Impact of Foliar Application of Amino Acids on Total Phenols, Phenolic Acids Content of Different Mints Varieties under the Field Condition
Abstract
1. Introduction
2. Results
2.1. Total Phenol Content
2.2. Total Phenolic Acids Content
2.3. Hydroxybenzoic Acids Content
2.4. Hydroxycinnamic Acids Content
2.5. Principal Component (PCA) and Hierarchical Clustering Analysis (HCA)
3. Discussion
4. Materials and Methods
4.1. Experimental Sites and Soil
4.2. Meteorological Conditions
4.3. Chemicals
4.4. Methods of Sample Preparation
4.5. Polyphenol Content
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tucker, A.O. Mentha: Economic Uses. In Mint, The Genus Mentha; Lawrence, B.M., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 519–522. [Google Scholar]
- Gulluce, M.; Shain, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguel, A.; Ozcan, H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. spp. longifolia. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [CrossRef]
- Nazem, V.; Sabzalian, R.M.; Saeidi, G.; Rahimmalek, M. Essential oil yield and composition and secondary metabolites in self- and open-pollinated populations of mint (Mentha spp.). Ind. Crops Prod. 2019, 130, 332–340. [Google Scholar] [CrossRef]
- Benabdallah, A.; Rahmoune, C.; Boumendjel, M.; Aissi, O.; Messaoud, C. Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of Algeria. Asian Pac. J. Trop. Biomed. 2016, 6, 760–766. [Google Scholar] [CrossRef]
- Hanafy, D.M.; Prenzler, P.D.; Burrows, G.E.; Ryan, D.; Nielsen, S.; El Sawi, S.A.; El Alfy, T.S.; Abdelrahman, E.H.; Obied, H.K. Biophenols of mints: Antioxidant, acetylcholinesterase, butyrylcholinesterase and histone deacetylase inhibition activities targeting Alzheimer’s disease treatment. J. Funct. Foods. 2017, 33, 345–362. [Google Scholar] [CrossRef]
- Atanassova, M.; Georgieva, S.; Ivancheva, K. Total phenolic and total flavonoids contents, antioxidant capacity and biological contaminants in medicinal herbs. J. Univ. Chem. Technol. Metall. 2011, 46, 81–88. [Google Scholar]
- Kapp, K. Polyphenolic and Essential Oil Composition of Mentha and Their Antimicrobial Effect. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2015. [Google Scholar]
- Kivilompolo, M.; Hyotylainen, T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J. Chromatogr. 2007, 1145, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Mekinic, I.G.; Skroza, D.; Ljubenkov, I.; Simat, V.; Mozina, S.S.; Katalinic, V. In vitro antioxidant and antibacterial activity of Lamiaceae phenolic extracts: A correlation study. Food Technol. Biotechnol. 2014, 52, 119–127. [Google Scholar]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Thomas, J.; Mandal, A.K.A.; Kumar, R.R.; Chordia, A. Role of biologically active amino acid formulations on quality and crop productivity of tea (Camellia sp.). Int. J. Agric. Res. 2009, 4, 228–236. [Google Scholar] [CrossRef]
- Mehrafarin, A.; Qavami, N.; Tahmasebi, Z.; Badi, H.N.; Abdossi, V.; Sahandi, M.S. Phytochemical and morpho-physiological responses of Lemon Balm (Melissa officinalis L.) to biostimulants application. J. Med. Plants 2015, 14, 29–42. [Google Scholar]
- Azza, S.M.; Yousef, R.S. Response of Basil Plant (Ocimum sanctum L.) to foliar spray with amino acids or seaweed extract. JHSOP 2015, 7, 94–106. [Google Scholar]
- Nia, A.F.; Hassanali, N.B.; Mehrafarin, A.; Bahman, S.; Sahandi, M.S. Changes in the essential oil content and terpene composition of rosemary (Rosmarinus officinalis L.) by using plant biostimulants. Acta Agric. Slov. 2016, 107, 147–157. [Google Scholar]
- Reham, M.S.; Khattab, M.E.; Ahmed, S.S.; Kandil, M.A.M. Influence of foliar spray with phenylalanine and nickel on growth, yield quality and chemical composition of genoveser basil plant. Afr. J. Agric. Res. 2016, 11, 1348–1410. [Google Scholar] [CrossRef]
- Knaggs, A.R. The biosynthesis of shikimate metabolites. Nat. Prod. Rep. 2003, 20, 119–136. [Google Scholar] [CrossRef]
- Wink, M. Biochemistry, Physiology and Ecological Functions of Secondary Metabolites. In Biochemistry of Plant Secondary Metabolism, 2nd ed.; Wink, M., Ed.; Wiley–Blackwell Publishing: New York, NY, USA, 2010; pp. 1–17. [Google Scholar]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Ardebili, Z.O.; Ladan Moghadam, A.R.; Ardebili, N.O.; Pashaie, A.R. The induced physiological changes by foliar application of amino acids in Aloe vera L. plants. J. Plant Biol. Omics. 2012, 5, 279–284. [Google Scholar]
- Watts, K.T.; Mijts, B.N.; Lee, P.C.; Manning, A.J.; Schmidt-Dannert, C. Discovery of a substrate selectivity switch in tyrosine ammonia-lyase, a member of the aromatic amino acid lyase family. Chem. Biol. 2006, 13, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Musbah, H.M.; Ibrahim, K.M. Effects of feeding tyrosine or phenylalanine on the accumulation of polyphenols in Coleus Blumei in Vivo and in Vitro. J. Biotechnol. Res. Center 2019, 13, 35–43. [Google Scholar]
- Spaepen, S.; Vanderleyden, J. Auxin and Plant-Microbe Interactions. Cold Spring Harb Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Bujor, O.C.; Popa, V. Phenolic and Natural Compounds and Their Influence on Physiological Processes in Plants. In Polyphenols in Plants; Watson, R.R., Ed.; Academic Press: London, UK; Oxford, UK; San Diego, CA, USA; Cambridge, MA, USA, 2019; pp. 45–58. [Google Scholar]
- Lattanzio, V.; Cardinali, A.; Ruta, C.; Fortunato, I.M.; Lattanzio, V.M.T.; Linsalata, V.; Cicco, N. Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ. Exp. Bot. 2009, 65, 54–62. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Akashdeep, P.R.; Soni, G. Effect of cultural manipulations on antioxidant potential of tissue cultured Mentha spicata (SPR 8). J. Pharm. Res. 2011, 4, 3368–3371. [Google Scholar]
- Debleena, R.; Sandip, M. Enhanced rosmarinic acid production in cultured plants of two species of Mentha. IJEB 2012, 50, 817–825. [Google Scholar]
- Debleena, R.; Bidisha, M.; Dipu, S. Augmentation of antioxidative potential of in vitro propagated Mentha piperita L. IJEB 2020, 58, 131–137. [Google Scholar]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Marchiosi, R.; Dantas dos Santos, W.; Constantin, R.P.; Barbosa de Lima, R.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; de Paiva Foletto-Felipe, M.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Manela, N.; Oliva, M.; Ovadia, R.; Sikron-Persi, N.; Ayenew, B.; Fait, A.; Galili, G.; Perl, A.; Weiss, D.; Oren-Shamir, M. Phenylalanine and tyrosine levels are rate-limiting factors in production of health promoting metabolites in Vitis vinifera cv. Gamay Red cell suspension. Front. Plant Sci. 2015, 6, 538. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agar-Wal, C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm. Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.J.; Choi, C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Khadem, S.; Marles, R.J. Monocyclic Phenolic Acids; Hydroxy- and Polyhydroxybenzoic Acids: Occurrence and Recent Bioactivity Studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef]
- Tuteja, A.N.; Gill, S.S.; Kumar Trivedi, P.; Asif, M.H.; Nath, P. Plant growth regulators and their role in stress tolerance. Plant Stress 2010, 4, 1–18. [Google Scholar]
- Singh, H.; Bhat, J.A.; Singh, V.P.; Corpas, F.J.; Yadav, S.R. Auxin metabolic network regulates the plant response to metalloids stress. J. Hazard. Mater. 2021, 405, 124250. [Google Scholar] [CrossRef]
- AL Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, M.A. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef]
- Macoy, D.M.; Kim, W.Y.; Lee, S.Y.; Kim, M.G. Biosynthesis, physiology, and functions of hydroxycinnamic acid amides in plants. Plant. Biotechnol. Rep. 2015, 9, 269–278. [Google Scholar] [CrossRef]
- Granataa, G.; Consolia, G.M.L.; Lo Nigrob, R.; Geraci, C. Hydroxycinnamic acids loaded in lipid-core nanocapsules. Food Chem. 2018, 245, 551–556. [Google Scholar] [CrossRef]
- Barros, J.; Serrani-Yarce, J.C.; Chen, F.; Baxter, D.; Venables, B.J.; Dixon, R.A. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat. Plants 2016, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ipson, B.R.; Fisher, A.L. Roles of the tyrosine isomers meta-tyrosine and ortho-tyrosine in oxidative stress. Ageing Res. Rev. 2016, 27, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Hack, H.; Bleiholder, H.; Buhr, L.; Meier, U.; Schnock-Fricke, U.; Weber, E.; Witzenberger, A. Einheitliche Codierung der Phanologischen Entwicklungsstadien Mono und Dikotyler Pflanzen–Entweirterte BBCH-Skala. Allg. Nachr. Deut Pflanzenschutzd. 1992, 44, 265–270. [Google Scholar]
- Hallmann, E. The influence of organic and conventional cultivation systems on the nutritional value and content of bioactive compounds in selected tomato types. J. Sci. Food Agric. 2012, 92, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
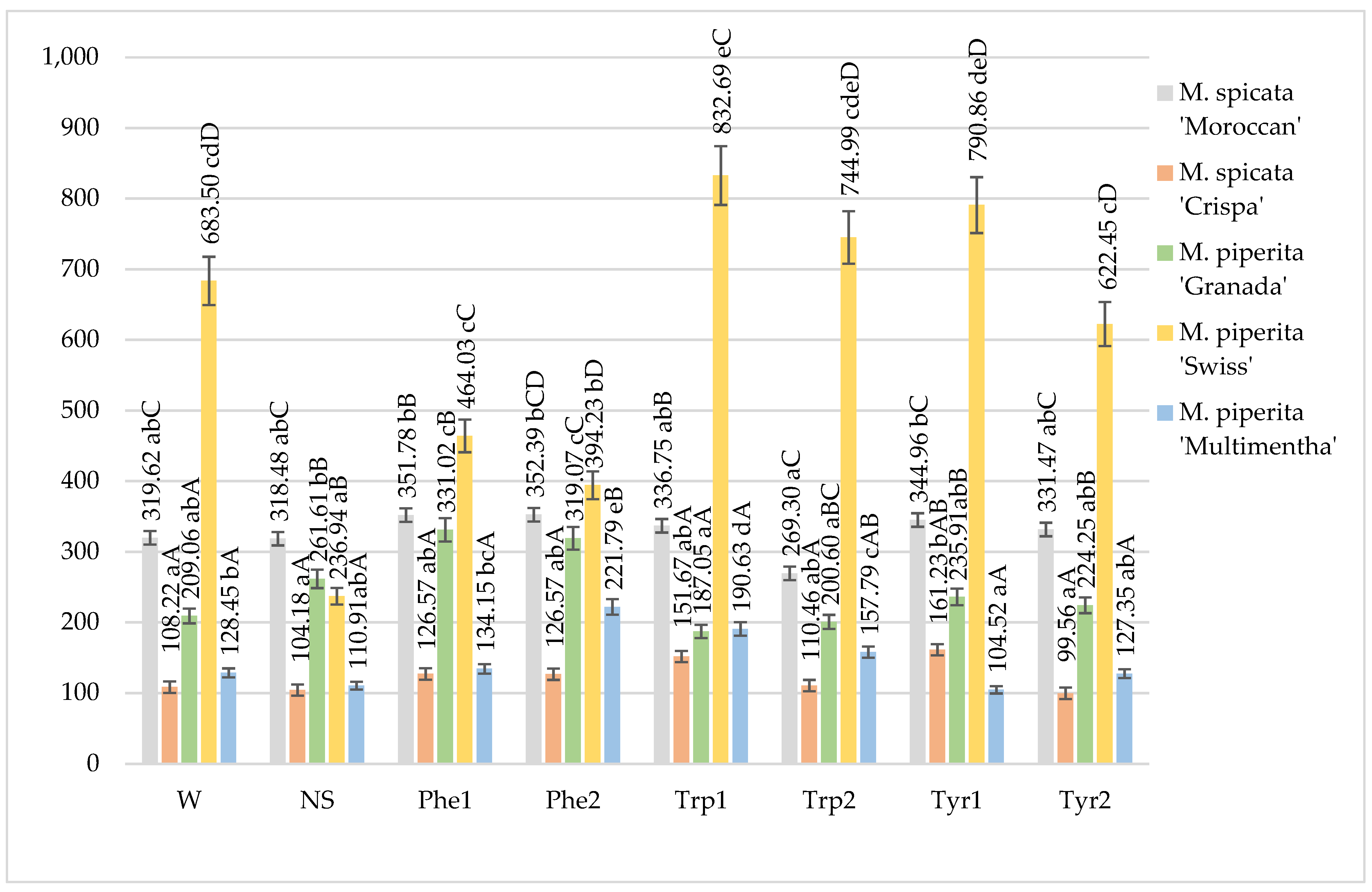

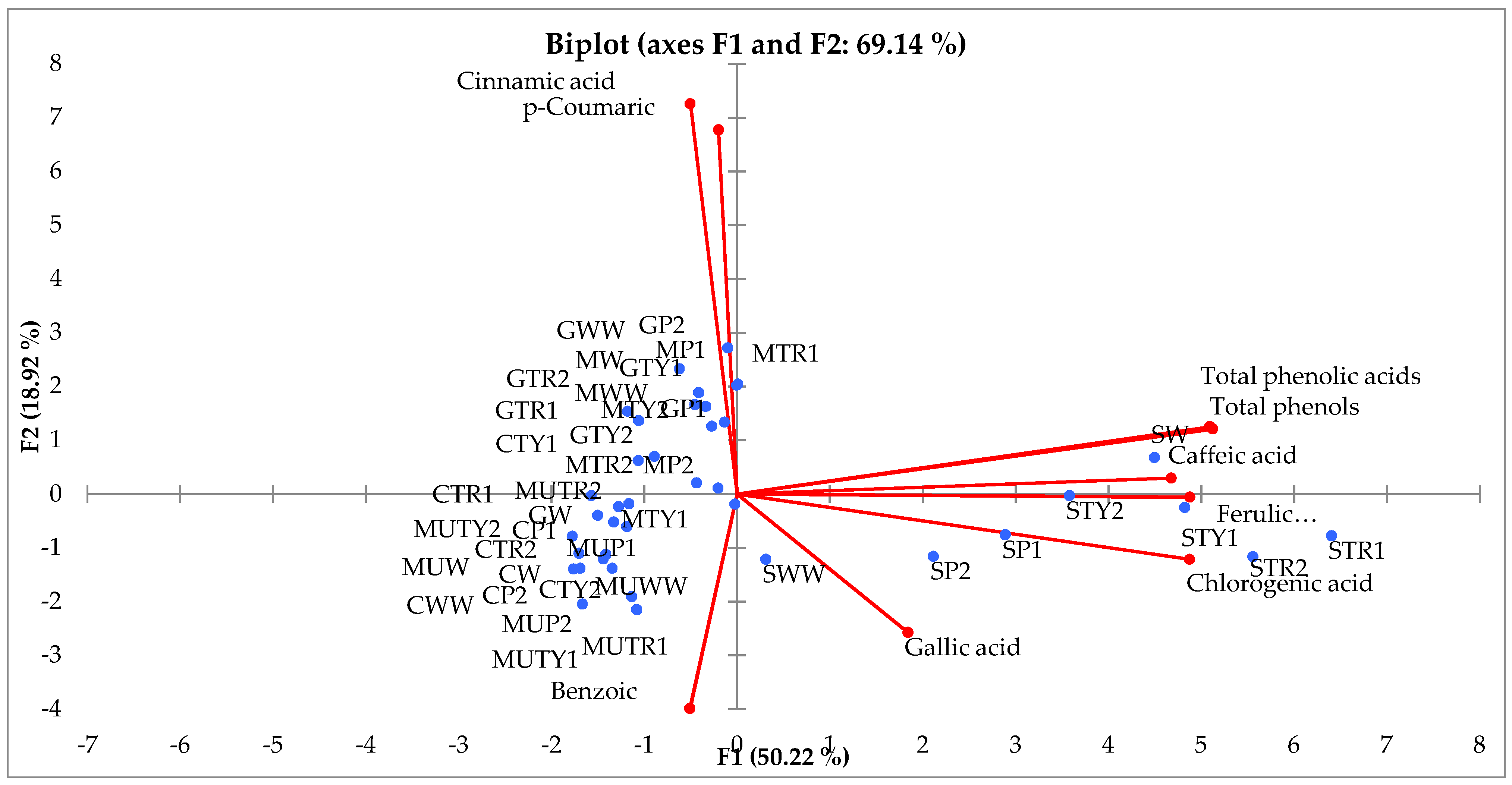


| Treatment | Species/Variety | ||||
|---|---|---|---|---|---|
| M. spicata “Moroccan” | M. spicata “Crispa” | M. piperita “Granada” | M. piperita “Swiss” | M. piperita “Multimentha” | |
| Water | 6.82 * ± 0.34 abcBC | 7.60 ± 0.52 cC | 6.08 ± 0.18 aC | 4.75 ± 0.16 aA | 11.21 ± 0.53 dD |
| Non-sprayed | 6.57 ± 0.08 abcA | 7.23 ± 0.42 cA | 6.34 ± 0.25 aA | 22.02 ± 2.84 cC | 13.69 ± 0.69 eB |
| Phenylalanine 100 mg L−1 | 7.12 ± 0.08 cB | 8.95 ± 0.53 dA | 8.66 ± 0.84 bA | 8.40 ± 0.27 abA | 6.39 ± 0.07 abB |
| Phenylalanine 200 mg L−1 | 7.16 ± 0.30 cA | 7.58 ± 0.09 cA | 13.84 ± 0.42 cC | 5.91 ± 0.23 abB | 7.62 ± 0.31 bA |
| Tryptophan 100 mg L−1 | 6.79 ± 0.27 abcA | 5.98 ± 0.16 bA | 6.82 ± 0.18 aA | 24.81 ± 1.39 cC | 16.70 ± 1.29 fB |
| Tryptophan 200 mg L−1 | 6.22 ± 0.33 aB | 4.94 ± 0.10 aA | 8.06 ± 0.18 bC | 23.07 ± 0.73 cD | 5.32 ± 0.29 aAB |
| Tyrosine 100 mg L−1 | 6.98 ± 0.08 bcC | 7.31 ± 0.24 cC | 5.97 ± 0.13 aB | 4.11 ± 0.30 aA | 9.46 ± 0.37 cD |
| Tyrosine 200 mg L−1 | 6.45 ± 0.17 abB | 6.96 ± 0.13 cB | 6.82 ± 0.31 aB | 4.74 ± 0.53 aA | 13.19 ± 0.32 eC |
| Treatment | Species/Variety | ||||
|---|---|---|---|---|---|
| M. spicata “Moroccan” | M. spicata “Crispa” | M. piperita “Granada” | M. piperita “Swiss” | M. piperita “Multimentha” | |
| Water | 2.03 * ± 0.38 abAB | 2.96 ± 0.41 bB | 5.36 ± 0.39 dC | 1.91 ± 0.38 abA | 4.96 ± 0.43 cdC |
| Non-sprayed | 4.30 ± 0.39 eBC | 5.13 ± 1.07 cC | 2.44 ± 0.38 bcAB | 0.92 ± 0.31 aA | 3.42 ± 1.59 bcBC |
| Phenylalanine 100 mg L−1 | 3.02 ± 0.38 bcB | 0.89 ± 0.37 aA | 2.91 ± 0.41 cB | 1.12 ± 0.37 aA | 0.93 ± 0.33 aA |
| Phenylalanine 200 mg L−1 | 1.62 ± 0.41aA | 5.35 ± 0.40 cBC | 1.92 ± 0.31 bA | 5.24 ± 0.40 eBC | 7.17 ± 0.41 deC |
| Tryptophan 100 mg L−1 | 3.13 ± 0.38 cdAB | 2.38 ± 0.44 bAB | 2.17 ± 0.29 bcA | 3.42 ± 0.44 cdB | 8.90 ± 0.78 eC |
| Tryptophan 200 mg L−1 | 1.99 ± 0.38 abA | 2.04 ± 0.39 abA | 2.10 ± 0.40 bcA | 2.28 ± 0.40 bA | 1.46 ± 0.39 abA |
| Tyrosine 100 mg L−1 | 4.13 ± 0.39 deB | 2.05 ± 0.38 abA | 0.53 ± 0.37 aA | 4.34 ± 0.55 deB | 7.82 ± 1.36 eC |
| Tyrosine 200 mg L−1 | 3.03 ± 0.38 bcC | 2.23 ± 0.38 abBC | 1.929 ± 0.35 bcB | 2.58 ± 0.38 bcBC | 0.92 ± 0.44 aA |
| Treatment | Species/Variety | ||||
|---|---|---|---|---|---|
| M. spicata “Moroccan” | M. spicata “Crispa” | M. piperita “Granada” | M. piperita “Swiss” | M. piperita “Multimentha” | |
| Water | 11.15 * ± 2.68 aA | 2.14 ± 0.04 aA | 7.61 ± 0.19 abA | 130.27 ± 14.23 bB | 6.13 ± 0.30 abA |
| Non-sprayed | 14.93 ± 0.36 bcA | 2.09 ± 0.05 aA | 7.09 ± 0.18 abA | 48.28 ± 14.63 aB | 8.25 ± 2.64 abA |
| Phenylalanine 100 mg L−1 | 16.29 ± 1.50 bcC | 2.02 ± 0.10 aA | 10.43 ± 0.59 cB | 123.63 ± 4.57 bD | 9.54 ± 0.65 bcB |
| Phenylalanine 200 mg L−1 | 15.75 ± 0.59 bcAB | 2.35 ± 0.16 aA | 12.47 ± 0.30 dA | 67.92 ± 12.19 aC | 28.10 ± 5.57 dB |
| Tryptophan 100 mg L−1 | 16.79 ± 0.56 cB | 1.93 ± 0.05 aA | 6.89 ± 0.43 aAB | 114.43 ± 12.45 bC | 15.49 ± 3.05 cAB |
| Tryptophan 200 mg L−1 | 13.46 ± 0.33 abA | 3.55 ± 0.09 aA | 11.35 ± 0.20 cdA | 103.68 ± 13.04 bB | 9.63 ± 0.76 bcA |
| Tyrosine 100 mg L−1 | 15.40 ± 0.42 bcB | 12.54 ± 0.33 bB | 19.42 ± 1.09 eC | 73.86 ± 2.44 aD | 3.87 ± 1.69 abA |
| Tyrosine 200 mg L−1 | 14.93 ± 0.37 bcB | 14.71 ± 1.74 cB | 8.33 ± 0.23 bAB | 64.38 ± 5.65 aC | 2.51 ± 0.80 aA |
| Treatment | Species/Variety | ||||
|---|---|---|---|---|---|
| M. spicata “Moroccan” | M. spicata “Crispa” | M. piperita “Granada” | M. piperita “Swiss” | M. piperita “Multimentha” | |
| Water | 53.34 * ± 12.04 aB | 9.26 ± 1.15 aA | 46.24 ± 7.51 bcB | 90.55 ± 15.44 aC | 9.21 ± 1.75 abA |
| Non-sprayed | 55.93 ± 9.80 aB | 7.64 ± 1.17 aA | 47.87 ± 8.37 bcB | 22.59 ± 13.64 aA | 7.50 ± 0.94 abA |
| Phenylalanine 100 mg L−1 | 77.97 ± 15.72 aA | 11.39 ± 1.86 aB | 56.55 ± 15.98 cA | 58.79 ± 8.24 aA | 5.18 ± 0.56 aB |
| Phenylalanine 200 mg L−1 | 83.01 ± 14.13 aC | 6.41 ± 0.89 aA | 46.81 ± 8.79 bcB | 55.47 ± 9.51 aB | 6.88 ± 1.33 aA |
| Tryptophan 100 mg L−1 | 82.66 ± 13.43 aB | 4.65 ± 0.52 aA | 19.82 ± 3.30 aAB | 340.87 ± 61.93 bC | 7.14 ± 0.82 abA |
| Tryptophan 200 mg L−1 | 62.41 ± 10.96 aA | 5.25 ± 0.68 aA | 20.02 ± 3.34 aA | 300.32 ± 53.78 bB | 12.02 ± 4.29 bA |
| Tyrosine 100 mg L−1 | 82.56 ± 17.22 aB | 40.56 ± 7.08 bAB | 25.17 ± 9.82 abAB | 338.18 ± 60.58 bC | 7.87 ± 1.64 abA |
| Tyrosine 200 mg L−1 | 65.76 ± 11.57 aB | 6.56 ± 1.12 aA | 48.38 ± 7.02 bcAB | 277.64 ± 44.96 bC | 6.05 ± 0.83 aA |
| Treatment | Species/Variety | ||||
|---|---|---|---|---|---|
| M. spicata “Moroccan” | M. spicata “Crispa” | M. piperita “Granada” | M. piperita “Swiss” | M. piperita “Multimentha” | |
| Water | 104.45 * ± 8.45 bC | 28.41 ± 4.57 abA | 58.53 ± 5.38 abB | 65.54 ± 5.72 dB | 48.30 ± 10.22 bB |
| Non-sprayed | 117.27 ± 11.05 bB | 33.61 ± 2.34 bA | 105.84 ± 9.91 cdB | 38.10 ± 3.07 bA | 30.16 ± 1.95 aA |
| Phenylalanine 100 mg L−1 | 120.79 ± 11.62 bB | 48.89 ± 4.83 cdA | 134.99 ± 14.99 dB | 28.85 ± 1.90 bA | 28.51 ± 1.57 aA |
| Phenylalanine 200 mg L−1 | 65.66 ± 5.85 aC | 37.68 ± 3.49 bcB | 134.87 ± 18.28 dD | 15.14 ± 0.76 aA | 28.26 ± 1.79 aAB |
| Tryptophan 100 mg L−1 | 120.10 ± 11.38 bC | 76.82 ± 8.98 eB | 53.21 ± 5.95 abA | 64.47 ± 6.38 dAB | 48.84 ± 4.16 bA |
| Tryptophan 200 mg L−1 | 97.72 ± 9.08 bC | 31.34 ± 2.40 bA | 51.37 ± 3.62 abB | 29.47 ± 3.26 bA | 55.55 ± 5.27 bB |
| Tyrosine 100 mg L−1 | 111.65 ± 12.67 bC | 60.01 ± 5.71 dB | 44.22 ± 3.04 aAB | 28.54 ± 1.93 bA | 30.07 ± 5.38 aA |
| Tyrosine 200 mg L−1 | 98.57 ± 9.16 bD | 16.22 ± 1.62 aA | 76.85 ± 15.51 bcC | 51.71 ± 3.75 cB | 52.65 ± 3.93 bB |
| Treatment | Species/Variety | ||||
|---|---|---|---|---|---|
| M. spicata “Moroccan” | M. spicata “Crispa” | M. piperita “Granada” | M. piperita “Swiss” | M. piperita “Multimentha” | |
| Water | 17.34 * ± 1.65 bcdB | 2.36 ± 0.18 aA | 5.19 ± 0.37 bA | 176.13 ± 9.57 dC | 4.18 ± 0.32 abA |
| Non-sprayed | 19.26 ± 0.99 cdeC | 3.16 ± 0.27 aA | 22.79 ± 1.06 aD | 27.10 ± 1.41 aE | 7.22 ± 0.50 cB |
| Phenylalanine 100 mg L−1 | 20.59 ± 1.19 dcA | 2.64 ± 0.24 aB | 24.07 ± 1.61 aD | 113.38 ± 12.62 bcC | 30.79 ± 1.91 eA |
| Phenylalanine 200 mg L−1 | 7.34 ± 0.42 aAB | 3.53 ± 0.28 aA | 20.42 ± 1.51 aCD | 98.17 ± 11.90 bcC | 2.98 ± 0.34 aA |
| Tryptophan 100 mg L−1 | 22.11 ± 2.17 eB | 2.67 ± 0.29 aA | 18.12 ± 1.66 cdBC | 115.80 ± 13.14 cC | 2.29 ± 0.19 aA |
| Tryptophan 200 mg L−1 | 15.64 ± 0.93 bB | 2.41 ± 0.22 aA | 16.28 ± 0.98 cB | 112.15 ± 6.18 bcD | 23.22 ± 1.00 dC |
| Tyrosine 100 mg L−1 | 18.08 ± 1.21 bcdA | 7.82 ± 0.51 bA | 47.98 ± 2.12 eE | 116.21 ± 12.16 cC | 7.04 ± 0.47 cA |
| Tyrosine 200 mg L−1 | 17.04 ± 1.01 bcB | 15.76 ± 1.14 cB | 7.34 ± 0.49 bA | 88.43 ± 4.17 bC | 6.12 ± 0.77 bcA |
| Treatment | Species/Variety | ||||
|---|---|---|---|---|---|
| M. spicata “Moroccan” | M. spicata “Crispa” | M. piperita “Granada” | M. piperita “Swiss” | M. piperita “Multimentha” | |
| Water | 14.86 * ± 1.73 cD | 0.30 ± 0.05 aA | 4.80 ± 0.21 aB | 7.18 ± 0.39 cC | 1.03 ± 0.96 aA |
| Non-sprayed | 13.97 ± 0.81 cB | 0.34 ± 0.03 aA | 20.74 ± 1.23 bC | 0.23 ± 0.03 aA | 1.00 ± 0.92 aA |
| Phenylalanine 100 mg L−1 | 14.88 ± 1.11 cC | 0.19 ± 0.03 aA | 4.44 ± 0.25 aB | 0.45 ± 0.02 aA | 1.00 ± 0.93 aA |
| Phenylalanine 200 mg L−1 | 1.23 ± 0.08 aA | 0.16 ± 0.04 aA | 21.64 ± 1.28 bC | 3.51± 0.19 bB | 0.07 ± 0.05 aA |
| Tryptophan 100 mg L−1 | 15.39 ± 1.26 cB | 0.07 ± 0.04 aA | 23.40 ± 1.80 bC | 0.23 ± 0.06 aA | 1.15 ± 0.09 aA |
| Tryptophan 200 mg L−1 | 11.08 ± 0.65 bB | 0.08 ± 0.04 aA | 22.32 ± 1.41 bC | 0.63 ± 0.98 aA | 0.48 ± 0.39 aA |
| Tyrosine 100 mg L−1 | 14.05 ± 1.17 cD | 11.22 ± 0.64 cC | 22.55 ± 1.60 bE | 4.48 ± 0.21 bB | 0.48 ± 0.46 aA |
| Tyrosine 200 mg L−1 | 10.64 ± 0.61 bD | 1.14 ± 0.08 bB | 7.27 ± 0.40 aC | 0.18 ± 0.05 aA | 0.26 ± 0.03 aA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasevičienė, Ž.; Velička, A.; Paulauskienė, A. Impact of Foliar Application of Amino Acids on Total Phenols, Phenolic Acids Content of Different Mints Varieties under the Field Condition. Plants 2021, 10, 599. https://doi.org/10.3390/plants10030599
Tarasevičienė Ž, Velička A, Paulauskienė A. Impact of Foliar Application of Amino Acids on Total Phenols, Phenolic Acids Content of Different Mints Varieties under the Field Condition. Plants. 2021; 10(3):599. https://doi.org/10.3390/plants10030599
Chicago/Turabian StyleTarasevičienė, Živilė, Aloyzas Velička, and Aurelija Paulauskienė. 2021. "Impact of Foliar Application of Amino Acids on Total Phenols, Phenolic Acids Content of Different Mints Varieties under the Field Condition" Plants 10, no. 3: 599. https://doi.org/10.3390/plants10030599
APA StyleTarasevičienė, Ž., Velička, A., & Paulauskienė, A. (2021). Impact of Foliar Application of Amino Acids on Total Phenols, Phenolic Acids Content of Different Mints Varieties under the Field Condition. Plants, 10(3), 599. https://doi.org/10.3390/plants10030599







