Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Dry Matter and Total Soluble Solids Content in Nettle Leaves
2.2. Ascorbic Acid Content and Titratable Acidity of Nettle Leaves
2.3. Total Carotenoid, Chlorophyll a, and Chlorophyll b Content of Nettle Leaves
2.4. Total Phenolic Content of Nettle Leaves
2.5. The Antioxidant Activity of Nettle Leaves
2.6. Crude Ash and Mineral Elements in Nettle Leaves
3. Materials and Methods
3.1. Plant Material Collection and Soil Properties
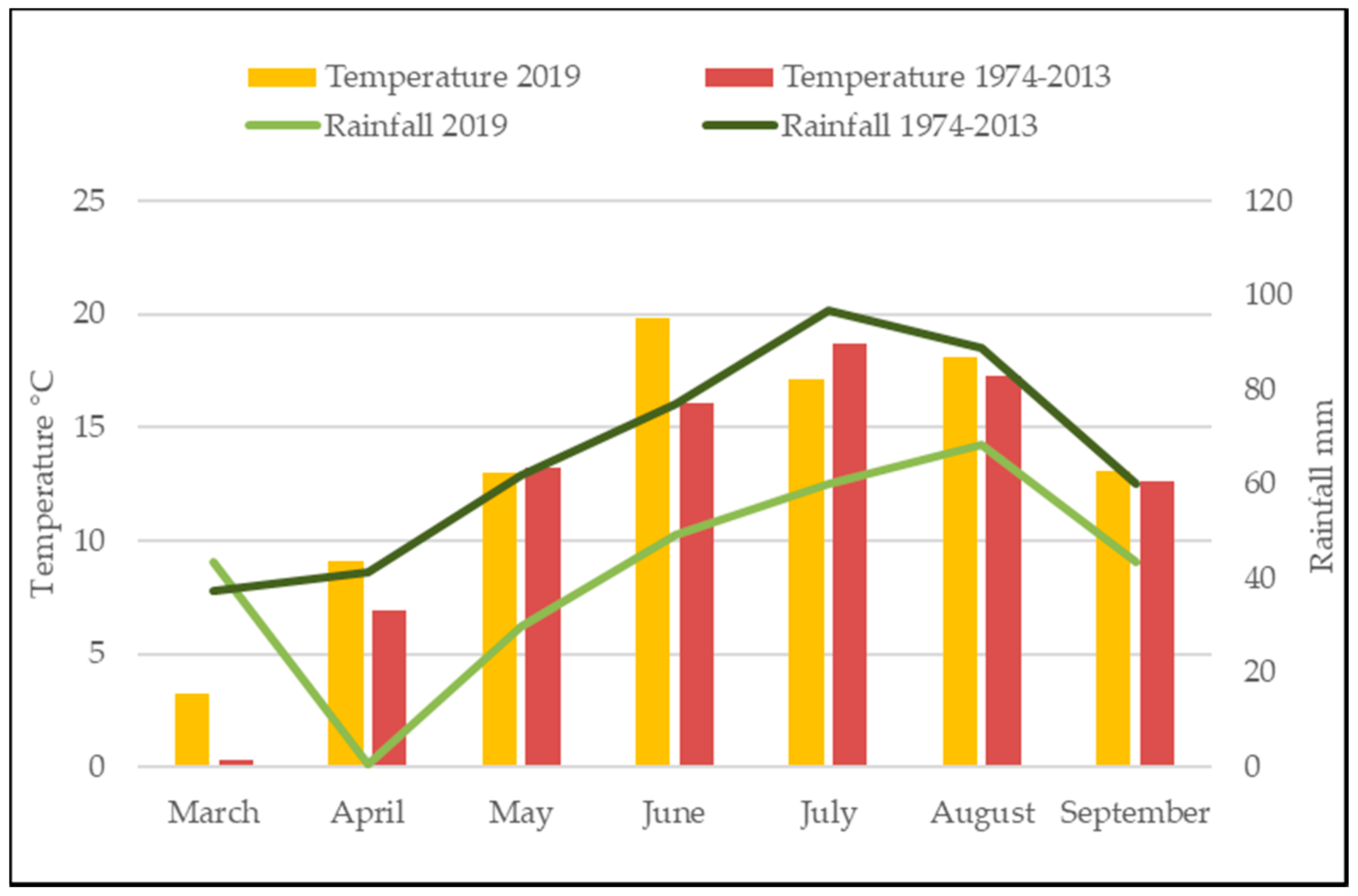
3.2. Meteorological Conditions
3.3. Chemical Analysis
3.3.1. Chemical Analysis of Soil
3.3.2. Dry Matter and Total Soluble Solids Content
3.3.3. Ascorbic Acid Content and Titratable Acidity
3.3.4. Total Carotenoid, Chlorophyll a, and Chlorophyll b Content
3.3.5. Total Phenolic Content
3.3.6. Antioxidant Activity
3.3.7. Crude Ash and Mineral Elements
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jankauskienė, Z.; Gruzdevienė, E. Changes in the productivity of wild and cultivated stinging nettle (Urtica dioica L.) as influenced by the planting density and crop age. Zemdirbyste 2015, 102, 31–40. [Google Scholar] [CrossRef][Green Version]
- Biesiada, A.; Kurcharska, A.; Sokól-Lêtowska, A.; Kus, A. Effect of the age of plantation and harvest term on chemical composition and antioxidant activity of stinging nettle (Urtica Dioica L.). Ecol. Chem. Eng. 2010, 17, 1061–1067. Available online: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BPG8-0060-0014 (accessed on 28 March 2021).
- Rafajlovska, V.; Kavrakovski, Z.; Siminovska, J.; Srbinoska, M. Determination of protein and mineral contents in stinging nettle. Qual. Life 2013, 4, 26–30. [Google Scholar] [CrossRef]
- Rutto, L.K.; Xu, Y.; Ramirez, E.; Brandt, M. Mineral Properties and Dietary Value of Raw and Processed Stinging Nettle (Urtica dioica L.). Int. J. Food Sci. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Said, A.A.H.; Otmani, I.S.E.; Derfoufi, S.; Benmoussa, A. Highlights on nutritional and therapeutic value of stinging nettle (Urtica Dioica). Int. J. Pharm. Pharm. Sci. 2015, 7, 8–14. Available online: https://innovareacademics.in/journals/index.php/ijpps/article/view/8165 (accessed on 28 March 2021).
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacognostical review of Urtica dioica L. Int. J. Gren Pharm. 2014, 201–209. [Google Scholar] [CrossRef]
- Upton, R. Stinging nettles leaf (Urtica dioica L.): Extraordinary vegetable medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Otles, S.; Yalcin, B. Phenolic Compounds Analysis of Root, Stalk, and Leaves of Nettle. Sci. World J. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zeković, Z.; Cvetanović, A.; Švarc-Gajić, J.; Gorjanović, S.; Sužnjević, D.; Mašković, P.; Savić, S.; Radojković, M.; Đurović, S. Chemical and biological screening of stinging nettle leaves extracts obtained by modern extraction techniques. Ind. Crops Prod. 2017, 108, 423–430. [Google Scholar] [CrossRef]
- Vajić, U.J.; Grujić-Milanović, J.; Živković, J.; Šavikin, K.; Godevac, D.; Miloradović, Z.; Bugarski, B.; Mihailović-Stanojević, N. Optimization of extraction of stinging nettle leaf phenolic compounds using response surface methodology. Ind. Crop. Prod. 2015, 74, 912–917. [Google Scholar] [CrossRef]
- Kavalali, G.M. Urtica: The Genus Urtica, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Chandra, S.; Rawat, D.S. Medicinal plants of the family Caryophyllaceae: A review of ethno-medicinal uses and pharmacological properties. Integr. Med. Res. 2015, 4, 123–131. [Google Scholar] [CrossRef]
- Zeipina, S.; Alsina, I.; Lepse, L.; Dūma, M. Antioxidant activity in nettle (Urtica dioica L.) and garden orache (Atriplex hortensis L.) leaves during vegetation period. Cheminė Technol. 2015, 1, 29–33. [Google Scholar] [CrossRef]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.H.; Bhat, T.M.; Sharma, P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.K.K.; Parasuraman, S. Urtica dioica L., (Urticaceae): A Stinging Nettle. Syst. Rev. Pharm. 2014, 5, 6–8. [Google Scholar] [CrossRef]
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging nettle, Urtica dioica L.: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Cummings, A.J.; Olsen, M. Mechanism of Action of Stinging Nettles. Wilderness Environ. Med. 2011, 22, 136–139. [Google Scholar] [CrossRef]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrients Sources Added to Food). Scientific Opinion on the re-evaluation of chlorophylls (E 140(i)) as food additives. EFSA J. 2015, 13, 1–51. [Google Scholar] [CrossRef]
- Skalozubova, T.A.; Reshetova, V.O. Leaves of Common Nettle (Urtica dioica L.) As a Source of Ascorbic Acid (Vitamin C). World Appl. Sci. J. 2013, 28, 250–253. [Google Scholar] [CrossRef]
- Radman, S.; Žutić, I.; Fabek, S.; Žlabur, J.Š.; Benko, B.; Toth, N.; Čoga, L. Influence of nitrogen fertilization on chemical composition of cultivated nettle. Emir. J. Food. Agric. 2015, 27, 889–896. [Google Scholar] [CrossRef]
- Shonte, T.T.; Duodu, K.G.; de Kock, H.L. Effect of drying methods on chemical composition and antioxidant activity of underutilized stinging nette leaves. Heliyon 2020, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Assessment Report for Herbal Substance(s), Herbal Preparation(s) or Combinations thereof with Tradicional Use Urtica dioica L., Urtica urens L., herba; European Medicines Agency: London, UK, 2008; Available online: http://www.emea.europa.eu (accessed on 28 March 2021).
- Drincovich, M.F.; Voll, L.M.; Maurino, V.G. Editorial: On the Diversity of Roles of Organic Acids. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef]
- Truică, G.; Teodor, E.D.; Radu, G.L. Organic acids assesments in medicinal plants by capillary electrophoresis. Rev. Roum. Chim. 2013, 58, 809–814. Available online: http://revroum.lew.ro/wp-content/uploads/2013/9/Art%2011.pdf (accessed on 28 March 2021).
- Guil-Guerrero, J.L.; Rebolloso-Fuentes, M.M.; Torija Isasa, M.E. Fatty acids and carotenoids from Stinging Nettle (Urtica dioica L.). J. Food Compost. Anal. 2003, 16, 111–119. [Google Scholar] [CrossRef]
- Kukrić, Z.Z.; Topalić-Trivunović, L.N.; Kukavica, B.M.; Matoš, S.B.; Pavičić, S.S.; Boroja, M.M.; Savić, A.S. Characterization of antioxidant and antimicrobial activities of nettle leaves (Urtica dioica L.). Acta Period. Technol. 2012, 43, 257–272. [Google Scholar] [CrossRef]
- Šircelj, H.; Mikulic-Petkovsek, M.; Veberič, R.; Hudina, M.; Slatnar, A. Lipophilic antioxidants in edible weeds from agricultural areas. Turk. J. Agric. For. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Grevsen, K.; Frette, X.C.; Christensen, L.P. Concentration and Composition of Flavonol Glycosides and Phenolic Acids in Aerial Parts of Stinging Nettle (Urtica dioica L.) are Affected by Nitrogen Fertilization and by Harvest Time. Europ. J. Hort. Sci. 2008, 73, 20–27. Available online: https://www.pubhort.org/ejhs/2008/file_539290.pdf (accessed on 28 March 2021).
- Biesiada, A.; Woloszczak, E.; Sokól-Lêtowska, A.; Kurcharska, A.Z.; Nawirska-Olszanska, A. The effect of nitrogen form and dose on yield, chemical composition and antioxidant avctivity of stinging Nettle (Urtica dioica L.). Herba Pol. 2009, 55, 84–93. Available online: https://www.herbapolonica.pl/magazines-files/1406128-Pages%20from%20Herba_3-10.pdf (accessed on 28 March 2021).
- Kőszegi, K.; Békássy-Molnár, E.; Koczka, N.; Kerner, T.; Stefanovits-Bányai, É. Changes in Total Polyphenol Content and Antioxidant Capasity of Stinging Nettle (Urtica dioica L.) from Spring to Autumn. Period. Polytech. Chem. Eng. 2020, 64, 548–554. [Google Scholar] [CrossRef]
- Carvalho, A.R.; Costa, G.; Figueirinha, A.; Liberal, J.; Prior, J.A.V.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Urtica spp.: Phenolic composition, safety, antioxidant and anti-inflammatory activities. Food Res. Int. 2017, 99, 485–494. [Google Scholar] [CrossRef]
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging nettle (Urtica diodica L.): A reservoir of nutrition and bioactive components with great functional potential. J. Food Meas. Charact. 2017, 11, 423–433. [Google Scholar] [CrossRef]
- ISO 10390. Soil Quality–Determination of pH; International Organization for Standardization: Geneva, Switzerland, 2005; p. 5. [Google Scholar]
- Égnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen uber Die Chemische Bodenanalyse Als Grundlage fur Die Beurteilung Des Nahrstoffzustandes der Boden, II: Chemische Extraktionsmethoden zur Phosphor, und Kalium-Bestimmung; Annaler 26; Kungliga Lantbrukshogskolans: Stockholm, Sweden, 1960; pp. 199–215. [Google Scholar]
- ISO 11261. Soil Quality–Detrmination of Total Nitrogen–Modified Kjeldahl Method; International Organization for Standardization: Geneva, Switzerland, 1995; p. 4. [Google Scholar]
- Latimer, G.W. Official Methods of Analysis of AOAC International, 20th ed.; Latimer, G.W., Jr., Ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Straumite, E.; Kruma, Z.; Galoburda, R. Pigments in mint leaves and stems. Agron. Res. 2015, 13, 1104–1111. Available online: https://agronomy.emu.ee/vol134/13_4_24_B5.pdf (accessed on 28 March 2021).
- First Commission Directive of 15 June 1971 Establishing Community Methods of Analysis for the Official Control of Feeding-Stuffs (71/250/EEC). p. 22. Available online: http://data.europa.eu/eli/dir/1971/250/2005-02-16 (accessed on 28 March 2021).
- Second Commission Directive of 18 November 1971 Establishing Community Methods of Analysis for the Official Control of Feedingstuffs (71/393/EEC). p. 12. Available online: http://data.europa.eu/eli/dir/1971/393/1998-10-09 (accessed on 28 March 2021).
- Fourth Commission Directive of 5 December 1972 Establishing Community Methods of Analysis for the Official Control of Feedingstuffs (73/46/EEC). p. 7. Available online: http://data.europa.eu/eli/dir/1973/46/1999-05-26 (accessed on 28 March 2021).

| April | May | June | July | August | September | |
|---|---|---|---|---|---|---|
| Dry matter, % | 21.24 ± 0.53 bc* | 21.57 ± 0.15 bc | 24.41 ± 0.15 a | 21.13 ± 0.18 bc | 22.61 ± 0.53 ab | 20.48 ± 0.43 c |
| Total soluble solids, % | 11.70 ± 0.49 a | 3.47 ± 0.26 d | 1.47 ± 0.09 f | 4.60 ± 0.15 c | 2.57 ± 0.03 e | 8.07 ± 0.26 b |
| Ascorbic acid. mg 100 g−1 | 8.17 ± 0.59 b | 6.45 ± 0.29 c | 1.31 ± 0.25 d | 9.02 ± 0.29 b | 25.66 ± 1.04 a | 0.58 ± 0.15 d |
| Total carotenoids, mg 100 g−1 | 12.68 ± 0.15 cd | 28.41 ± 0.45 a | 6.46 ± 0.82 e | 15.70 ± 0.79 c | 19.52 ± 0.89 b | 9.68 ± 0.86 d |
| Chlorophyll a, mg 100 g−1 | 40.47 ± 0.59 d | 161.02 ± 1.86 a | 18.05 ± 0.41 e | 64.31 ± 1.01 c | 80.46 ± 2.15 b | 39.43 ± 1.71 d |
| Chlorophyll b, mg 100 g−1 | 16.40 ± 0.95 c | 67.69 ± 1.96 a | 6.48 ± 0.15 d | 32.60 ± 0.74 b | 36.97 ± 1.74 b | 14.67 ± 1.46 cd |
| Titratable acidity, % | 1.27 ± 0.32 ab | 1.91 ± 0.28 a | 1.10 ± 0.01 b | 0.96 ± 0.16 b | 1.60 ± 0.16 ab | 1.43 ± 0.27 ab |
| Total phenolics, mg GAE g−1 | 7.96 ± 0.02 c | 3.28 ± 0.76 e | 3.62 ± 0.01 e | 5.32 ± 0.01 d | 19.07 ± 0.92 a | 13.96 ± 0.02 b |
| Antioxidant activity, % | 63.35 ± 0.06 d | 95.17 ± 0.01 a | 57.32 ± 0.01 e | 52.92 ± 0.30 f | 68.99 ± 0.23 c | 84.44 ± 0.01 b |
| April | May | June | July | August | September | |
|---|---|---|---|---|---|---|
| Crude ash, % | 3.06 ± 0.10 c* | 3.14 ± 0.07 c | 3.48 ± 0.02 bc | 4.37 ± 0.14 a | 4.70 ± 0.20 a | 3.73 ± 0.19 b |
| Phosphorus, % | 1.02 ± 0.01 a | 0.83 ± 0.01 b | 0.76 ± 0.01 d | 0.72 ± 0.01 e | 0.80 ± 0.01 bc | 0.77 ± 0.02 cd |
| Potassium, % | 3.60 ± 0.01 a | 3.51 ± 0.01 b | 2.98 ± 0.01 d | 2.59 ± 0.01 e | 3.41 ± 0.01 c | 2.98 ± 0.00 d |
| Calcium, % | 2.21 ± 0.01 f | 2.85 ± 0.01 d | 2.82 ± 0.01 e | 3.32 ± 0.02 b | 3.05 ± 0.01 c | 3.97 ± 0.01 a |
| Magnesium, % | 0.42 ± 0.01 f | 0.46 ± 0.01 d | 0.67 ± 0.01 b | 0.68 ± 0.02 b | 0.60 ± 0.01 c | 0.81 ± 0.01 a |
| Iron, mg kg−1 | 526.20 ± 0.12 a | 148.37 ± 0.09 e | 172.53 ± 0.09 c | 165.40 ± 0.15 d | 112.60 ± 0.12 f | 223.60 ± 0.12 b |
| Copper, mg kg−1 | 13.40 ± 0.15 d | 17.53 ± 0.09 b | 18.43 ± 0.12 a | 13.87 ± 0.09 c | 11.90 ± 0.12 e | 10.23 ± 0.09 f |
| Manganese, mg kg−1 | 40.90 ± 0.06 e | 48.13 ± 0.09 c | 48.87 ± 0.15 b | 57.40 ± 0.06 a | 30.50 ± 0.06 f | 42.11 ± 0.02 d |
| Zinc, mg kg−1 | 34.30 ± 0.12 a | 17.93 ± 0.09 b | 16.60 ± 0.12 d | 17.40± 0.06 c | 12.70 ± 0.12 f | 14.20 ± 0.12 e |
| Boron, mg kg−1 | 40.07 ± 0.12 e | 31.93 ± 0.15 f | 50.20 ± 0.12 d | 58.40 ± 0.12 b | 52.77 ± 0.09 c | 62.13 ± 0.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulauskienė, A.; Tarasevičienė, Ž.; Laukagalis, V. Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.). Plants 2021, 10, 686. https://doi.org/10.3390/plants10040686
Paulauskienė A, Tarasevičienė Ž, Laukagalis V. Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.). Plants. 2021; 10(4):686. https://doi.org/10.3390/plants10040686
Chicago/Turabian StylePaulauskienė, Aurelija, Živilė Tarasevičienė, and Valdas Laukagalis. 2021. "Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.)" Plants 10, no. 4: 686. https://doi.org/10.3390/plants10040686
APA StylePaulauskienė, A., Tarasevičienė, Ž., & Laukagalis, V. (2021). Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.). Plants, 10(4), 686. https://doi.org/10.3390/plants10040686






