Polyphenol Diversity and Antioxidant Activity of European Cistus creticus L. (Cistaceae) Compared to Six Further, Partly Sympatric Cistus Species
Abstract
1. Introduction
2. Results
2.1. Extract Composition and Chemotypes
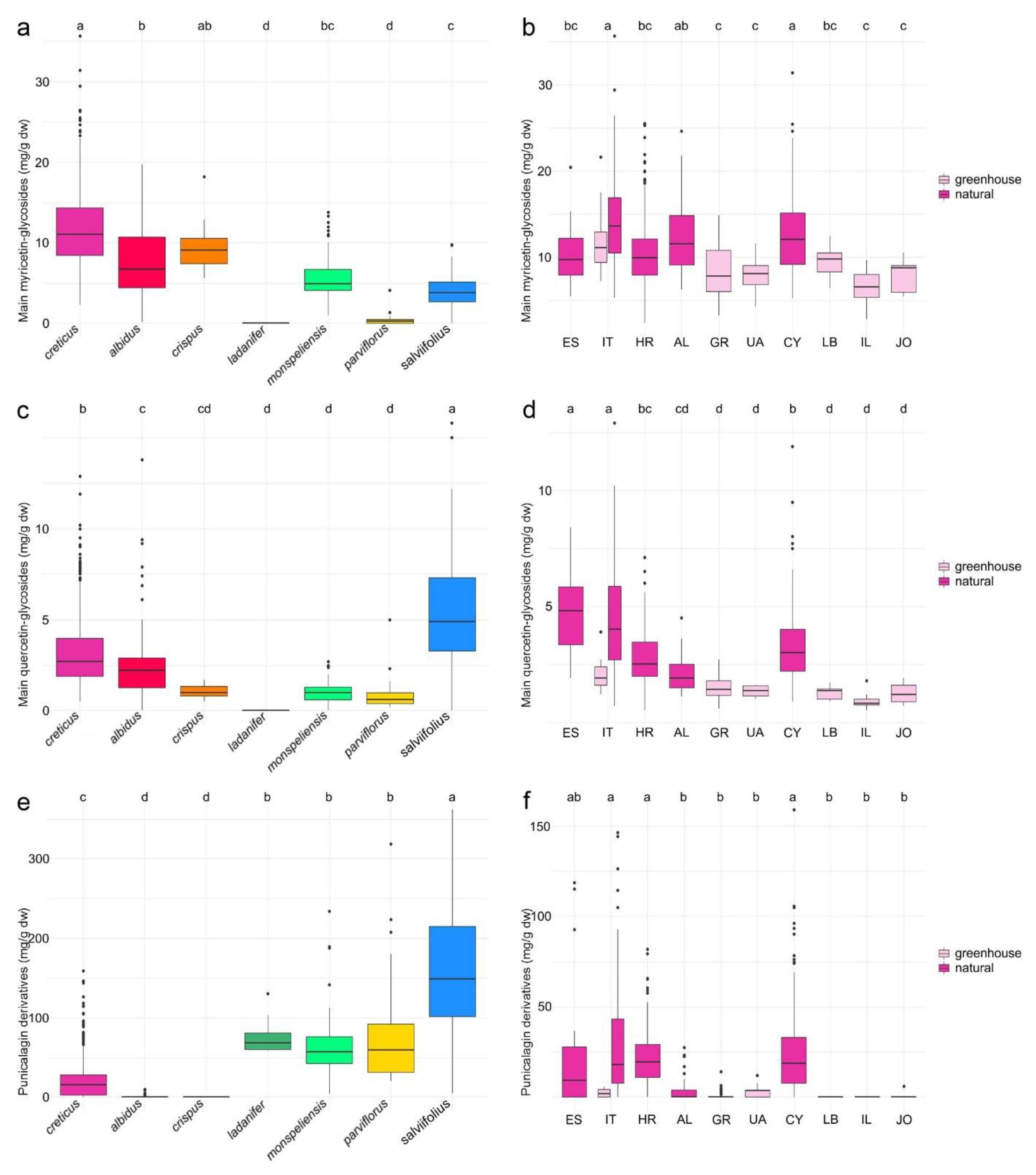
2.1.1. Myricetin Glycosides
2.1.2. Quercetin Glycosides
2.1.3. Punicalagin Derivatives
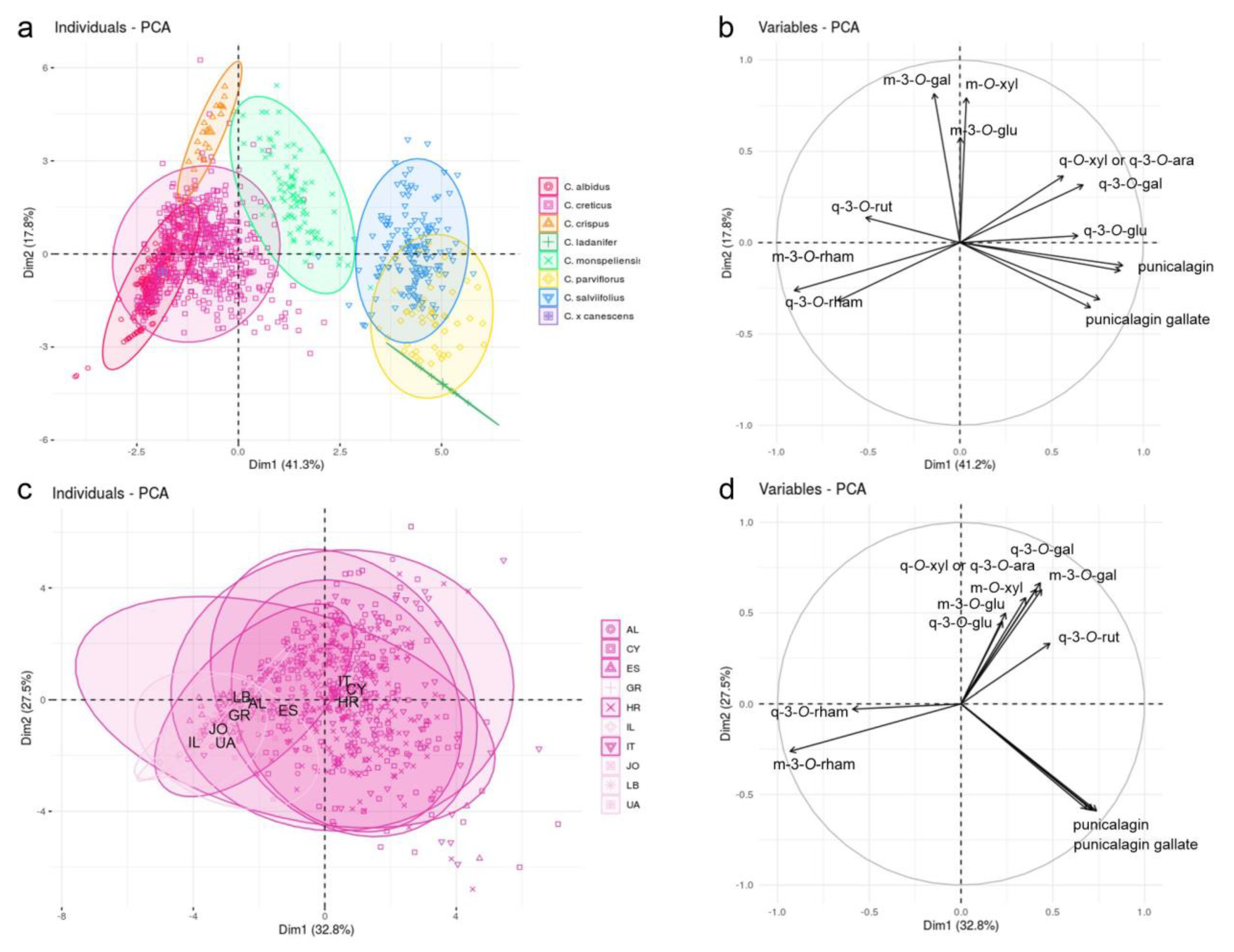
2.2. Sample Classification
2.3. Extract Composition and Classification of Trade Samples
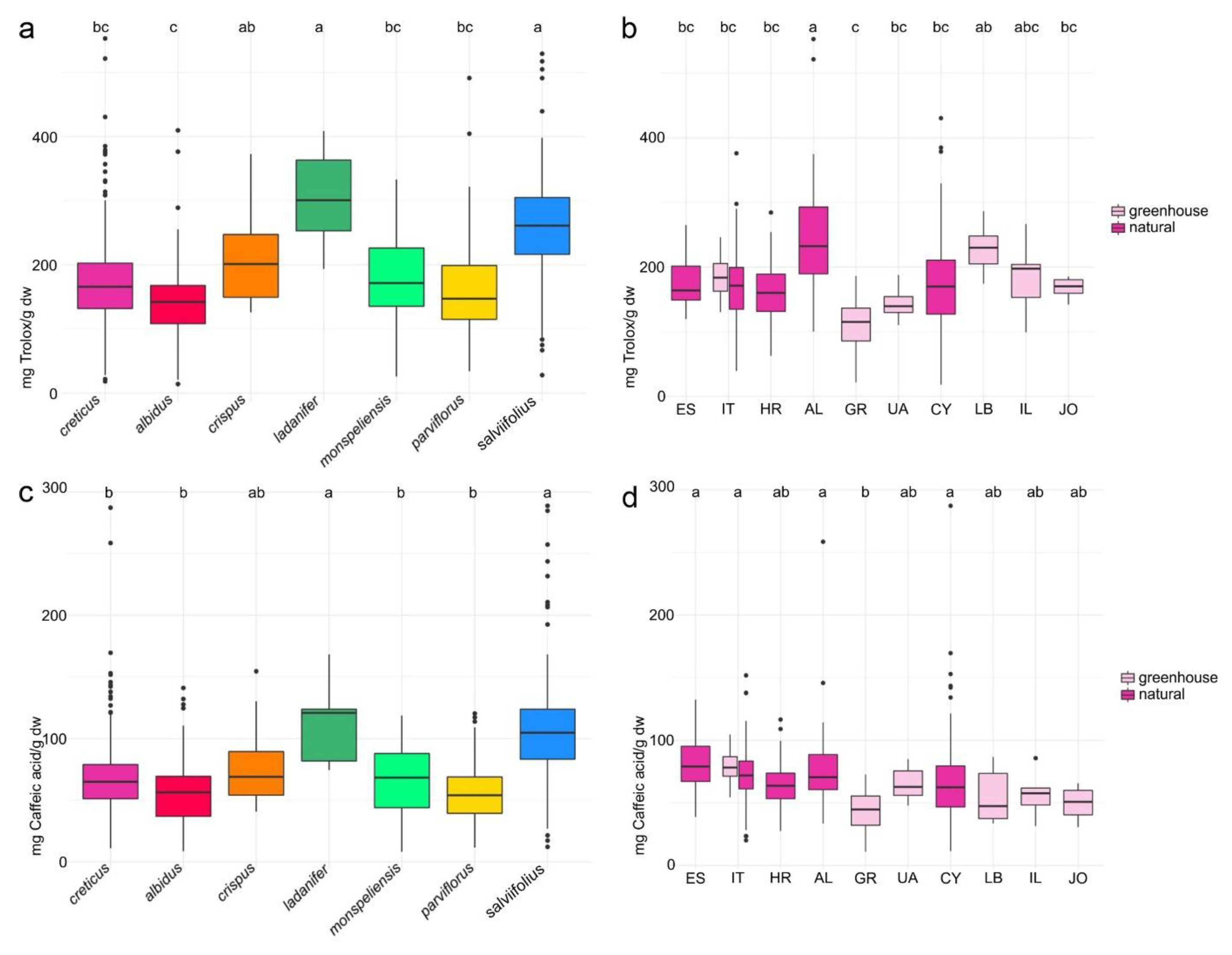
2.4. Antioxidant Activity and Total Phenolics
3. Discussion
4. Materials and Methods
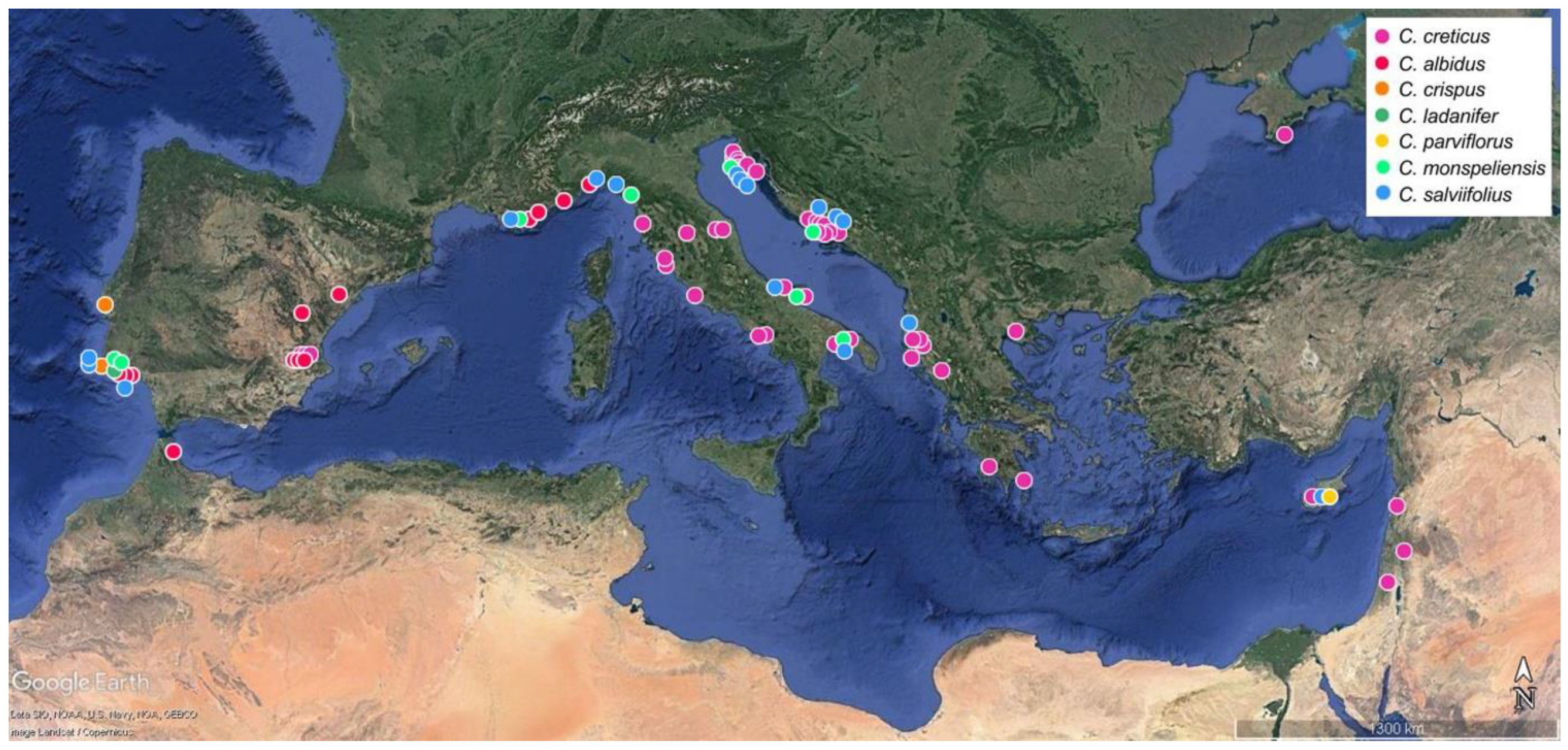
4.1. Plant Material
4.2. Sampling Procedure and Handling of Plant Material
4.3. Extractions
4.4. HPLC and HPLC-MS Analysis
4.5. Total Phenolics
4.6. DPPH Radical Scavenging Activity
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzman, B.; Vargas, P. Systematics, character evolution, and biogeography of Cistus L. (Cistaceae) based on ITS, trnL-trnF, and matK sequences. Mol. Phylogenet. Evol. 2005, 37, 644–660. [Google Scholar] [CrossRef]
- Civeyrel, L.; Leclercq, J.; Demoly, J.-P.; Agnan, Y.; Quebre, N.; Pelissier, C.; Otto, T. Molecular systematics, character evolution, and pollen morphology of Cistus and Halimium (Cistaceae). Plant Syst. Evol. 2011, 295, 23–54. [Google Scholar] [CrossRef]
- Von Raab-Straube, E. Cistaceae. Euro+Med Plantbase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 5 March 2021).
- Greuter, W.; Burdet, H.M.; Long, G. Med Checklist. A Critical Inventory of Vascular Plants of the Circum-Mediterranean Countries; Conservatoire et Jardin Botanique de la Ville de Geneve: Geneve, Switzerland; Available online: http://ww2.bgbm.org/mcl/home.asp (accessed on 5 March 2021).
- Demetzos, C.; Anastasaki, T.; Perdetzoglou, D.A. Chemometric Interpopulation Study of the Essential Oils of Cistus creticus L. Growing in Crete (Greece). Z. Naturforsch. C 2002, 57, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Paolini, J.; Falchi, A.; Quilichini, Y.; Desjobert, J.-M.; De Cian, M.-C.; Varesi, L.; Costa, J. Morphological, chemical and genetic differentiation of two subspecies of Cistus creticus L. (C. creticus subsp. eriocephalus and C. creticus subsp. corsicus). Phytochemistry 2009, 70, 1146–1160. [Google Scholar] [CrossRef] [PubMed]
- Brussel, D.E. Medicinal plants of Mt. Pelion, Greece. Econ. Bot. 2004, 58, 174–202. [Google Scholar] [CrossRef]
- Lardos, A.; Prieto-Garcia, J.; Heinrich, M. Resins and Gums in Historical Iatrosophia Texts from Cyprus—A Botanical and Medico-pharmacological Approach. Front. Pharmacol. 2011, 2, 32. [Google Scholar] [CrossRef]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Usai, M. Comparison of essential oils from Cistus species growing in Sardinia. Nat. Prod. Res. 2016, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Usai, M. Interpopulation Variability in the Essential Oil Composition of Cistus creticus subsp. eriocephalus from Sardinia. Chem. Biodivers. 2018, 15, e1800151. [Google Scholar] [CrossRef]
- Barrajon-Catalan, E.; Fernandez-Arroyo, S.; Roldan, C.; Guillen, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A Systematic Study of the Polyphenolic Composition of Aqueous Extracts Deriving from Several Cistus Genus Species: Evolutionary Relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef]
- Gori, A.; Ferrini, F.; Marzano, M.C.; Tattini, M.; Centritto, M.; Baratto, M.C.; Pogni, R.; Brunetti, C. Characterisation and Antioxidant Activity of Crude Extract and Polyphenolic Rich Fractions from C. incanus Leaves. Int. J. Mol. Sci. 2016, 17, 1344. [Google Scholar] [CrossRef]
- Maggi, F.; Lucarini, D.; Papa, F.; Peron, G.; Dall’Acqua, S. Phytochemical analysis of the labdanum-poor Cistus creticus subsp. eriocephalus (Viv.) Greuter et Burdet growing in central Italy. Biochem. Syst. Ecol. 2016, 66, 50–57. [Google Scholar] [CrossRef]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Juliano, C.C. Analysis and Potential Antimicrobial Activity of Phenolic Compounds in the Extracts of Cistus creticus Subspecies from Sardinia. Nat. Prod. J. 2018, 8, 166–174. [Google Scholar] [CrossRef]
- Barrajon-Catalan, E.; Fernandez-Arroyo, S.; Saura, D.; Guillen, E.; Fernandez-Guiterrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Petereit, F. Polyphenolische Inhaltsstoffe und Untersuchungen zur Entzündungshemmenden Aktivität der Traditionellen Arzneipflanze Cistus incanus L. (Cistaceae). Ph.D. Thesis, Westfälische Wilhelms-Universität, Münster, Germany, 1992. [Google Scholar]
- Droebner, K.; Ehrhardt, C.; Poetter, A.; Ludwig, S.; Planz, O. CYSTUS052, a polyphenol-rich plant extract, exerts anti-influenza activity in mice. Antivir. Res. 2007, 76, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, K.; Tung, N.H.; Ohta, T.; Uto, T.; Raekiansayh, M.; Grötzinger, K.; Rausch, H.; Shoyama, Y.; Rauwald, H.W.; Morita, K. The old pharmaceutical oleoresin labdanum of Cistus creticus L., exerts pronounced in vitro anti-dengue virus activity. J. Ethnopharm. 2020, 257, 112316. [Google Scholar] [CrossRef]
- Rebensburg, S.; Helfer, M.; Schneider, M.; Koppensteiner, H.; Eberle, J.; Schindler, M.; Gürtler, L.; Brack-Werner, R. Potent in vitro antiviral activity of Cistus incanus extracts against HIV and Filoviruses targets viral envelope proteins. Nat. Sci. Rep. 2016, 6, 20394. [Google Scholar] [CrossRef]
- Feng, J.; Leone, J.; Schweig, S.; Zhang, Y. Evaluation of Natural and Botanical Medicines for Activity against Growing and Non-growing Forms of B. burgdorferi. Front. Med. 2020, 7, 6. [Google Scholar] [CrossRef]
- Rauwald, H.; Liebo, T.; Grötzinger, K.; Lehmann, J.; Kuchta, K. Labdanum and Labdanes of Cistus creticus and C. ladanifer: Anti-Borrelia activity and its phytochemical profiling. Phytomedicine 2019, 60, 152977. [Google Scholar] [CrossRef]
- Tomas-Menor, L.; Morales-Soto, A.; Barrajon-Catalan, E.; Roldan-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the antibacterial activity and the composition of extracts derived from various Spanish Cistus species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef]
- Tomas-Menor, L.; Barrajon-Catalan, E.; Segura-Carretero, A.; Marti, N.; Saura, D.; Menendez, J.A.; Joven, J.; Micol, V. The Promiscuous and Synergic Molecular Interaction of Polyphenols in Bactericidal Activity: An Opportunity to Improve the Performance of Antibiotics? Phytother. Res. 2015, 29, 466–473. [Google Scholar] [CrossRef]
- Viapiana, A.; Konopacka, A.; Waleron, K.; Wesolowski, M. Cistus incanus L. commercial products as a good source of polyphenols in human diet. Ind. Crop. Prod. 2017, 107, 297–304. [Google Scholar] [CrossRef]
- Hatziantoniou, S.; Dimas, K.; Georgopoulos, A.; Sotiriadou, N.; Demetzos, C. Cytotoxic and antitumor activity of liposome-incorporated sclareol against cancer cell lines and human colon cancer xenografts. Pharmacol. Res. 2006, 53, 80–87. [Google Scholar] [CrossRef]
- Moreira, H.; Slezak, A.; Szyjka, A.; Oszmianski, J.; Gasiorowski, K. Antioxidant and Cancer Chemopreventive Activities of Cistus and Pomegranate Polyphenols. Acta Pol. Pharm. 2017, 74, 688–698. [Google Scholar] [PubMed]
- Skoric, M.; Todorovic, S.; Gligorijevic, N.; Jankovic, R.; Zivkovic, S.; Ristic, M.; Radulovic, M. Cytotoxic activity of ethanol extracts of in vitro grown Cistus creticus subsp. creticus L. on human cancer cell lines. Ind. Crop. Prod. 2012, 38, 153–159. [Google Scholar] [CrossRef]
- Kuchta, A.; Konopacka, A.; Waleron, K.; Viapiana, A.; Wesolowski, M.; Dabkowski, K.; Cwiklinska, A.; Mickiewicz, A.; Sledzinska, A.; Wieczorek, E.; et al. The effect of Cistus incanus herbal tea supplementation on oxidative stress markers and lipid profile in healthy adults. Cardiol. J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Attaguile, G.; Russo, A.; Campisi, A.; Savoca, F.; Acquaviva, R.; Ragusa, N.; Vanella, A. Antioxidant activity and protective effect on DNA cleavage of extracts from Cistus incanus L. and Cistus monspeliensis L. Cell Biol. Toxicol. 2000, 16, 83–90. [Google Scholar] [CrossRef]
- Gawel-Beben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzepek-Gomolka, M.; Antowiewicz, B. Characterization of Cistus x incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Wittpahl, G.; Kölling-Speer, I.; Basche, S.; Hermann, E.; Hannig, M.; Speer, K.; Hannig, C. The polyphenolic composition of Cistus incanus herbal tea and its antibacterial and anti-adherent activity against Streptococcus mutans. Planta Med. 2015, 81, 1727–1735. [Google Scholar] [CrossRef]
- Waed, A.; Ghalia, S.; Adawia, K. Evaluation of Radical Scavenging Activity, Total Phenolics and Total Flavonoids Contents of Cistus Species in Syria. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1071–1077. [Google Scholar]
- Dimcheva, V.; Karsheva, M. Cistus incanus from Strandja Mountain as a Source of Bioactive Antioxidants. Plants 2018, 7, 8. [Google Scholar] [CrossRef]
- Moosmang, S.; Sturm, S.; Novak, J.; Lukas, B.; Stuppner, H. Differentiation between Cistus L. (sub-)species (Cistaceae) using NMR metabolic fingerprinting. Planta Med. 2020, 86, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Lukas, B.; Bragagna, L.; Starzyk, K.; Labedz, K.; Stolze, K.; Novak, J. Polyphenol-Variabilität von zypriotischem Cistus creticus L. In Proceedings of the VIIIth Conference of Medicinal and Aromatic Plant Research, Bonn, Germany, 10–13 September 2018. [Google Scholar]
- Barros, L.; Duenas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antifungal activity and detailed chemical characterization of Cistus ladanifer phenolic extracts. Ind. Crop. Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Riehle, P.; Vollmer, M.; Rohn, S. Phenolic compounds in Cistus incanus herbal infusions—Antioxidant capacity and thermal stability during the brewing process. Food Res. Int. 2013, 53, 891–899. [Google Scholar] [CrossRef]
- Riehle, P. Phenolische Inhaltsstoffe in Cistus incanus Tee—Charakterisierung und Stabilität innerhalb der Teezubereitung. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2014. [Google Scholar]
- Sayah, K.; Marmouzi, I.; Naceiri Mrabti, H.; Cherrah, Y.; Abbes Faouzi, M. Antioxidant Activity and Inhibitory Potential of Cistus salviifolius (L.) and Cistus monspeliensis (L.) Aerial Parts Extracts against Key Enzymes Linked to Hyperglycemia. BioMed Res. Int. 2017, 2017, 2789482. [Google Scholar] [CrossRef] [PubMed]
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (antioxidant systems) in higher plants and their response to stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer: Cham, Switzerland, 2018; pp. 253–268. [Google Scholar]
- Bautista, I.; Boscaiu, M.; Lidon, A.; Llinares, J.V.; Lull, C.; Donat, M.P.; Vicente, O. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant. 2016, 38, 9. [Google Scholar] [CrossRef]
- Borges, C.V.; Minatel, I.O.; Gomez-Gomez, H.A.; Lima, G.P.P. Medicinal Plants: Influence of environmental factors on the content of secondary metabolites. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 259–277. [Google Scholar]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Nascimento, L.B.; Ferrini, F.; Centritto, M.; Brunetti, C. Seasonal and Diurnal Variation in Leaf Phenolics of Three Medicinal Mediterranean Wild Species: What Is the Best Harvesting Moment to Obtain the Richest and the Most Antioxidant Extracts? Molecules 2020, 25, 956. [Google Scholar] [CrossRef]
- Sebastini, F.; Torre, S.; Gori, A.; Brunetti, C.; Centritto, M.; Ferrini, F.; Tattini, M. Dissecting Adaptation Mechanisms to Contrasting Solar Irradiance in the Mediterranean Shrub Cistus incanus. Int. J. Mol. Sci. 2019, 20, 3599. [Google Scholar] [CrossRef] [PubMed]
- Hopia, A.; Heinonen, M. Antioxidant activity of flavonol aglycones and their glycosides in methyl linoleate. J. Am. Oil Chem. Soc. 1999, 76, 139–144. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Aouadhi, C.; Kaddour, R.; Gruber, M.; Zargouni, H.; Zaouali, W.; Ben Hamida, N.; Ben Nasri, M.; Ouerghi, Z.; Hosni, K. Comparison of antioxidant and antimicrobial activities of two cultivated Cistus species from Tunisia. Biosci. J. Uberlandia 2016, 32, 226–237. [Google Scholar] [CrossRef]
- Andrade, D.; Gil, C.; Breitenfeld, L.; Domingues, F.; Duarte, A.P. Bioactive extracts from Cistus ladanifer and Arbutus unedo L. Ind. Crop. Prod. 2009, 30, 165–167. [Google Scholar] [CrossRef]
- Rebaya, A.; Belghith, S.; Cherif, J.; Trabelsi-Ayadi, M. Total Phenolic Compounds and Antioxidant Potential of Rockrose (Cistus salviifolius) Leaves and Flowers Grown in Tunisia. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 327–331. [Google Scholar]
- Lamien-Meda, A.; Nell, M.; Lohwasser, U.; Börner, A.; Franz, C.; Novak, J. Investigation of antioxidant and rosmarinic acid variation in the sage collection of the genebank in Gatersleben. J. Agric. Food Chem. 2010, 58, 3813–3819. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R.; Michitsch, H.; Franz, C. Antioxidative properties of Thymus vulgaris leaves: Comparison of different extracts and essential oil chemotypes. J. Agric. Food Chem. 2008, 56, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- European Commission. Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R2470&from=EN (accessed on 5 March 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org (accessed on 15 September 2018).
| Peak | Rt (min) | [M-H]− (m/z) | MS/MS (m/z) | Proposed Compound | Literature | Rel. Area % |
|---|---|---|---|---|---|---|
| 1 | 10.1 | 1083 | 301/541 | Punicalagin, isomer 1 1 | [11,13,26,31,36] | 0–16% |
| 2 | 14.4 | 1083 | 301/541 | Punicalagin, isomer 2 1 | [11,13,26,31,36] | 0–19% |
| 3 | 14.8 | 1251 | 541/603 | Punicalagin gallate, isomer 1 | [11,26,31,36] | 0–10% |
| 4 | 22.1 | 1251 | 541/603 | Punicalagin gallate, isomer 2 | [11,26,31,36] | 0–14% |
| 5 | 32.1 | 479 | 316 | M-3-O-galactoside | [11,12,26,31,37,38] | 0–47% |
| 6 | 32.8 | 479 | 316 | M-3-O-glucoside | [11,12,13,26,31,37,38] | 0–6% |
| 7 | 37.3 | 449 | 316 | M-O-xyloside and/or m-3-O-arabinoside 2 | [26,31,37,38] | 0–50% |
| 8 | 38.4 | 463 | 316 | M-3-O-rhamnoside (myricitrin) 1 | [11,12,13,26,31,37,38] | 5–83% |
| 9 | 39.3 | 609 | 301 | Q-3-O-rutinoside (rutin) 1, 2 | [11,12,13,26,31,37,38] | 0–17% |
| 521 | 316 | Putative m-derivative, ev. an artifact | ||||
| 10 | 40.0 | 463 | 301 | Q-3-O-galactoside (hyperoside) 1 | [26,31,37,38] | 0–12% |
| 11 | 40.8 | 463 | 301 | Q-3-O-glucoside (isoquercetin) 1 | [11,13,26,31,36,37,38] | 0–3% |
| 12 | 46.0 | 433 | 301 | Q-O-xyloside and/or q-3-O-arabinoside 2 | [11,12,13,26,31,36,37,38] | 0–9% |
| 13 | 48.9 | 447 | 301 | Q-3-O-rhamnoside (quercitrin) 1 | [11,12,13,26,31,37,38] | 0–35% |
| Sample | Declaration | Origin | P-Derivatives | M-Glycosides | Q-Glycosides | Tentative Identification |
|---|---|---|---|---|---|---|
| (mg/g dry wt) | (mg/g dry wt) | (mg/g dry wt) | (via HPLC profile) | |||
| CHP01 1 | Cistus sp. | TR | 161.2 | 4.8 | 2.4 | White-flowered species |
| CHP02 1 | C. incanus L. | GR | <LOD | 4.0 | 0.8 | C. creticus |
| CHP03 1 | C. incanus | - | 16.8 | 10.0 | 2.5 | C. creticus |
| CHP04 1 | C. incanus L. | GR | <LOD | 0.7 | 0.2 | C. creticus |
| CHP05 2 | C. incanus | - | 81.1 | 4.1 | 1.5 | White-flowered species |
| CHP06 2 | C. incanuskbA | - | 33.1 | 0.5 | 0.2 | White-flowered species |
| CHP07 2 | C. incanuskbA | CY | 85.3 | 8.4 | 2.3 | C. creticus |
| CHP08 2 | C. incanus | TR | 22.1 | 8.6 | 2.0 | C. creticus (adulterated?) |
| CHP09 2 | C. incanus | - | 50.7 | 5.9 | 1.7 | White-flowered species |
| CHP10 2 | C. incanus | - | 61.5 | 4.0 | 1.3 | White-flowered species |
| CHP11 2 | C. incanus | - | 79.6 | 3.5 | 1.1 | White-flowered species |
| CHP12 2 | C. creticus | - | 123.3 | 3.3 | 3.0 | White-flowered species |
| CHP13 2 | C. incanuskbA | TR | 66.0 | 3.2 | 1.4 | White-flowered species |
| CHP14 2 | C. incanus | - | 82.9 | 5.1 | 2.7 | White-flowered species |
| CHP15 2 | Cistus sp. | TR | 29.4 | 6.5 | 1.5 | C. creticus (adulterated?) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukas, B.; Bragagna, L.; Starzyk, K.; Labedz, K.; Stolze, K.; Novak, J. Polyphenol Diversity and Antioxidant Activity of European Cistus creticus L. (Cistaceae) Compared to Six Further, Partly Sympatric Cistus Species. Plants 2021, 10, 615. https://doi.org/10.3390/plants10040615
Lukas B, Bragagna L, Starzyk K, Labedz K, Stolze K, Novak J. Polyphenol Diversity and Antioxidant Activity of European Cistus creticus L. (Cistaceae) Compared to Six Further, Partly Sympatric Cistus Species. Plants. 2021; 10(4):615. https://doi.org/10.3390/plants10040615
Chicago/Turabian StyleLukas, Brigitte, Laura Bragagna, Katharina Starzyk, Klaudia Labedz, Klaus Stolze, and Johannes Novak. 2021. "Polyphenol Diversity and Antioxidant Activity of European Cistus creticus L. (Cistaceae) Compared to Six Further, Partly Sympatric Cistus Species" Plants 10, no. 4: 615. https://doi.org/10.3390/plants10040615
APA StyleLukas, B., Bragagna, L., Starzyk, K., Labedz, K., Stolze, K., & Novak, J. (2021). Polyphenol Diversity and Antioxidant Activity of European Cistus creticus L. (Cistaceae) Compared to Six Further, Partly Sympatric Cistus Species. Plants, 10(4), 615. https://doi.org/10.3390/plants10040615







