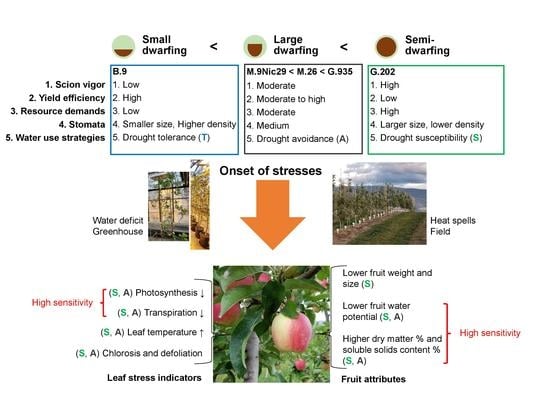
Rootstocks with Different Vigor Influenced Scion–Water Relations and Stress Responses in AmbrosiaTM Apple Trees (Malus Domestica var. Ambrosia)
Abstract
1. Introduction
2. Results
2.1. Greenhouse Experiment: Responses of Different Rootstocks to Water Deficit
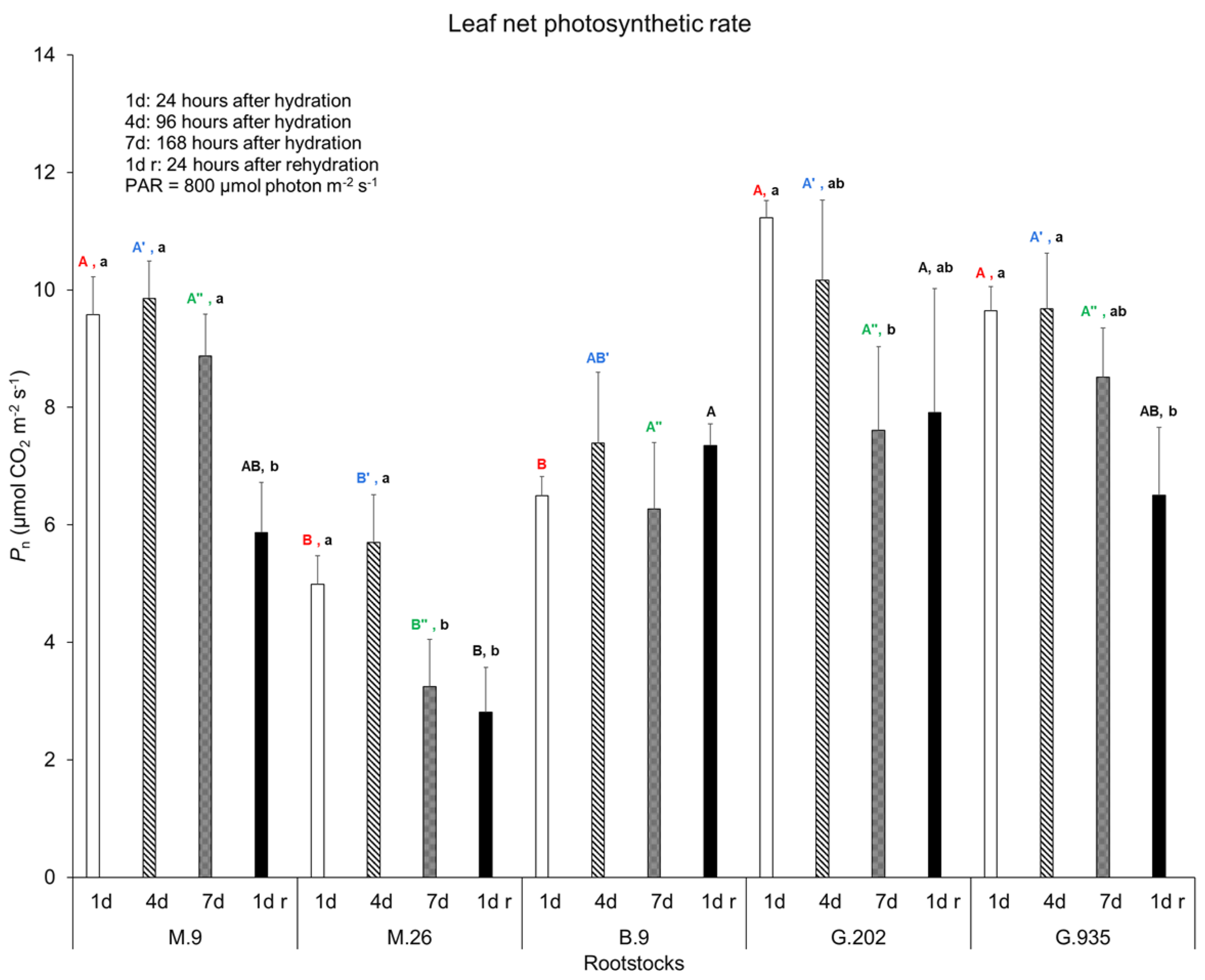
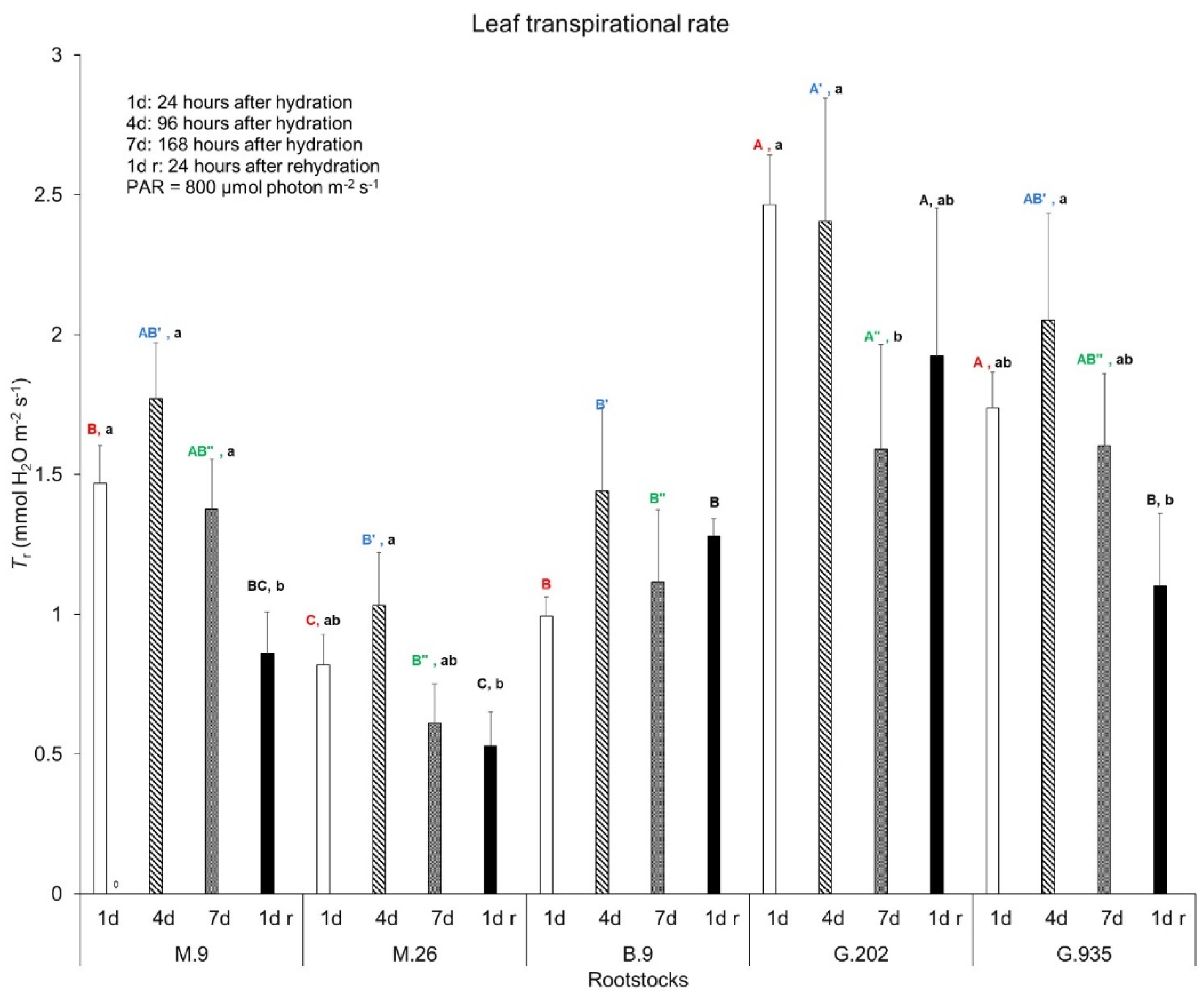
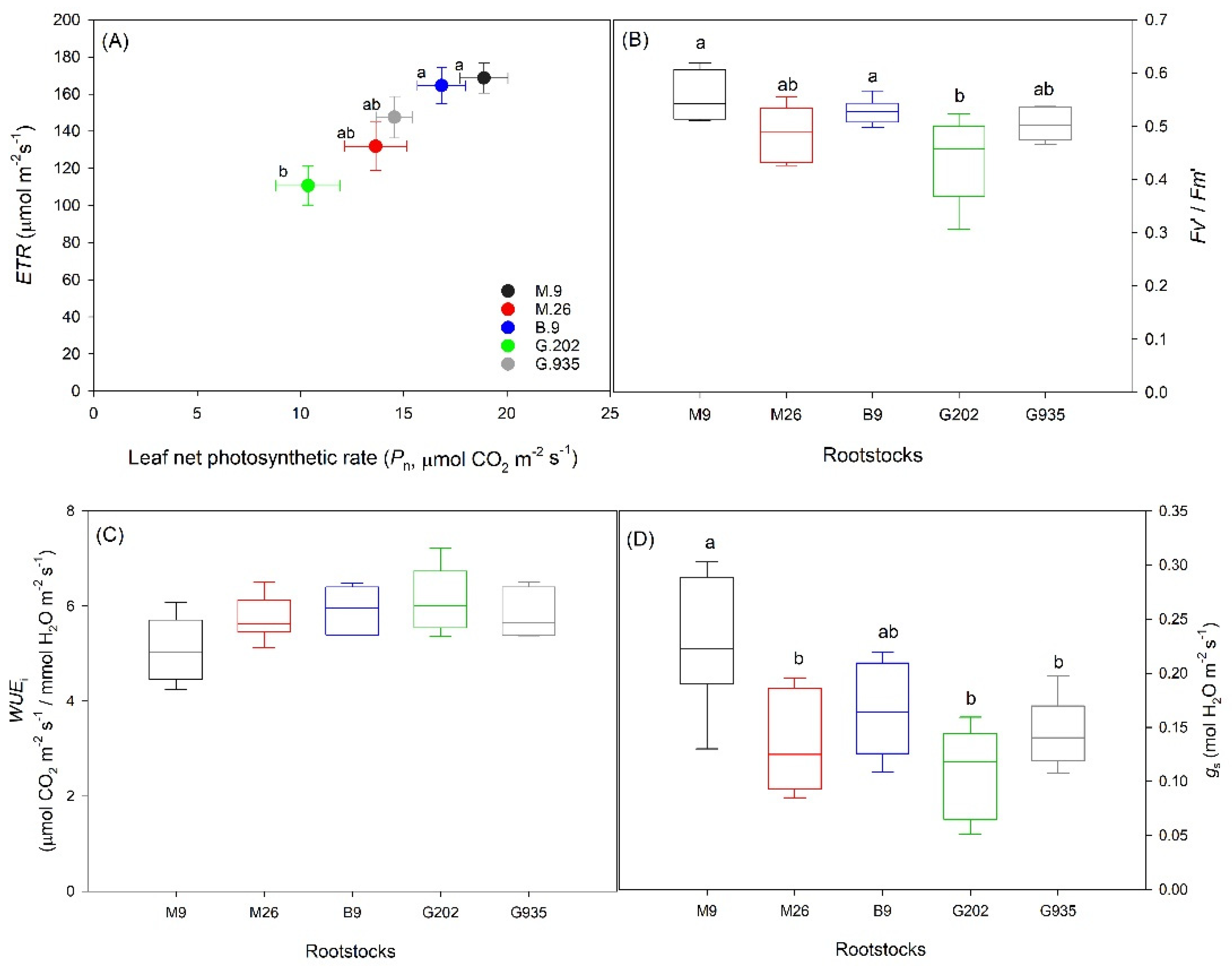
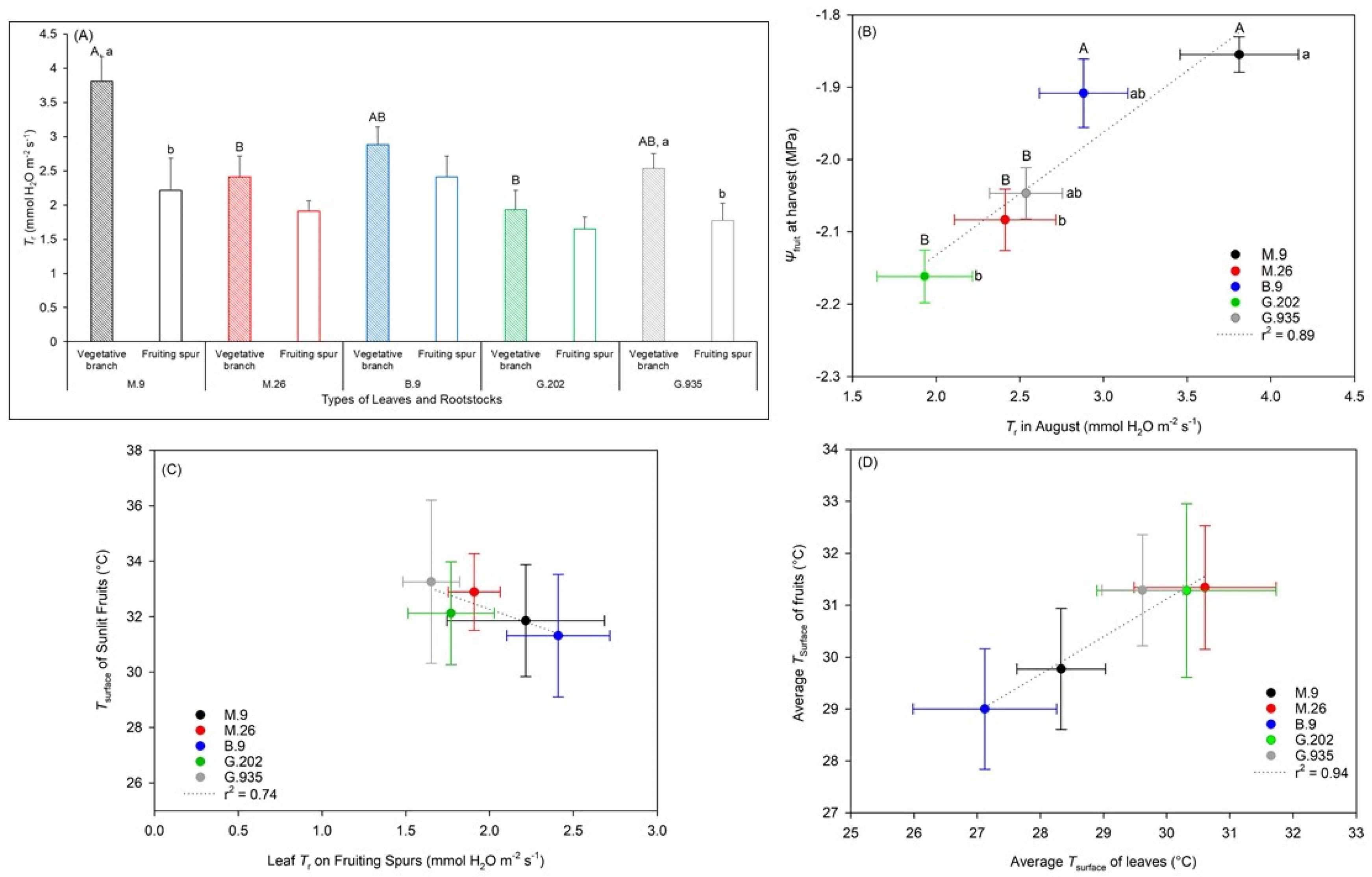
2.1.1. Soil Water Depletion and Leaf Gas Exchanges
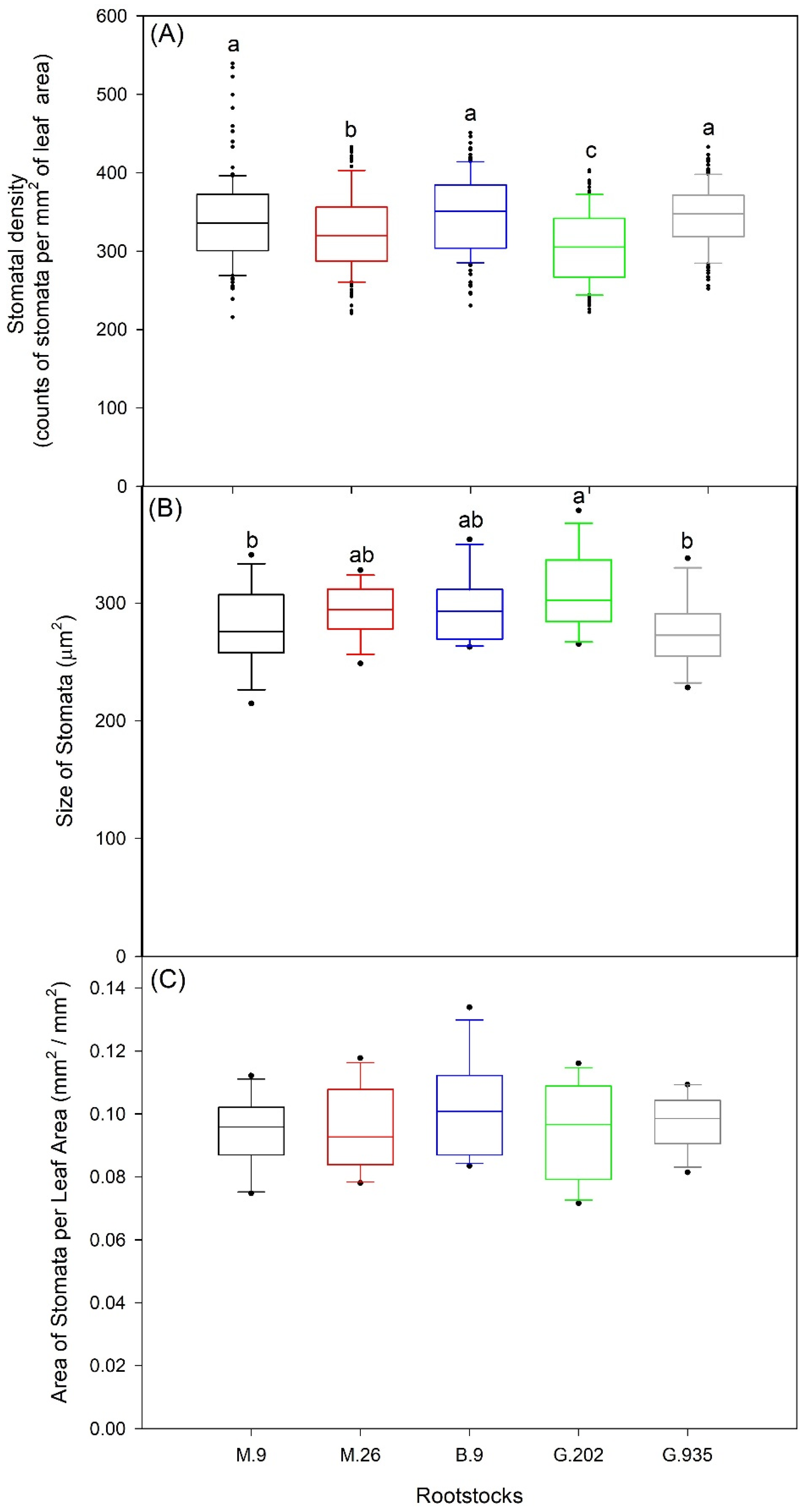
2.1.2. Stomatal Characteristics
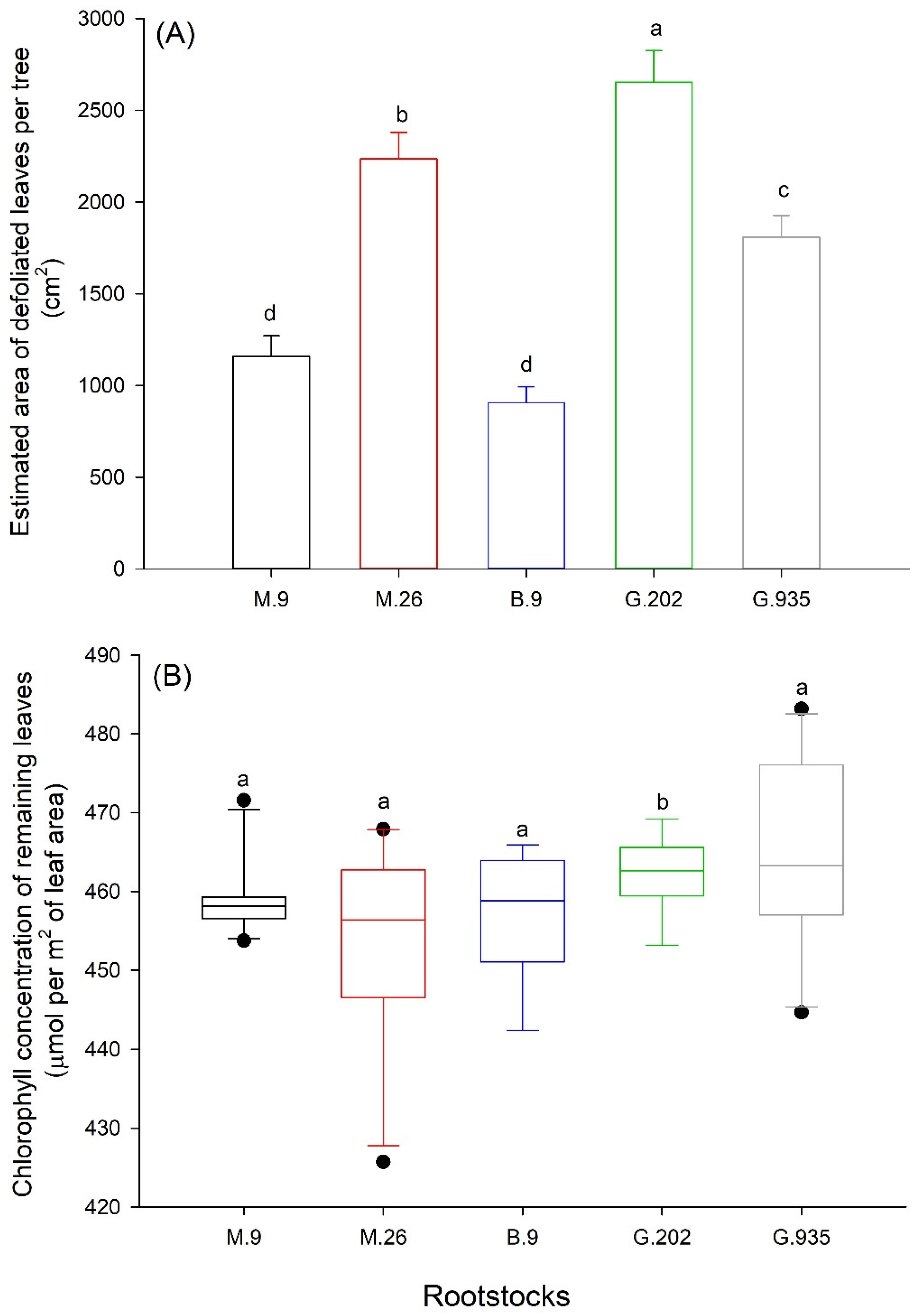
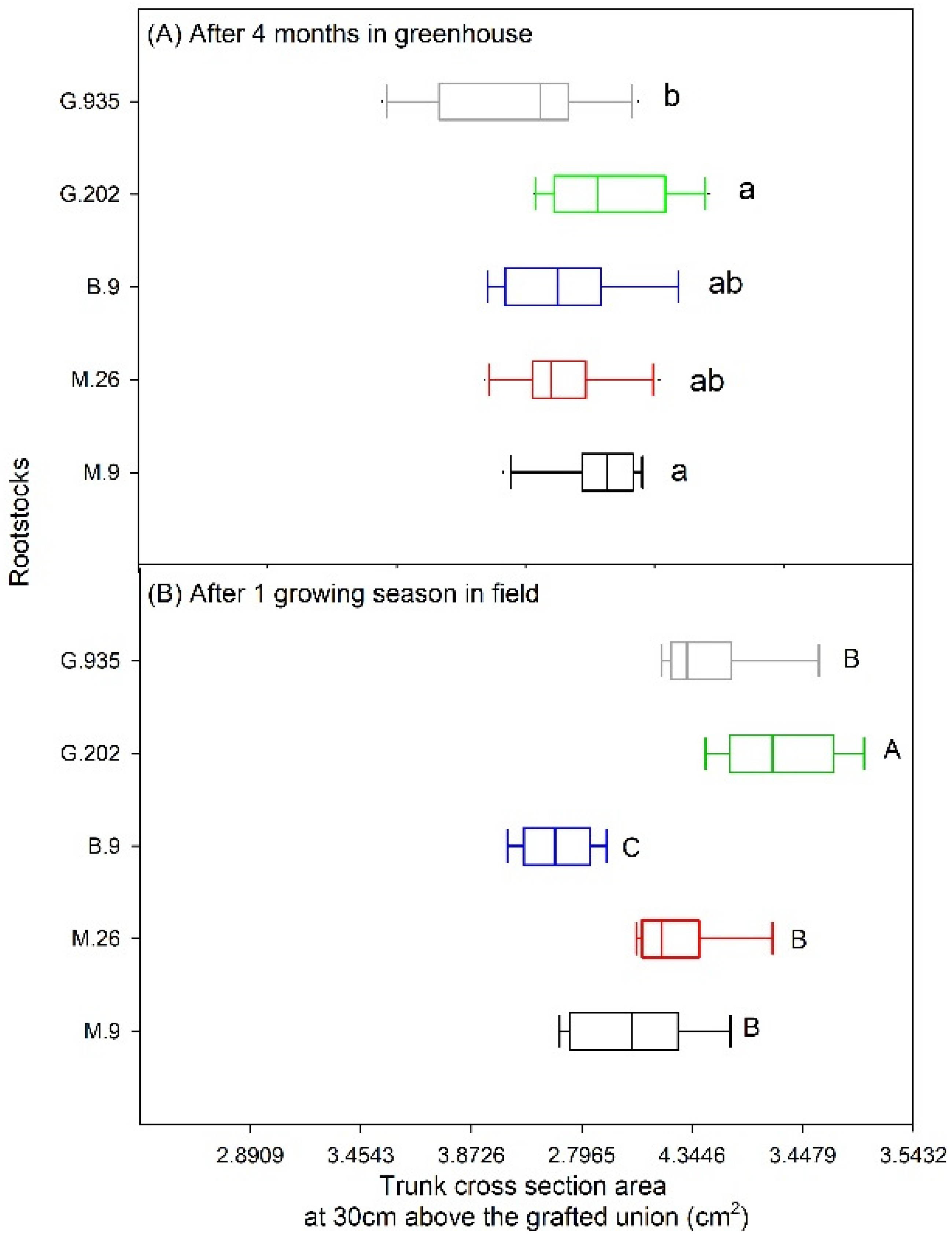
2.1.3. Leaf Defoliation, Chlorosis and Scion Trunk Growth
2.2. Field Trial: Rootstock Performances in the Second-Year Planting and Under Heat Stress
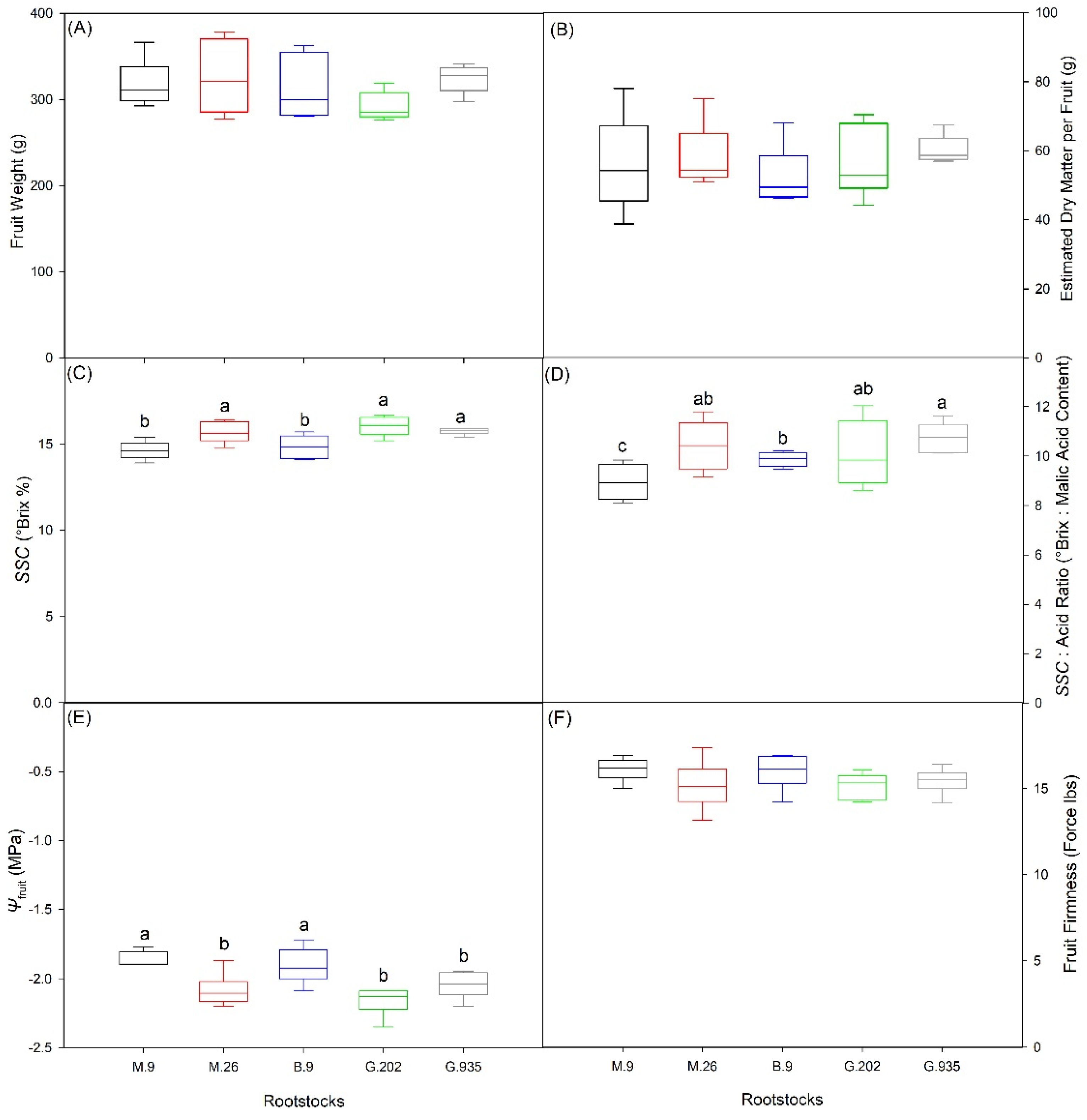
2.2.1. Scion Trunk Growth, Yield and Fruit Quality
2.2.2. Water Relationships in Response to the Onset of Heat Stress
3. Discussion
3.1. Rootstock Performances and Water Use Strategies
3.1.1. Semi-Dwarfing G.202
3.1.2. Large Dwarfing G.935, M.26 and M.9
3.1.3. Small Dwarfing B.9
3.2. Responses to Water Deficit and Heat Stress in Relation to Water Use Strategies
3.2.1. Drought Susceptibility versus Drought Resistance
3.2.2. Drought Tolerance and Avoidance
3.3. Stress Indicators for AmbrosiaTM Scion–Rootstock System
3.3.1. Photosynthesis, Transpiration, and Leaf Surface Temperatures
3.3.2. Water Potential and Quality Attributes of Fruits
3.4. Summary and Future Perspectives
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.1.1. Greenhouse Conditions of Water Deficit Experiment
4.1.2. Field Trial Conditions
4.2. Measurements of Stress Indicators of Plant–Water Relationships
4.2.1. Leaf Photosynthesis and Transpiration
4.2.2. Tissue Surface Temperature and Fruit Water Potential, Ψfruit
4.3. Leaf Chlorophyll Concentration and Stomatal Characteristics
4.4. Nutrient Use and Yield Efficiency
4.5. Fruit Quality Attributes
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Policarpo, M.; Webster, A.D.; Kuden, A.M. Drought tolerance of apple rootstocks: Production and partitioning of dry matter. Plant Soil 1999, 206, 223–235. [Google Scholar] [CrossRef]
- Beakbane, A.B.; Thompson, E.C. Anatomical studies of stems and roots of hardy fruit trees ii. The internal structure of the roots of some vigorous and some dwarfing apple rootstocks, and the correlation of structure with vigour. J. Pomol. Hortic. Sci. 1940, 17, 141–149. [Google Scholar] [CrossRef]
- Harrison, N.; Harrison, R.J.; Barber-Perez, N.; Cascant-Lopez, E.; Cobo-Medina, M.; Lipska, M.; Conde-Ruíz, R.; Brain, P.; Gregory, P.J.; Fernández-Fernández, F. A new three-locus model for rootstock-induced dwarfing in apple revealed by genetic mapping of root bark percentage. J. Exp. Bot. 2016, 67, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Naor, A.; Bennink, J.; Grava, A.; Tyree, M. Hydraulic resistance components of mature apple trees on rootstocks of different vigours. J. Exp. Bot. 2007, 58, 4213–4224. [Google Scholar] [CrossRef]
- Jones, H.G. How do rootstocks control shoot water relations? New Phytol. 2012, 194, 301–303. [Google Scholar] [CrossRef]
- Foster, T.M.; Celton, J.-M.; Chagné, D.; Tustin, D.S.; Gardiner, S.E. Two quantitative trait loci, dw1 and dw2, are primarily responsible for rootstock-induced dwarfing in apple. J. Hortic. Res. 2015, 2, 1–9. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.; Hassan, G.; Baba, T.; Alyemeni, M.; Alsahli, A.; El-Serehy, H.; Paray, B.; Ahmad, P. Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Olien, W.; Lakso, A.N. Effect of rootstock on apple (Malus domestica) tree water relations. Physiol. Planta 1986, 67, 421–430. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Webster, A.D.; Vaughan, S.; Lucas, A.S. Effects of root restriction on the physiology of apple tree growth. In Proceedings of the VI International Symposium on Integrated Canopy, Rootstock, Environmental Physiology in Orchard Systems, 17 July 1996; Volume 451, pp. 587–598. [Google Scholar]
- Webster, A.D. Rootstock and interstock effects on deciduous fruit tree vigour, precocity, and yield productivity. N. Z. J. Crop Hortic. Sci. 1995, 23, 373–382. [Google Scholar] [CrossRef]
- Fernandez, R.T.; Perry, R.L.; Flore, J.A. Drought response of young apple trees on three rootstocks. Ii. Gas exchange, chlorophyll fluorescence, water relations, and leaf abscisic acid. J. Am. Soc. Hortic. Sci. 1997, 122, 841–848. [Google Scholar] [CrossRef]
- Kosina, J. Effect of dwarfing and semi dwarfing apple rootstocks on growth and productivity of selected apple cultivars. Hortic. Sci. 2010, 37, 121–126. [Google Scholar] [CrossRef]
- Hampson, C.; McKenzie, D.L. Sensory characteristics of 21 new apple cultivars after short-term cold air storage. J. Am. Pomol. Soc. 2006, 60, 9–19. [Google Scholar]
- Neilsen, D.; Neilsen, G.; Herbert, L.; Guak, S. Effect of irrigation and crop load management on fruit nutrition and quality for Ambrosia/M. 9 apple. Acta Hortic. 2010, 868, 63–72. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D.; Guak, S.H.; Forge, T. The effect of deficit irrigation and crop load on leaf and fruit nutrition of fertigated ‘Ambrosia’/‘M. 9′apple. HortScience 2015, 50, 1387–1393. [Google Scholar] [CrossRef]
- Robinson, T.; Fazio, G.; Hoying, S.; Miranda, M.; Iungerman, K. Geneva® rootstocks for weak growing scion cultivars like ‘Honeycrisp’. N. Y. Fruit Q. 2011, 19, 10–16. [Google Scholar]
- Autio, W.; Robinson, T.; Black, B.; Crassweller, R.; Fallahi, E.; Hoying, S.; Parker, M.; Quezada, R.P.; Reig, G.; Wolfe, D. Budagovsky, Geneva, Pillnitz, and Malling apple rootstocks affect ‘Fuji’ performance over eight years in the 2010 NC-140 ‘Fuji’ apple rootstock trial. J. Am. Pomol. Soc. 2020, 74, 196–209. [Google Scholar]
- Autio, W.; Robinson, T.; Black, B.; Blatt, S.; Cochran, D.; Cowgill, W.; Hampson, C.; Hoover, E.; Lang, G.; Miller, D.; et al. Budagovsky, Geneva, Pillnitz, and Malling apple rootstocks affect ’Honeycrisp’ performance over the first five years of the 2010 NC-140 ‘Honeycrisp’ apple rootstock trial. J. Am. Pomol. Soc. 2017, 71, 149–166. [Google Scholar]
- Beakbane, A.B.; Majumder, P. A relationship between stomatal density and growth potential in apple rootstocks. J. Pomol. Hortic. Sci. 1975, 50, 285–289. [Google Scholar] [CrossRef]
- Pathak, R.; Pandey, D.; Pandey, V.S. Stomatal distribution as an index for predicting vigour of plum rootstocks. J. Hortic. Sci. 1977, 34, 117–119. [Google Scholar]
- Rankenberg, T.; Geldhof, B.; van Veen, H.; Holsteens, K.; Van de Poel, B.; Sasidharan, R. Age-dependent abiotic stress resilience in plants. Trends Plant Sci. 2021, in press. [Google Scholar] [CrossRef]
- Vangdal, E. Quality criteria for fruit for fresh consumption. Acta Agric. Scand. 1985, 35, 41–47. [Google Scholar] [CrossRef]
- Harker, F.R.; Marsh, K.B.; Young, H.; Murray, S.H.; Gunson, F.A.; Walker, S.B. Sensory interpretation of instrumental measurements 2: Sweet and acid taste of apple fruit. Postharvest Biol. Technol. 2002, 24, 241–250. [Google Scholar] [CrossRef]
- Elias, P. Stomata density and size of apple trees growing in irrigated and non irrigated conditions. Biol. Bratisl. 1995, 50, 115. [Google Scholar]
- Bacelar, E.L.; Moutinho-Pereira, J.M.; Gonçalves, B.M.; Brito, C.V.; Gomes-Laranjo, J.; Ferreira, H.M.; Correia, C.M. Water use strategies of plants under drought conditions. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–170. [Google Scholar]
- Kröber, W.; Plath, I.; Heklau, H.; Bruelheide, H. Relating stomatal conductance to leaf functional traits. J. Vis. Exp. 2015, 104, e52738. [Google Scholar] [CrossRef]
- Massonnet, C.; Costes, E.; Rambal, S.; Dreyer, E.; Regnard, J.L. Stomatal regulation of photosynthesis in apple leaves: Evidence for different water-use strategies between two cultivars. Ann. Bot. 2007, 100, 1347–1356. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G. Effects of size-controlling apple rootstocks on growth, abscisic acid, and hydraulic conductivity of scion of different vigor. Int. J. Fruit Sci. 2015, 15, 369–381. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.-M.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop. J. 2015, 3, 220–228. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Remorini, D.; Massai, R. Comparison of water status indicators for young peach trees. J. Irrig. Sci. 2003, 22, 39–46. [Google Scholar] [CrossRef]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap pressure in vascular plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Naor, A. Midday stem water potential as a plant water stress indicator for irrigation scheduling in fruit trees. In Proceedings of the III International Symposium on Irrigation of Horticultural Crops, Lisbon, Portugal, 28 June 1999; Volume 537, pp. 447–454. [Google Scholar]
- Gelly, M.; Marsal, J.; Mata, M.; Arbonès, A.; Rufat, J.; Girona, J. Fruit water potential: A comparison study to other water potential parameters. In Proceedings of the International Symposium on Irrigation and Water Relations in Grapevine and Fruit Trees, Mendoza, Argentina, 4 December 2001; Volume 646, pp. 35–40. [Google Scholar]
- Mpelasoka, B.S. Fruit Water Relations, Growth, Yield, and Quality of ‘Braeburn’ Apple in Response to Deficit Irrigation and to Crop Load. Ph.D. Thesis, Massey University, Auckland, New Zealand, 2001. [Google Scholar]
- Naor, A.; Naschitz, S.; Peres, M.; Gal, Y. Responses of apple fruit size to tree water status and crop load. Tree Physiol. 2008, 28, 1255–1261. [Google Scholar] [CrossRef]
- Genty, B.; Harbinson, J.; Baker, N.R. Relative quantum efficiencies of the two photosystems of leaves in photorespiratory and non-respiratory conditions. Plant Physiol. Biochem. 1990, 28, 1–10. [Google Scholar]
- Parry, C.; Blonquist, J.M., Jr.; Bugbee, B. In situ measurement of leaf chlorophyll concentration: Analysis of the optical/absolute relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Meth. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
| Rootstocks | ΔVWC Day 7–Day 1 (m3/m3) | ΔWUEi Day 7–Day 1 (µmol CO2/mmol H2O) | Δgs Day 7–Day 1 (mmol m−2 s−1) | ΔΦCO2 Day 7–Day 1 (mmol CO2 mol−1 Absorbed Quanta) |
|---|---|---|---|---|
| M.9 | −0.31 ± 0.03 a | 0.16 ± 0.19 b | −9.14 ± 3.61 b | −1.04 ± 0.37 b |
| M.26 | −0.14 ± 0.02 b | −0.83 ± 0.40 c | −21.72 ± 3.84 b | −2.59 ± 0.60 ab |
| B.9 | −0.25 ± 0.01 ab | −0.39 ±0.26 bc | 7.32 ± 12.31 b | −0.34 ± 1.26 b |
| G.202 | −0.25 ± 0.06 ab | 1.14 ± 0.34 a | −67.46 ± 12.30 a | −5.37 ± 1.82 a |
| G.935 | −0.3 ± 0.01 a | 0.21 ±0.21 b | −16.46 ± 12.40 b | −1.68 ± 0.72 b |
| Rootstocks | ||||||
|---|---|---|---|---|---|---|
| M.9 | M.26 | B.9 | G.202 | G.935 | ||
| Leaf Chlorophyll (µmol m−2) | 452.7 ± 2.7 a | 441.2 ± 2.7 a | 450.7 ± 1.5 a | 398.4 ± 9.1 b | 448.6 ± 1.7 a | |
| Fruit Nutrients | N | 7.91 ± 0.42 a | 5.54 ± 0.27 b | 6.00 ± 0.14 b | 4.96 ± 0.13 b | 9.13 ± 0.98 a |
| P | 2.00 ± 0.11 bc | 2.11 ± 0.04 b | 1.78 ± 0.08 c | 2.42 ± 0.05 a | 2.61 ± 0.06 a | |
| K | 25.66 ± 0.68 a | 24.54 ± 0.61 a | 22.62 ± 0.23 ab | 22.85 ± 2.41 ab | 18.36 ± 2.37 b | |
| Mg | 1.37 ± 0.05 a | 1.47 ± 0.07 a | 1.24 ± 0.11 ab | 1.18 ± 0.09 ab | 1.06 ± 0.11 b | |
| Ca | 0.84 ± 0.05 ab | 1.04 ± 0.03 a | 1.11 ± 0.04 a | 0.58 ± 0.04 bc | 0.31 ± 0.17 c | |
| B | 0.032 ± 0.001 c | 0.033 c | 0.028 c | 0.051 ± 0.001 b | 0.064 ± 0.007 a | |
| Zn | 0.003 | 0.004 | 0.005 ± 0.001 | 0.004 | 0.004 | |
| Yield Efficiency (kg/cm2 TCSA) | 0.44 ± 0.04 b | 0.38 ± 0.02 bc | 0.57 ± 0.03 a | 0.32 ± 0.03 c | 0.36 ± 0.02 bc | |
| Water Use Strategy | Growth Characteristics | Responses of Physiological Indicators to Stresses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TCSA | Yield Potential | Yield Efficiency | PSII Efficiency (ETR, Fv’/Fm’) | Pn, ΦCO2, Tr, gs | Average Tsurface of Leaves and Fruits | Ψfruit | Fruit Weight, DM%, SSC% | Leaf Chlorosis, Defoliation | |
| High Demand, Drought Susceptibility | Larger | High | Low | Low | Symptomatic reduction | More elevated | Low | Lower weight, higher DM% and SSC% | Severe |
| Reduction in Water Use, Drought Avoidance | Medium | Medium | Medium | Medium–normal | Rapid reduction to a lower level | More elevated | Low | Higher DM% and SSC% | Moderate defoliation |
| Less Water Use, Drought Tolerance | Smaller | Medium | High | Normal | Moderate and stable | Less elevated | High | Lower DM% and SSC% | Slight–none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Ediger, D. Rootstocks with Different Vigor Influenced Scion–Water Relations and Stress Responses in AmbrosiaTM Apple Trees (Malus Domestica var. Ambrosia). Plants 2021, 10, 614. https://doi.org/10.3390/plants10040614
Xu H, Ediger D. Rootstocks with Different Vigor Influenced Scion–Water Relations and Stress Responses in AmbrosiaTM Apple Trees (Malus Domestica var. Ambrosia). Plants. 2021; 10(4):614. https://doi.org/10.3390/plants10040614
Chicago/Turabian StyleXu, Hao, and Danielle Ediger. 2021. "Rootstocks with Different Vigor Influenced Scion–Water Relations and Stress Responses in AmbrosiaTM Apple Trees (Malus Domestica var. Ambrosia)" Plants 10, no. 4: 614. https://doi.org/10.3390/plants10040614
APA StyleXu, H., & Ediger, D. (2021). Rootstocks with Different Vigor Influenced Scion–Water Relations and Stress Responses in AmbrosiaTM Apple Trees (Malus Domestica var. Ambrosia). Plants, 10(4), 614. https://doi.org/10.3390/plants10040614