Evolutionary and Ecological Considerations on Nectar-Mediated Tripartite Interactions in Angiosperms and Their Relevance in the Mediterranean Basin
Abstract
1. Introduction
2. Nectar Sugars of Flowering Plants: An Evolutionary Hypothesis
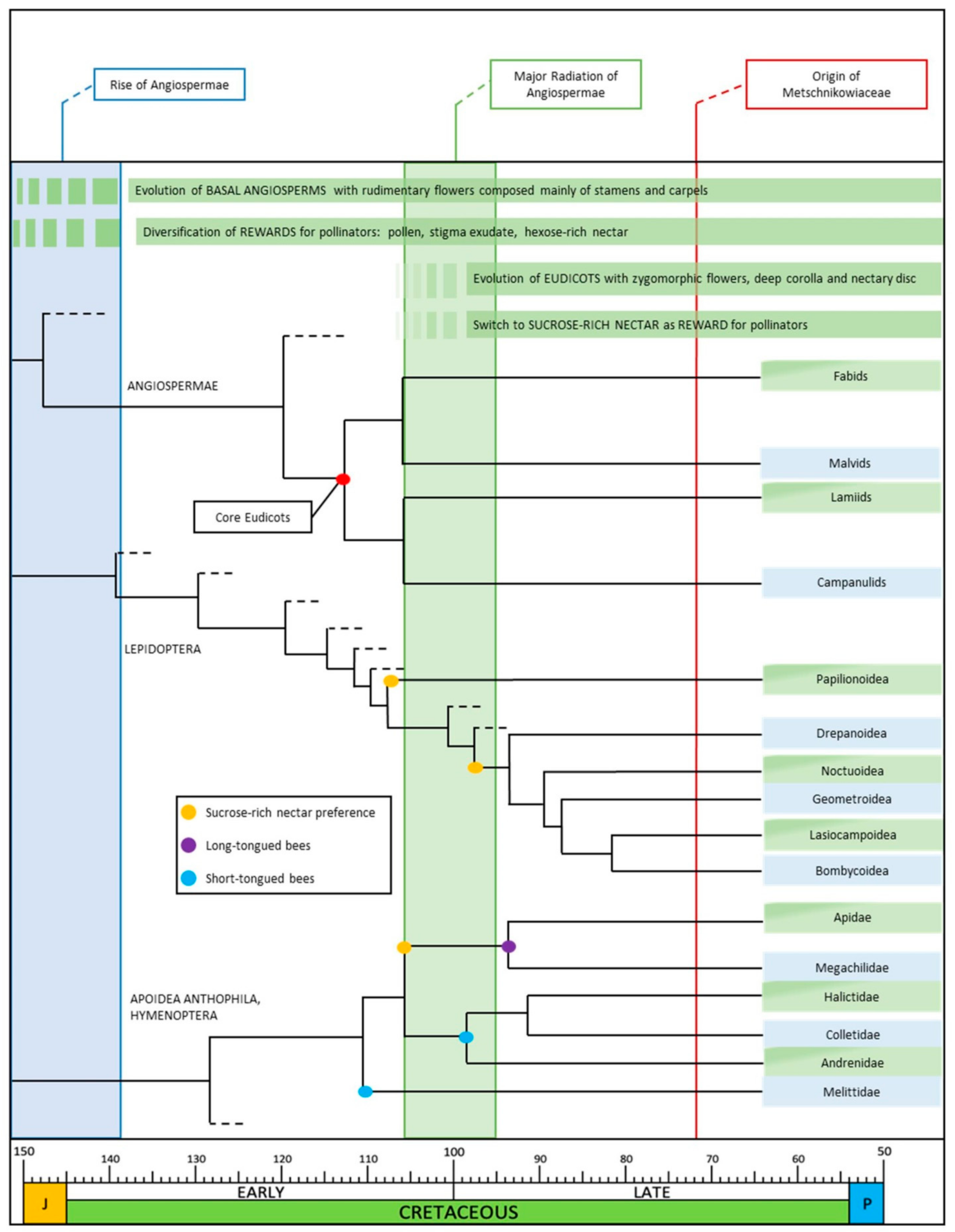
2.1. Rewards and Pollinators in the Mesozoic Era and in Early Angiosperms
2.2. Evolution of Rewards along the Cretaceous
3. Evolution of Sucrose-Dominant Nectar Sugar Profile
3.1. Climatic Changes in the Cretaceous Period and Effects on the Chemistry of Sugary Secretions and Flower Evolution
3.2. Evolution of New Groups of Insects and Their Foraging Behavior
4. Nectar Secondary Compounds and Their Importance in Mediterranean-Type Ecosystems
5. Nectar-Dwelling Microorganisms Contribute to Shaping Flower–Insect Relationships in Mediterranean Regions
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Blondel, J.; Aronson, J. Biology and Wildlife of the Mediterranean Region; Oxford University Press: Oxford, UK; New York, NY, USA, 1999. [Google Scholar]
- Michener, C.D. Biogeography of the bees. An. Mi. Bot. Gard. 1979, 66, 277–347. [Google Scholar] [CrossRef]
- Petanidou, T.; Lamborn, E. A land for flowers and bees: Studying pollination ecology in Mediterranean communities. Plant Biosyst. 2005, 139, 279–294. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2000. [Google Scholar]
- Petanidou, T.; Ellis, W.N. Pollinating fauna of a phryganic ecosystem: Composition and diversity. Biodivers. Lett. 1993, 1, 9–22. [Google Scholar] [CrossRef]
- Dafni, A.; O’Toole, C. Pollination syndromes in the Mediterranean: Generalizations and peculiarities. In Plant-Animal Interactions in Mediterranean-Type Ecosystems; Arianoutsou, M., Groves, R.H., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 125–135. [Google Scholar]
- Herrera, J. Nectar secretion patterns in southern Spanish Mediterranean scrublands. Isr. J. Bot. 1985, 34, 47–58. [Google Scholar]
- Petanidou, T.; Goethals, V.; Smets, E. The effects of nutrient and water availability in the nectar production and nectar structure of the dominant Labiatae species of phrygana. Syst. Geogr. Pl. 1999, 68, 233–244. [Google Scholar] [CrossRef]
- Petanidou, T. Ecological and evolutionary aspects of floral nectars in Mediterranean habitats. In Nectaries and Nectar; Nicolson, S., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 343–374. [Google Scholar]
- Dupont, Y.L.; Hansen, D.M.; Rasmussen, J.T.; Olesen, J.M. Evolutionary changes in nectar sugar composition associated with switches between bird and insect pollination: The Canarian bird-flower element revisited. Funct. Ecol. 2004, 18, 670–676. [Google Scholar] [CrossRef]
- Galetto, L.; Bernardello, G. Floral nectaries, nectar production dynamics and chemical composition in six Ipomoea species (Convolvulaceae) in relation to pollinators. Ann. Bot. 2004, 94, 269–280. [Google Scholar] [CrossRef]
- Kaczorowski, R.L.; Gardener, M.C.; Holtsford, T.P. Nectar traits in Nicotiana section Alatae (Solanaceae) in relation to floral traits, pollinators, and mating system. Am. J. Bot. 2005, 92, 1270–1283. [Google Scholar] [CrossRef]
- Ackermann, M.; Weigend, M. Nectar, floral morphology and pollination syndrome in Loasaceae subfam. Loasoideae (Cornales). Ann. Bot. 2006, 98, 503–514. [Google Scholar] [CrossRef]
- Petanidou, T.; Van Laere, A.N.; Ellis, W.; Smets, E. What shapes amino acid and sugar composition in Mediterranean floral nectars? Oikos 2006, 115, 155–169. [Google Scholar] [CrossRef]
- Nicolson, S.W. Nectar consumers. In Nectaries and Nectar; Nicolson, S., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 289–342. [Google Scholar]
- Schmidt-Lebuhn, A.N.; Schwerdtfeger, M.; Kessler, M.; Lohaus, G. Phylogenetic conservatism vs. ecology in the nectar composition of Acanthaceae. Flora 2007, 202, 62–69. [Google Scholar] [CrossRef]
- Krömer, T.; Kessler, M.; Lohaus, G.; Schmidt-Lebuhn, A.N. Nectar sugar composition and concentration in relation to pollination syndromes in Bromeliaceae. Plant Biol. 2008, 10, 502–511. [Google Scholar] [CrossRef]
- Nocentini, D.; Pacini, E.; Guarnieri, M.; Martelli, D.; Nepi, M. Intrapopulation heterogeneity in floral nectar attributes and foraging insects of an ecotonal Mediterranean species. Plant Ecol. 2013, 214, 799–809. [Google Scholar] [CrossRef]
- Rodríguez-Riaño, T.; Ortega-Olivencia, A.; López, J.; Pérez-Bote, J.L.; Navarro-Pérez, M.L. Main sugar composition of floral nectar in three species groups of Scrophularia (Scrophulariaceae) with different principal pollinators. Plant Biol. 2014, 16, 1075–1086. [Google Scholar] [PubMed]
- Tiedge, K.; Lohaus, G. Nectar sugars and amino acids in day-and night-flowering Nicotiana species are more strongly shaped by pollinators’ preferences than organic acids and inorganic ions. PLoS ONE 2017, 12, e0176865. [Google Scholar] [CrossRef]
- Roguz, K.; Bajguz, A.; Chmur, M.; Gołębiewska, A.; Roguz, A.; Zych, M. Diversity of nectar amino acids in the Fritillaria (Liliaceae) genus: Ecological and evolutionary implications. Sci. Rep. 2019, 9, 15209. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.G.; Baker, I. Floral nectar sugar constituents in relation to pollinator type. In Handbook of Pollination Biology; Little, R.J., Jones, C.E., Eds.; Scientific and Academic Editions: New York, NY, USA, 1983; pp. 117–141. [Google Scholar]
- Van Wyk, B.-E. Nectar sugar composition in southern African Papilionoideae (Fabaceae). Biochem. Syst. Ecol. 1993, 21, 271–277. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Whitehead, C.S.; Glen, H.F.; Hardy, D.S.; van Jaarsveld, E.J.; Smiths, G.F. Nectar sugar composition in the subfamily Alooideae (Asphodelaceae). Biochem. Syst. Ecol. 1993, 21, 249–253. [Google Scholar] [CrossRef]
- Nicolson, S.W.; van Wyk, B.-E. Nectar sugars in Proteaceae: Patterns and processes. Austr. J. Bot. 1998, 46, 489–504. [Google Scholar] [CrossRef]
- Petanidou, T. Sugars in Mediterranean floral nectars: An ecological and evolutionary approach. J. Chem. Ecol. 2005, 31, 1065–1088. [Google Scholar] [CrossRef]
- Nepi, M.; von Aderkas, P.; Wagner, R.; Mugnaini, S.; Coulter, A.; Pacini, E. Nectar and pollination drops: How different are they? Ann. Bot. 2009, 104, 205–219. [Google Scholar] [CrossRef]
- Nepi, M.; Little, S.; Guarnieri, M.; Nocentini, D.; Prior, N.; Gill, J.; Tomlinson, P.B.; Ickert-Bond, S.R.; Pirone, C.; Pacini, E.; et al. Phylogenetic and functional signals in gymnosperm ovular secretions. Ann. Bot. 2017, 120, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Nepi, M.; Grasso, D.A.; Mancuso, S. Nectar in plant–insect mutualistic relationships: From food reward to partner manipulation. Front. Plant Sci. 2018, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.A. Neuroactive nectar: Compounds in nectar that interact with neurons. Arthropod-Plant Interact. 2020, 14, 151–159. [Google Scholar] [CrossRef]
- Nepi, M. New perspectives in nectar evolution and ecology: Simple alimentary reward or a complex multi-organismic interaction? Acta Agrobot. 2017, 70, 1704. [Google Scholar] [CrossRef]
- Herrera, C.M.; Pérez, R.; Alonso, C. Extreme intraplant variation in nectar sugar composition in an insect-pollinated perennial herb. Am. J. Bot. 2006, 93, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Nepi, M.; Selvi, F.; Pacini, E. Variation in nectar–sugar profile of Anchusa and allied genera (Boraginaceae). Bot. J. Linn. Soc. 2010, 162, 616–627. [Google Scholar] [CrossRef]
- Pozo, M.I.; Lievens, B.; Jacquemyn, H. Impact of microorganisms on nectar chemistry, pollinator attraction and plant fitness. In Nectar: Production, Chemical Composition and Benefits to Animals and Plants; Peck, R.L., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 1–40. [Google Scholar]
- Friis, E.M.; Crane, P.R.; Pedersen, K.R. Early Flowers and Angiosperm Evolution; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Erbar, C. Nectar secretion and nectaries in basal angiosperms, magnoliids and non-core eudicots and a comparison with core eudicots. Plant Div. Evol. 2014, 131, 63–143. [Google Scholar] [CrossRef]
- Gottsberger, G. How diverse are Annonaceae with regard to pollination? Bot. J. Linn. Soc. 2012, 169, 245–261. [Google Scholar] [CrossRef]
- Gottsberger, G. Generalist and specialist pollination in basal angiosperms (ANITA grade, basal monocots, magnoliids, Chloranthaceae and Ceratophyllaceae): What we know now. Plant Div. Evol. 2015, 131, 263–362. [Google Scholar] [CrossRef]
- Bernardello, G. A systematic survey of floral nectar. In Nectaries and Nectar; Nicolson, S., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 19–128. [Google Scholar]
- Endress, P.K. Floral structure and evolution of primitive angiosperms: Recent advances. Plant Syst. Evol. 1994, 192, 79–97. [Google Scholar] [CrossRef]
- Von Balthazar, M.; Crane, P.R.; Pedersen, K.R.; Friis, E.M.; Wanntorp, L.; Ronse De Craene, L.P. New flowers of Laurales from the Early Cretaceous (early to middle Albian) of eastern North America. In Flowers on the Tree of Life; Wannthorp, L., Ronse De Craene, L.P., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 49–87. [Google Scholar]
- Labandeira, C.C.; Kvaček, J.; Mostovski, M.B. Pollination drops, pollen, and insect pollination of Mesozoic gymnosperms. Taxon 2007, 56, 663–695. [Google Scholar] [CrossRef]
- Dong, R.; Labandeira, C.C.; Santiago-Blay, J.A.; Rasnitsyn, A.; Shih, C.; Bashkuev, A.; Logan, M.A.; Hotton, C.L.; Dilcher, D. A probable pollination mode before angiosperms: Eurasian, long-proboscid scorpionflies. Science 2009, 326, 840–847. [Google Scholar]
- Labandeira, C.C. The pollination of Mid Mesozoic seed plants and the early history of long-proboscid insects. Ann. Missouri Bot. Gard. 2010, 97, 469–513. [Google Scholar] [CrossRef]
- Labandeira, C.C.; Yang, Q.; Santiago-Blay, J.A.; Hotton, C.L.; Monteiro, A.; Wang, Y.J.; Goreva, Y.; Shih, C.K.; Siljeström, S.; Rose, T.R.; et al. The evolutionary convergence of mid-Mesozoic lacewings and Cenozoic butterflies. Proc. R. Soc. B-Biol. Sci. 2016, 283, 20152893. [Google Scholar] [CrossRef] [PubMed]
- Khramov, A.V.; Lukashevich, E.D. A Jurassic dipteran pollinator with an extremely long proboscis. Gondwana Res. 2019, 71, 210–215. [Google Scholar] [CrossRef]
- Lau, J.Y.; Pang, C.C.; Ramsden, L.; Saunders, R.M. Stigmatic exudate in the Annonaceae: Pollinator reward, pollen germination medium or extragynoecial compitum? J. Integr. Plant Biol. 2017, 59, 881–894. [Google Scholar] [CrossRef]
- Steenhuisen, S.L.; Johnson, S.D. Evidence for beetle pollination in the African grassland sugarbushes (Protea: Proteaceae). Plant Syst. Evol. 2012, 298, 857–869. [Google Scholar] [CrossRef]
- Peñalver, E.; Labandeira, C.C.; Barrón, E.; Delclòs, X.; Nel, P.; Nel, A.; Tafforeau, P.; Soriano, C. Thrips pollination of Mesozoic gymnosperms. Proc. Nat. Acad. Sci. USA 2012, 109, 8623–8628. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.J. Carbohydrates, pollinators, and cycads. Commun. Integr. Biol. 2014, 8, e1017162. [Google Scholar] [CrossRef] [PubMed]
- Von Aderkas, P.; Prior, N.A.; Little, S.A. The evolution of sexual fluids in gymnosperms from pollination drops to nectar. Front. Plant Sci. 2018, 9, 1844. [Google Scholar] [CrossRef]
- Baum, S.F.; Eshed, Y.; Bowman, J.L. The Arabidopsis nectary is an ABC-independent floral structure. Development 2001, 128, 4657–4667. [Google Scholar]
- Willis, K.J.; McElwain, J.C. The Evolution of Plants, 2nd ed.; Oxford University Press: Oxford, UK, 2014; pp. 179–224. [Google Scholar]
- Abrahamczyk, S.; Kessler, M.; Hanley, D.; Karger, D.N.; Müller, M.P.J.; Knauer, A.C.; Keller, F.; Schwerdtfeger, M.; Humphreys, A.M. Pollination adaptation and the evolution of floral nectar sugar composition. J. Evol. Biol. 2017, 30, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.H.; Berg-Sørensen, K.; Bruus, H.; Holbrook, N.M.; Liesche, J.; Schulz, A.; Zwieniecki, M.A.; Bohr, T. Sap flow and sugar transport in plant. Rev. Mod. Phys. 2016, 88, 035007. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, L.; Lu, Y.; Wang, D.; Jiang, X.X.; Zhang, M.; Wang, L. The mechanism of pollination drop withdrawal in Ginkgo biloba L. BMC Plant Biol. 2012, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Navascues, G. Liquid surfaces: Theory of surface tension. Rep. Prog. Phy. 2011, 42, 1131–1186. [Google Scholar] [CrossRef]
- Atkins, P.; De Paula, J. Physical Chemistry, 11th ed.; Oxford University Press: Oxford, UK, 2018; pp. 40–42. [Google Scholar]
- Telis, V.R.N.; Telis-Romero, J.; Mazzotti, H.B.; Gabas, A.L. Viscosity of aqueous carbohydrate solutions at different temperatures and concentrations. Int. J. Food Prop. 2007, 10, 185–195. [Google Scholar] [CrossRef]
- Docoslis, A.; Giese, R.F.; van Oss, C.J. Influence of the water–air interface on the apparent surface tension of aqueous solutions of hydrophilic solutes. Colloids Surf. B 2000, 19, 147–162. [Google Scholar] [CrossRef]
- Matubayasi, N. Surface Tension and Related Thermodynamic Quantities of Aqueous Electrolyte Solutions, 1st ed.; Taylor and Francis: Boca Raton, FL, USA, 2014; pp. 30–37. [Google Scholar]
- Jenkyns, H.C. Transient cooling episodes during Cretaceous Oceanic Anoxic Events with special reference to OAE 1a (Early Aptian). Phil. Trans. R. Soc. A 2018, 376, 20170073. [Google Scholar] [CrossRef]
- Heimhofer, I.; Wucherpfennig, N.; Adatte, T.; Schouten, S.; Schneebeli-Hermann, E.; Gardin, S.; Keller, G.; Kentsch, S.; Kujau, A. Vegetation response to exceptional global warmth during Oceanic Anoxic Event 2. Nat. Commun. 2018, 9, 3832. [Google Scholar] [CrossRef]
- Raguso, R.A. Why are some floral nectars scented? Ecology 2004, 85, 1486–1494. [Google Scholar] [CrossRef]
- Southwick, E.E. Photosynthate allocation to floral nectar: A neglected energy investment. Ecology 1984, 65, 1775–1779. [Google Scholar] [CrossRef]
- Pyke, G.H. What does it cost a plant to produce floral nectar? Nature 1991, 350, 58–59. [Google Scholar] [CrossRef]
- Nepi, M.; Stpiczyńska, M. The complexity of nectar: Secretion and resorption dynamically regulate nectar features. Naturwissenschaften 2008, 95, 177–184. [Google Scholar] [CrossRef]
- Friis, E.M.; Pedersen, K.R.; Crane, P.R. Cretaceous angiosperm flowers: Innovation and evolution in plant reproduction. Palaeogeog. Paleocl. 2006, 232, 251–293. [Google Scholar] [CrossRef]
- Kawahara, A.Y.; Plotkin, D.; Espeland, M.; Meusemann, K.; Toussaint, E.F.; Donath, A.; Gimniche, F.; Frandsenh, P.B.; Zwickf, A.; dos Reisj, M.; et al. Phylogenomics reveals the evolutionary timing and pattern of butterflies and moths. Proc. Nat. Acad. Sci. USA 2019, 116, 22657–22663. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.S.; Krogmann, L.; Mayer, C.; Donath, A.; Gunkel, S.; Meusemann, K.; Kozlov, A.; Podsiadlowski, L.; Petersen, M.; Lanfear, R.; et al. Evolutionary history of the Hymenoptera. Curr. Biol. 2017, 27, 1013–1018. [Google Scholar] [CrossRef]
- Cole, T.C.H.; Hilger, H.H.; Stevens, P.F. Angiosperm Phylogeny Poster—Flowering Plant Systematics. Peer J. Prepr. 2019, 7, e2320v6. [Google Scholar]
- Grimaldi, D. The co-radiations of pollinating insects and angiosperms in the Cretaceous. Ann. Miss. Bot. Gard. 1999, 86, 373–406. [Google Scholar] [CrossRef]
- Hernández-Hernández, T.; Wiens, J.J. Why are there so many flowering plants? A multiscale analysis of plant diversification. Am. Nat. 2020, 195, 948–963. [Google Scholar] [CrossRef]
- Crepet, M.L. Timing in the evolution of derived floral characters: Upper Cretaceous (Turonian) taxa with tricolpate and tricolpate-derived pollen. Rev. Palaeobot. Palynol. 1996, 90, 339–359. [Google Scholar] [CrossRef]
- Johnson, S.D.; Anderson, B. Coevolution between food-rewarding flowers and their pollinators. Evol. Edu. Outreach 2010, 3, 32–39. [Google Scholar] [CrossRef]
- Cardinal, S.; Danforth, B.N. Bees diversified in the age of eudicots. Proc. R. Soc. B 2013, 280, 20122686. [Google Scholar] [CrossRef]
- Miller-Struttmann, N.E.; Galen, C. High-altitude multi-tasker: Bumble bee food plant use broadens along an altitudinal productivity gradient. Oecologia 2014, 176, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.S.; Rasmussen, C.; Gonzalez, V.H. Bees. In Encyclopedia of Social Insects; Starr, C., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Heil, M.; Büchler, R.; Boland, W. Quantification of invertase activity in ants under field conditions. J. Chem. Ecol. 2005, 31, 431–437. [Google Scholar] [CrossRef]
- Kubota, M.; Tsuji, M.; Nishimoto, M.; Wongchawalit, J.; Okuyama, M.; Mori, H.; Matsui, H.; Surarit, R.; Svasti, J.; Kimura, A.; et al. Localization of α-glucosidases I, II, and III in organs of European honeybees, Apis mellifera L.; and the origin of α-glucosidase in honey. Biosci. Biotech. Biochem. 2004, 68, 2346–2352. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microb. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef] [PubMed]
- Alberoni, D.; Baffoni, L.; Gaggiìa, F.; Ryan, P.M.; Murphy, K.; Ross, P.R.; Santon, C.; Di Gioia, D. Impact of beneficial bacteria supplementation on the gut microbiota, colony development and productivity of Apis mellifera L. Benef. Microbes 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Calcagno, V.; Thomas, Y.; Bourguet, D. Sympatric host races of the European corn borer: Adaptation to host plants and hybrid performance. J. Evol. Biol. 2007, 20, 1720–1729. [Google Scholar] [CrossRef]
- Taylor, M.A.; Robertson, A.W.; Biggs, P.J.; Richards, K.K.; Jones, D.F.; Parkar, S.G. The effect of carbohydrate sources: Sucrose, invert sugar and components of mānuka honey, on core bacteria in the digestive tract of adult honey bees (Apis mellifera). PLoS ONE 2019, 14, e0225845. [Google Scholar] [CrossRef]
- Roy, R.; Schmitt, A.J.; Thomas, J.B.; Carter, C.J. Nectar biology: From molecules to ecosystems. Plant Sci. 2017, 262, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.C.; Nicolson, S.W.; Wright, G.A. Plant secondary metabolites in nectar: Impacts on pollinators and ecological functions. Funct. Ecol. 2017, 31, 65–75. [Google Scholar] [CrossRef]
- Manson, J.S.; Cook, D.; Gardner, D.R.; Irwin, R.E. Dose-dependent effects of nectaralkaloids in a montane plant-pollinator community. J. Ecol. 2013, 101, 1604–1612. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; van Loon, J.J.A.; Dicke, M. Insect—Plant Biology; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Wright, G.A.; Baker, D.D.; Palmer, M.J.; Stabler, D.; Mustard, J.A.; Power, E.F.; Borland, A.M.; Stevenson, P.C. Caffeine in floral nectar enhances a pollinator’s memory of reward. Science 2013, 339, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Baracchi, D.; Marples, A.; Jenkins, A.J.; Leitch, A.R.; Chittka, L. Nicotine in floral nectar pharmacologically influences bumblebee learning of floral features. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nepi, M. Beyond nectar sweetness: The hidden ecological role of non-protein amino acids in nectar. J. Ecol. 2014, 102, 108–115. [Google Scholar] [CrossRef]
- Nocentini, D.; Pacini, E.; Guarnieri, M.; Nepi, M. Flower morphology, nectar traits and pollinators of Cerinthe major (Boraginaceae-Lithospermeae). Flora 2012, 207, 186–196. [Google Scholar] [CrossRef]
- Nocentini, D. Chemical Diversity of Floral Nectar in the Tribe Lithospermeae. Ph.D. Thesis, University of Siena, Siena, Italy, 2014. [Google Scholar]
- Forlani, G.; Bertazzini, M.; Giberti, S. Differential accumulation of γ–aminobutyric acid in elicited cells of two rice cultivars showing contrasting sensitivity to the blast pathogen. Plant. Biol. 2014, 16, 1127–1132. [Google Scholar] [CrossRef]
- Ramos-Ruiz, R.; Martinez, F.; Knauf-Beiter, G. The effects of GABA in plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Biancucci, M.; Mattioli, R.; Forlani, G.; Funck, D.; Costantino, P.; Trovato, M. Role of proline and GABA in sexual reproduction of angiosperms. Front. Plant Sci. 2015, 6, 680. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Thornburg, R.W. Nectar chemistry. In Nectaries and Nectar; Nicolson, S., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 215–264. [Google Scholar]
- Bogo, G.; Bortolotti, L.; Sagona, S.; Felicioli, A.; Galloni, M.; Barberis, M.; Nepi, M. Effects of non-protein amino acids in nectar on bee survival and behavior. J. Chem. Ecol. 2019, 45, 278–285. [Google Scholar] [CrossRef]
- Vannette, R.L. The floral microbiome: Plant, pollinator, and microbial perspectives. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 363–386. [Google Scholar] [CrossRef]
- Francis, J.S.; Tatarko, A.R.; Richman, S.K.; Vaudo, A.D.; Leonard, A.S. Microbes and Pollinator Behavior in the Floral Marketplace. Curr. Opin. Insect Sci. 2021, 44, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Nat. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg-Gershtein, Y.; Izhaki, I.; Halpern, M. Do honeybees shape the bacterial community composition in floral nectar? PLoS ONE 2013, 8, e67556. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.S.; Irwin, R.E.; McArt, S.H.; Vannette, R.L. Floral traits affecting the transmission of beneficial and pathogenic pollinator-associated microbes. Curr. Opin. Insect Sci. 2021, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.M.; Frixione, N.J.; Burkert, A.C.; Dinsdale, E.A.; Vannette, R.L. Microbial abundance, composition, and function in nectar are shaped by flower visitor identity. FEMS Microbiol. Ecol. 2020, 96, fiaa003. [Google Scholar] [CrossRef] [PubMed]
- Mittelbach, M.; Yurkov, A.M.; Nocentini, D.; Nepi, M.; Weigend, M.; Begerow, D. Nectar sugars and bird visitation define a floral niche for basidiomycetous yeast on the Canary Islands. BMC Ecol. 2015, 15, 2. [Google Scholar] [CrossRef]
- Canto, A.; Pérez, R.; Medrano, M.; Castellanos, M.C.; Herrera, C.M. Intra-plant variation in nectar sugar composition in two Aquilegia species (Ranunculaceae): Contrasting patterns under field and glasshouse conditions. Ann. Bot. 2007, 99, 653–660. [Google Scholar] [CrossRef]
- Canto, A.; Herrera, C.M.; Medrano, M.; Pérez, R.; García, I.M. Pollinator foraging modifies nectar sugar composition in Helleborus foetidus (Ranunculaceae): An experimental test. Am. J. Bot. 2008, 95, 315–320. [Google Scholar] [CrossRef]
- Herrera, C.M.; de Vega, C.; Canto, A.; Pozo, M.I. Yeasts in floral nectar: A quantitative survey. Ann. Bot. 2009, 103, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, S.; Lievens, B.; Fukami, T. Yeast–bacterium interactions: The next frontier in nectar research. Trends Plant Sci. 2019, 24, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, S.; Herrera, C.M. Composition, richness and nonrandom assembly of culturable bacterial–microfungal communities in floral nectar of Mediterranean plants. FEMS Microbiol. Ecol. 2013, 83, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Cullen, N.; Fetters, A.; Ashman, T.L. Integrating microbes into pollination. Curr. Opin. Insect Sci. 2021, 44, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Vannette, R.L.; Fukami, T. Nectar microbes can reduce secondary metabolites in nectar and alter effects on nectar consumption by pollinators. Ecology 2016, 97, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M.; Pozo, M.I.; Medrano, M. Yeasts in nectar of an early-blooming herb: Sought by bumble bees, detrimental to plant fecundity. Ecology 2013, 94, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Rering, C.C.; Beck, J.J.; Hall, G.W.; McCartney, M.M.; Vannette, R.L. Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol. 2018, 220, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M.; Pozo, M.I. Nectar yeasts warm the flowers of a winter-blooming plant. Proc. R. Soc. B Biol. Sci. 2010, 277, 1827–1834. [Google Scholar] [CrossRef]
- Jacquemin, H.; Pozo, M.; Álvarez-Pérez, S.; Lievens, B.; Fukami, T. Yeast-nectar interactions: Metacommunities and effects on pollinators. Curr. Opin. Insect Sci. 2021, 44, 35–40. [Google Scholar] [CrossRef]
- Sáez, A.; Morales, C.L.; Ramos, L.Y.; Aizen, M.A. Extremely frequent bee visits increase pollen deposition but reduce drupelet set in raspberry. J. Appl. Ecol. 2014, 51, 1603–1612. [Google Scholar] [CrossRef]
- Pozo, M.I.; Herrera, C.M.; Bazaga, P. Species richness of yeast communities in floral nectar of southern Spanish plants. Microb. Ecol. 2011, 61, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, R.N.; Vannette, R.L.; Irwin, R.E. Nectar yeasts in Delphinium nuttallianum (Ranunculaceae) and their effects on nectar quality. Fungal Ecol. 2015, 18, 100–106. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Baets, D.; Goelen, T.; Herrera-Malaver, B.; Bosmans, L.; Van den Ende, W.; Verstrepen, K.J.; Wäkers, F.; Jaquemyn, H.; Lievens, B. Sweet scents: Nectar specialist yeasts enhance nectar attraction of a generalist aphid parasitoid without affecting survival. Front. Plant Sci. 2018, 9, 1009. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, B.; Lachance, M.A.; Herrera, C.M. Phylogenetic analysis of the angiosperm-floricolous insect–yeast association: Have yeast and angiosperm lineages co-diversified? Mol. Phylogenet. Evol. 2013, 68, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Baracchi, D. Cognitive ecology of pollinators and the main determinants of foraging plasticity. Curr. Zool. 2019, 65, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, P.L.; Fernández-Díaz, P.; Pareja, D.; Escudero, M.; Arista, M. Do visual traits honestly signal floral rewards at community level? Funct. Ecol. 2020. [Google Scholar] [CrossRef]
- Zhao, C.; Doucet, D.; Mittapalli, O. Characterization of horizontally transferred β-fructofuranosidase (ScrB) genes in Agrilus planipennis. Insect Mol. Biol. 2014, 23, 821–832. [Google Scholar] [CrossRef]
- Rebolleda-Gómez, M.; Forrester, N.J.; Russell, A.L.; Wei, N.; Fetters, A.M.; Stephens, J.D.; Ashman, T.L. Gazing into the anthosphere: Considering how microbes influence floral evolution. New Phytol. 2019, 224, 1012–1020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nepi, M.; Calabrese, D.; Guarnieri, M.; Giordano, E. Evolutionary and Ecological Considerations on Nectar-Mediated Tripartite Interactions in Angiosperms and Their Relevance in the Mediterranean Basin. Plants 2021, 10, 507. https://doi.org/10.3390/plants10030507
Nepi M, Calabrese D, Guarnieri M, Giordano E. Evolutionary and Ecological Considerations on Nectar-Mediated Tripartite Interactions in Angiosperms and Their Relevance in the Mediterranean Basin. Plants. 2021; 10(3):507. https://doi.org/10.3390/plants10030507
Chicago/Turabian StyleNepi, Massimo, Daniele Calabrese, Massimo Guarnieri, and Emanuele Giordano. 2021. "Evolutionary and Ecological Considerations on Nectar-Mediated Tripartite Interactions in Angiosperms and Their Relevance in the Mediterranean Basin" Plants 10, no. 3: 507. https://doi.org/10.3390/plants10030507
APA StyleNepi, M., Calabrese, D., Guarnieri, M., & Giordano, E. (2021). Evolutionary and Ecological Considerations on Nectar-Mediated Tripartite Interactions in Angiosperms and Their Relevance in the Mediterranean Basin. Plants, 10(3), 507. https://doi.org/10.3390/plants10030507






