The Short-Term Metabolic Modulation of Basil (Ocimum basilicum L. cv. ‘Genovese’) after Exposure to Cold or Heat
Abstract
1. Introduction
2. Results
2.1. Content of Photosynthetic Pigments and Soluble Proteins
2.2. Activity of Antioxidant Enzymes
2.3. Content of Secondary Metabolites
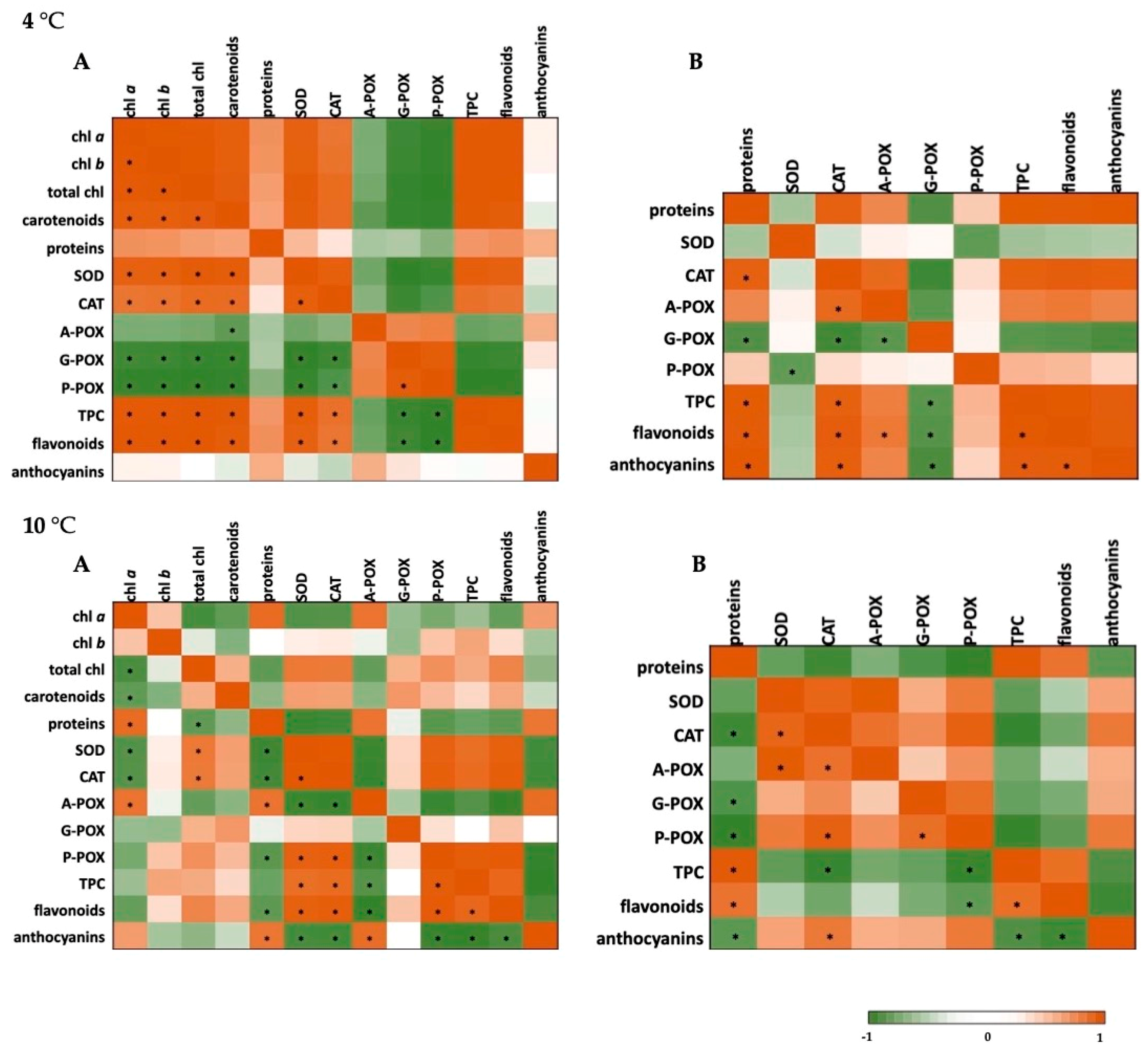
2.4. Correlation Analysis and Heat Maps
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Design and Stress Conditions
5.2. Determination of Photosynthetic Pigments and Soluble Proteins Content
5.3. Enzyme Extraction and Measurements
5.4. Total Phenolic and Flavonoid Content
5.5. Total Anthocyanin Content
5.6. Statistical Testing and Data Visualization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, M.; Kim, J.W.; Begum, M.; Sohel, M.; Taher, A.; Lim, Y.S. Physiological and biochemical changes in sugar beet seedlings to confer stress adaptability under drought condition. Plants 2020, 9, 1511. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Pazouki, L.; Niinemets, Ü. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant Physiol. 2012, 169, 664–672. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Ihsan, M.Z. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643. [Google Scholar] [CrossRef] [PubMed]
- Rooy, S.S.B.; Salekdeh, G.H.; Ghabooli, M.; Gholami, M.; Karimi, R. Cold-induced physiological and biochemical responses of three grapevine cultivars differing in cold tolerance. Acta Physiol. Plant. 2017, 39, 264. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef]
- Ren, R.; Li, Z.; Zhang, L.; Zhou, H.; Jiang, X.; Liu, Y. Enzymatic and nonenzymatic antioxidant systems impact the viability of cryopreserved Paeonia suffruticosa pollen. Plant Cell Tiss Organ Cult. 2020. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Babula, P.; Jarošová, M. Variation of antioxidants and secondary metabolites in nitrogen-deficient barley plants. J. Plant Physiol. 2014, 171, 260–268. [Google Scholar] [CrossRef]
- Jakovljević, D.; Topuzović, M.; Stanković, M. Nutrient limitation as a tool for the induction of secondary metabolites with antioxidant activity in basil cultivars. Ind. Crops Prod. 2019, 138, 111462. [Google Scholar] [CrossRef]
- Kalisz, A.; Jezdinský, A.; Pokluda, R.; Sękara, A.; Grabowska, A.; Gil, J. Impacts of chilling on photosynthesis and chlorophyll pigment content in juvenile basil cultivars. Hortic. Environ. Biotechnol. 2016, 57, 330–339. [Google Scholar] [CrossRef]
- Al-Huqail, A.; El-Dakak, R.M.; Sanad, M.N.; Badr, R.H.; Ibrahim, M.M.; Soliman, D.; Khan, F. Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in sweet basil (Ocimum basilicum L.) leafy vegetable. Scientifica 2020. [Google Scholar] [CrossRef] [PubMed]
- Renard, D.; Tilman, D. National food production stabilized by crop diversity. Nature 2019, 571, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; West, P.C.; Clark, M.; Gerber, J.S.; Prishchepov, A.V.; Chatterjee, S. Climate change has likely already affected global food production. PLoS ONE 2019, 14, e0217148. [Google Scholar] [CrossRef]
- Voss-Fels, K.P.; Stahl, A.; Hickey, L.T. Q&A: Modern crop breeding for future food security. BMC Biol. 2019, 17, 18. [Google Scholar]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.S.; Abdelaal, K.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; El Nahhas, N. Exogenous ascorbic acid induced chilling tolerance in tomato plants through modulating metabolism, osmolytes, antioxidants, and transcriptional regulation of catalase and heat shock proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Guo, S.; An, Y.; Shu, S.; Lu, N.; Sun, J. Exogenous spermidine maintains the chloroplast structure of cucumber seedlings and inhibits the degradation of photosynthetic protein complexes under high-temperature stress. Acta Physiol. Plant. 2018, 40, 47. [Google Scholar] [CrossRef]
- Haldimann, P. How do changes in temperature during growth affect leaf pigment composition and photosynthesis in Zea mays genotypes differing in sensitivity to low temperature? J. Exp. Bot. 1999, 50, 543–550. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Jakovljević, D. Intraspecijska varijabilnost primarnog i sekundarnog metabolizma nutritivno depriviranih klijanaca vrste Ocimum basillcum L. (Lamiaceae). Ph.D. Thesis, University of Kragujevac, Faculty of Science, Kragujevac, Serbia, 2018. [Google Scholar]
- Lara, I.; Drincovich, M.F.; Beckles, D.M.; Cao, S. Physiological, molecular and genetic perspectives of chilling tolerance in horticultural crops. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Nahakpam, S. Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol. Biochem. 2012, 57, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhu, X.; Xiang, S.; Zhang, X.; Liu, Z.; Zhu, L.; Lai, J. Comparative proteomic analysis reveals differential protein and energy metabolisms from two tobacco cultivars in response to cold stress. Acta Physiol. Plant. 2018, 40, 19. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.; Liu, C.; Liu, G.; Li, S.; Wang, L. Integrating omics and alternative splicing reveals insights into grape response to high temperature. Plant Physiol. 2017, 173, 1502–1518. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S.; Genisel, M.; Atici, O.; Demir, Y.; Yanmis, D. The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant Growth Regul. 2014, 74, 139–152. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Zaid, A.; Abo-Baker, A.B.A.E.; Salem, W.; Abu Alhmad, M.F. Mitigation of copper stress in maize by inoculation with Paenibacillus polymyxa and Bacillus circulans. Plants 2020, 9, 1513. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Kalisz, A.; Pokluda, R.; Jezdinský, A.; Sękara, A.; Grabowska, A.; Gil, J.; Neugebauerová, J. Chilling-induced changes in the antioxidant status of basil plants. Acta Physiol. Plant. 2016, 38, 196. [Google Scholar] [CrossRef]
- Jakovljević, D.Z.; Topuzović, M.D.; Stanković, M.S.; Bojović, B.M. Changes in antioxidant enzyme activity in response to salinity-induced oxidative stress during early growth of sweet basil. Hortic. Environ. Biotechnol. 2017, 58, 240–246. [Google Scholar] [CrossRef]
- Ara, N.; Nakkanong, K.; Lv, W.; Yang, J.; Hu, Z.; Zhang, M. Antioxidant enzymatic activities and gene expression associated with heat tolerance in the stems and roots of two cucurbit species (“Cucurbita maxima” and “Cucurbita moschata”) and their interspecific inbred line “Maxchata”. Int. J. Mol. Sci. 2013, 14, 24008–24028. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Khanna-Chopra, R.; Semwal, V.K. Superoxide dismutase and ascorbate peroxidase are constitutively more thermotolerant than other antioxidant enzymes in Chenopodium album. Physiol. Mol. Biol. Plants 2011, 17, 339. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Front. Plant Sci. 2017, 8, 953. [Google Scholar] [CrossRef]
- Sarkar, J.; Chakraborty, B.; Chakraborty, U. Temperature stress induced antioxidative and biochemical changes in wheat (Triticum aestivum L.) cultivars. J. Plant Stress Physiol. 2016, 2, 22–30. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef] [PubMed]
- Lüthje, S.; Martinez-Cortes, T. Membrane-bound class III peroxidases: Unexpected enzymes with exciting functions. Int. J. Mol. Sci. 2018, 19, 2876. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.A.; Jan, N.; Qazi, H.A.; Andrabi, K.I.; John, R. Cold stress induces biochemical changes, fatty acid profile, antioxidant system and gene expression in Capsella bursa pastoris L. Acta Physiol. Plant. 2018, 40, 167. [Google Scholar] [CrossRef]
- Rezaie, R.; Mandoulakani, B.A.; Fattahi, M. Cold stress changes antioxidant defense system, phenylpropanoid contents and expression of genes involved in their biosynthesis in Ocimum basilicum L. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Rastogi, S.; Shah, S.; Kumar, R.; Vashisth, D.; Akhtar, M.Q.; Kumar, A.; Shasany, A.K. Ocimum metabolomics in response to abiotic stresses: Cold, flood, drought and salinity. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhai, J.; Shao, L.; Lin, W.; Peng, C. Accumulation of anthocyanins: An adaptation strategy of Mikania micrantha to low temperature in winter. Front. Plant Sci. 2019, 10, 1049. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, A.; Wu, X.; Zhu, Z.; Yang, Z.; Zhu, Y.; Zha, D. Transcriptome analysis revealed expression of genes related to anthocyanin biosynthesis in eggplant (Solanum melongena L.) under high-temperature stress. BMC Plant Biol. 2019, 19, 387. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, D.; Stanković, M.; Bojović, B.; Topuzović, M. Regulation of early growth and antioxidant defense mechanism of sweet basil seedlings in response to nutrition. Acta Physiol. Plant. 2017, 39, 243. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Jia, L.; Xu, W.; Li, W.; Ye, N.; Liu, R.; Shi, L.; Bin Rubaiyath, A.N.M.; Fan, M.; Zhang, J. Class III peroxidases are activated in proanthocyanidin-deficient Arabidopsis thaliana seeds. Ann. Bot. 2013, 111, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Kukavica, B.; Morina, F.; Janjić, N.; Boroja, M.; Jovanović, L.; Veljović- Jovanović, S. Effects of mixed saline and alkaline stress on the morphology and anatomy of Pisum sativum L.: The role of peroxidase and ascorbate oxidase in growth regulation. Arch. Biol. Sci. 2013, 65, 265–278. [Google Scholar]
- Mihailović, V.; Mišić, D.; Matić, S.; Mihailović, M.; Stanić, S.; Vrvić, M.; Katanić, J.; Mladenović, M.; Stanković, N.; Boroja, T.; et al. Comparative phyto-chemical analysis of Gentiana cruciata L. roots and aerial parts, and their biological activities. Ind. Crops Prod. 2015, 73, 49–62. [Google Scholar] [CrossRef]
- Inácio, M.R.C.; de Lima, K.M.G.; Lopes, V.G.; Pessoa, J.D.C.; de Almeida Teixeira, G.H. Total anthocyanin content determination in intact açaí (Euterpe oleracea Mart.) and palmitero-juçara (Euterpe edulis Mart.) fruit using near infrared spectroscopy (NIR) and multivariate calibration. Food Chem. 2013, 136, 1160–1164. [Google Scholar] [CrossRef] [PubMed]


| Temperature Treatment (°C) | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Carotenoids | Protein (Leaves) | Protein (Roots) |
|---|---|---|---|---|---|---|
| control | 0.034 ± 0.003 c 1 | 0.024 ± 0.00 c | 0.066 ± 0.007 b | 0.016 ± 0.001 b | 2.54 ± 0.03 a | 3.52 ± 0.04 b |
| 4 | 0.052 ± 0.000 b | 0.037 ± 0.00 b | 0.101 ± 0.002 a | 0.024 ± 0.000 a | 2.94 ± 0.09 a | 4.84 ± 0.15 a |
| 10 | 0.032 ± 0.007 c | 0.023 ± 0.00 c | 0.070 ± 0.017 b | 0.017 ± 0.001 b | 1.77 ± 0.02 b | 2.06 ± 0.03 c |
| 30 | 0.076 ± 0.001 a | 0.047 ± 0.00 a | 0.140 ± 0.001 a | 0.030 ± 0.003 a | 0.81 ± 0.01 c | 4.30 ± 0.11 a |
| 40 | 0.063 ± 0.001 a | 0.032 ± 0.02 b | 0.109 ± 0.021 a | 0.030 ± 0.000 a | 1.31 ± 0.01 b | 4.34 ± 0.15 a |
| Temperature Treatment (°C) | Total Phenolic Content | Flavonoids | Total Anthocyanin Content | |||
|---|---|---|---|---|---|---|
| Leaves | Roots | Leaves | Roots | Leaves | Roots | |
| control | 2.35 ± 0.07 d 1 | 452.00 ± 0.94 c | 19.69 ± 0.01 d | 11.96 ± 0.00 b | 8.35 ± 0.00 d | 4.18 ± 0.05 b |
| 4 | 227.29 ± 0.52 a | 761.41 ± 0.53 a | 22.80 ± 0.00 c | 13.71 ± 0.01 a | 13.87 ± 0.02 b | 10.02 ± 0.07 a |
| 10 | 34.25 ± 0.53 c | 314.35 ± 0.06 d | 27.51 ± 0.01 a | 11.43 ± 0.01 b | 11.19 ± 0.08 c | 5.51 ± 0.05 b |
| 30 | 80.24 ± 0.35 b | 449.65 ± 0.11 c | 25.83 ± 0.01 b | 12.19 ± 0.00 b | 17.69 ± 0.03 a | 4.68 ± 0.00 b |
| 40 | 70.82 ± 0.12 b | 470.82± 0.94 b | 27.31 ± 0.00 a | 12.43 ± 0.00 a | 13.03 ± 0.17 b | 3.34 ± 0.03 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovljević, D.; Momčilović, J.; Bojović, B.; Stanković, M. The Short-Term Metabolic Modulation of Basil (Ocimum basilicum L. cv. ‘Genovese’) after Exposure to Cold or Heat. Plants 2021, 10, 590. https://doi.org/10.3390/plants10030590
Jakovljević D, Momčilović J, Bojović B, Stanković M. The Short-Term Metabolic Modulation of Basil (Ocimum basilicum L. cv. ‘Genovese’) after Exposure to Cold or Heat. Plants. 2021; 10(3):590. https://doi.org/10.3390/plants10030590
Chicago/Turabian StyleJakovljević, Dragana, Jovana Momčilović, Biljana Bojović, and Milan Stanković. 2021. "The Short-Term Metabolic Modulation of Basil (Ocimum basilicum L. cv. ‘Genovese’) after Exposure to Cold or Heat" Plants 10, no. 3: 590. https://doi.org/10.3390/plants10030590
APA StyleJakovljević, D., Momčilović, J., Bojović, B., & Stanković, M. (2021). The Short-Term Metabolic Modulation of Basil (Ocimum basilicum L. cv. ‘Genovese’) after Exposure to Cold or Heat. Plants, 10(3), 590. https://doi.org/10.3390/plants10030590








