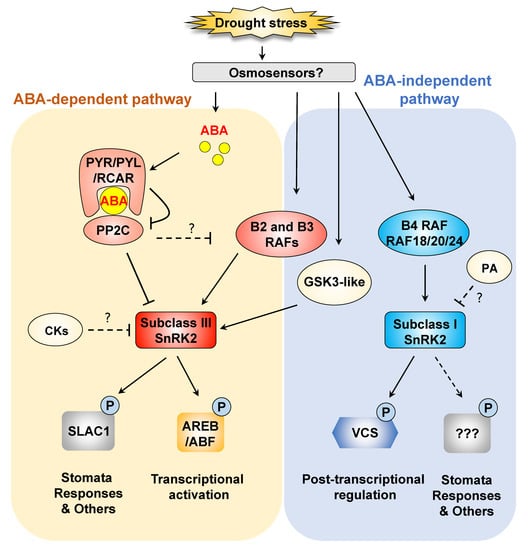
Figure 1.
SnRK2-mediated drought stress signaling and gene expression. Subclass III SNF1-related protein kinases 2 (SnRK2s) play key roles in abscisic acid (ABA) signaling under osmotic stress. Negative regulation of subclass III SnRK2s is released via inhibition of protein phosphatases 2C (PP2C) by ABA-pyrabactin resistance1/pyr1-like/regulatory components of ABA receptor (ABA-PYR/PYL/RCAR) complexes under osmotic stress. Subclass III SnRK2s are phosphorylated and activated by B-type Raf-like kinases and GSK3-like kinases in an ABA-independent manner. Activated subclass III SnRK2s phosphorylate a variety of substrates, including ABA-responsive element binding proteins/ABA-responsive element binding factor (AREB/ABF), to induce the expression of stress-responsive genes. ABA-unresponsive subclass I SnRK2s are quickly activated in response to osmotic stress in an ABA-independent manner. Osmotic stress-activated subclass I SnRK2s regulate mRNA decay by phosphorylating the mRNA-decapping activator VCS. The B4 Raf-like kinases RAF18, RAF20 and RAF24 are responsible for the activation of subclass I SnRK2s. Dashed lines indicate possible but unconfirmed routes.
One of the phosphorylation targets of subclass III SnRK2s is the group A bZIP transcription factor ABA-RESPONSIVE ELEMENT BINDING PROTEIN/ABA-RESPONSIVE ELEMENT BINDING FACTOR (AREB/ABF), which regulates the expression of ABA-inducible genes [28,29] in response to drought stress or ABA [30,31]. AREB/ABF is activated through multisite phosphorylation of conserved domains by SnRK2s [31]. In particular, AREB1/ABF2, AREB2/ABF4, ABF3 and ABF1 redundantly regulate stress-inducible gene expression [5,32]. These ABA-SnRK2-AREB/ABF signaling modules can be formed in vitro or in yeast cells [20,33].
Phosphoproteomics studies using srk2dei triple mutants lacking all subclass III SnRK2s have been performed to investigate SnRK2-mediated phosphorylation signaling [34,35]. MITOGEN-ACTIVATED PROTEIN KINASE (MPK) 1, MPK2, SnRK2 SUBSTRATE 1 (SNS1) and bZIP transcription factors, including AREB1/ABF2, AREB3 and EEL, were identified as candidate substrates of subclass III SnRK2s by these analyses [34,35]. SNS1 is an unknown protein, but an sns1 mutant showed an ABA-hypersensitive phenotype in the post-germination stage [34]. Although these factors may be involved in SnRK2-mediated phosphorylation signaling, further analyses are required to reveal whether these factors are directly or indirectly regulated by SnRK2s. Phosphoproteomic analyses also revealed that the subclass III SnRK2-AREB/ABF phosphorylation signaling module is well conserved in Oryza sativa [36], Brachypodium distachyon [37], and Solanum lycopersicum [38]. Recently, a new approach was reported to integrate in vivo and in vitro phosphoproteomic information to show the direct targets of protein kinases [39]. Extensive analyses of Arabidopsis proteins were performed using genome-wide phosphoproteomics data from plant cells, which is an important basis for phosphorylation signaling in Arabidopsis [40].
2.2. Regulatory Mechanisms of Subclass III SnRK2s
Subclass III SnRK2s are activated in ABA-deficient and ABA-insensitive mutants, suggesting that SnRK2s can be activated by upstream kinases in an ABA-independent manner under drought stress [17,27,41,42]. B-type MPK KINASE KINASE (MAPKKK) Raf-like kinases have been shown to directly regulate the activity of SnRK2s under osmotic stress [12,43,44,45]. An ABA-insensitive Physcomitrella patens mutant was isolated by a forward genetic approach and was shown to have a mutation in a gene for ABA AND ABIOTIC STRESS-RESPONSIVE RAF-LIKE KINASE/ABA NON-RESPONSIVE (ARK/ANR), one of the B3-MAPKKK Raf-like kinases [46,47]. This gene was previously identified as a PpCTR1 that regulates ABA and ethylene responses in Physcomitrella patens [48]. The ARK/ANR kinase directly phosphorylates and activates SnRK2s [46,49]. Three ARK/ANR homologs, M3Kδ1/RAF3, M3Kδ6/RAF5/SIS8 and M3Kδ7/RAF4, which are classified as B3 Raf-like kinases, were identified as activators of subclass III SnRK2s in Arabidopsis [50]. RAF6, which is the closest homolog of CTR1/RAF1 and a member of B3 Raf-like kinases, also regulates subclass III SnRK2s under osmotic stresses [50,51]. RAF10, one of the B2 Raf-like kinases, which are the closest family members of B3 Raf-like kinases, can also phosphorylate and activate subclass III SnRK2s by releasing them from PP2Cs [52,53] (Figure 1). These reports suggest that B2 Raf-like kinases may also be involved in the activation of subclass III SnRK2s. Recently, three B4 Raf-like kinases, RAF18, RAF20 and RAF24, were identified as activators of ABA-unresponsive subclass I SnRK2s under osmotic stress [54]. In addition, the activities of SnRK2s were significantly decreased in a multiple mutant lacking B2, B3 and B4 Raf-like kinases under osmotic stress [55]. It is necessary to elucidate different roles between B2 and B3 Raf-like kinases to reveal the physiological and functional roles of subclass III SnRK2 activation by Raf-like kinases.
In maize, CASEIN KINASES 2 (CK2s) phosphorylate SnRK2s, increasing their binding to PP2Cs [56]. However, CK2-knockout mutants show reduced ABA sensitivity [57]. CK2 catalytic subunits (CK2αs) positively regulate ABA-dependent gene expression, but the CK2 regulatory subunit (CK2β) negatively regulates ABA-dependent gene expression [58]. These findings suggest that the balance of CK2 subunits determines ABA responses (Figure 1). Subclass III SnRK2s are also phosphorylated by BRASSINOSTEROID INSENSITIVE 2 (BIN2), a GLYCOGEN SYNTHASE KINASE 3 (GSK3)-like kinase [59]. BIN2 interacts with and phosphorylates subclass III SnRK2s to promote their activity [59] (Figure 1). Taken together, crosstalk between ABA and brassinosteroids may occur under stress conditions via subclass III SnRK2s.
The activity of subclass III SnRK2s is also regulated by their stability. AtPP2-B11 and HOS15, which are components of E3 ligase complexes, promote the degradation of SnRK2s in the presence of ABA [60,61]. SnRK2 INTERACTING CALCIUM SENSOR (SCS) inhibits the activity of SnRK2s in the presence of Ca2+ in plant cells [62,63]. As the cytosolic concentration of Ca2+ is significantly increased in response to osmotic stress, SCS may regulate the activity of SnRK2s in the later stage of osmotic stress signaling.
2.3. Other Regulatory Mechanisms of ABA Receptors and Coreceptors
The activities of subclass III SnRK2s are also controlled by mediators other than ABA receptors. Degradation of PP2Cs is an additional regulatory mechanism for the inhibition of PYR/PYL/RCAR receptor activities. Among clade A, PP2Cs, PP2C, ABI1, ABI2, PP2CA and HAB1 are essential for osmotic stress responses [64]. ABI1 is degraded by two U-box E3 ligases, PUB12 and PUB13, and this degradation is promoted by ABA [65]. On the other hand, PP2CA is degraded by several protease systems, such as the E3 ubiquitin ligases RGLG1, RGLG5 [66], PIR1.1, and PIR1.2 [67] and the BPM-CUL3 E3 ligases BPM3 and BPM5 [68], to enhance ABA responses. DELAY OF GERMINATION 1 (DOG1), which encodes a protein of unknown function, suppresses ABA-HYPERSENSITIVE GERMINATION (AHG) 1/3 encoding a clade A PP2C [69,70] in the germination stage. Because DOG1 positively regulates drought tolerance [71], DOG1-mediated PP2C suppression may function in the drought stress response. These findings suggest that PP2Cs are regulated by several mechanisms other than PYR/PYL/RCAR-dependent regulation by ABA to promote survival under changing environmental conditions by precisely controlling ABA responses.
Regulation of ABA receptors is also involved in ABA signaling under drought stress. CARK1, a putative receptor-like cytoplasmic kinase, phosphorylates PYL8/RCAR3 and PYR1/RCAR11 to promote its stability and ability to inhibit ABI1, leading to the activation of ABA signaling in the presence of ABA [72]. On the other hand, Arabidopsis EL1-LIKE (AEL) phosphorylates PYR/PYL/RCAR to decrease its stability, leading to suppression of ABA responses under non-stress conditions [73]. These findings suggest that ABA signaling is precisely regulated by controlling the stability of PYR/PYL/RCAR.
Dehydration stress-resistant crops can be produced by overexpression or mutation of ABA receptors. ABA sensitivity and drought tolerance are significantly improved in PYL-overexpressing wheat plants (TaPYLox) compared with wild-type plants [74]. Biomass production and seed production are also improved by ABA receptor overexpression because of the reduction in transpiration and enhancement of photosynthetic activity in wheat [74]. ABA signaling suppresses plant growth under non-stress conditions [18,75]. Rice growth and productivity can be improved by CRISPR/Cas9-mediated mutations in rice PYR/PYL/RCAR [76]. Several combinations of PYL mutations have been constructed and analyzed in rice. Among them, pyl1/4/6 exhibited the best growth and improved grain productivity under field conditions [76]. These methods provide novel insight into approaches to improve drought stress tolerance and plant growth in crops by applying the factors involved in ABA responses in Arabidopsis.
3. ABA-Independent Signaling in Response to Dehydration Stress: Important Roles of Subclass I SnRK2s
In drought stress responses, ABA-independent regulatory systems have been identified as playing important roles in addition to ABA-dependent systems. Under drought stress, plants sense and respond to water deficit signals in the roots; then, the stress signal is transported to aerial parts, including the leaves, to synthesize ABA and close stomata to reduce water loss. ABA also induces many stress genes with various functions to cope with dehydration stress. In the early processes of drought stress responses before the accumulation of endogenous ABA, ABA-independent processes function in sensing and signaling to activate regulatory systems to respond to water deficit stress, including osmotic stress, ROS stress, mechanical stress and other stress signals. Then, these early stress signals upregulate ABA biosynthesis by the induction of the 9-CIS-EPOXYCAROTENOID DIOXYGENASE 3 (NCED3) gene [6]. Therefore, for a proper understanding of plant responses to drought stress, the early processes of the ABA-independent regulatory system must be included. In cellular signal transduction, subclass I SnRK2 protein kinases play important roles in ABA-independent signaling pathways. In this section, we discuss the phosphorylation processes and focus on the roles of subclass I SnRK2 protein kinases.
3.1. Subclass I SnRK2-Mediated Dehydration Stress Signaling
Four of the ten SnRK2s, SRK2A/SnRK2.6, SRK2B/SnRK2.10, SRK2.1/SnRK2.10 and SRK2H/SnRK2.5, which are classified as subclass I SnRK2s in Arabidopsis, are strongly activated in response to osmotic stress but not ABA [17]. The kinase activities of subclass I SnRK2s are enhanced by dehydration stress prior to ABA accumulation and in ABA-deficient plants [11,17]. Although subclass I SnRK2s are involved in the osmotic stress response and cadmium response, the substrates of these kinases have not been elucidated [11,77]. Interactome analyses have revealed that subclass I SnRK2s interact with VARICOSE (VCS), which is a scaffold protein of mRNA-decapping complexes [9]. The expression of many stress-repressive genes was similarly upregulated in srk2abgh, a mutant lacking all functional subclass I SnRK2s, and in VCS-knockdown plants under osmotic stress. Additionally, mRNA decay of the transcripts of these genes was impaired in these plants under osmotic stress. On the other hand, the expression of many stress-induced genes decreased in srk2abgh and VCS-knockdown plants compared with wild-type plants under osmotic stress. These observations suggest that the expression of drought stress-responsive genes is positively regulated by posttranslational regulation mediated by the subclass I SnRK2-VCS signaling module [9] (Figure 1). The subclass I SnRK2s-VCS signaling module is also involved in modifying root architecture and development in response to salt stress [10]. DCP1, one of the mRNA decapping activators, is also phosphorylated by MPK6 in response to dehydration stress [78]. These reports suggest that phosphorylation of decapping complexes is important for the activation of mRNA decapping and decay. Phosphoproteomic analyses revealed that SRK2B/SnRK2.10 phosphorylates two dehydrins, EARLY RESPONSIVE TO DEHYDRATION (ERD)10 and ERD14 [79]. Phosphorylation of ERDs is involved in the regulation of subcellular localization to acquire drought tolerance [79] (Figure 1). Subclass I SnRK2s also regulate the expression of several genes responsible for ROS generation and removal under salt stress [80].
3.2. Regulatory Components of Subclass I SnRK2s
All functional subclass I SnRK2s are phosphorylated in the early stage of osmotic stress [81]. The Ser-154 residue of subclass I SnRK2s is phosphorylated by unknown kinases in response to osmotic stress [27,42]. Recently, three B4 Raf-like kinases, RAF18, RAF20 and RAF24, were identified as protein kinases responsible for phosphorylating Ser-154 of subclass I SnRK2s and activating them in response to osmotic stress [54]. The activities of subclass I SnRK2s under osmotic stress were largely decreased in a raf18/20/24 triple mutant [54]. Considering that the three Raf-like kinases and subclass I SnRK2s are well conserved in seed plants but not in moss [9,54], the RAF18/20/24-subclass I SnRK2-VCS signaling module may have evolved in seed plants to control mRNA populations and to enhance adaptability to stress conditions (Figure 1). Another report of a genetic approach using a septuple mutant lacking all B4 Raf-like kinases also suggested that B4 Raf-like kinase is responsible for the activation of subclass I SnRK2s [55]. The growth retardation of the septuple mutant was more severe than that of the raf18/20/24 or srk2abgh mutant under osmotic stress, indicating the existence of other substrates of B4 Raf-like kinases. Further analyses are required to reveal the other substrates of B4 Raf-like kinases.
The activity of subclass I SnRK2s is also controlled by other mediators in addition to RAF18/20/24 kinases. The activities of SRK2A/SnRK2.4 and SRK2B/SnRK2.10 are regulated by ABI1 and PP2CA under osmotic stress [82]. Because of the existence of a phosphatidic acid (PA)-binding domain in subclass I SnRK2s [83], PA is thought to regulate the kinase activity of these kinases (Figure 1). As osmotic stress-induced accumulation of PA in roots is dependent on PHOSPHOLIPASE Dα (PLDα1) [84], the activity of SnRK2s might be regulated by PLDα1.
In summary, the importance of ABA-activated and ABA-independent SnRK2s in dehydration stress responses has been discussed (Figure 1). ABA-activated subclass III SnRK2s regulate the expression of many stress-responsive genes and stomatal responses under dehydration stress. On the other hand, ABA-independent and dehydration-activated subclass I SnRK2s regulate the mRNA population to correctly control stress-induced gene expression. However, the osmosensors that receive an osmotic signal and induce ABA accumulation or directly activate RAF-SnRK2 in an ABA-independent manner are largely unknown. There are many reports on candidates for sensing molecules of dehydration stress [85,86]. However, major sensory systems have not yet been identified. In the next step, it is important to analyze upstream factors of Raf1 and subclass I SnRK2 to identify sensors of dehydration stress signals.
4. Transcriptional Regulation of Cellular Signaling in Drought Stress Responses
Various types of transcription factors mediate the regulation of gene expression in response to drought stress. Drought stress-responsive genes play crucial roles in the drought stress tolerance of plants [8,87]. Higher plants have more transcription factors than animals do to adapt to environmental stresses, thereby indicating that the networks for transcriptional regulation in plants are more complicated than those in animals, which can move to reduce or avoid environmental stresses. In plants, ABA plays important roles in drought stress-responsive gene expression. ABA-independent transcriptional regulatory systems also function in dehydration stress responses in addition to ABA-dependent systems [87]. In this section, we summarize the functions of transcription factors that mediate drought, heat and ABA responses in plants.
4.1. Transcription Factors That Mediate ABA-Responsive Gene Expression
Many drought stress-responsive genes have cis-acting ABREs (PyACGTGGC) in their promoter regions [87,88]. The ABA-RESPONSIVE ELEMENT (ABRE) motif is mainly recognized by bZIP-type transcription factors, AREBs/ABFs (Figure 2). There are nine subfamily members of AREBs/ABF in Arabidopsis. Among them, AREB1/ABF2, AREB2/ABF4 and ABF3 have conserved amino acid sequences that are phosphorylated by subclass III SnRK2s (SRK2D/SnRK2.2, SRK2E/SnRK2.6 and SRK2I/SnRK2.3) and mediate the expression of drought- and ABA-responsive genes through binding to the ABRE motif in their promoter region [21,89]. The substitution of phosphorylated amino acids with aspartic acid led to the enhancement of AREB1 transcriptional activity even without ABA treatment, indicating that phosphorylation modification is required for the regulation of transcriptional activity in AREBs/ABF under stress conditions [31]. Triple mutants of AREB1, AREB2 and ABF3 showed a reduction in drought- and ABA-responsive gene expression and drought stress-sensitive phenotypes [90]. These results indicate that AREB1/ABF2, AREB2/ABF4 and ABF3 are key transcription factors that regulate drought stress responses through an ABA-dependent pathway. Remarkably, COUPLING ELEMENT 3 (CE3) is often located in the vicinity of ABREs on the promoters of ABA-responsive genes [91]. Further analysis will reveal the functional relationship between CE3 and ABRE that regulates the binding of transcription factors under drought stress.
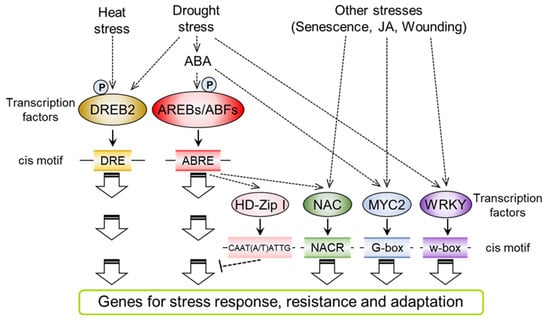
Figure 2.
A schematic model of transcriptional regulatory networks under abiotic stress, including drought, heat and other stresses. The ellipsoids show transcription factors that mainly mediate drought stress and/or ABA responses. The oblong boxes indicate the cis motifs that exist in the promoter region of stress-induced genes. ABREs/ABFs function mainly in drought- and ABA-dependent pathways. DREB2 mediates ABA-independent and heat stress signals. Other transcription factors, including NAC, MYC2 and WRKY, mediate crosstalk signals between ABA and other stresses in plants..
4.2. Transcription Factors for Drought- and Heat-Responsive Gene Expression in ABA-Independent Signaling
The DREB2A transcription factor is expressed under dehydration and osmotic stress [92]. DREB2A binds to the DRE motif (A/GCCGAC) present in the promoter regions of drought stress-responsive genes [88] (Figure 2). Domain analysis of DREB2A revealed that a negative regulatory domain (NRD) located in the central region of DREB2A is important for the regulation of DREB2A transcriptional activity [93]. Transgenic plants overexpressing constitutively active forms of DREB2A (DREB2A CA) that lack the NRD show enhancement of both stress-inducible gene expression and dehydration stress resistance. The abiotic stress-induced overexpression of DREB2A CA improved the phenotype of drought stress resistance in transgenic Arabidopsis and soybean without growth defects [93,94]. The stability of the DREB2A protein is regulated by modification of the NRD. DREB2A-INTERACTING PROTEIN 1 (DRIP1) and DRIP2 were isolated as E3 ubiquitin ligases that mediate the ubiquitination of DREB2A [95]. Ubiquitinated DREB2A is degraded through the 26S proteasome pathway under control conditions.
Drought stress responses are known as complex physiological responses that include not only dehydration stress responses but also heat stress responses. Other research indicated that overexpression of DREB2A CA in plants mediated the enhancement of heat-induced gene expression and resulted in thermotolerance [96]. The stability of DREB2A is regulated by other degradation pathways under heat stress. BTB/POZ AND MATH DOMAIN PROTEINS (BPMs) encode substrate adaptors of the CULLIN3 (CUL3)-based E3 ligase and bind to the NRD of DREB2A [97]. BPM-knockdown plants accumulated the DREB2A protein and showed enhanced expression of DREB2A target genes under heat stress and improved heat stress tolerance. DNA POLYMERASE II SUBUNIT B3-1 (DBP3-1)/NUCLEAR FACTOR-Y SUBUNIT C10 (NF-YC10), NF-YA and NY-YB interact with DREB2A and enhance the transcriptional activity of DREB2A under heat stress [98]. The inhibition of phosphorylation in the NRD through CASEIN KINASE 1 (CK1) stabilizes and activates DREB2A in response to heat stress [99]. These results indicate that DREB2A mediates drought and heat stress signaling via the different regulatory mechanisms of the NRD in DREB2A.
4.3. Crosstalk in Transcriptional Regulation between Drought and Other Stress Responses
Cellular responses to drought stress are regulated through various types of transcription factors [8]. AREB/ABFs partially mediate the expression of DREB2A under dehydration stress [100], suggesting crosstalk between early processes of dehydration stress before the accumulation of ABA and slow adaptive ABA-dependent processes of stress-responsive gene expression. Moreover, various types of transcription factors have been reported to function in dehydration-responsive transcription. Most of them function mainly in crosstalk between drought and other stress responses, such as wounding, active oxygen, pathogen infection and other environmental stress signals. In this section, we focus on HOMEODOMAIN-LEUCINE ZIPPER (HD-Zip), NAM, ATAF1, 2 and CUC2 (NAC) transcription factors in the crosstalk between drought and other stress responses.
An HD-Zip transcription factor, HOMEOBOX PROTEINS 6 (HB6), negatively regulates ABA responses [101]. HB6 interacts with ABA INSENSITIVE 1 (ABI1) and binds to the core motif (CAATTATTA) that ABA-responsive genes have in their promoter regions. Transgenic plants overexpressing HB6 show an ABA-insensitive phenotype during seed germination. HB6 inhibits stomatal closure in detached leaves. These observations support the role of HB6 mainly in the crosstalk between ABA responses under drought stress.
The NAM, ATAF1, 2 and CUC2 (NAC) genes are plant-specific transcription factors that bind to the consensus NAC recognition site (NACR, CGTG/A) [102] (Figure 2). Previous studies indicate that a subfamily of stress-responsive NAC transcription factors (SNACs) mediate environmental stress responses [103,104]. ANAC096 is expressed in response to drought stress and enhances the expression of drought-induced genes. Specifically, ANAC096 mediates the expression of the RESPONSIVE-TO-DEHYDRATION 29A (RD29A) gene, which is one of the marker genes in drought stress signaling, through interaction with AREB1/ABF2 and AREB2/ABF4. anac096 mutants show a dehydration-sensitive phenotype [105]. These results indicate that ANAC096 functions as a positive regulator in drought stress signaling. On the other hand, ANAC016 negatively regulates the expression of drought stress-induced genes, including AREB1/ABF2, although the expression of the ANAC016 gene is enhanced in response to drought stress [106], which may cause the drought stress-resistant phenotype of anac016 mutants. Detailed phylogenetic analysis has revealed that SNACs are divided into two major branches, SNAC-A and SNAC-B. Seven SNACs, ANAC002/ATAF1, ANAC019, ANAC032, ANAC055, ANAC072/RD26, ANAC081/ATAF2 and ANAC102, were included in the SNAC-A group and expressed under drought and ABA treatment. These seven SNACs mediate ABA-dependent leaf senescence under drought stress [107]. The septuple mutant of SNACs showed a delayed leaf senescence phenotype in response to ABA treatment. Comparative transcriptome analysis revealed that major senescence-related genes were repressed in the SNAC septuple mutants.
5. ABA Crosstalk with Other Hormones under Drought Stress
Transcriptional regulation via transcription factors mediates the crosstalk between ABA and other hormone signaling pathways. Among them, the regulatory roles of MYC, MYB, WRKY and ERF transcription factors are discussed in this section. AtMYC2, encoding a basic helix loop helix (bHLH) transcription factor, is induced by ABA and regulates ABA-inducible transcription of the drought-inducible RD22 gene and AtADH1 gene [108,109]. AtMYC2 is also a master regulator of jasmonate (JA) signaling pathways and controls many JA-dependent processes, from insect and pathogen defense to plant development [110]. A unique function of MYC2 is its regulatory role in the crosstalk between hormone signaling pathways involving JA and those involving ABA, salicylic acid (SA), gibberellin (GA) and auxin (IAA) [110,111]. AtMYB2, encoding an R2R3-type MYB-related transcription factor, regulates RD22 together with AtMYC2 [109], which indicates that the interaction between AtMYC2 and MYB2 determines their functions in ABA-inducible transcription. Another MYB transcription factor, MYB96, mediates dehydration stress responses through the regulation of crosstalk between ABA and auxin signals [112]. MYB96 is expressed not only in the vasculature of leaves and roots but also in stomata in response to ABA and dehydration stress. Transgenic plants overexpressing MYB96 show reduced shoot growth and fewer lateral roots, thereby enhancing dehydration stress resistance.
The WRKY transcription factors that bind to the W-box (TTGACC/T) mediate various abiotic and biotic stress responses and act as activators or repressors of particular physiological responses [113] (Figure 2). Previous studies indicate that WRKY18, WRKY40 and WRKY60, which belong to the group IIa WRKY family, are involved in ABA signaling [114]. WRKY40 directly binds to the promoters of the AREB2/ABF4, ABI4, ABI5, MYB2, DREB1A and RAB18 genes under control conditions [115,116]. This suppressive effect of WRKY40 is eliminated in response to ABA treatment. Triple knockout mutants of WRKY18, WRKY40 and WRKY60 showed an ABA-hypersensitive phenotype, such as growth arrest during ABA-mediated seed germination prior to the post-germination stage. These results indicate that WRKY18, WRKY40 and WRKY60 act as repressors in ABA responses. ABA overly sensitive 3 (ABO3)/WRKY63 was identified as the causative gene of both root growth inhibition and germination inhibition in ABA hypersensitive mutants [117]. abo3 mutants showed impaired ABA-induced stomatal closure, thereby showing a dehydration-sensitive phenotype. ABO3/WRKY63 is able to bind the W-box in the AREB1/ABF2 promoter. The expression of the AREB1/ABF2, RD29A and COR47 genes was partially repressed in the abo3 mutants under ABA treatment. On the other hand, areb1/abf2 mutants show the opposite phenotype in ABA-mediated germination inhibition compared to that of abo3 mutants. AREB1/ABF2 does not mediate ABA-induced stomatal closure. These results indicate that ABO3/WRKY63 mediates not only AREB1/ABF2-mediated signaling but also other transcriptional cascades in ABA and dehydration stress responses.
ETHYLENE RESPONSE FACTOR1 (ERF1) is a transcription factor that mediates pathogen responses through ethylene and jasmonate signaling. ERF1 is also able to bind to the DRE motif in the promoter regions of genes downstream of ERF1. ERF1 regulates stomatal closure to prevent water loss from guard cells. ERF1-overexpressing plants show a dehydration resistance phenotype. These results indicate that ERF1 activates specific sets of stress genes and functions in crosstalk between biotic and abiotic stress responses.
6. Conclusions and Future Perspectives
Plants have a sophisticated stress response mechanism that provides a balance between stress tolerance and optimal growth under drought stress. The phytohormone ABA mediates various physiological responses under dehydration stress, including stomatal closure in guard cells and stress-mediated gene expression in various plant tissues. ABA strongly accumulates in response to drought stress through peptide signaling, phosphorylation signaling and gene expression. In addition to an ABA-dependent regulatory system in dehydration responses, an ABA-independent regulatory system including signal transduction and transcription also plays important roles in early processes of the stress response before the accumulation of ABA. In an ABA-dependent pathway, subclass III SnRK2s and AREB/ABF are the main positive regulators of dehydration stress responses. ABA-activated subclass III SnRK2s mediate the expression of stress-responsive genes and enhance stress tolerance through the phosphorylation of AREB/ABF transcription factors. In addition to the subclass III SnRK2-AREB/ABF pathway, plants have evolved a subclass I SnRK2-VCS pathway. The subclass I SnRK2-VCS pathway regulates changes in the transcriptome by activating mRNA decay under dehydration stress via an ABA-independent pathway. The fine tuning of transcriptomic changes is required for optimal growth without growth retardation under drought stress because some stress-mediated genes adversely affect both plant growth and yield.
Recent phosphoproteomics analyses revealed that there are many regulators of subclass III SnRK2s, ABA receptors or coreceptors that mediate the regulation of gene expression through the AREB/ABF transcriptional pathway. Among them, plant Raf-like kinases have been newly identified as upstream factors of SnRK2s. Considering that Raf-like kinases phosphorylate and activate almost all subclass III SnRK2s under osmotic stress, we need to redefine the physiological relationship between group A PP2Cs and Raf-like kinases with regard to SnRK2 activation.
Furthermore, an ABA-independent regulatory system functions in early processes of dehydration stress responses before the accumulation of ABA. In the early processes of dehydration responses, the identification of osmosensors that may directly regulate the activity of Raf-like kinases will provide new insights into how phosphorylation signaling pathways are activated quickly by dehydration stress. Further studies will also reveal the downstream regulation of SnRK2s through transcription factors such as AREB/ABF, DREB2A and other transcription factors in dehydration responses. A comprehensive understanding of regulatory mechanisms through gene expression will contribute to the discovery of useful genes for the improvement of yield productivity and optimal growth under drought stress.
Author Contributions
Writing—original draft preparation, F.S. and F.T.; writing—review and editing, K.Y.-S., K.S.; supervision, K.Y.-S., K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants-in-aid, BRAIN, the Project of the JSPS KAKENHI under grant numbers JP15H05960 (to K.Y.-S.), JP18H03996 (to K.Y.-S), JP19H03255 (to F.T.), JP20K21437 (to F.T.) and JP20K15496 (to F.S.), research grants from the Toray Science Foundation (to F.T.) and grants-in-aid for JSPS Fellows under grant number 18J13854 (to F.S.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Takashi Kuromori and Kaoru Urano for discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Fujita, M.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Engineering drought tolerance in plants: Discovering and tailoring genes to unlock the future. Curr. Opin. Biotechnol. 2006, 17, 113–122. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef]
- Umezawa, T.; Nakashima, K.; Miyakawa, T.; Kuromori, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol. 2010, 51, 1821–1839. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Kuromori, T.; Sato, H.; Shinozaki, K. Regulatory Gene Networks in Drought Stress Responses and Resistance in Plants. Adv. Exp. Med. Biol. 2018, 1081, 189–214. [Google Scholar] [CrossRef]
- Soma, F.; Mogami, J.; Yoshida, T.; Abekura, M.; Takahashi, F.; Kidokoro, S.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-unresponsive SnRK2 protein kinases regulate mRNA decay under osmotic stress in plants. Nat. Plants 2017, 3, 16204. [Google Scholar] [CrossRef]
- Kawa, D.; Meyer, A.J.; Dekker, H.L.; Abd-El-Haliem, A.; Gevaert, K.; Van De Slijke, E.; Maszkowska, J.; Bucholc, M.; Dobrowolska, G.; De Jaeger, G.; et al. SnRK2 protein kinases and mRNA decapping machinery control root development and response to salt. Plant Physiol. 2019, 182, 361–377. [Google Scholar] [CrossRef]
- McLoughlin, F.; Galvan-Ampudia, C.S.; Julkowska, M.M.; Caarls, L.; van der Does, D.; Laurière, C.; Munnik, T.; Haring, M.A.; Testerink, C. The Snf1-related protein kinases SnRK2.4 and SnRK2.10 are involved in maintenance of root system architecture during salt stress. Plant J. 2012, 72, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Fabregas, N.; Yoshida, T.; Fernie, A.R. Role of Raf-like kinases in SnRK2 activation and osmotic stress response in plants. Nat. Commun. 2020, 11, 6184. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, M.; Mueller, H.M.; Bauer, H.; Peirats-Llobet, M.; Rodriguez, P.L.; Geilfus, C.M.; Carpentier, S.C.; Al Rasheid, K.A.S.; Kollist, H.; Merilo, E.; et al. The role of Arabidopsis ABA receptors from the PYR/PYL/RCAR family in stomatal acclimation and closure signal integration. Nat. Plants 2019, 5, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Mustilli, A.C.; Merlot, S.; Vavasseur, A.; Fenzi, F.; Giraudat, J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 2002, 14, 3089–3099. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Hobo, T.; Ichimura, K.; Mizoguchi, T.; Takahashi, F.; Aronso, J.; Ecker, J.R.; Shinozaki, K. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 2002, 43, 1473–1483. [Google Scholar] [CrossRef]
- Yoshida, R.; Umezawa, T.; Mizoguchi, T.; Takahashi, S.; Takahashi, F.; Shinozaki, K. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J. Biol. Chem. 2006, 281, 5310–5318. [Google Scholar] [CrossRef]
- Boudsocq, M.; Droillard, M.J.; Barbier-Brygoo, H.; Laurière, C. Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Mol. Biol. 2007, 63, 491–503. [Google Scholar] [CrossRef]
- Mizoguchi, M.; Umezawa, T.; Nakashima, K.; Kidokoro, S.; Takasaki, H.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two closely related subclass II SnRK2 protein kinases cooperatively regulate drought-inducible gene expression. Plant Cell Physiol. 2010, 51, 842–847. [Google Scholar] [CrossRef]
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.K. In vitro reconstitution of an abscisic acid signalling pathway. Nature 2009, 462, 660–664. [Google Scholar] [CrossRef]
- Fujita, Y.; Nakashima, K.; Yoshida, T.; Katagiri, T.; Kidokoro, S.; Kanamori, N.; Umezawa, T.; Fujita, M.; Maruyama, K.; Ishiyama, K.; et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol. 2009, 50, 2123–2132. [Google Scholar] [CrossRef]
- Nakashima, K.; Fujita, Y.; Kanamori, N.; Katagiri, T.; Umezawa, T.; Kidokoro, S.; Maruyama, K.; Yoshida, T.; Ishiyama, K.; Kobayashi, M.; et al. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol. 2009, 50, 1345–1363. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Sugiyama, N.; Mizoguchi, M.; Hayashi, S.; Myouga, F.; Yamaguchi-Shinozaki, K.; Ishihama, Y.; Hirayama, T.; Shinozaki, K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 17588–17593. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; Miyakawa, T.; Sawano, Y.; Kubota, K.; Kang, H.J.; Asano, A.; Miyauchi, Y.; Takahashi, M.; Zhi, Y.; Fujita, Y.; et al. Structural basis of abscisic acid signalling. Nature 2009, 462, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamamoto, S.; Minami, H.; Kagaya, Y.; Hattori, T. Differential activation of the rice sucrose nonfermenting1–related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 2004, 16, 1163–1177. [Google Scholar] [CrossRef]
- Choi, H.; Hong, J.; Ha, J.; Kang, J.; Kim, S.Y. ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem. 2000, 275, 1723–1730. [Google Scholar] [CrossRef]
- Uno, Y.; Furihata, T.; Abe, H.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc. Natl. Acad. Sci. USA 2000, 97, 11632–11637. [Google Scholar] [CrossRef]
- Fujii, H.; Verslues, P.E.; Zhu, J.K. Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell 2007, 19, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Furihata, T.; Maruyama, K.; Fujita, Y.; Umezawa, T.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc. Natl. Acad. Sci. USA 2006, 103, 1988–1993. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2015, 38, 35–49. [Google Scholar] [CrossRef]
- Ruschhaupt, M.; Mergner, J.; Mucha, S.; Papacek, M.; Doch, I.; Tischer, S.V.; Hemmler, D.; Chiasson, D.; Edel, K.H.; Kudla, J.; et al. Rebuilding core abscisic acid signaling pathways of Arabidopsis in yeast. EMBO J. 2019, 38, e101859. [Google Scholar] [CrossRef]
- Umezawa, T.; Sugiyama, N.; Takahashi, F.; Anderson, J.C.; Ishihama, Y.; Peck, S.C.; Shinozaki, K. Genetics and phosphoproteomics reveal a protein phosphorylation network in the abscisic acid signaling pathway in Arabidopsis thaliana. Sci. Signal. 2013, 6, rs8. [Google Scholar] [CrossRef]
- Wang, P.; Xue, L.; Batelli, G.; Lee, S.; Hou, Y.J.; Van Oosten, M.J.; Zhang, H.; Tao, W.A.; Zhu, J.K. Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc. Natl. Acad. Sci. USA 2013, 110, 11205–11210. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, X.; Qiu, J.; Li, Z.; Zhao, J.; Hou, Y.; Tang, L.; Zhang, J. A phosphoproteomic landscape of rice (Oryza sativa) tissues. Physiol. Plant. 2017, 160, 458–475. [Google Scholar] [CrossRef]
- Lv, D.W.; Subburaj, S.; Cao, M.; Yan, X.; Li, X.; Appels, R.; Sun, D.F.; Ma, W.; Yan, Y.M. Proteome and phosphoproteome characterization reveals new response and defense mechanisms of Brachypodium distachyon leaves under salt stress. Mol. Cell. Proteom. 2014, 13, 632–652. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Zhu, Y.; Arrington, J.V.; Paez, J.S.; Wang, P.; Zhu, P.; Chen, I.H.; Zhu, J.K.; Tao, W.A. Universal Plant Phosphoproteomics Workflow and Its Application to Tomato Signaling in Response to Cold Stress. Mol. Cell. Proteom. 2018, 17, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hsu, C.C.; Du, Y.; Zhu, P.; Zhao, C.; Fu, X.; Zhang, C.; Paez, J.S.; Macho, A.P.; Tao, W.A.; et al. Mapping proteome-wide targets of protein kinases in plant stress responses. Proc. Natl. Acad. Sci. USA 2020, 117, 3270–3280. [Google Scholar] [CrossRef] [PubMed]
- Mergner, J.; Frejno, M.; List, M.; Papacek, M.; Chen, X.; Chaudhary, A.; Samaras, P.; Richter, S.; Shikata, H.; Messerer, M.; et al. Mass-spectrometry-based draft of the Arabidopsis proteome. Nature 2020, 579, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Boudsocq, M.; Barbier-Brygoo, H.; Laurière, C. Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J. Biol. Chem. 2004, 279, 41758–41766. [Google Scholar] [CrossRef]
- Vlad, F.; Droillard, M.J.; Valot, B.; Khafif, M.; Rodrigues, A.; Brault, M.; Zivy, M.; Rodriguez, P.L.; Merlot, S.; Laurière, C. Phospho-site mapping, genetic and in planta activation studies reveal key aspects of the different phosphorylation mechanisms involved in activation of SnRK2s. Plant J. 2010, 63, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Juste, J.; Alrefaei, A.F.; Rodriguez, P.L. Plant Osmotic Stress Signaling: MAPKKKs Meet SnRK2s. Trends Plant Sci. 2020, 25, 1179–1182. [Google Scholar] [CrossRef]
- Komatsu, K.; Takezawa, D.; Sakata, Y. Decoding ABA and osmostress signalling in plants from an evolutionary point of view. Plant Cell Environ. 2020, 43, 2894–2911. [Google Scholar] [CrossRef]
- Hsu, C.C.; Tsai, C.F.; Tao, W.A.; Wang, P. Phosphoproteomic Strategy for Profiling Osmotic Stress Signaling in Arabidopsis. J. Vis. Exp. 2020, 160, e61489. [Google Scholar] [CrossRef]
- Saruhashi, M.; Kumar Ghosh, T.; Arai, K.; Ishizaki, Y.; Hagiwara, K.; Komatsu, K.; Shiwa, Y.; Izumikawa, K.; Yoshikawa, H.; Umezawa, T.; et al. Plant Raf-like kinase integrates abscisic acid and hyperosmotic stress signaling upstream of SNF1-related protein kinase2. Proc. Natl. Acad. Sci. USA 2015, 112, E6388–E6396. [Google Scholar] [CrossRef]
- Stevenson, S.R.; Kamisugi, Y.; Trinh, C.H.; Schmutz, J.; Jenkins, J.W.; Grimwood, J.; Muchero, W.; Tuskan, G.A.; Rensing, S.A.; Lang, D.; et al. Genetic Analysis of Physcomitrella patens Identifies ABSCISIC ACID NON-RESPONSIVE, a Regulator of ABA Responses Unique to Basal Land Plants and Required for Desiccation Tolerance. Plant Cell 2016, 28, 1310–1327. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, Y.; Pierik, R.; Kelly, S.; Sakuta, M.; Voesenek, L.A.; Harberd, N.P. An Ancestral Role for CONSTITUTIVE TRIPLE RESPONSE1 Proteins in Both Ethylene and Abscisic Acid Signaling. Plant Physiol. 2015, 169, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Shinozawa, A.; Otake, R.; Takezawa, D.; Umezawa, T.; Komatsu, K.; Tanaka, K.; Amagai, A.; Ishikawa, S.; Hara, Y.; Kamisugi, Y.; et al. SnRK2 protein kinases represent an ancient system in plants for adaptation to a terrestrial environment. Commun. Biol. 2019, 2, 30. [Google Scholar] [CrossRef]
- Takahashi, Y.; Zhang, J.; Hsu, P.K.; Ceciliato, P.H.O.; Zhang, L.; Dubeaux, G.; Munemasa, S.; Ge, C.; Zhao, Y.; Hauser, F.; et al. MAP3Kinase-dependent SnRK2-kinase activation is required for abscisic acid signal transduction and rapid osmotic stress response. Nat. Commun. 2020, 11, 12. [Google Scholar] [CrossRef]
- Katsuta, S.; Masuda, G.; Bak, H.; Shinozawa, A.; Kamiyama, Y.; Umezawa, T.; Takezawa, D.; Yotsui, I.; Taji, T.; Sakata, Y. Arabidopsis Raf-like kinases act as positive regulators of subclass III SnRK2 in osmostress signaling. Plant J. 2020, 103, 634–644. [Google Scholar] [CrossRef]
- Nguyen, Q.T.C.; Lee, S.J.; Choi, S.W.; Na, Y.J.; Song, M.R.; Hoang, Q.T.N.; Sim, S.Y.; Kim, M.S.; Kim, J.I.; Soh, M.S.; et al. Arabidopsis Raf-Like Kinase Raf10 Is a Regulatory Component of Core ABA Signaling. Mol. Cells 2019, 42, 646–660. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, M.H.; Kim, J.I.; Kim, S.Y. Arabidopsis putative MAP kinase kinase kinases Raf10 and Raf11 are positive regulators of seed dormancy and ABA response. Plant Cell Physiol. 2015, 56, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Soma, F.; Takahashi, F.; Suzuki, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Plant Raf-like kinases regulate the mRNA population upstream of ABA-unresponsive SnRK2 kinases under drought stress. Nat. Commun. 2020, 11, 1373. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Zhang, Z.; Liu, X.; Hsu, C.C.; Du, Y.; Sang, T.; Zhu, C.; Wang, Y.; Satheesh, V.; et al. A RAF-SnRK2 kinase cascade mediates early osmotic stress signaling in higher plants. Nat. Commun. 2020, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Vilela, B.; Nájar, E.; Lumbreras, V.; Leung, J.; Pagès, M. Casein Kinase 2 Negatively Regulates Abscisic Acid-Activated SnRK2s in the Core Abscisic Acid-Signaling Module. Mol. Plant 2015, 8, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, H.; Hu, S.; Lu, X.; Yuan, C.; Zhang, C.; Wang, P.; Xiao, W.; Xiao, L.; Xue, G.P.; et al. Plastid casein kinase 2 knockout reduces abscisic acid (ABA) sensitivity, thermotolerance, and expression of ABA- and heat-stress-responsive nuclear genes. J. Exp. Bot. 2014, 65, 4159–4175. [Google Scholar] [CrossRef] [PubMed]
- Nagatoshi, Y.; Fujita, M.; Fujita, Y. Casein kinase 2 α and β subunits inversely modulate ABA signal output in Arabidopsis protoplasts. Planta 2018, 248, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, J.; Wang, H.; Yang, C.; Chen, Y.; Li, Y.; Pan, S.; Dong, R.; Tang, G.; Barajas-Lopez, J.E.D.; et al. GSK3-like kinases positively modulate abscisic acid signaling through phosphorylating subgroup III SnRK2s in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 9651–9656. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Z.; Ren, Z.; Zhi, L.; Yao, B.; Su, C.; Liu, L.; Li, X. SCFAtPP2-B11 modulates ABA signaling by facilitating SnRK2.3 degradation in Arabidopsis thaliana. PLoS Genet. 2017, 13, e1006947. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Kim, J.K.; Jan, M.; Khan, H.A.; Khan, I.U.; Shen, M.; Park, J.; Lim, C.J.; Hussain, S.; Baek, D.; et al. Rheostatic Control of ABA Signaling through HOS15-Mediated OST1 Degradation. Mol. Plant 2019, 12, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, K.; Klimecka, M.; Ciesielski, A.; Goch, G.; Kulik, A.; Fedak, H.; Poznanski, J.; Lichocka, M.; Pierechod, M.; Engh, R.A.; et al. Two SnRK2-interacting Calcium Sensor Isoforms Negatively Regulate SnRK2 Activity by Different Mechanisms. Plant Physiol. 2019, 182, 1142–1160. [Google Scholar] [CrossRef] [PubMed]
- Bucholc, M.; Ciesielski, A.; Goch, G.; Anielska-Mazur, A.; Kulik, A.; Krzywińska, E.; Dobrowolska, G. SNF1-related protein kinases 2 are negatively regulated by a plant-specific calcium sensor. J. Biol. Chem. 2011, 286, 3429–3441. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Gonzalez-Guzman, M.; Rodriguez, L.; Peirats-Llobet, M.; Pizzio, G.A.; Fernandez, M.A.; De Winne, N.; De Jaeger, G.; Dietrich, D.; Bennett, M.J.; et al. PYRABACTIN RESISTANCE1-LIKE8 plays an important role for the regulation of abscisic acid signaling in root. Plant Physiol. 2013, 161, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Cheng, J.; Zhu, Y.; Ding, Y.; Meng, J.; Chen, Z.; Xie, Q.; Guo, Y.; Li, J.; Yang, S.; et al. Degradation of the ABA co-receptor ABI1 by PUB12/13 U-box E3 ligases. Nat. Commun. 2015, 6, 8630. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, X.; Peirats-Llobet, M.; Belda-Palazon, B.; Wang, X.; Cui, S.; Yu, X.; Rodriguez, P.L.; An, C. Ubiquitin Ligases RGLG1 and RGLG5 Regulate Abscisic Acid Signaling by Controlling the Turnover of Phosphatase PP2CA. Plant Cell 2016, 28, 2178–2196. [Google Scholar] [CrossRef]
- Baek, W.; Lim, C.W.; Luan, S.; Lee, S.C. The RING finger E3 ligases PIR1 and PIR2 mediate PP2CA degradation to enhance abscisic acid response in Arabidopsis. Plant J. 2019, 100, 473–486. [Google Scholar] [CrossRef]
- Julian, J.; Coego, A.; Lozano-Juste, J.; Lechner, E.; Wu, Q.; Zhang, X.; Merilo, E.; Belda-Palazon, B.; Park, S.Y.; Cutler, S.R.; et al. The MATH-BTB BPM3 and BPM5 subunits of Cullin3-RING E3 ubiquitin ligases target PP2CA and other clade A PP2Cs for degradation. Proc. Natl. Acad. Sci. USA 2019, 116, 15725–15734. [Google Scholar] [CrossRef]
- Nishimura, N.; Tsuchiya, W.; Moresco, J.J.; Hayashi, Y.; Satoh, K.; Kaiwa, N.; Irisa, T.; Kinoshita, T.; Schroeder, J.I.; Yates, J.R.; et al. Control of seed dormancy and germination by DOG1-AHG1 PP2C phosphatase complex via binding to heme. Nat. Commun. 2018, 9, 2132. [Google Scholar] [CrossRef]
- Née, G.; Kramer, K.; Nakabayashi, K.; Yuan, B.; Xiang, Y.; Miatton, E.; Finkemeier, I.; Soppe, W.J.J. DELAY OF GERMINATION1 requires PP2C phosphatases of the ABA signalling pathway to control seed dormancy. Nat. Commun. 2017, 8, 72. [Google Scholar] [CrossRef]
- Yatusevich, R.; Fedak, H.; Ciesielski, A.; Krzyczmonik, K.; Kulik, A.; Dobrowolska, G.; Swiezewski, S. Antisense transcription represses. EMBO Rep. 2017, 18, 2186–2196. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Li, D.; Sun, Y.; Li, Y.; Luo, Q.; Liu, Z.; Wang, J.; Zhang, H.; Lou, Z.; et al. CARK1 mediates ABA signaling by phosphorylation of ABA receptors. Cell Discov. 2018, 4, 30. [Google Scholar] [CrossRef]
- Chen, H.H.; Qu, L.; Xu, Z.H.; Zhu, J.K.; Xue, H.W. EL1-like Casein Kinases Suppress ABA Signaling and Responses by Phosphorylating and Destabilizing the ABA Receptors PYR/PYLs in Arabidopsis. Mol. Plant 2018, 11, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Mega, R.; Abe, F.; Kim, J.S.; Tsuboi, Y.; Tanaka, K.; Kobayashi, H.; Sakata, Y.; Hanada, K.; Tsujimoto, H.; Kikuchi, J.; et al. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat. Plants 2019, 5, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, Y.; Li, Z.; Hsu, C.C.; Liu, X.; Fu, L.; Hou, Y.J.; Du, Y.; Xie, S.; Zhang, C.; et al. Reciprocal Regulation of the TOR Kinase and ABA Receptor Balances Plant Growth and Stress Response. Mol. Cell 2018, 69, 100–112.e106. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Xiao, L.; Hua, K.; Zou, C.; Zhao, Y.; Bressan, R.A.; Zhu, J.K. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc. Natl. Acad. Sci. USA 2018, 115, 6058–6063. [Google Scholar] [CrossRef]
- Kulik, A.; Anielska-Mazur, A.; Bucholc, M.; Koen, E.; Szymanska, K.; Zmienko, A.; Krzywinska, E.; Wawer, I.; McLoughlin, F.; Ruszkowski, D.; et al. SNF1-related protein kinases type 2 are involved in plant responses to cadmium stress. Plant Physiol. 2012, 160, 868–883. [Google Scholar] [CrossRef]
- Xu, J.; Chua, N.H. Dehydration stress activates Arabidopsis MPK6 to signal DCP1 phosphorylation. EMBO J. 2012, 31, 1975–1984. [Google Scholar] [CrossRef]
- Maszkowska, J.; Dębski, J.; Kulik, A.; Kistowski, M.; Bucholc, M.; Lichocka, M.; Klimecka, M.; Sztatelman, O.; Szymańska, K.P.; Dadlez, M.; et al. Phosphoproteomic Analysis Reveals that Dehydrins ERD10 and ERD14 are Phosphorylated by SNF1-related Protein Kinase 2.10 in Response to Osmotic Stress. Plant Cell Environ. 2018, 42, 931–946. [Google Scholar] [CrossRef]
- Szymańska, K.P.; Polkowska-Kowalczyk, L.; Lichocka, M.; Maszkowska, J.; Dobrowolska, G. SNF1-Related Protein Kinases SnRK2.4 and SnRK2.10 Modulate ROS Homeostasis in Plant Response to Salt Stress. Int. J. Mol. Sci. 2019, 20, 143. [Google Scholar] [CrossRef]
- Stecker, K.E.; Minkoff, B.B.; Sussman, M.R. Phosphoproteomic Analyses Reveal Early Signaling Events in the Osmotic Stress Response. Plant Physiol. 2014, 165, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Krzywińska, E.; Kulik, A.; Bucholc, M.; Fernandez, M.A.; Rodriguez, P.L.; Dobrowolska, G. Protein phosphatase type 2C PP2CA together with ABI1 inhibits SnRK2.4 activity and regulates plant responses to salinity. Plant Signal. Behav. 2016, 11, e1253647. [Google Scholar] [CrossRef] [PubMed]
- Julkowska, M.M.; McLoughlin, F.; Galvan-Ampudia, C.S.; Rankenberg, J.M.; Kawa, D.; Klimecka, M.; Haring, M.A.; Munnik, T.; Kooijman, E.E.; Testerink, C. Identification and functional characterization of the Arabidopsis Snf1-related protein kinase SnRK2.4 phosphatidic acid-binding domain. Plant Cell Environ. 2015, 38, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Song, T.; Wallrad, L.; Kudla, J.; Wang, X.; Zhang, W. Tissue-specific accumulation of pH-sensing phosphatidic acid determines plant stress tolerance. Nat. Plants 2019, 5, 1012–1021. [Google Scholar] [CrossRef]
- Takahashi, F.; Kuromori, T.; Urano, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Drought Stress Responses and Resistance in Plants: From Cellular Responses to Long-Distance Intercellular Communication. Front. Plant Sci. 2020, 11, 556972. [Google Scholar] [CrossRef]
- Yoshida, T.; Fernie, A.R.; Shinozaki, K.; Takahashi, F. Long-distance stress and developmental signals associated with abscisic acid signaling in environmental responses. Plant J. 2021, 105, 477–488. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 1994, 6, 251–264. [Google Scholar] [CrossRef]
- Maruyama, K.; Todaka, D.; Mizoi, J.; Yoshida, T.; Kidokoro, S.; Matsukura, S.; Takasaki, H.; Sakurai, T.; Yamamoto, Y.Y.; Yoshiwara, K.; et al. Identification of cis-acting promoter elements in cold- and dehydration-induced transcriptional pathways in Arabidopsis, rice, and soybean. DNA Res. 2012, 19, 37–49. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, M.; Satoh, R.; Maruyama, K.; Parvez, M.M.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 2005, 17, 3470–3488. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, P.; Ho, T.H. Modular nature of abscisic acid (ABA) response complexes: Composite promoter units that are necessary and sufficient for ABA induction of gene expression in barley. Plant Cell 1996, 8, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef]
- Engels, C.; Fuganti-Pagliarini, R.; Marin, S.R.; Marcelino-Guimaraes, F.C.; Oliveira, M.C.; Kanamori, N.; Mizoi, J.; Nakashima, K.; Yamaguchi-Shinozaki, K.; Nepomuceno, A.L. Introduction of the rd29A:AtDREB2A CA gene into soybean (Glycine max L. Merril) and its molecular characterization in leaves and roots during dehydration. Genet. Mol. Biol. 2013, 36, 556–565. [Google Scholar] [CrossRef]
- Qin, F.; Sakuma, Y.; Tran, L.S.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Fujita, M.; Umezawa, T.; Sawano, Y.; Miyazono, K.; et al. Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress-responsive gene expression. Plant Cell 2008, 20, 1693–1707. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Qin, F.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18822–18827. [Google Scholar] [CrossRef]
- Morimoto, K.; Ohama, N.; Kidokoro, S.; Mizoi, J.; Takahashi, F.; Todaka, D.; Mogami, J.; Sato, H.; Qin, F.; Kim, J.S.; et al. BPM-CUL3 E3 ligase modulates thermotolerance by facilitating negative regulatory domain-mediated degradation of DREB2A in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E8528–E8536. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Mizoi, J.; Tanaka, H.; Maruyama, K.; Qin, F.; Osakabe, Y.; Morimoto, K.; Ohori, T.; Kusakabe, K.; Nagata, M.; et al. Arabidopsis DPB3-1, a DREB2A interactor, specifically enhances heat stress-induced gene expression by forming a heat stress-specific transcriptional complex with NF-Y subunits. Plant Cell 2014, 26, 4954–4973. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, J.; Kanazawa, N.; Kidokoro, S.; Takahashi, F.; Qin, F.; Morimoto, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Heat-induced inhibition of phosphorylation of the stress-protective transcription factor DREB2A promotes thermotolerance of Arabidopsis thaliana. J. Biol. Chem. 2019, 294, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Mizoi, J.; Yoshida, T.; Fujita, Y.; Nakajima, J.; Ohori, T.; Todaka, D.; Nakashima, K.; Hirayama, T.; Shinozaki, K.; et al. An ABRE Promoter Sequence is Involved in Osmotic Stress-Responsive Expression of the DREB2A Gene, Which Encodes a Transcription Factor Regulating Drought-Inducible Genes in Arabidopsis. Plant Cell Physiol. 2011, 52, 2136–2146. [Google Scholar] [CrossRef]
- Himmelbach, A.; Hoffmann, T.; Leube, M.; Hohener, B.; Grill, E. Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis. EMBO J. 2002, 21, 3029–3038. [Google Scholar] [CrossRef]
- Puranik, S.; Sahu, P.P.; Srivastava, P.S.; Prasad, M. NAC proteins: Regulation and role in stress tolerance. Trends Plant Sci. 2012, 17, 369–381. [Google Scholar] [CrossRef]
- Tran, L.S.; Nakashima, K.; Sakuma, Y.; Simpson, S.D.; Fujita, Y.; Maruyama, K.; Fujita, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 2004, 16, 2481–2498. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, H.; Maruyama, K.; Kidokoro, S.; Ito, Y.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; Nakashima, K. The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol. Genet. Genom. 2010, 284, 173–183. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Kim, S.Y.; Hyeon do, Y.; Kim, D.H.; Dong, T.; Park, Y.; Jin, J.B.; Joo, S.H.; Kim, S.K.; Hong, J.C.; et al. The Arabidopsis NAC transcription factor ANAC096 cooperates with bZIP-type transcription factors in dehydration and osmotic stress responses. Plant Cell 2013, 25, 4708–4724. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Kim, Y.S.; Han, S.H.; Lee, B.D.; Paek, N.C. The Arabidopsis Transcription Factor NAC016 Promotes Drought Stress Responses by Repressing AREB1 Transcription through a Trifurcate Feed-Forward Regulatory Loop Involving NAP. Plant Cell 2015, 27, 1771–1787. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, H.; Maruyama, K.; Takahashi, F.; Fujita, M.; Yoshida, T.; Nakashima, K.; Myouga, F.; Toyooka, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. SNAC-As, stress-responsive NAC transcription factors, mediate ABA-inducible leaf senescence. Plant J. 2015, 84, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Yamaguchi-Shinozaki, K.; Urao, T.; Iwasaki, T.; Hosokawa, D.; Shinozaki, K. Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 1997, 9, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. MYC2: The master in action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Seo, P.J.; Xiang, F.; Qiao, M.; Park, J.Y.; Lee, Y.N.; Kim, S.G.; Lee, Y.H.; Park, W.J.; Park, C.M. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sunarti, S.; Kissoudis, C.; Visser, R.G.F.; van der Linden, C.G. The Role of Tomato WRKY Genes in Plant Responses to Combined Abiotic and Biotic Stresses. Front. Plant Sci. 2018, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Rushton, D.L.; Tripathi, P.; Rabara, R.C.; Lin, J.; Ringler, P.; Boken, A.K.; Langum, T.J.; Smidt, L.; Boomsma, D.D.; Emme, N.J.; et al. WRKY transcription factors: Key components in abscisic acid signalling. Plant. Biotechnol. J. 2012, 10, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Yan, L.; Liu, Z.Q.; Cao, Z.; Mei, C.; Xin, Q.; Wu, F.Q.; Wang, X.F.; Du, S.Y.; Jiang, T.; et al. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell 2010, 22, 1909–1935. [Google Scholar] [CrossRef]
- Chen, H.; Lai, Z.; Shi, J.; Xiao, Y.; Chen, Z.; Xu, X. Roles of Arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol. 2010, 10, 281. [Google Scholar] [CrossRef]
- Ren, X.; Chen, Z.; Liu, Y.; Zhang, H.; Zhang, M.; Liu, Q.; Hong, X.; Zhu, J.K.; Gong, Z. ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J. 2010, 63, 417–429. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).