Nitrogen Deficiency and Synergism between Continuous Light and Root Ammonium Supply Modulate Distinct but Overlapping Patterns of Phytohormone Composition in Xylem Sap of Tomato Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment with Strong Stress
2.2. Experiment with Mild Stress
2.3. Measurements of Plant Growth
2.4. One-Step Extraction of Chlorophyll, Anions, Cations and Sugars
2.5. Quantitative Determination of Pigments
2.6. Chlorophyll Index
2.7. Analysis of Nonstructural Carbohydrates from the Water:Methanol Phase
2.8. Extraction of Starch
2.9. Ion Chromatographic Determination of Anions and Cations
2.10. Determination of Total Nitrogen by Alkaline Persulfate Digestion
2.11. Xylem Sap Collection
2.12. Hormone Analysis
2.13. Statistics
3. Results
3.1. Interaction of Continuous Light with N Forms on Tomato Plant Growth Traits
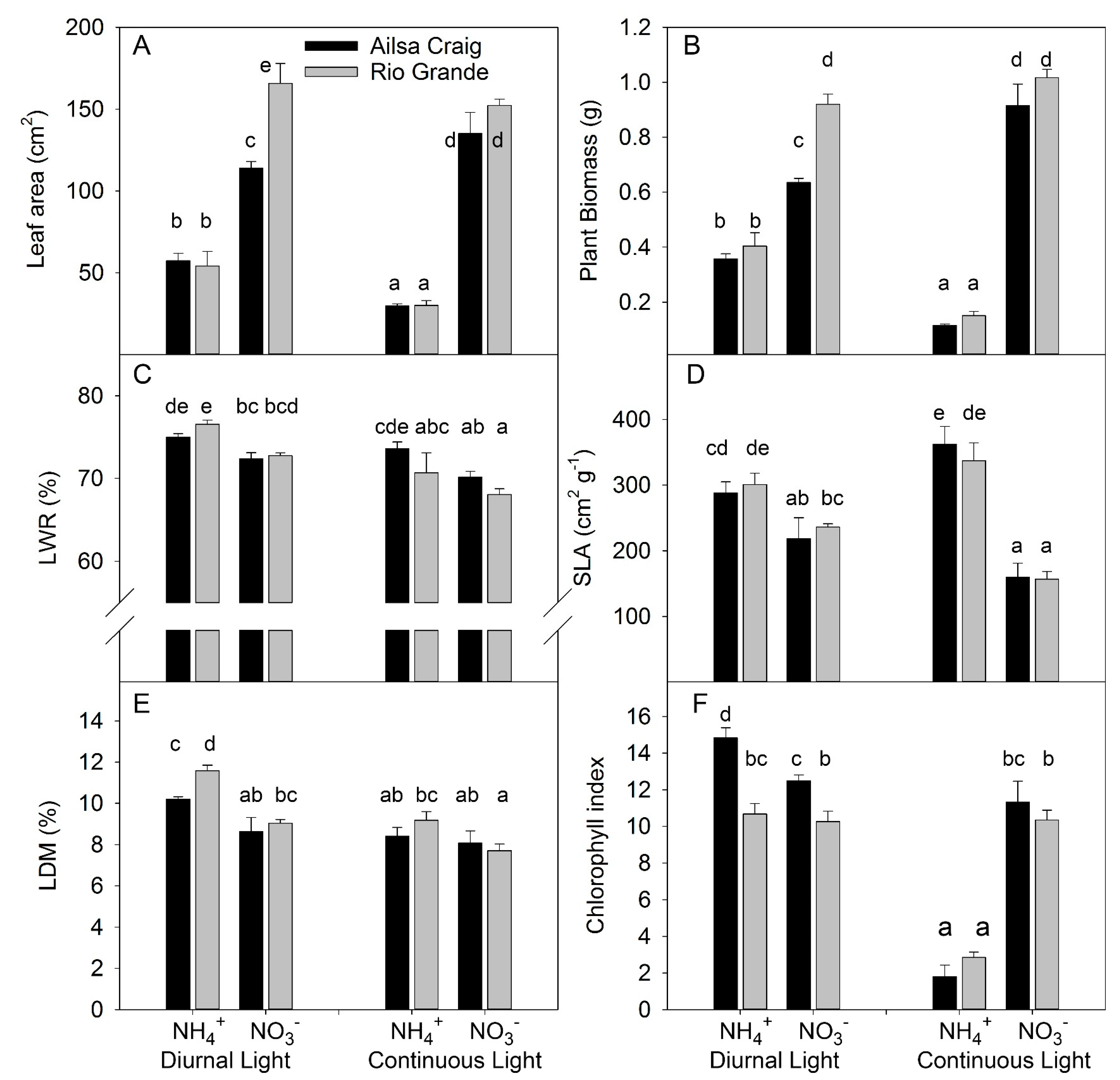
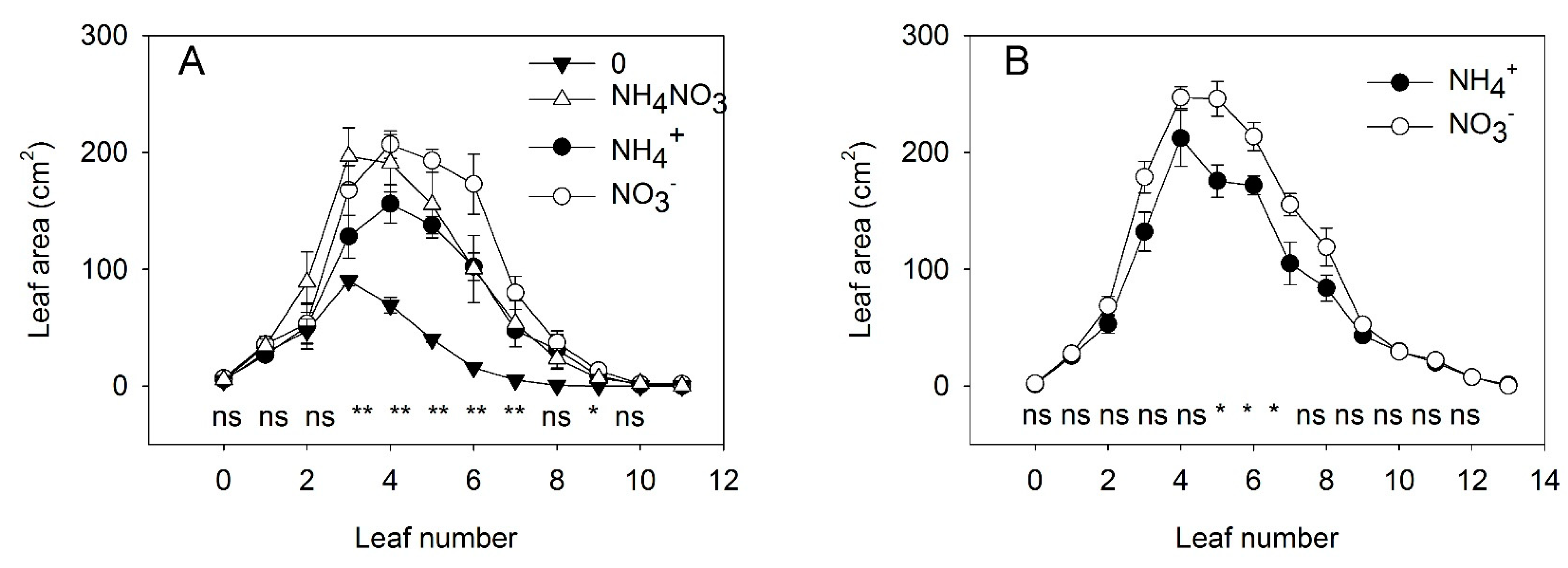
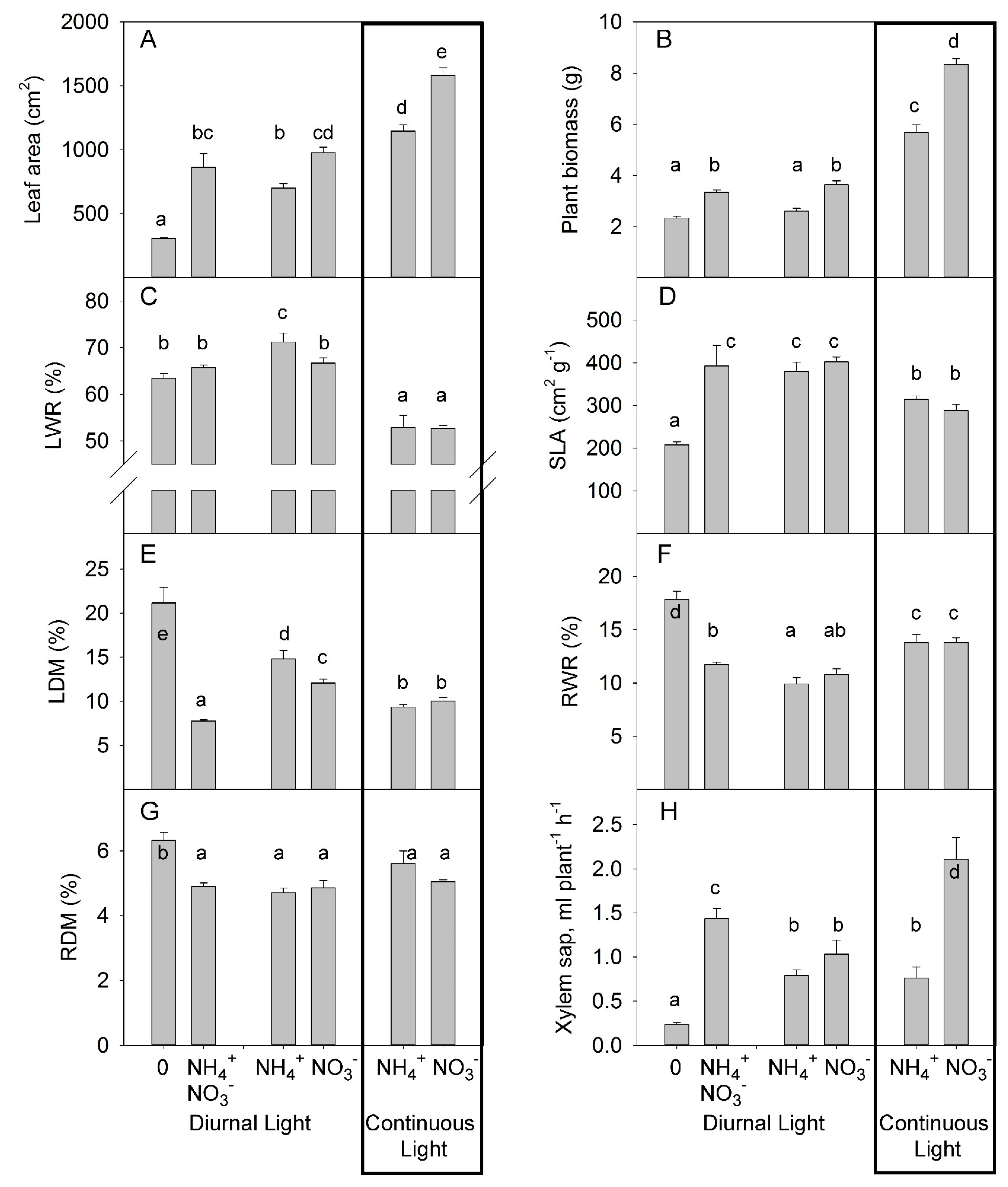
3.1.1. Experiment with Strong Stress
3.1.2. Experiment with Mild Stress
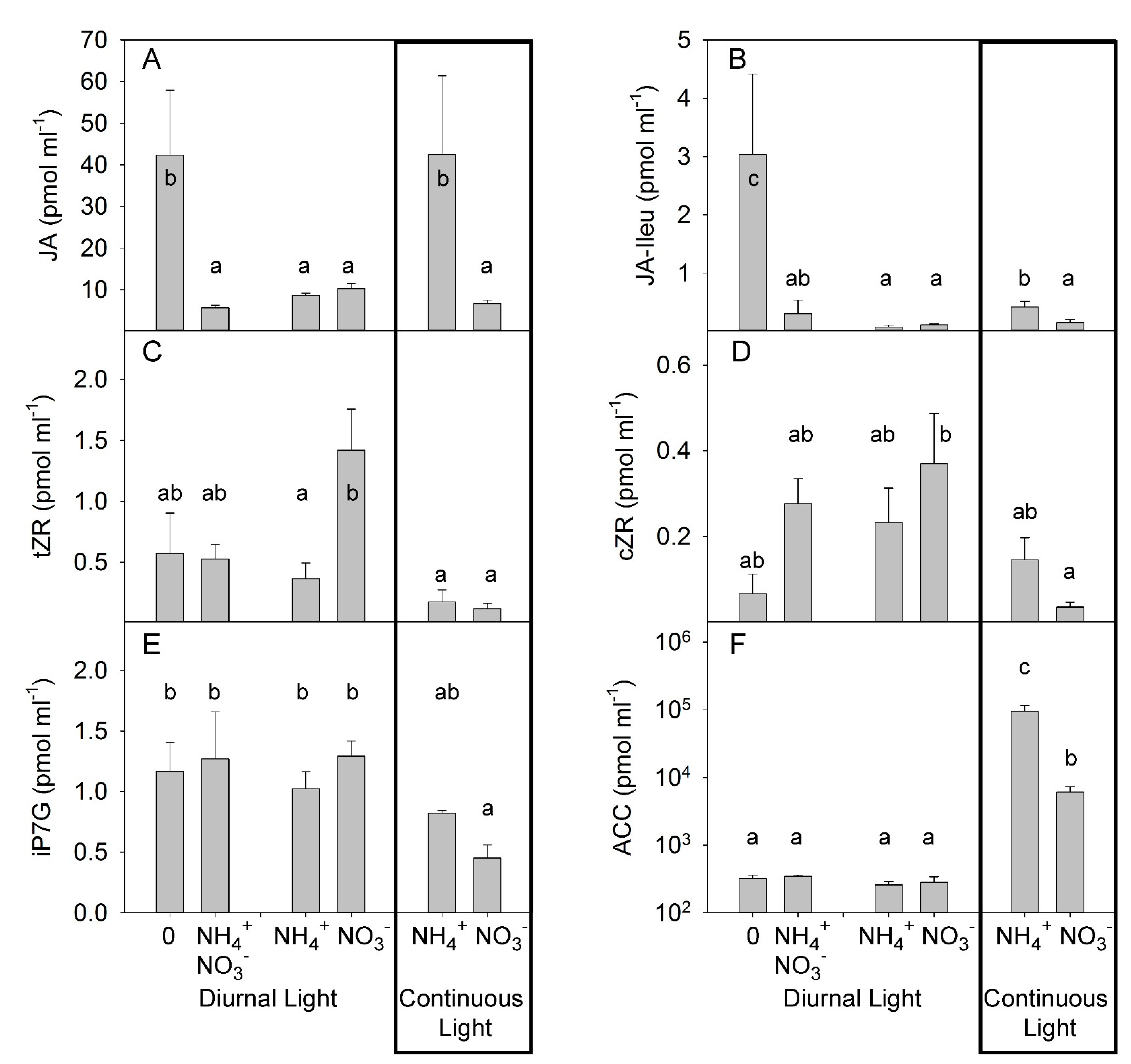
3.2. Effects of Different N Forms on the Hormonal Composition of Xylem Sap
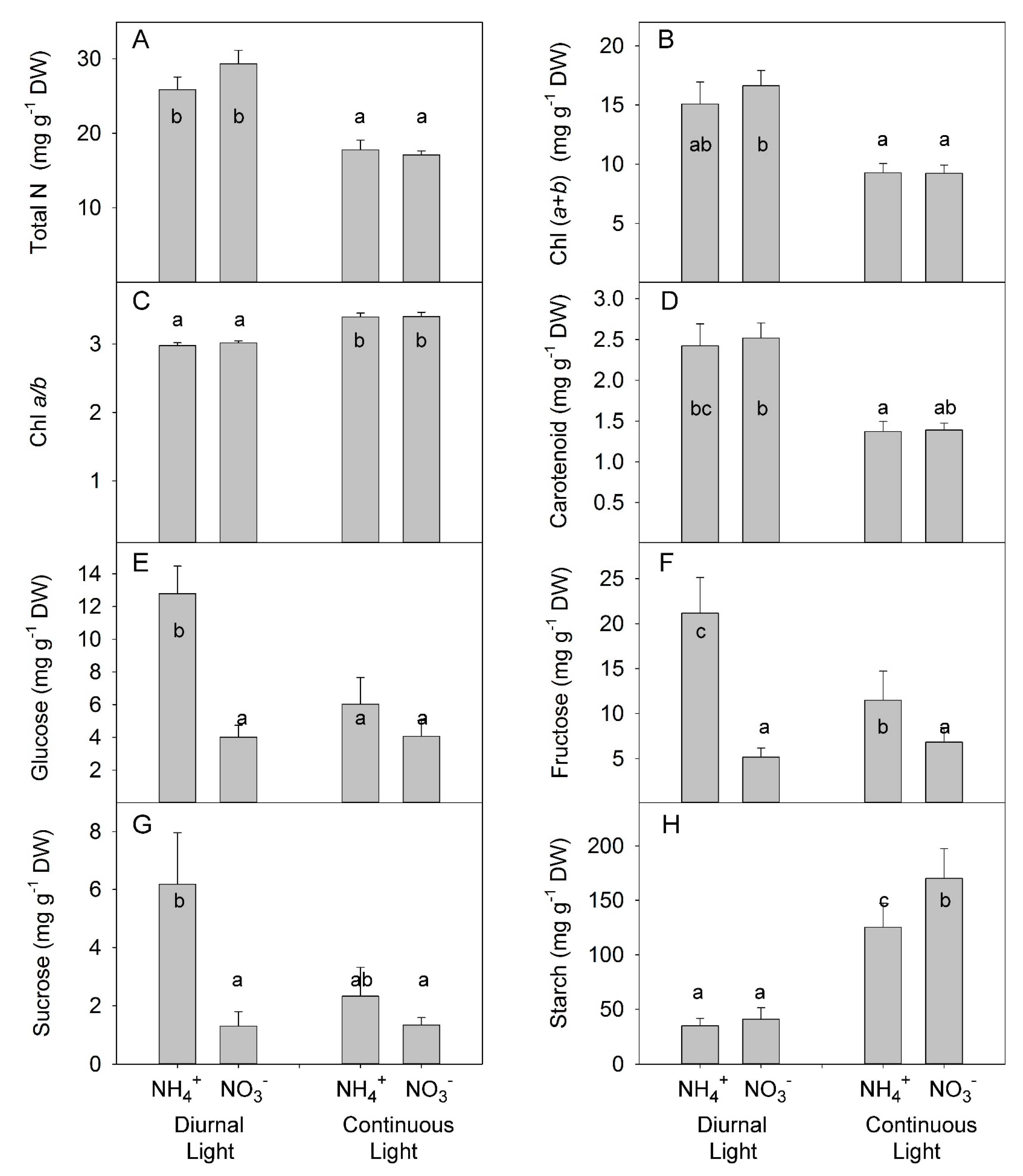
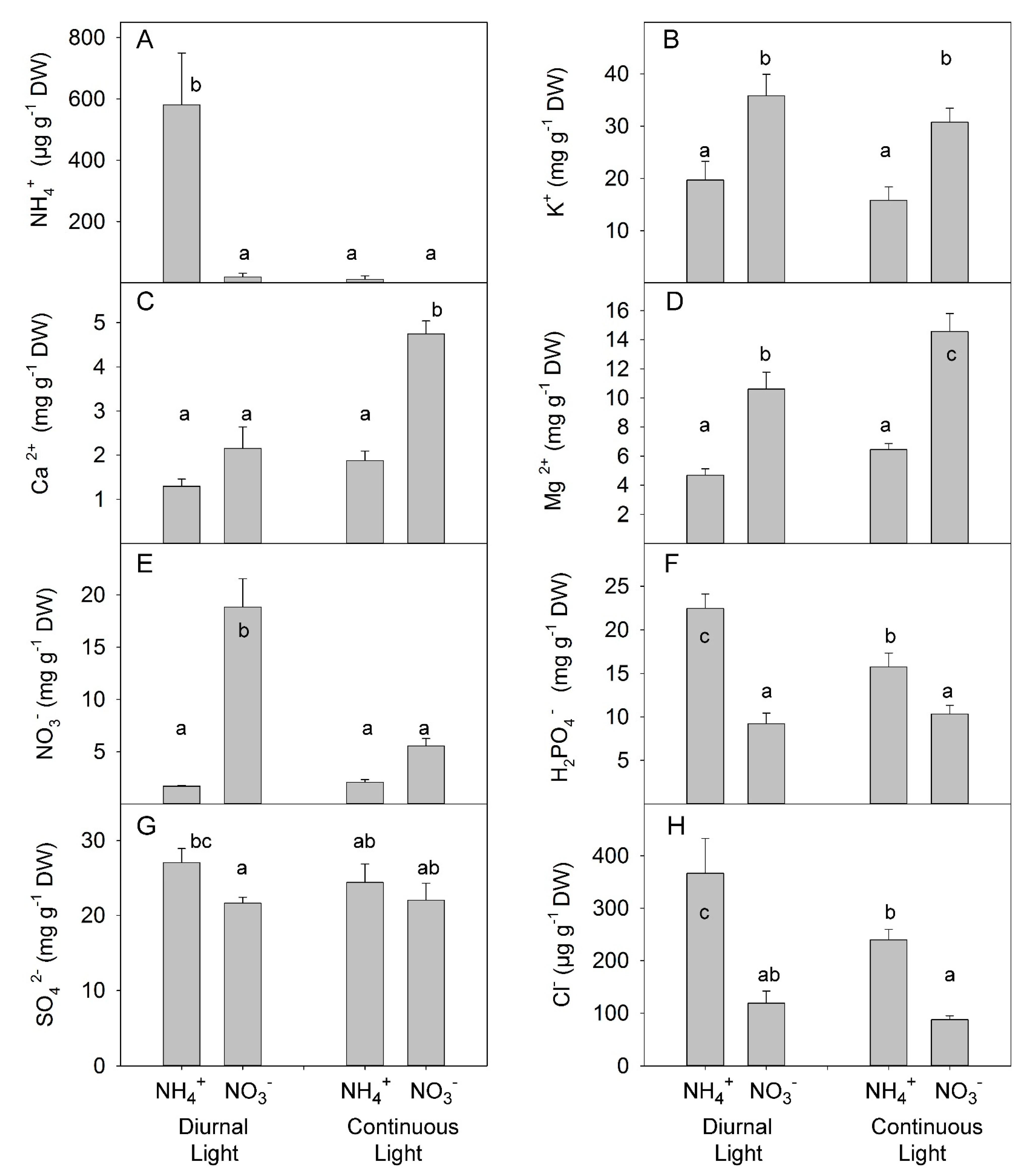
3.3. Interaction between N Forms and Light on Total Nitrogen, Leaf Pigment, Non-Structural Carbohydrate, and Mineral Ion Concentrations in Leaves
4. Discussion
4.1. Synergetic Effect of Combined Application of CL and NH4+
4.2. The Absence of Synergetic Effects of Combined CL and NH4+ Application for Soluble Carbohydrates and Mineral ion Accumulation in Leaves
4.3. Plant Adaptation to N-Deficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oda, M.; Aoki, S.; Nagaoka, M.; Tsuji, K. Nutrient solution culture of leaf lettuce under artificial light. II. Growth promotion induced by continuous illumination with low light Intensity. Environ. Control Biol. 1989, 27, 75–82. [Google Scholar] [CrossRef]
- Ohyama, K.; Manabe, K.; Omura, Y.; Kozai, T.; Kubota, C. Potential use of a 24-h photoperiod (continuous light) with alternating air temperature for production of tomato plug transplants in a closed system. Hortscience 2005, 40, 374–377. [Google Scholar] [CrossRef]
- Lillo, C. Signalling cascades integrating light-enhanced nitrate metabolism. Biochem. J. 2008, 415, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Suzuki, A. Exploration of nitrate-to-glutamate assimilation in non-photosynthetic roots of higher plants by studies of N-15-tracing, enzymes involved, reductant supply, and nitrate signaling: A review and synthesis. Plant Physiol. Bioch. 2019, 136, 245–254. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Sysoeva, M.I.; Markovskaya, E.F.; Shibaeva, T.G. Plants under continuous light: A review. Plant Stress 2010, 5, 5–17. [Google Scholar]
- Horchani, F.; Hajri, R.; Aschi-Smiti, S. Effect of ammonium or nitrate nutrition on photosynthesis, growth, and nitrogen assimilation in tomato plants. J. Plant Nutr. Soil Sc. 2010, 173, 610–617. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; van Poppel, P.; Heuvelink, E.; Millenaar, F.F. A single locus confers tolerance to continuous light and allows substantial yield increase in tomato. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Velez-Ramirez, A.I.; Dunner-Planella, G.; Vreugdenhil, D.; Millenaar, F.F.; van Ieperen, W. On the induction of injury in tomato under continuous light: Circadian asynchrony as the main triggering factor. Funct. Plant Biol. 2017, 44, 597–611. [Google Scholar] [CrossRef]
- Drath, M.; Kloft, N.; Batschauer, A.; Marin, K.; Novak, J.; Forchhammer, K. Ammonia triggers photodamage of photosystem II in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol. 2008, 147, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Yoon, G.M. Light-induced stabilization of ACS contributes to hypocotyl elongation during the dark-to-light transition in Arabidopsis seedlings. Plant J. 2019, 98, 898–911. [Google Scholar] [CrossRef]
- Vinterhalter, D.; Savic, J.; Stanisic, M.; Vinterhalter, B.; Dobrev, P.I.; Motyka, V. Diurnal rhythmicity of endogenous phytohormones and phototropic bending capacity in potato (Solanum tuberosum L.) shoot cultures. Plant Growth Regul. 2020, 90, 151–161. [Google Scholar] [CrossRef]
- Nitschke, S.; Cortleven, A.; Iven, T.; Feussner, I.; Havaux, M.; Riefler, M.; Schmulling, T. Circadian stress regimes affect the circadian clock and cause jasmonic acid dependent cell death in cytokinin deficient Arabidopsis Plants. Plant Cell 2016, 28, 1616–1639. [Google Scholar] [CrossRef]
- Ueda, Y.; Konishi, M.; Yanagisawa, S. Molecular basis of the nitrogen response in plants. Soil Sci. Plant Nutr. 2017, 63, 329–341. [Google Scholar] [CrossRef]
- Ruffel, S.; Poitout, A.; Krouk, G.; Coruzzi, G.M.; Lacombe, B. Long-distance nitrate signaling displays cytokinin dependent and independent branches. J. Integr. Plant Biol. 2016, 58, 226–229. [Google Scholar] [CrossRef]
- Ruffel, S. Nutrient-related long-distance signals: Common players and possible cross-talk. Plant Cell Physiol. 2018, 59, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; O’Brien, J.A.; Gutierrez, R.A. Nitrate and hormonal signaling crosstalk for plant growth and development. Curr. Opin. Plant Biol. 2019, 52, 155–163. [Google Scholar] [CrossRef]
- Walch-Liu, P.; Neumann, G.; Bangerth, F.; Engels, C. Rapid effects of nitrogen form on leaf morphogenesis in tobacco. J. Exp. Bot. 2000, 51, 227–237. [Google Scholar] [CrossRef]
- Ruffel, S.; Krouk, G.; Ristova, D.; Shasha, D.; Birnbaum, K.D.; Coruzzi, G.M. Nitrogen economics of root foraging: Transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc. Natl. Acad. Sci. USA 2011, 108, 18524–18529. [Google Scholar] [CrossRef]
- Jia, W.S.; Davies, W.J. Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals. Plant Physiol. 2007, 143, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Hartung, W.; Sauter, A.; Hose, E. Abscisic acid in the xylem: Where does it come from, where does it go to? J. Exp. Bot. 2002, 53, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; von Wirén, N. Ammonium as a signal for physiological and morphological responses in plants. J. Exp. Bot. 2017, 68, 2581–2592. [Google Scholar] [CrossRef]
- Peuke, A.D.; Jeschke, W.D.; Hartung, W. Foliar application of nitrate or ammonium as sole nitrogen supply in Ricinus communis II. The flows of cations, chloride and abscisic acid. New Phytol. 1998, 140, 625–636. [Google Scholar] [CrossRef]
- Li, B.H.; Li, Q.; Xiong, L.M.; Kronzucker, H.J.; Kramer, U.; Shi, W.M. Arabidopsis plastid AMOS1/EGY1 integrates abscisic acid signaling to regulate global gene expression response to ammonium stress. Plant Physiol. 2012, 160, 2040–2051. [Google Scholar] [CrossRef]
- Carlisle, E.; Myers, S.; Raboy, V.; Bloom, A. The effects of inorganic nitrogen form and CO2 concentration on wheat yield and nutrient accumulation and distribution. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef]
- Salem, M.A.; Juppner, J.; Bajdzienko, K.; Giavalisco, P. Protocol: A fast, comprehensive and reproducible one-step extraction method for the rapid preparation of polar and semi-polar metabolites, lipids, proteins, starch and cell wall polymers from a single sample. Plant Methods 2016, 12. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Warren, C.R. Rapid measurement of chlorophylls with a microplate reader. J. Plant Nutr. 2008, 31, 1321–1332. [Google Scholar] [CrossRef]
- Zhao, D.; MacKown, C.T.; Starks, P.J.; Kindiger, B.K. Rapid analysis of nonstructural carbohydrate components in grass forage using microplate enzymatic assays. Crop Sci. 2010, 50, 1537–1545. [Google Scholar] [CrossRef]
- Hendrix, D.L. apid extraction and analysis of nonstructural carbohydrates in plant-tissues. Crop Sci. 1993, 33, 1306–1311. [Google Scholar] [CrossRef]
- Paponov, M.; Kechasov, D.; Lacek, J.; Verheul, M.J.; Paponov, I.A. Supplemental light-emitting diode inter-lighting increases tomato fruit growth through enhanced photosynthetic light use efficiency and modulated root activity. Front. Plant Sci. 2020, 10, 1656. [Google Scholar] [CrossRef]
- Purcell, L.C.; King, C.A. Total nitrogen determination in plant material by persulfate digestion. Agron. J. 1996, 88, 111–113. [Google Scholar] [CrossRef]
- Alexou, M.; Peuke, A.D. Methods for xylem sap collection. In Plant Mineral Nutrients: Methods and Protocols, Methods in Molecular Biology; Maathuis, F.J.M., Ed.; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2013; Volume 953, pp. 195–207. [Google Scholar]
- Dobrev, P.I.; Kaminek, M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Vankova, R. Quantification of abscisic acid, cytokinin, and auxin content in salt-stressed plant tissues. Methods Mol. Biol. 2012, 913, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, E.A.; Mengel, K. Ionic balance in different tissues of tomato plant in relation to nitrate urea or ammonium nutrition. Plant Physiol. 1967, 42, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, D.W. Optimization of plant root:shoot ratios and internal nitrogen concentration. Ann. Bot. 1990, 66, 91–99. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Vile, D.; Garnier, E.; Shipley, B.; Laurent, G.; Navas, M.L.; Roumet, C.; Lavorel, S.; Diaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- Pettigrew, W.T.; Heitholt, J.J.; Vaughn, K.C. Gas-exchange differences and comparative anatomy among cotton leaf-type isolines. Crop Sci. 1993, 33, 1295–1299. [Google Scholar] [CrossRef]
- Pettigrew, W.T.; Meredith, W.R. Leaf gas-exchange parameters vary among cotton genotypes. Crop Sci. 1994, 34, 700–705. [Google Scholar] [CrossRef]
- Lecoeur, J.; Wery, J.; Turc, O.; Tardieu, F. Expansion of pea leaves subjected to short water-deficit: Cell number and cell size are sensitive to stress at different periods of leaf development. J. Exp. Bot. 1995, 46, 1093–1101. [Google Scholar] [CrossRef]
- Olff, H. Effects of light and nutrient availability on dry-matter and N allocation in six successional grassland species: Testing for resource ratio effects. Oecologia 1992, 89, 412–421. [Google Scholar] [CrossRef]
- Kepka, M.; Benson, C.L.; Gonugunta, V.K.; Nelson, K.M.; Christmann, A.; Grill, E.; Abrams, S.R. Action of natural abscisic acid precursors and catabolites on abscisic acid receptor complexes. Plant Physiol. 2011, 157, 2108–2119. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Crespo, E.; Camanes, G.; Garcia-Agustin, P. Ammonium enhances resistance to salinity stress in citrus plants. J. Plant Physio 2012, 169, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, N.C.; Nesbit, A.D.; Gordon, E.P.; Green, C.; Pare, P.W.; Thompson, L.; Peffley, E.B.; Tissue, D.T. Continuous light may induce photosynthetic downregulation in onion—Consequences for growth and biomass partitioning. Physiol. Plantarum 2005, 125, 235–246. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, U.; Ntagkas, N.; Siebenkas, A.; Maenpaa, M.; Matsubara, S.; Pons, T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- ten Hoopen, F.; Cuin, T.A.; Pedas, P.; Hegelund, J.N.; Shabala, S.; Schjoerring, J.K.; Jahn, T.P. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: Molecular mechanisms and physiological consequences. J. Exp. Bot. 2010, 61, 2303–2315. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. A re-evaluation of the ATP: NADPH budget during C(3) photosynthesis: A contribution from nitrate assimilation and its associated respiratory activity? J. Exp. Bot. 1998, 49, 1895–1908. [Google Scholar] [CrossRef]
- Haque, M.S.; de Sousa, A.; Soares, C.; Kjaer, K.H.; Fidalgo, F.; Rosenqvist, E.; Ottosen, C.O. Temperature variation under continuous light restores tomato leaf photosynthesis and maintains the diurnal pattern in stomatal conductance. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic acid: A key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2020. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.Y.; Song, S.S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef]
- Pandey, D.; Rajendran, S.; Gaur, M.; Sajeesh, P.K.; Kumar, A. Plant defense signaling and responses against necrotrophic fungal pathogens. J. Plant Growth Regul. 2016, 35, 1159–1174. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, M.I.R.; Anjum, N.A.; Masood, A.; Hussain, S.J.; Khan, N.A. Jasmonates in plants under abiotic stresses: Crosstalk with other phytohormones matters. Environ. Exp. Bot. 2018, 145, 104–120. [Google Scholar] [CrossRef]
- Abouelsaad, I.; Renault, S. Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J. Plant Physiol. 2018, 226, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Laxmi, A. Jasmonates: Emerging players in controlling temperature stress tolerance. Front. Plant Sci. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Campos, M.L.; Yoshida, Y.; Major, I.T.; de Oliveira Ferreira, D.; Weraduwage, S.M.; Froehlich, J.E.; Johnson, B.F.; Kramer, D.M.; Jander, G.; Sharkey, T.D.; et al. Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat. Commun. 2016, 7, 12570. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.H.; Li, C.Q.; Liu, L.; Han, G.Y.; Zhang, Y.L.; Wang, C.M. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.-J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Demers, D.A.; Gosselin, A. Growing greenhouse tomato and sweet pepper under supplemental lighting: Optimal photoperiod, negative effects of long photoperiod and their causes. Acta Hortic. 2002, 580, 83–88. [Google Scholar] [CrossRef]
- Globig, S.; Rosen, I.; Janes, H.W. Continuous light effects on photosynthesis and carbon metabolism in tomato. Acta Hortic. 1997, 418, 141–151. [Google Scholar] [CrossRef]
- Schjoerring, J.K.; Husted, S.; Mack, G.; Mattsson, M. The regulation of ammonium translocation in plants. J. Exp. Bot. 2002, 53, 883–890. [Google Scholar] [CrossRef]
- Miflin, B.J.; Lea, P.J. Ammonia assimilation. In The Biochemistry of Plants; Miflin, B.J., Ed.; Academic Press: New York, NY, USA, 1980; Volume 5, pp. 169–202. [Google Scholar]
- Paponov, I.A.; Lebedinskai, S.; Koshkin, E.I. Growth analysis of solution culture-grown winter rye, wheat and triticale at different relative rates of nitrogen supply. Ann. Bot. 1999, 84, 467–473. [Google Scholar] [CrossRef]
- Stitt, M. Nitrate regulation of metabolism and growth. Curr. Opin. Plant Biol. 1999, 2, 178–186. [Google Scholar] [CrossRef]
- Vega-Mas, I.; Marino, D.; Sanchez-Zabala, J.; Gonzalez-Murua, C.; Estavillo, J.M.; Gonzalez-Moro, M.B. CO2 enrichment modulates ammonium nutrition in tomato adjusting carbon and nitrogen metabolism to stomatal conductance. Plant Sci. 2015, 241, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Raven, J.A.; Lea, P.J.; Sprent, J.I. A role for shoot protein in shoot-root dry matter allocation in higher plants. Ann. Bot. 2006, 97, 3–10. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic Acid synthesis and response. Arab. Book 2013, 11, e0166. [Google Scholar] [CrossRef]
- Goldbach, E.; Goldbach, H.; Wagner, H.; Michael, G. Influence of N deficiency on abscisic acid content of sunflower plants. Physiol. Plantarum 1975, 34, 138–140. [Google Scholar] [CrossRef]
- Krauss, A. Tuberization and abscisic acid content in Solanum tuberosum as affected by nitrogen nutrition. Potato Res. 1978, 21, 183–193. [Google Scholar] [CrossRef]
- Palmer, S.J.; Berridge, D.M.; McDonald, A.J.S.; Davies, W.J. Control of leaf expansion in sunflower (Helianthus annuus L) by nitrogen nutrition. J. Exp. Bot. 1996, 47, 359–368. [Google Scholar] [CrossRef]
- Zdunek, E.; Lips, S.H. Transport and accumulation rates of abscisic acid and aldehyde oxidase activity in Pisum sativum L. in response to suboptimal growth conditions. J. Exp. Bot. 2001, 52, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C.; Tan, L.P.; He, J. Do increases in xylem sap pH and/or ABA concentration mediate stomatal closure following nitrate deprivation? J. Exp. Bot. 2003, 54, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, E.A.; Armstrong, M.J. Nitrate uptake by roots as regulated by nitrate assimilation in the shoot of castor oil plants. Plant Physiol. 1980, 65, 286–290. [Google Scholar] [CrossRef]
- Chapin, F.S.; Walter, C.H.S.; Clarkson, D.T. Growth-response of barley and tomato to nitrogen stress and its control by abscisic-acid, water relations and photosynthesis. Planta 1988, 173, 352–366. [Google Scholar] [CrossRef]
- Else, M.A.; Hall, K.C.; Arnold, G.M.; Davies, W.J.; Jackson, M.B. Export of abscisic-acid, 1-aminocyclopropane-1-carboxylic acid, phosphate, and nitrate from roots to shoots of flooded tomato plants (accounting for effects of xylem sap flow rate on concentration and delivery. Plant Physiol. 1995, 107, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Schurr, U.; Schulze, E.D. The concentration of xylem sap constituents in root exudate, and in sap from intact, transpiring castor bean plants (Ricinus communis L.). Plant Cell Environ. 1995, 18, 409–420. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Xu, J.; Hofte, M. Making sense of hormone-mediated defense networking: From rice to Arabidopsis. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Yaeno, T.; Iba, K. BAH1/NLA, a RING-type ubiquitin E3 ligase, regulates the accumulation of salicylic acid and immune responses to Pseudomonas syringae DC3000. Plant Physiol. 2008, 148, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Alborn, H.T.; Engelberth, J.; Tumlinson, J.H. Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize. Plant Physiol. 2003, 133, 295–306. [Google Scholar] [CrossRef]
- Hsieh, P.-H.; Kan, C.-C.; Wu, H.-Y.; Yang, H.-C.; Hsieh, M.-H. Early molecular events associated with nitrogen deficiency in rice seedling roots. Sci. Rep. 2018, 8, 12207. [Google Scholar] [CrossRef]
- Sheflin, A.M.; Chiniquy, D.; Yuan, C.H.; Goren, E.; Kumar, I.; Braud, M.; Brutnell, T.; Eveland, A.L.; Tringe, S.; Liu, P.; et al. Metabolomics of sorghum roots during nitrogen stress reveals compromised metabolic capacity for salicylic acid biosynthesis. Plant Direct 2019, 3. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, X.G.; Yang, J.; Liu, W.W.; Du, Q.G.; Wang, H.Q.; Fu, C.X.; Li, W.X. MicroRNA528 affects lodging resistance of maize by regulating lignin biosynthesis under nitrogen luxury conditions. Mol. Plant 2018, 11, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Camargo, E.L.O.; Nascimento, L.C.; Soler, M.; Salazar, M.M.; Lepikson-Neto, J.; Marques, W.L.; Alves, A.; Teixeira, P.; Mieczkowski, P.; Carazzolle, M.F.; et al. Contrasting nitrogen fertilization treatments impact xylem gene expression and secondary cell wall lignification in Eucalyptus. BMC Plant Biol. 2014, 14. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paponov, M.; Arakelyan, A.; Dobrev, P.I.; Verheul, M.J.; Paponov, I.A. Nitrogen Deficiency and Synergism between Continuous Light and Root Ammonium Supply Modulate Distinct but Overlapping Patterns of Phytohormone Composition in Xylem Sap of Tomato Plants. Plants 2021, 10, 573. https://doi.org/10.3390/plants10030573
Paponov M, Arakelyan A, Dobrev PI, Verheul MJ, Paponov IA. Nitrogen Deficiency and Synergism between Continuous Light and Root Ammonium Supply Modulate Distinct but Overlapping Patterns of Phytohormone Composition in Xylem Sap of Tomato Plants. Plants. 2021; 10(3):573. https://doi.org/10.3390/plants10030573
Chicago/Turabian StylePaponov, Martina, Aleksandr Arakelyan, Petre I. Dobrev, Michel J. Verheul, and Ivan A. Paponov. 2021. "Nitrogen Deficiency and Synergism between Continuous Light and Root Ammonium Supply Modulate Distinct but Overlapping Patterns of Phytohormone Composition in Xylem Sap of Tomato Plants" Plants 10, no. 3: 573. https://doi.org/10.3390/plants10030573
APA StylePaponov, M., Arakelyan, A., Dobrev, P. I., Verheul, M. J., & Paponov, I. A. (2021). Nitrogen Deficiency and Synergism between Continuous Light and Root Ammonium Supply Modulate Distinct but Overlapping Patterns of Phytohormone Composition in Xylem Sap of Tomato Plants. Plants, 10(3), 573. https://doi.org/10.3390/plants10030573






