Coordination of Cyclic Electron Flow and Water–Water Cycle Facilitates Photoprotection under Fluctuating Light and Temperature Stress in the Epiphytic Orchid Dendrobium officinale
Abstract
1. Introduction
2. Results
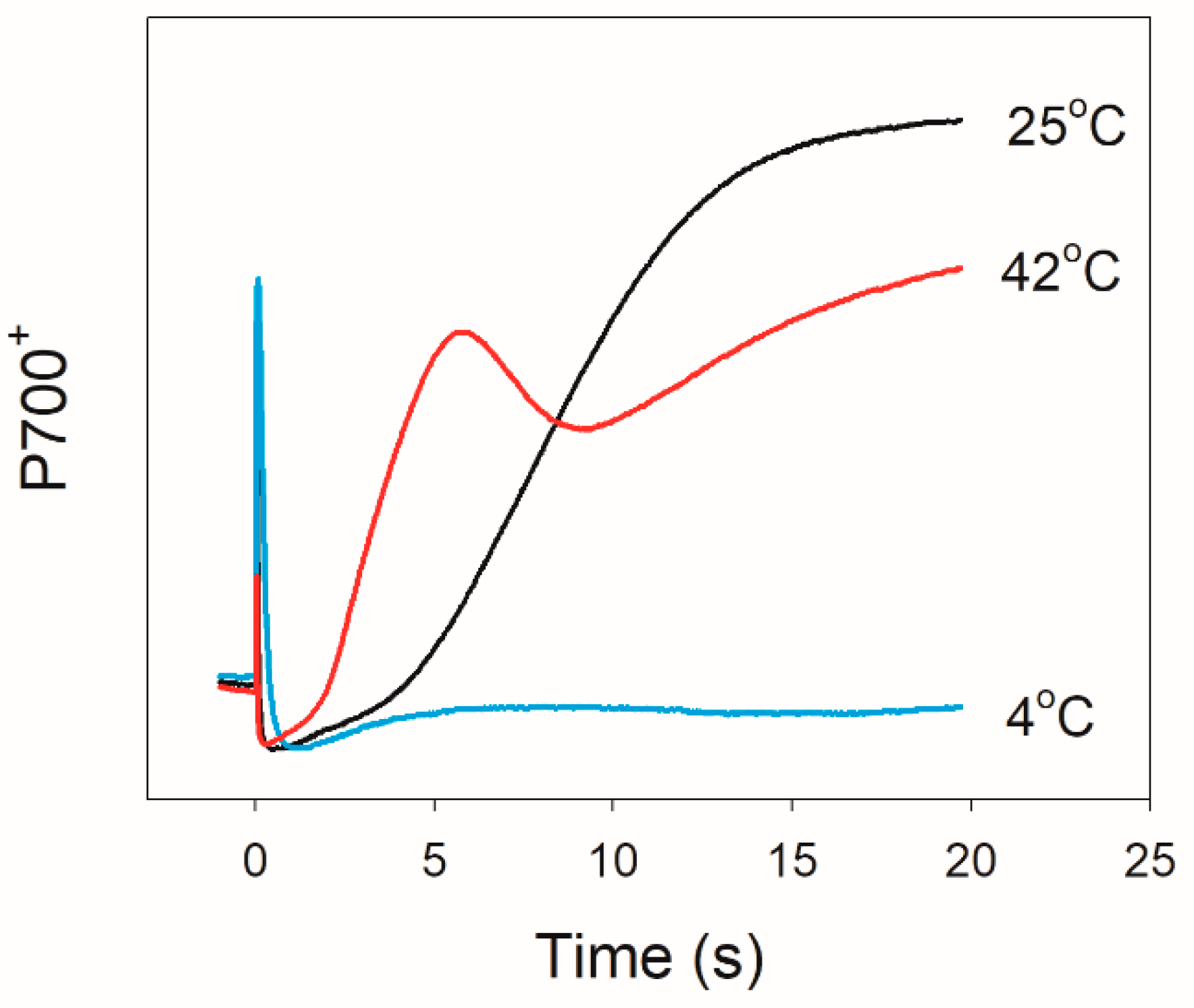
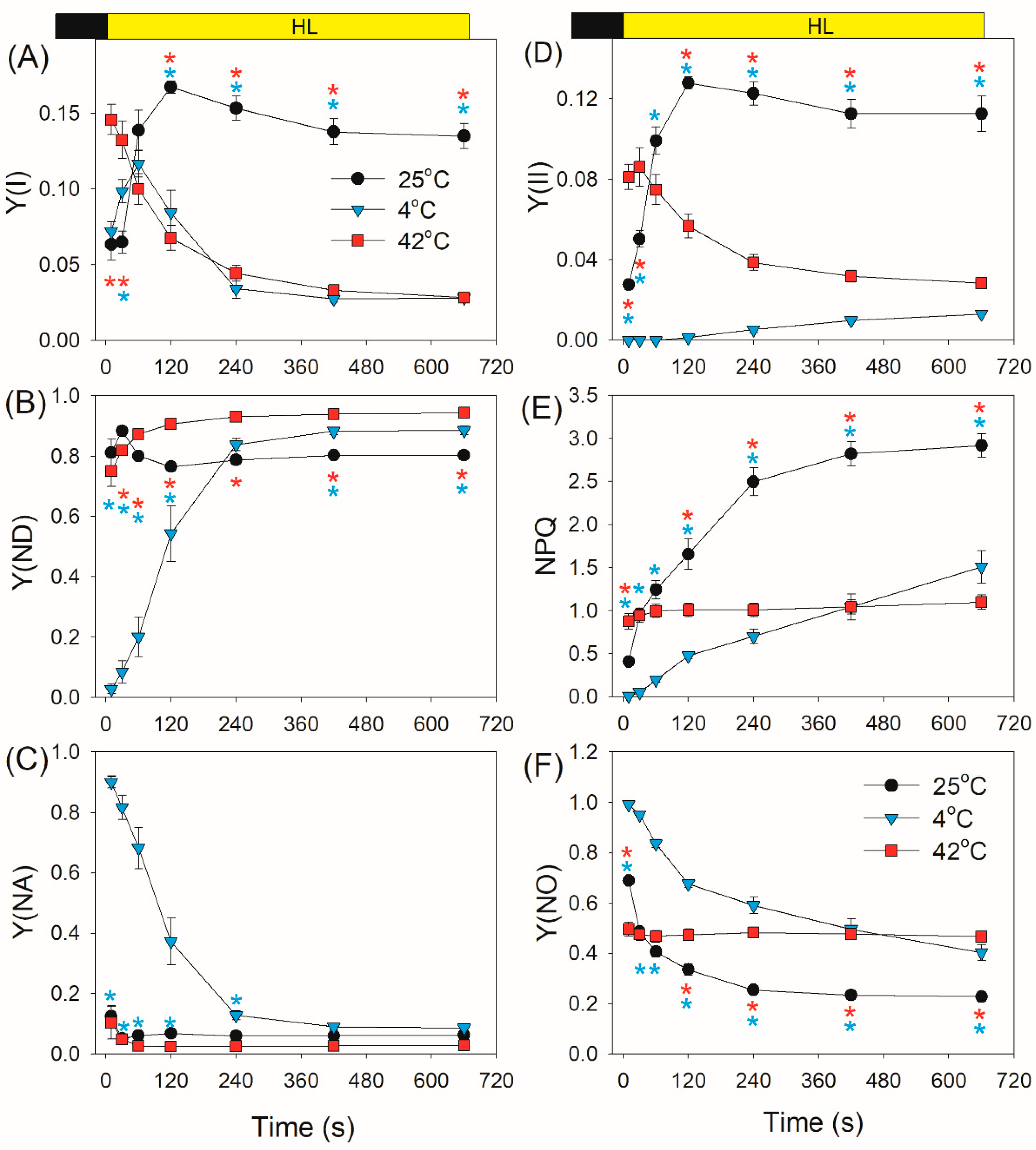
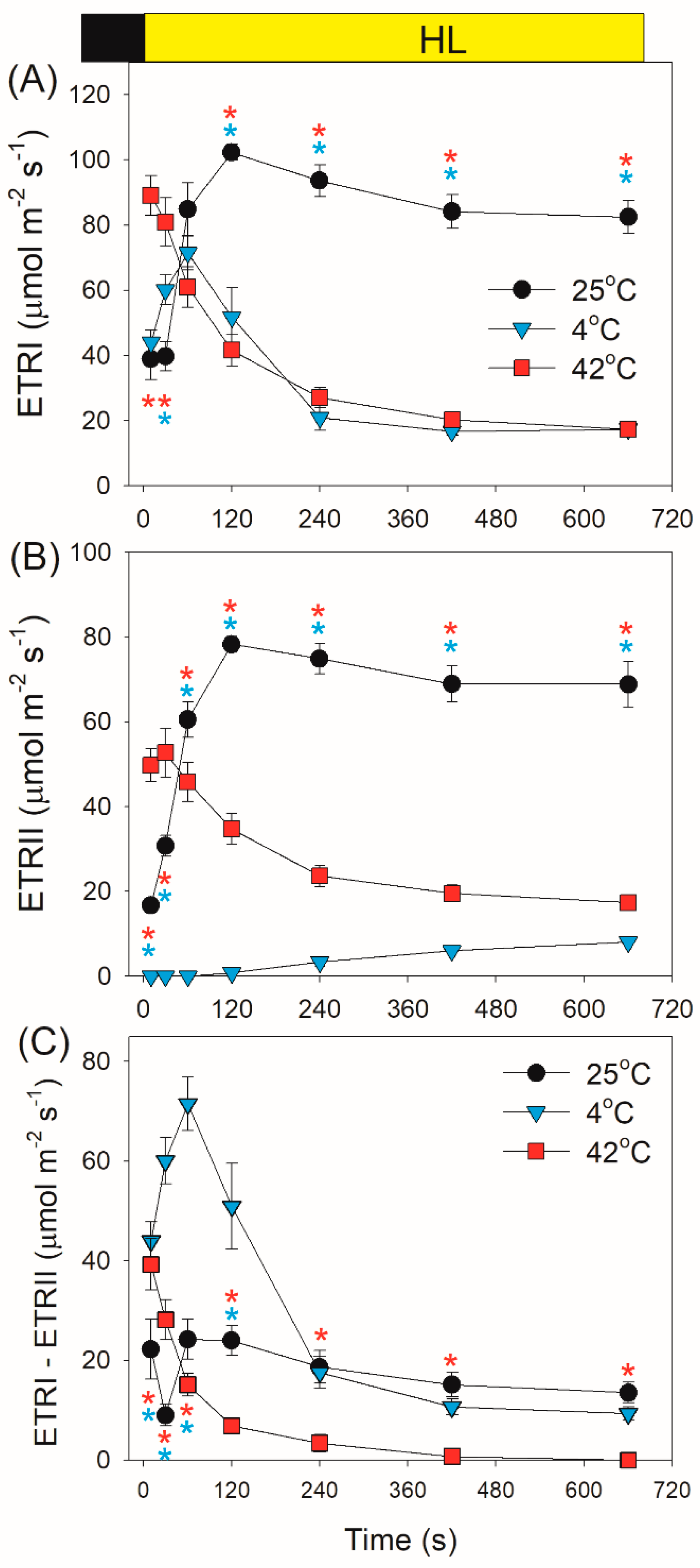
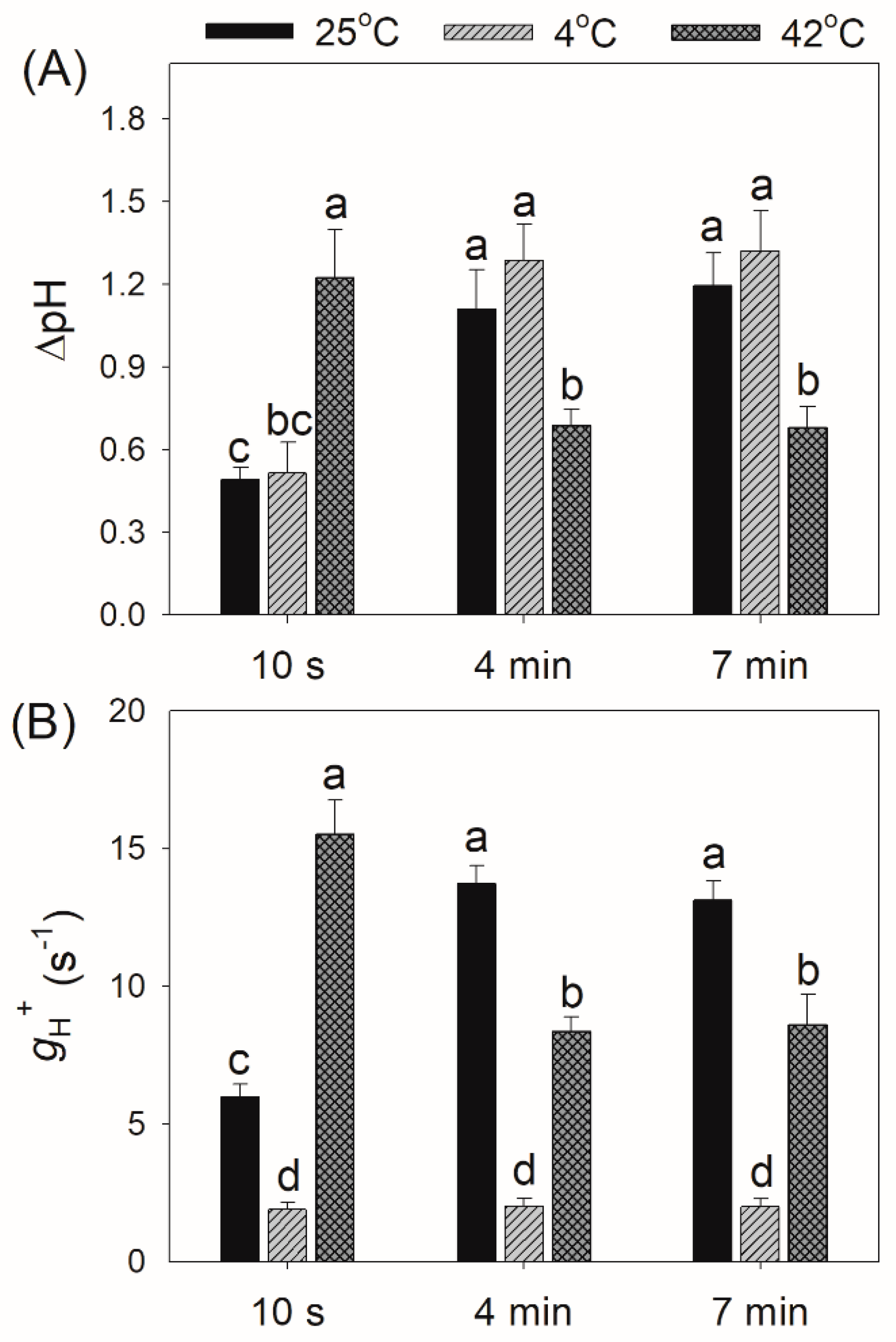
2.1. Photosynthetic Regulation after Transition from Dark to Light
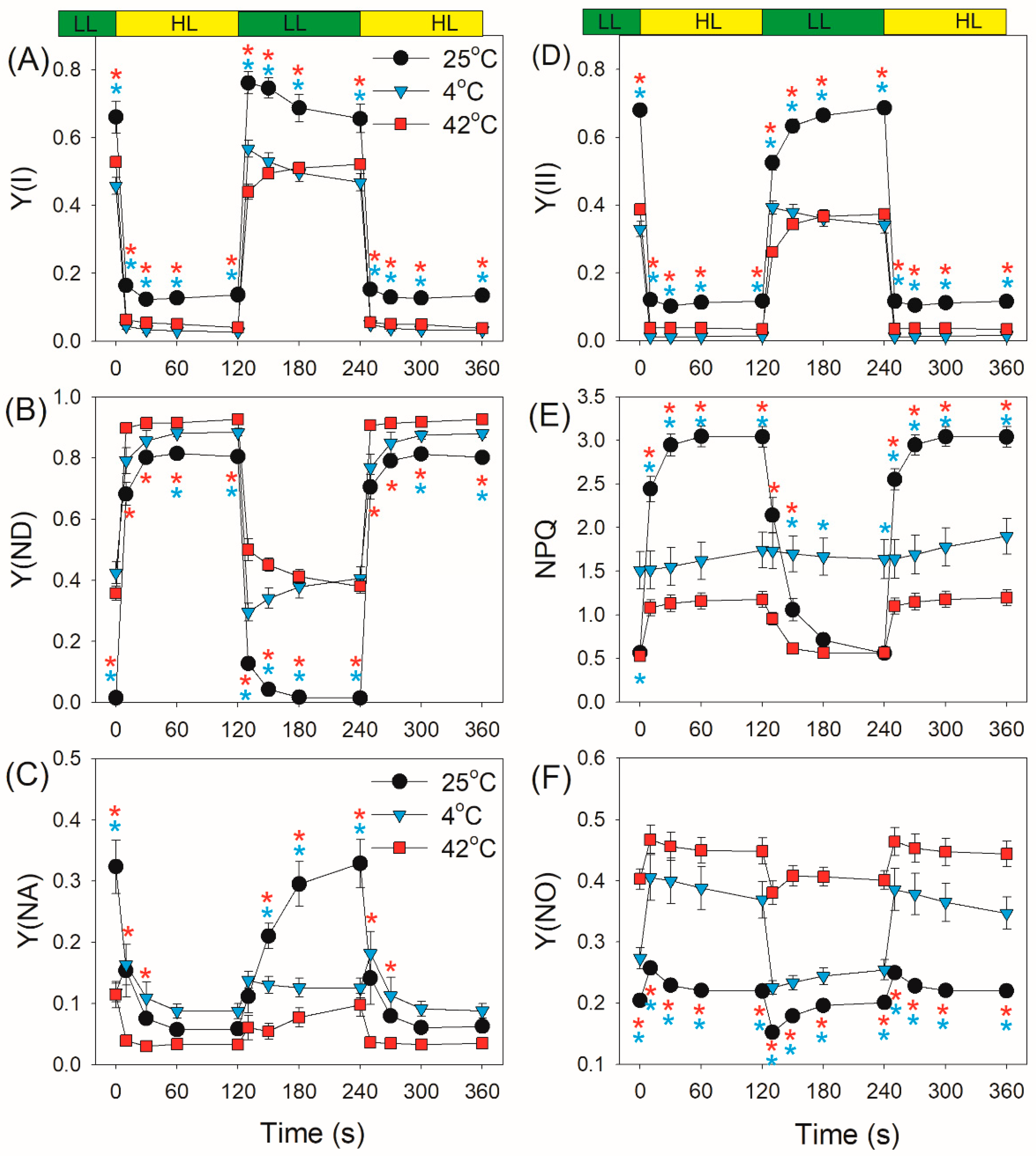
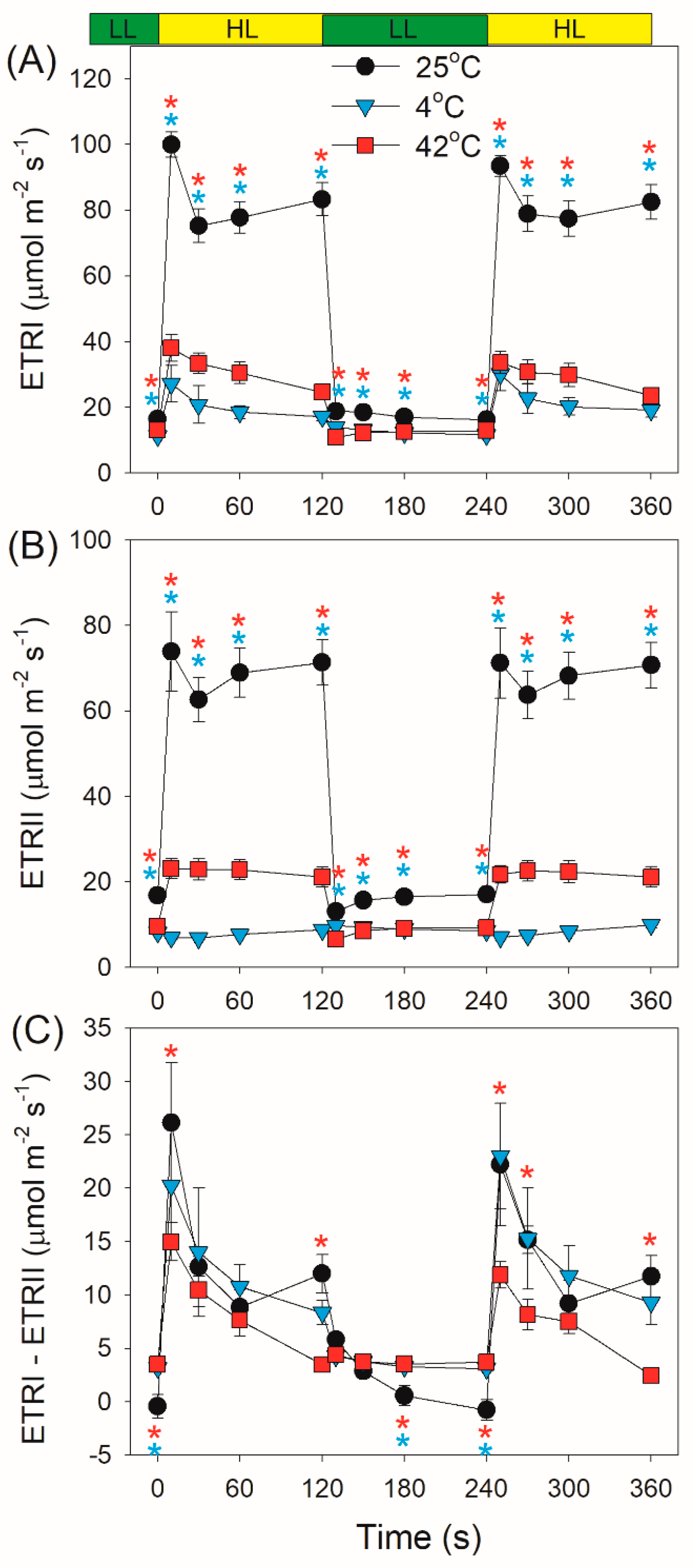
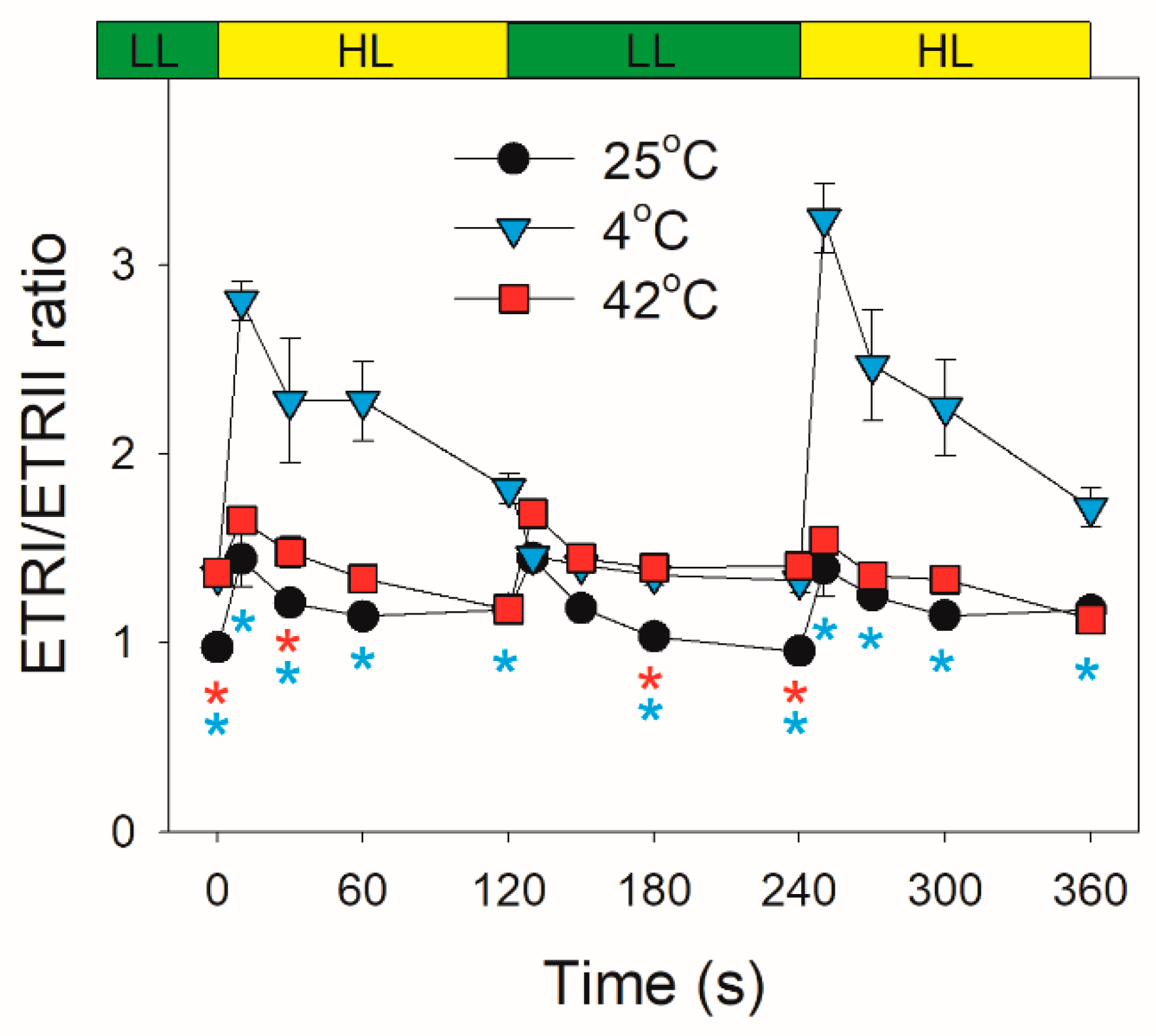
2.2. Photosynthetic Regulation after Transition from Low to High Light
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. P700 Redox Kinetics Measurement
4.3. PSI and PSII Measurements
4.4. Proton Motive Force Measurement
4.5. FL Treatment
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Slattery, R.A.; Walker, B.J.; Weber, A.P.M.; Ort, D.R. The impacts of fluctuating light on crop performance. Plant. Physiol. 2018, 176, 990–1003. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Zhang, S.-B.; Wang, J.-H.; Huang, W. Photosynthetic regulation under fluctuating light in field-grown Cerasus cerasoides: A comparison of young and mature leaves. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 148073. [Google Scholar] [CrossRef]
- Yamori, W.; Kusumi, K.; Iba, K.; Terashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant. Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Gerotto, C.; Alboresi, A.; Meneghesso, A.; Jokel, M.; Suorsa, M.; Aro, E.-M.; Morosinotto, T. Flavodiiron proteins act as safety valve for electrons in Physcomitrella patens. Proc. Natl. Acad. Sci. USA 2016, 113, 12322–12327. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Noguchi, K.; Terashima, I. Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant. Cell Physiol. 2014, 55, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Shikanai, T. PGR5-dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides. Plant. Physiol. 2019, 179, 588–600. [Google Scholar] [CrossRef]
- Takagi, D.; Takumi, S.; Hashiguchi, M.; Sejima, T.; Miyake, C. Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant. Physiol. 2016, 171, 1626–1634. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Sytar, O.; Allakhverdiev, S.I. Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth. Res. 2015, 126, 449–463. [Google Scholar] [CrossRef]
- Sejima, T.; Takagi, D.; Fukayama, H.; Makino, A.; Miyake, C. Repetitive short-pulse light mainly inactivates photosystem i in sunflower leaves. Plant. Cell Physiol. 2014, 55, 1184–1193. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Sytar, O.; Shao, H.; Kalaji, H.M.; Allakhverdiev, S.I. Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2015, 125, 151–166. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Allakhverdiev, S.I. High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2016, 130, 251–266. [Google Scholar] [CrossRef]
- Lima-Melo, Y.; Gollan, P.J.; Tikkanen, M.; Silveira, J.A.G.; Aro, E.M. Consequences of photosystem-I damage and repair on photosynthesis and carbon use in Arabidopsis thaliana. Plant. J. 2019, 97, 1061–1072. [Google Scholar] [CrossRef]
- Storti, M.; Segalla, A.; Mellon, M.; Alboresi, A.; Morosinotto, T. Regulation of electron transport is essential for photosystem I stability and plant growth. New Phytol. 2020, 228, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Shimakawa, G.; Miyake, C. What quantity of photosystem I is optimum for safe photosynthesis? Plant. Physiol. 2019, 179, 1479–1485. [Google Scholar] [CrossRef]
- Alboresi, A.; Storti, M.; Morosinotto, T. Balancing protection and efficiency in the regulation of photosynthetic electron transport across plant evolution. New Phytol. 2019, 221, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; Roustan, V.; Dermendjiev, G.; Rantala, S.; Jain, A.; Leonardelli, M.; Neumann, K.; Berger, V.; Engelmeier, D.; Bachmann, G.; et al. Adjustment of photosynthetic activity to drought and fluctuating light in wheat. Plant. Cell Environ. 2020, 43, 1484–1500. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyeva, Y.; Suorsa, M.; Tikkanen, M.; Aro, E.M. Photoprotection of photosystems in fluctuating light intensities. J. Exp. Bot. 2015, 66, 2427–2436. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.J.; Zhang, S.B. The role of water-water cycle in regulating the redox state of photosystem I under fluctuating light. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, Y.-J.; Huang, W. The water-water cycle is more effective in regulating redox state of photosystem I under fluctuating light than cyclic electron transport. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148235. [Google Scholar] [CrossRef]
- Suorsa, M.; Jarvi, S.; Grieco, M.; Nurmi, M.; Pietrzykowska, M.; Rantala, M.; Kangasjarvi, S.; Paakkarinen, V.; Tikkanen, M.; Jansson, S.; et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant. Cell 2012, 24, 2934–2948. [Google Scholar] [CrossRef]
- Joliot, P.; Johnson, G.N. Regulation of cyclic and linear electron flow in higher plants. Proc. Natl. Acad. Sci. USA 2011, 108, 13317–13322. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C. Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: Molecular mechanisms and physiological functions. Plant. Cell Physiol. 2010, 51, 1951–1963. [Google Scholar] [CrossRef]
- Kono, M.; Terashima, I. Elucidation of photoprotective mechanisms of PSI against fluctuating light photoinhibition. Plant. Cell Physiol. 2016, 57, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, U.; Correa Galvis, V.; Kunz, H.H.; Strand, D.D. The regulation of the chloroplast proton motive force plays a key role for photosynthesis in fluctuating light. Curr. Opin. Plant. Biol. 2017, 37, 56–62. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Botyanszka, L.; Chen, Y.E.; Allakhverdiev, S.I. Phenotyping of isogenic chlorophyll-less bread and durum wheat mutant lines in relation to photoprotection and photosynthetic capacity. Photosynth. Res. 2019, 139, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Živčak, M.; Sytar, O.; Kovár, M.; Watanabe, N.; Pancaldi, S.; Baldisserotto, C.; Brestič, M. Chlorophyll-depleted wheat mutants are disturbed in photosynthetic electron flow regulation but can retain an acclimation ability to a fluctuating light regime. Environ. Exp. Bot. 2020, 178, 104156. [Google Scholar] [CrossRef]
- Walker, B.J.; Strand, D.D.; Kramer, D.M.; Cousins, A.B. The response of cyclic electron flow around photosystem I to changes in photorespiration and nitrate assimilation. Plant. Physiol. 2014, 165, 453–462. [Google Scholar] [CrossRef]
- Avenson, T.J.; Cruz, J.A.; Kanazawa, A.; Kramer, D.M. Regulating the proton budget of higher plant photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9709–9713. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Tan, S.-L.; Yang, Y.-J.; Huang, W. Moderate heat stress accelerates photoinhibition of photosystem I under fluctuating light in tobacco young leaves. Photosynth. Res. 2020, 144, 373–382. [Google Scholar] [CrossRef]
- Tan, S.-L.; Yang, Y.-J.; Liu, T.; Zhang, S.-B.; Huang, W. Responses of photosystem I compared with photosystem II to combination of heat stress and fluctuating light in tobacco leaves. Plant. Sci. 2020, 292, 110371. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Zhang, S.-B.; Huang, W. Photosynthetic regulation under fluctuating light in young and mature leaves of the CAM plant Bryophyllum pinnatum. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Tan, S.-L.; Huang, J.-L.; Zhang, S.-B.; Huang, W. The water-water cycle facilitates photosynthetic regulation under fluctuating light in the epiphytic orchid Dendrobium officinale. Environ. Exp. Bot. 2020, 180, 104238. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Ding, X.-X.; Huang, W. Stimulation of cyclic electron flow around photosystem I upon a sudden transition from low to high light in two angiosperms Arabidopsis thaliana and Bletilla striata. Plant. Sci. 2019, 287, 110166. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.-J.; Zhang, S.-B. Photoinhibition of photosystem I under fluctuating light is linked to the insufficient ΔpH upon a sudden transition from low to high light. Environ. Exp. Bot. 2019, 160, 112–119. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Tan, S.-L.; Sun, H.; Huang, J.-L.; Huang, W.; Zhang, S.-B. Photosystem I is tolerant to fluctuating light under moderate heat stress in two orchids Dendrobium officinale and Bletilla striata. Plant. Sci. 2021, 303, 110795. [Google Scholar] [CrossRef]
- Huang, W.; Sun, H.; Tan, S.-L.; Zhang, S.-B. The water-water cycle is not a major alternative sink in fluctuating light at chilling temperature. Plant. Sci. 2021, 305, 110828. [Google Scholar] [CrossRef]
- Tan, S.-L.; Huang, J.-L.; Zhang, F.-P.; Zhang, S.-B.; Huang, W. Photosystem I photoinhibition induced by fluctuating light depends on background low light irradiance. Environ. Exp. Bot. 2021, 181, 104298. [Google Scholar] [CrossRef]
- Yamamoto, H.; Takahashi, S.; Badger, M.R.; Shikanai, T. Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nat. Plants 2016, 2, 16012. [Google Scholar] [CrossRef]
- Yamori, W.; Makino, A.; Shikanai, T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef]
- Jokel, M.; Johnson, X.; Peltier, G.; Aro, E.M.; Allahverdiyeva, Y. Hunting the main player enabling Chlamydomonas reinhardtii growth under fluctuating light. Plant. J. 2018, 94, 822–835. [Google Scholar] [CrossRef]
- Allahverdiyeva, Y.; Mustila, H.; Ermakova, M.; Bersanini, L.; Richaud, P.; Ajlani, G.; Battchikova, N.; Cournac, L.; Aro, E.-M. Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc. Natl. Acad. Sci. USA 2013, 110, 4111–4116. [Google Scholar] [CrossRef]
- Shimakawa, G.; Ishizaki, K.; Tsukamoto, S.; Tanaka, M.; Sejima, T.; Miyake, C. The liverwort, Marchantia, drives alternative electron flow using a flavodiiron protein to protect PSI. Plant. Physiol. 2017, 173, 1636–1647. [Google Scholar] [CrossRef]
- Chaux, F.; Burlacot, A.; Mekhalfi, M.; Auroy, P.; Blangy, S.; Richaud, P.; Peltier, G. Flavodiiron proteins promote fast and transient O2 photoreduction in Chlamydomonas. Plant. Physiol. 2017, 174, 1825–1836. [Google Scholar] [CrossRef]
- Sonoike, K. Selective photoinhibition of photosystem I in isolated thylakoid membranes from cucumber and spinach. Plant. Cell Physiol. 1995, 36, 825–830. [Google Scholar] [CrossRef]
- Terashima, I.; Noguchi, K.; Itoh-Nemoto, T.; Park, Y.M.; Kubo, A.; Tanaka, K. The cause of PSI photoinhibition at low temperatures in leaves of Cucumis sativus, a chilling-sensitive plant. Physiol. Plant. 1998, 103, 295–303. [Google Scholar] [CrossRef]
- Kudoh, H.; Sonoike, K. Irreversible damage to photosystem I by chilling in the light: Cause of the degradation of chlorophyll after returning to normal growth temperature. Planta 2002, 215, 541–548. [Google Scholar] [CrossRef]
- Zhang, S.; Scheller, H.V. Photoinhibition of photosystem I at chilling temperature and subsequent recovery in Arabidopsis thaliana. Plant. Cell Physiol. 2004, 45, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, S.-B.; Cao, K.-F. The different effects of chilling stress under moderate light intensity on photosystem II compared with photosystem I and subsequent recovery in tropical tree species. Photosynth. Res. 2010, 103, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I.; Murata, N. Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage–repair cycle of Photosystem II in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta Bioenerg. 2004, 1657, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cheng, L.; Gao, H.; Jiang, C.; Peng, T. Heterogeneous behavior of PSII in soybean (Glycine max) leaves with identical PSII photochemistry efficiency under different high temperature treatments. J. Plant. Physiol. 2009, 166, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Gong, H.; Lu, C. Heat stress induces a reversible inhibition of electron transport at the acceptor side of photosystem II in a cyanobacterium Spirulina platensis. Plant. Sci. 2005, 168, 1471–1476. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Zhang, T.; Chen, X.; Li, C.; Liu, Y.; Brestic, M.; Chen, T.H.H.; Yang, X. Glycinebetaine mitigated the photoinhibition of photosystem II at high temperature in transgenic tomato plants. Photosynth. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, S.; Paunov, M.; Pavlova, B.; Dankov, K.; Kouzmanova, M.; Velikova, V.; Tsonev, T.; Kalaji, H.M.; Goltsev, V. Special issue in honour of Prof. Reto J. Strasser—Photosynthetic efficiency of two Platanus orientalis L. ecotypes exposed to moderately high temperature—JIP-test analysis. Photosynthetica 2020, 58, 657–670. [Google Scholar] [CrossRef]
- Pšidová, E.; Živčák, M.; Stojnić, S.; Orlović, S.; Gömöry, D.; Kučerová, J.; Ditmarová, Ľ.; Střelcová, K.; Brestič, M.; Kalaji, H.M. Altitude of origin influences the responses of PSII photochemistry to heat waves in European beech (Fagus sylvatica L.). Environ. Exp. Bot. 2018, 152, 97–106. [Google Scholar] [CrossRef]
- Zhang, R.; Cruz, J.A.; Kramer, D.M.; Magallanes-Lundback, M.E.; Dellapenna, D.; Sharkey, T.D. Moderate heat stress reduces the pH component of the transthylakoid proton motive force in light-adapted, intact tobacco leaves. Plant. Cell Environ. 2009, 32, 1538–1547. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Wiese, C.; Neimanis, S.; Heber, U. Heat sensitivity of chloroplasts and leaves: Leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth. Res. 1999, 59, 81–93. [Google Scholar] [CrossRef]
- Chovancek, E.; Zivcak, M.; Botyanszka, L.; Hauptvogel, P.; Yang, X.; Misheva, S.; Hussain, S.; Brestic, M. Transient Heat Waves May Affect the Photosynthetic Capacity of Susceptible Wheat Genotypes Due to Insufficient Photosystem I Photoprotection. Plants 2019, 8, 282. [Google Scholar] [CrossRef]
- Schreiber, U.; Klughammer, C. Non-photochemical fluorescence quenching and quantum yields in PS I and PS II: Analysis of heat-induced limitations using Maxi-Imaging- PAM and Dual-PAM-100. PAM Appl. Notes 2008, 1, 15–18. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of Q A redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Siebke, K.; Schreiber, U. Continuous ECS-indicated recording of the proton-motive charge flux in leaves. Photosynth. Res. 2013, 117, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yamamoto, H.; Shikanai, T. Role of cyclic electron transport around photosystem I in regulating proton motive force. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Amako, K.; Hashiguchi, M.; Fukaki, H.; Ishizaki, K.; Goh, T.; Fukao, Y.; Sano, R.; Kurata, T.; Demura, T.; et al. Chloroplastic ATP synthase builds up a proton motive force preventing production of reactive oxygen species in photosystem I. Plant. J. 2017, 91, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Sacksteder, C.A.; Kanazawa, A.; Jacoby, M.E.; Kramer, D.M. The proton to electron stoichiometry of steady-state photosynthesis in living plants: A proton-pumping Q cycle is continuously engaged. Proc. Natl. Acad. Sci. USA 2000, 97, 14283–14288. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Shi, Q.; Zhang, S.-B.; Huang, W. Coordination of Cyclic Electron Flow and Water–Water Cycle Facilitates Photoprotection under Fluctuating Light and Temperature Stress in the Epiphytic Orchid Dendrobium officinale. Plants 2021, 10, 606. https://doi.org/10.3390/plants10030606
Sun H, Shi Q, Zhang S-B, Huang W. Coordination of Cyclic Electron Flow and Water–Water Cycle Facilitates Photoprotection under Fluctuating Light and Temperature Stress in the Epiphytic Orchid Dendrobium officinale. Plants. 2021; 10(3):606. https://doi.org/10.3390/plants10030606
Chicago/Turabian StyleSun, Hu, Qi Shi, Shi-Bao Zhang, and Wei Huang. 2021. "Coordination of Cyclic Electron Flow and Water–Water Cycle Facilitates Photoprotection under Fluctuating Light and Temperature Stress in the Epiphytic Orchid Dendrobium officinale" Plants 10, no. 3: 606. https://doi.org/10.3390/plants10030606
APA StyleSun, H., Shi, Q., Zhang, S.-B., & Huang, W. (2021). Coordination of Cyclic Electron Flow and Water–Water Cycle Facilitates Photoprotection under Fluctuating Light and Temperature Stress in the Epiphytic Orchid Dendrobium officinale. Plants, 10(3), 606. https://doi.org/10.3390/plants10030606





