Abstract
The soil–root interface is the micro-ecosystem where roots uptake metals. However, less than 10% of hyperaccumulators’ rhizosphere has been examined. The present study evaluated the root and shoot response to nickel in hyperaccumulator and non-hyperaccumulator species, through the analysis of root surface and biomass and the ecophysiological response of the related aboveground biomass. Ni-hyperaccumulators Alyssoides utriculata (L.) Medik. and Noccaea caerulescens (J. Presl and C. Presl) F.K. Mey. and non-hyperaccumulators Alyssum montanum L. and Thlaspi arvense L. were grown in pot on Ni-spiked soil (0–1000 mg Ni kg−1, total). Development of root surfaces was analysed with ImageJ; fresh and dry root biomass was determined. Photosynthetic efficiency was performed by analysing the fluorescence of chlorophyll a to estimate the plants’ physiological conditions at the end of the treatment. Hyperaccumulators did not show a Ni-dependent decrease in root surfaces and biomass (except Ni 1000 mg kg−1 for N. caerulescens). The non-hyperaccumulator A. montanum suffers metal stress which threatens plant development, while the excluder T. arvense exhibits a positive ecophysiological response to Ni. The analysis of the root system, as a component of the rhizosphere, help to clarify the response to soil nickel and plant development under metal stress for bioremediation purposes.
1. Introduction
The root system provides the structural element of the rhizospheric microenvironment [1] determining a plant’s access to soil-borne elements [2]. The root surface plays a significant role in element uptake through membrane transporters and in some hyperaccumulators the root grows towards trace elements in soil [2]. This metallophilic behaviour allows plants to mainly develop roots towards metal-rich patches [3]. The induction of root proliferation (i.e., root foraging) in response to Ni, Cd and Zn in soils were reported in few hyperaccumulators, like Noccaea caerulescens (J. Presl and C. Presl) F.K. Mey., Thlaspi goesingense Halácsy, Sedum alfredii Hance and Streptanthus polygaloides Gray. [4,5,6,7]. Nonetheless, other hyperaccumulators do not show the same positive chemotropism towards metal-spiked soil [8]. A recent study shows that the polymetallic hyperaccumulator of Zn, Cd and Pb Noccaea rotundifolia (L.) Moench ssp. cepaeifolia has a larger root and shoot biomass in soils where there is a heterogeneous distribution of the metal in the growth substrate, with a prevalence of the avoidance strategy against metal-rich patches in the soil [9].
Depth and root morphology are also important traits in relation to uptake, although little is known about the relationship between root morphology and metal accumulation [2]. Many hyperaccumulators have been described as shallow-rooted (<0.5 m width) and with a high proportion of fine roots for the accumulation of elements [5,10], but deep-rooted herbaceous species (2 m width) exist [11] and the roots of many arboreal hyperaccumulator species have not yet been examined [2].
At the root surface, specific membrane transporters provide metal uptake sites for soil metals such as Ni [12] which bind metal chelators; some of which can facilitate root-to-shoot translocation or be involved in the metal tolerance [2,13].
Among natural metalliferous soils, serpentine soils have nutrient deficiency and a toxic concentration of metals such as Ni which usually ranges from 500 to 8000 (bioavailable Ni: 7 to >100 mg kg−1) [14,15,16,17,18,19,20]). Worldwide, researchers use the term “serpentine” to define abiotic factors such as rocks, soils, but also biotic components such as vegetation and other biota associated with ultramafic outcrops [21]. Serpentine soils provide particularly harsh and hostile conditions for most plant species [22], except for some endemic and threatened species [23] and the presence of some tolerant hyperaccumulator species [24,25,26,27]. Hyperaccumulation is most probably a defense strategy against pathogens [28] or competitors [29] that allows plant species to thrive on harsh serpentine soils [22], enabling them to accumulate more than 1000 mg kg−1 dry weight of metals such as Ni, Co, Cu, Pb, Zn, Mn in their aboveground tissues [30].
This “serpentine factor” as mentioned by [31] is caused by peculiar edaphic conditions such as the lack of nutrients (N, P, S, K, Na, Ca) and the high concentration of phytotoxic elements (Ni, Fe, Cu, Co, Cr, etc., [32], wide temperature ranges, occurrence of thin soil layer and consequently low organic content, combined with high surface runoff [24]. Due to their mineralogical and chemical properties, serpentinitic soils have often been overexploited to extract metals from their ultramafic rocks [33] causing a serious threat to the surrounding ecosystem. In particular, mining activity is responsible for soil degradation and groundwater pollution [34], due to metal leaching and active acid mine drainage AMD [35]. Although root depth, morphology and the preferential metal allocation in roots may partially explain the high concentration of trace elements in some species of hyperaccumulator [2], the relation between the root system and the metal accumulation need to be further examined. Furthermore, metal stress affects the light phase of photosynthesis with negative impacts on the performance of the photosystems [36]. Recent reports on plant physiological activity under metal stress [36,37,38,39,40] highlight adverse effects on photosynthesis caused by high concentrations of metal. However, few detailed studies investigated hyperaccumulators ecophysiological response to metal stress [41,42].
A previous study [43] suggests that the rhizospheric micro ecosystem complex may be a fundamental model for better understanding the dynamics of plant development and the Ni uptake for soil bioremediation purposes.
Therefore, can we assume a nickelophilic root development and nickel foraging and related shoot response in nickel-hyperaccumulators? To evaluate these traits, the main aim of the study was to assess possible alterations in the development of the root system (morpho-functional response to Ni) in terms of biomass and surface area and how this behaviour could affect plant ecophysiology in terms of photosynthetic performance.
2. Results
2.1. Evaluation of Root Area, Biomass, and Plant Water Content
The red colour intensity of the leaves shown by the 1% colorimetric dimethylglyoxime test (DMG) test enhances with increasing Ni concentration, suggesting a nickel accumulation in the leaf epidermis for both the hyperaccumulator species starting from 100 mg kg−1 of Ni concentration in pot.
The qualitative observation of the roots of the test species scanned with the ImageJ software does not clarify any relationship between the root surface area and the increasing concentrations of Ni from 0 mg kg−1 to 1000 mg kg−1 (Figures S1–S4). However, the analysis in non-accumulator T. arvense (Figure 1D and Figure 2D) show an increase in terms of root surface area and root dry weight (p < 0.01) at increasing Ni concentrations, which is less evident for the aboveground organs (p < 0.05). It is interesting to note the significant increase in root (p < 0.01) and shoots (p < 0.01) biomass of the facultative Ni-hyperaccumulator A. utriculata (Figure 2A), although this is not supported by an increase of the root surface (Figure 1A).
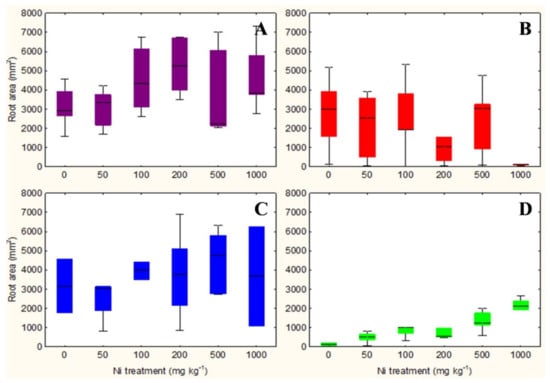
Figure 1.
Box-and-whisker plots showing the root surface area (mm2) of test hyperaccumulator species at increasing Ni concentrations (0–1000 mg kg−1): (A) A. utriculata, (B) N. caerulescens and non-hyperaccumulator species (C) A. montanum, (D) T. arvense. In each box, the central line marks the median of the data; the box edges represent the first and third quartiles; whiskers show non-outlier range. n = 20 each treatment.
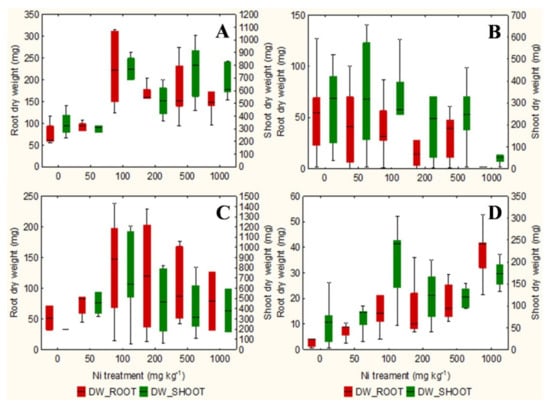
Figure 2.
Box-and-whisker plots showing the root and shoot dry biomass (mg) of test hyperaccumulator species at increasing Ni concentrations (0–1000 mg kg−1): (A) A. utriculata, (B) N. caerulescens and non-hyperaccumulator species (C) A. montanum, (D) T. arvense. In each box, the central line marks the median of the data; the box edges represent the first and third quartiles; whisker show non-outlier range. n = 20 each treatment.
N. caerulescens and A. montanum do not exhibit significant differences in terms of root surface area and biomass, except for N. caerulescens at Ni 1000 (Figure 1B,C and Figure 2B,C).
In the facultative Ni-hyperaccumulator A. utriculata, the Ni concentration is positively correlated with fresh and dry shoot biomass (p < 0.001) and with the root dry biomass (p < 0.01). In N. caerulescens Ni seems to positively affects the root water content (p < 0.01) (Table 1).

Table 1.
Spearman’s rank correlations coefficients between Ni concentration and biological parameters of test species: root surface area, fresh and dry weight (FW, DW) of root and shoot, root/shoot ratio (R/S) and water content (%). n = 20 each treatment. Au: A. utriculata, Nc: N. caerulescens, Am: A. montanum, Ta: T. arvense. ++ hyperaccumulator species, + non-hyperaccumulator species. * p < 0.05, ** p < 0.01, *** p < 0.001, NS: not significant.
Non-hyperaccumulator species behave differently: while in A. montanum the presence of Ni does not seem to affect the considered biological parameters, T. arvense shows a positive correlation between Ni and the root surface area and biomass, the root: shoot biomass ratio, the water content of the aerial organs (p < 0.001) and the shoot dry weight (p < 0.01) (Table 1).
A clear difference in terms of Ni uptake between the hyperaccumulator species exists, as indicated in Table 2. At the maximum Ni concentration, the facultative hyperaccumulator A. utriculata accumulates ~1000 mg Ni kg−1 in the aboveground biomass, while N. caerulescens can accumulate seven times higher Ni concentration (6798 mg Ni kg−1) compared to the other species.

Table 2.
X-ray fluorescens chemical analysis of Ni concentration measured on shoots of test species A. utriculata, N. caerulescens, A. montanum and T. arvense. n = 10 each species. ++ hyperaccumulator species, + non-hyperaccumulator species.
In the non-hyperaccumulator species, the shoot metal uptake at Ni 1000 is significantly lower compared hyperaccumulators and the range varies between 100 and 200 mg kg−1.
2.2. Photosynthetic Efficiency
The curves in Figure 3 are plotted on a logarithmic axis to observe the fluorescence over time up to the maximum fluorescence value. On the other hand, spider (or radar) plots (Figure 4) provide a method of comparing the spread of individual parameter values for selected records within a data set and a visualisation of which parameters have greater sensitivity to certain types of stress.
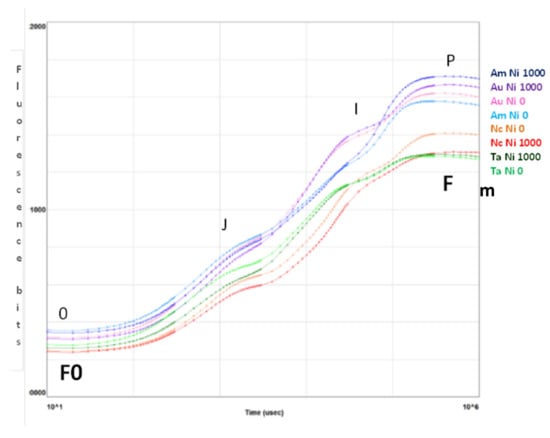
Figure 3.
Fluorescence transient analysis of test species distinguished by colour of the curves (purple: A. uticulata, red: N. caerulescens, blue: A. montanum, green: T. arvense) at Ni 0 mg kg−1 (light colour) and Ni 1000 mg kg−1 (dark colour). The peaks are denoted by letters 0, J, I, P and correspond to fluorescence values measured at 50 ms (F0, step 0), 2 ms (step J), 30 ms (step I), and maximal (Fm, step P). Data are the mean of ten measurements per plant. n = 100 each species.
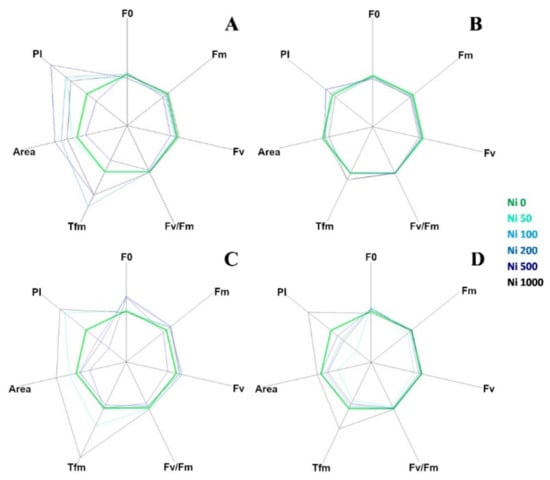
Figure 4.
Fluorescence transient analysis of test species represented by spider plots: (A) A. utriculata, (B) N. caerulescens, (C) A. montanum, (D) T. arvense. Parameters considered: F0: dark level fluorescence, Fm: maximum fluorescence, Fv: variable fluorescence, Fv/Fm: maximum efficiency of photosystem II (PSII), Tfm: time (ms) to reaching Fm, Area: area above the fluorescence curve between F0 and Fm, P.I. performance index. Increasing concentrations of nickel correspond to darker shades of blue; green identifies the control. Data are standardized for Ni 0 mg kg−1. Data are the mean of ten measurements per plant. n = 300 each species.
Figure 3 summarizes the transient fluorescence analysis for controls and treatments at Ni 1000 mg kg−1. The curves represent the average values of samples measured for the indicated species at Ni 0 (light colour) and Ni 1000 mg kg−1 (dark colour). The peaks represent the fluorescence values measured at 50 µs (0), 2 ms (J), 30 ms (I) and maximal (P) [44].
Through the evaluation of the Fv/Fm ratio, the Photosystem II efficiency was assessed for the test species. Instead, P.I. is an index of the vitality of the sample: it expresses the potential capacity for energy conservation.
Only N. caerulescens shows a greater Fm in the control than at the maximum Ni concentration, while A. utriculata and A. montanum exhibit the highest values of Fm.
Figure 4 represents a set of spider plots showing the differences between the test species at increasing Ni concentration in terms of recorded parameters.
Each axis of the plot corresponded to one of the seven parameters (F0, Fv, Fm, Fv/Fm, Tfm, Area and P.I.) and the average value of each parameter for the control species was used as the comparative value.
In A. utriculata (Figure 4A), Tfm, Area and P.I. increase in treatment (p < 0.001, p < 0.001 and p < 0.05 respectively). The parameters do not seem to differ considerably in N. caerulescens, while in non-hyperaccumulators there is a reduction in the P.I. except for Ni 50 mg kg−1 (A. montanum, Figure 4C) and Ni 1000 mg kg−1 (both species, Figure 4C,D). Clear differences are observed between the Ni treatments in non-accumulators. In particular, Tfm and P.I. reach the maximum value at Ni 1000 mg kg−1.
Data summarized in Table 3 show that in A. utriculata, Performance Index (P.I., p < 0.05) and the time to reaching Fm (Tfm, p < 0.001) increase at increasing Ni concentrations. Similarly, the latter enhances (p < 0.001) in N. caerulescens, although the maximum fluorescence (Fm) decrease (p < 0.01), and there is no significant difference in terms of P.I. In A. montanum a positive correlation between dark level fluorescence (F0) and Ni (p < 0.001) and between the latter and Fm (p < 0.05) is observed. On the other hand, in the same conditions, T. arvense exhibits a sensible reduction of F0 (p < 0.01), but a highly significant positive correlation (p < 0.001) between Ni and the maximum efficiency of photosystem II (Fv/Fm), the P.I. and the Tfm.

Table 3.
Spearman’s rank correlations coefficients between Ni concentration and fluorescence parameters of test species (F0: dark level fluorescence, Fm: maximum fluorescence, Fv/Fm: maximum efficiency of PSII, Tfm: time (ms) to reaching Fm, P.I. Performance Index). n = 300 each species. Au: A. utriculata, Nc: N. caerulescens, Am: A. montanum, Ta: T. arvense. ++ hyperaccumulator species, + non-hyperaccumulator species. * p < 0.05, ** p < 0.01, *** p < 0.001, NS: Not Significant.
3. Discussion
3.1. Evaluation of Root Area, Biomass, and Plant Water Content
The root surface and biomass development highlighted that at increasing available Ni concentration, both the polymetallic hyperaccumulator of Ni, Cd and Zn N. caerulescens [45,46] and the Ni-facultative hyperaccumulator A. utriculata [47] did not exhibit a clear dose-response effect by Ni as demonstrated by Whiting et al. in N. caerulescens [48]. The same response has been already highlighted for the hyperaccumulator N. caerulescens grown even at 100 μM Ni concentration without significant alterations of root dry weight [49].
Efficient sequestration and detoxification and high metal mobility in the root tissues in hyperaccumulators were well-known phenomena [2,49,50]. In addition, the root surfaces of hyperaccumulator species in response to Ni, even at a different level of affinity (higher for obligate hyperaccumulator and lower for facultative hyperaccumulator), supported their specific root response to metal, as demonstrated for Zn [51].
The response of the root system is interesting with respect to the tested concentrations, that seems to be comparable with natural serpentine soils where bioavailable Ni ranges from 7 to >100 mg kg−1 [15,18,19], while total Ni ranges between 500 and 8000 mg kg−1 [14,16,17].
The effects of metal concentration on plant growth and biomass are often used to assess phytotoxicity in non-hyperaccumulator species [52] or to evaluate differences in trace element concentration from inoculated and uninoculated plants [53,54].
In the pot test the non-hyperaccumulator A. montanum shows constant root area and plant biomass. Surprisingly, root biomass and root area of T. arvense significantly increase with Ni treatments (but 2.5 times less than hyperaccumulators) despite other studies showing a disrupted growth in non-accumulator plants, i.e., Lactuca sativa L. [25], or severe root biomass reduction in non-accumulator Cicer arietinum L. [8]. A possible explanation is that the pot test lasted long time causing a sort of adaptation of the involved species, but further investigations will clarify this point.
At the highest Ni concentration in pots, both hyperaccumulator species gave a positive reaction to the DMG test that highlighted an evident Ni translocation to aboveground biomass as expected [55]. In addition, A. utriculata significantly increased aboveground biomass while N. caerulescens was comparable to the control, as indicated by Pollard et al. [24] and similar to what was reported by Moradi et al. [8] for Berkheya coddii Roessler.
The phenotypic plasticity of the facultative hyperaccumulator species A. utriculata may depend on epigenetic modifications enabling a rapid adaptation to metal stress [56,57,58] or adaptation to edaphic conditions [59]. This may lead to a greater development of A. utriculata in the aboveground biomass where Ni accumulates. The significant increase of root biomass at increasing Ni treatment was not yet appreciated, even if A. utriculata had no negative effects on biomass, development and plant water content under Ni stress [60]. This aspect can probably be related to the species ability to concentrate metals in its aboveground biomass without display toxicity symptoms [60].
Considering that Ni-hyperaccumulators sequester Ni in shoots and by contrast Ni-excluders store Ni in roots, we could hypothesize in agreement with Seregin et al. [49] that roots systems of both hyperaccumulators A. utriculata and N. caerulescens do not show significant differences in terms of root surface and biomass at increasing Ni (except N. caerulescens, which suffered Ni 1000 mg kg−1) because they are more able to tolerate the metal stress while T. arvense specifically act as an excluder, increasing root area and biomass to sequester metal (phytostabilisation).
3.2. Photosynthetic Efficiency
Consistent with the biomass results, and consistent with other plants able to accumulate metals [61,62], the maximum primary photochemical efficiency of PSII, Fv/Fm, and the performance index (PI) [63] do not differ in the hyperaccumulator species at increasing Ni concentrations, while it significantly declines in A. montanum. This could be due to the great sensitivity to the fluctuations of environmental abiotic factors [64], such as Ni stress. Both parameters increase in T. arvense, although Fm is lower than the other species. A low Tfm may indicate sample stress [63] which causes the Fm to be achieved much earlier than expected, similar to what happens in A. montanum.
However, as confirmed by some studies [65,66], our results suggest that photosynthetic efficiency is less sensitive than the morpho-functional responses to Ni in terms of biomass and surface. The maximum efficiency of PSII (Fv/Fm) appears to be strictly dependent on the plant species tested, since the use of tolerant species might reduce their sensitivity to metal stress in the soil. Further studies on different species will be needed to clarify these aspects.
A comprehensive explanation could concern a Ni adaptation of A. utriculata to Ni input during the plant growth due to a possible phenotypic plasticity [67]. The same will probably occur to T. arvense, specifically at the root level where root surface and biomass increase to cope with a high level of Ni. However, epigenetic aspects involved in plant adaptation to strongly stressed environments and detoxification mechanisms need to be verified by further investigations [68].
In addition, the previous study [43] demonstrates that bacterial and fungal microbial communities isolated from the rhizosphere of hyperaccumulator species are subjected to selective pressure due to the ‘rhizophere effect’ [69,70,71,72,73,74] and to the high soil metal content [75,76,77,78,79]. Indeed, bacteria and fungi mainly thrive in rhizospheric serpentinitic soils compared to bare and non-metalliferous ones [43].
On the basis of plant growth-promoting (PGP) traits of many microorganisms [43,80,81,82,83,84,85,86,87], we can hypothesize the key role of the culturable rhizospheric microbiota to ameliorate the performance of metal hyperaccumulator species for soil remediation purposes.
Future research will better clarify the mutual behaviour of the rhizobiota associated with the root system of hyperaccumulators in the future perspective of the development of a holistic bioremediation system (plant–bacteria–fungi).
4. Materials and Methods
4.1. Plant Species
Alyssoides utriculata (Ni in leaves 36–2236 mg kg−1 DW, Figure 5A) is an evergreen shrub with good biomass. It is a Ni facultative hyperaccumulator [18,35] occurring on both metalliferous and ‘normal’ soils. The species ranges primarily in the north-eastern Mediterranean region [88]. The greater abundance of A. utriculata in low-competition serpentine soils compared to adjacent non-serpentine sites, suggests preadaptation tolerance traits [18]. It is able to accumulate greater quantities of Ni in the aboveground biomass even in soils with low metal level, compared to the typical serpentine non-hyperaccumulator species [60]. Despite the medium-high ability to concentrate Ni in shoots, A. utriculata species is of a key importance because it is a native Mediterranean hyperaccumulator that can be exploited for improved phytoremediation purposes in this climate.

Figure 5.
Test species: Ni-hyperaccumulator (A) A. utriculata and (B) N. caerulescens and non-hyperaccumulator (C) A. montanum and (D) T. arvense, photographs by S. Marsili (A,C), N. Mazzoni (B), G. Nicolella (D).
N. caerulescens (Ni in the shoots 1000–30000 mg kg−1 DW, Figure 5B) is a herbaceous biennial plant, found in Europe and in the USA [46]. It has been studied extensively for its ability to hyperaccumulate several metals [46]. Some populations of the genus Noccaea (syn. Thlaspi) hyperaccumulate Ni in the serpentinitic soil, whereas other populations are capable to uptake Zn and Cd [89], suggesting that hyperaccumulation is monophyletic [90]. N. caerulescens also known as ‘montane crucifer’ [91] or ‘alpine pennycress’ [92] includes populations that differ in morphological and physiological characteristics, exhibiting a wide range of accumulation and metal tolerance [93]. In Europe, N. caerulescens shows three ecological groups that correspond to three edaphic environments [94]. Two ecological groups are typical of metalliferous soils: the Calamine group develops in soils rich in Zn, Cd and Pb, while the Serpentine group is characterized by populations that thrive in Ni-rich soils derived from serpentinite ultramafic rocks. Finally, the third group includes non-metalliferous populations [95].
Alyssum montanum L. (Figure 5C) and Thlaspi arvense L. (Figure 5D) were the related non-hyperaccumulator species used for comparison, as in other experiments [45,46,96,97,98].
A. montanum, the ‘mountain gold’ [97] is a perennial herb belonging to the Brassicaceae family [99]. It is known to be a monophyletic polyploid complex [100] diversified in the last 2 million years [101] and characterized by a wide geographical and ecological range including more than 30 species and subspecies [99]. It is located in Europe, western Asia and northern Africa, with a clear species diversity in the Mediterranean area [102,103,104,105].
Thlaspi arvense L. (field pennycress, pennycress herein [106] is an oilseed-producing plant [107] member of Thlaspide, a tribe of Brassicaceae [108]. Pennycress is a winter annual with a short life cycle [109] native to Eurasia and it is widespread in temperate regions of the northern hemisphere [110]. It is closely related to the model Arabidopsis thaliana (L.) Heynh. [111]. Pennycress exhibits winter growth and extreme cold tolerance traits [112] and it is known to provide ecosystem services in the cold season such as habitat and food source for pollinator insects, weed suppression, and reduction of soil erosion and nutrients leaching [109,113,114,115,116].
4.2. Seed Collection
Hyperaccumulator plants A. utriculata and N. caerulescens (Figure 5A,B) were grown from seeds collected according to international guidelines (ENSCONET, 2009), from serpentine soils in Liguria (NW Italy) in July 2016. Samples were harvested from the eastern Ligurian Alps (Voltri Massif, 44°28′49″ N, 8°40′44″ E). The presence of Ni in the mother plants was assessed by means of a colorimetric field dimethylglyoxime test [117,118]. All plants yielded a dimethylglyoxime-positive reaction.
The related non-hyperaccumulator species A. montanum L. (Figure 5C) and T. arvense L. (Figure 5D) were collected from ‘normal’ soil and used for comparison. Seeds were provided by herbarium specimens: A. montanum from the Jardin Botanique de Bordeaux FR0BORD120310-Causse–Méjean, and T. arvense were provided by the Botanischer Garten Ulm IPEN XX-0-ULM-1998-F-152.
4.3. Pot Experiment
4.3.1. Evaluation of Root Area, Biomass, and Plant Water Content
The peat–sand mix (2:1) was chosen as a growing substrate; the substrate was sterilized at 120 °C for 20′, and oven dried at 60 °C. The final pH of substrate was measured by mixing an aliquot of soil with deionized water (ratio 1:3), and to obtain a pH of 6–6.5, slaked lime with Ca and Mg was added to the dry soil. After the assessment of pH, the soil water content at field capacity on a volume basis [119] was assessed to calculate the water holding capacity (WHC): 100 mL of water was added to 100 mL of dry soil placed in a funnel on a graduated cylinder. After waiting at least 1 h until the last drop, the WHC (%) was calculated based on the volume of water retained by the soil. Finally, the soil was transferred to 10 cm Ø pot.
To evaluate the root surface response to increasing concentration of available Ni, soil was homogeneously hydrated with a 70% WHC solution of 1/4 strength Hoagland’s basal salt mixture n.2 (Sigma-Aldrich, St. Louis, MO, USA) and metallic salt (NiSO4*6H2O) [4,8] was added to obtain increasing concentrations of available Ni: 0, 50, 100, 200, 500, 1000 mg L−1 respectively. Afterwards, seeds were surface-sterilized with sodium hypochlorite (NaClO) 10% for 10′ [120] and placed in pot (one seed per box, five replicates each concentration).
Pots were transferred to a greenhouse and the plants were grown in semi-natural conditions at controlled temperature (T = 19–22 °C) for 120 days, replenishing the plants with deionized water and monitoring plant growth two times a week.
During the third month of growth, the water-soluble fertilizer Leader N-P-K (20-10-20 + MgO + Me) was solubilised in deionised water at the concentration of 0.5 g L−1 and supplied for each pot once a week for one month.
At the end of the test, Ni accumulation in leaves was evaluated via a colorimetric dimethylglyoxime test (DMG 1%, Sigma-Aldrich, in ethanol 95%, [117] for each species and treatment. One mature leaf each plant was collected and placed in a solution of 1% DMG in ethanol 95°. Leaves turn red when a positive reaction occurs (high amount of Ni is stored in leaf epidermis).
Each plant was gently removed from the substrate, washed with tap water and then with deionised water, divided into root and shoot and weighed for fresh biomass. Roots were scanned and the resulting images were processed and analysed with ImageJ software to assess the root surface area. ImageJ is designed for scientific multidimensional images. It allows the user to create binary images (i.e., black and white), calibrate and analyse them, distinguish root from the background, and analyse the surfaces obtained to obtain the whole root area.
First, the measurement scale was set using a graduated scale in each scanned image. The root image was then converted to 8-bit images. Finally, images were converted into a black and white image by thresholding to evaluate the surface area.
Finally, DW were determined after oven-drying (60 °C, 48 h). Leaves were powdered using a ball mill (Retsch MM2000, Haan, Germany), preceding XRF analysis.
The chemical characterization of the dried samples was carried out on the granulometric fraction <2 mm by using an X-ray field portable spectrophotometer (X-MET7500 FP-EDXRF Analyser, Oxford Instruments, Abingdon-on-Thames, UK) that allows non-invasive and non-destructive analyses, providing information about chemical composition of the shoots. Quantitative analyses were obtained from trace level (ppm) to 100% for elements with atomic number ≥12 and the data quality level of the analyses was defined according to the Method 6200 of the U.S. Environmental Protection Agency [121]. This procedure was performed thanks to the collaboration with the Geospectra s.r.l, spin off of the University of Genoa. It represents an efficient, alternative approach to traditional laboratory analysis, allowing measurement of the concentration of a wide range of chemical elements.
4.3.2. Photosynthethic Efficiency
Chlorophyll fluorescence is a simple and non-invasive measurement technique of photosystem activity (PSII) in which the light energy absorbed by chlorophyll is partly re-emitted as light [122].
To estimate the plant physiological condition at the end of the growth period, 10 measurements of photosynthetic efficiency in each plant were performed on leaves with the digital fluorimeter Handy-Pea (Hansatech Instruments, King’s Lynn, UK). It provides the high time resolution essential to perform measurements of fast chlorophyll fluorescence induction kinetics. Leaf samples were covered with the leaf clip which has a small shutter plate to keep it close when the clip is attached so that light is excluded, and dark adaptation takes place. After 20 min the chlorophyll fluorescence signal received by the sensor head during recording is digitised within the Handy PEA control unit using a fast Analogue/Digital converter.
The fluorescence transient is a tool to characterize and screen photosynthetic samples [63]. General parameters recorded by fluorimeter are:
- F0 is the minimum fluorescence value and represents emission by the excited chlorophyll a molecule in the antenna structure of Photosystem II.
- Fm is the maximum fluorescence value obtained after the application of a saturation pulse to the dark-adapted leaf.
- Fv is the variable fluorescence, and it denotes the variable component of the recording and relates to the maximum capacity for photochemical quenching.
- Fv/Fm is widely used to indicate the maximum quantum efficiency of Photosystem II. It is a sensitive indication of plant photosynthetic performance.
- Tfm is used to express the time at which the maximum fluorescence value (Fm) was reached.
- Area above the fluorescence curve between F0 and Fm is proportional to the pool size of electron acceptors.
- P.I. (Performance Index) is essentially an indicator of sample vitality.
The excitation light consisted of a 1 s pulse of ultra-bright continuous red radiation (650 nm peak wavelength), provided by an array of three light-emitting diodes focused on a leaf surface of 5 mm at an intensity of 3500 μmol photons per square meter (m2) per second (s). The analysis of the transient was based on the fluorescence values measured at 50 µs (F0), 2 ms, 30 ms, and maximal (Fm) after about 300 ms [60,63]. The fluorescence induction is well represented by the Kautsky induction curve [123].
The fluorescence emission provides information about the phytochemical efficiency, the heat dissipation and the electron transfer reactions [124,125,126,127]; therefore, the presence of any type of stress results in photoinhibition and a low Fv/Fm ratio [122]. Therefore, a quick screening of the photosynthetic efficiency shows the trend of growth and plant yield [128,129].
Data on photosynthetic efficiency and plant physiological performance, obtained from the averages of measurements on the test species, have been processed with PEA-Plus software (Hansatech Instruments).
4.4. Data Analysis
The statistical analyses were performed with Statistica 8.0 (Statsoft Inc., Tulsa, OK, USA) software.
The averages were presented with their standard deviations (SD). Non-parametric tests were used to avoid data transformation. Normality of parameters were evaluated with the Shapiro–Wilk test. Correlations between variables were analysed using Spearman’s correlation coefficient (ρ) using different level of significance (α: 0.05, 0.01, 0.001), since data exhibit a non-normal statistical distribution.
Moreover, the R/S ratio for both fresh and dry biomass and the water content (100 * DW/FW) in the root and shoot were evaluated [60].
The open-source software ImageJ ([130]; http://imagej.nih.gov/ij/ accessed October 2019) was used to determine the root surface on the whole roots of each species and treatment (n = 90).
5. Conclusions
The evaluation of the morpho-functional and ecophysiological alterations of hyperaccumulator and non-hyperaccumulator species under Ni stress allows us to better clarify the behaviour of plant species suitable for soil bioremediation at the root and shoot level.
We highlighted a nickelophilic behaviour and Ni foraging of the root system mainly in the facultative hyperaccumulator A. utriculata that may be due to its phenotypic plasticity combined with epigenetic aspects that make this species an excellent plant model for studying the Ni uptake. Non-hyperaccumulators show symptoms of metal stress or nickel avoiding strategies, as in the case of T. arvense. This latter increases both root surface and overall biomass. Perhaps its Ni-excluder traits could explain this response since metal is immobilized at the root level to avoid translocation to shoot biomass and possible related toxicity symptoms. However, further studies are required to better understand its ecophysiological behaviour under Ni stress.
This study provides the evidence for a careful selection of the best performing species for phytoremediation purposes considering root metallophilic behaviour and consequent better adaptation on metal-polluted soils.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/10/3/508/s1, Figure S1. Root system of A. utriculata scanned and processed with ImageJ software at different concentrations of Ni (mg kg−1); (A) Ni 0, (B) Ni 50, (C) Ni 100, (D) Ni 200, (E) Ni 500, (F) Ni 1000. n = 20 each treatment. Figure S2. Root system of N. caerulescens scanned and processed with ImageJ software at different concentrations of Ni (mg kg−1); (A) Ni 0, (B) Ni 50, (C) Ni 100, (D) Ni 200, (E) Ni 500, (F) Ni 1000. n = 20 each treatment. Figure S3. Root system of A. montanum scanned and processed with ImageJ software at different concentrations of Ni (mg kg−1); (A) Ni 0, (B) Ni 50, (C) Ni 100, (D) Ni 200, (E) Ni 500, (F) Ni 1000. n = 20 each treatment. Figure S4. Root system of T. arvense scanned and processed with ImageJ software at different concentrations of Ni (mg kg−1); (A) Ni 0, (B) Ni 50, (C) Ni 100, (D) Ni 200, (E) Ni 500, (F) Ni 1000. n = 20 each treatment.
Author Contributions
Conceptualization, E.R. and S.R. (Stefano Rosatto); Methodology, S.R. (Stefano Rosatto) and E.R.; Validation, E.R.; Formal Analysis, E.R. and S.R. (Sara Romeo); Investigation, S.R. (Stefano Rosatto) and S.R. (Sara Romeo); Resources, E.R. and S.R. (Stefano Rosatto); Data Curation, S.R. (Stefano Rosatto); Writing—Original Draft Preparation, S.R. (Stefano Rosatto); Writing—Review and Editing, E.R.; Visualization, E.R. and M.M.; Supervision, M.M.; Project Administration, M.M.; Funding Acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PhD in Biology applied to Agriculture and Environment, (DISTAV-Department of Earth, Environmental and Life Sciences University of Genoa, Italy), XXXI and XXXIV cycle.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors wish to thank E. Mora, curator at Genoa Botanic Garden for the technical support in the greenhouse experiments. P. Marescotti and M. Brancucci are warmly thank for the XRF analytical measurements. S. Marsili, M. Mazzotti and G. Nicolella are thank for the field photographs kindly provided.
Conflicts of Interest
The authors declare no conflict of interest.
Consulted Websites
- Acta Plantarum-Flora delle Regioni italiane https://www.actaplantarum.org./ (accessed 11 December 2020).
- ENSCOBASE: the ENSCONET Virtual Seed Bank http://enscobase.maich.gr/ (accessed 8 October 2018).
- Global Hyperaccumulator Database http://hyperaccumulators.smi.uq.edu.au/collection/ (accessed 1 February 2021).
- KEW Royal Botanic Gardens http://data.kew.org/sid/ (accessed 9 November 2020).
References
- Wenzel, W.W.; Lombi, E.; Adriano, D.C. Root and Rhizosphere Processes in Metal Hyperaccumulation and Phytoremediation Technology. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2004; pp. 313–344. [Google Scholar] [CrossRef]
- Alford, É.R.; Pilon-Smits, E.A.H.; Paschke, M.W. Metallophytes—A View from the Rhizosphere. Plant Soil 2010, 337, 33–50. [Google Scholar] [CrossRef]
- Hodge, A. The Plastic Plant: Root Responses to Heterogeneous Supplies of Nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Dechamps, C.; Noret, N.; Mozek, R.; Draye, X.; Meerts, P. Root Allocation in Metal-Rich Patch by Thlaspi caerulescens from Normal and Metalliferous Soil—New Insights into the Rhizobox Approach. Plant Soil 2008, 310, 211. [Google Scholar] [CrossRef]
- Himmelbauer, M.L.; Puschenreiter, M.; Schnepf, A.; Loiskandl, W.; Wenzel, W.W. Root Morphology of Thlaspi goesingense Hálácsy Grown on a Serpentine Soil. J. Plant Nutr. Soil Sci. 2005, 168, 138–144. [Google Scholar] [CrossRef]
- Liu, F.; Tang, Y.; Du, R.; Yang, H.; Wu, Q.; Qiu, R. Root Foraging for Zinc and Cadmium Requirement in the Zn/Cd Hyperaccumulator Plant Sedum alfredii. Plant Soil 2010, 327, 365–375. [Google Scholar] [CrossRef]
- Mincey, K. Ecological and Genetic Investigations of the Nickel Hyperaccumulator Streptanthus polygaloides (Brassicaceae). Ph.D. Dissertation, Auburn University, Auburn, AL, USA, 2018. [Google Scholar]
- Moradi, A.B.; Conesa, H.M.; Robinson, B.H.; Lehmann, E.; Kaestner, A.; Schulin, R. Root Responses to Soil Ni Heterogeneity in a Hyperaccumulator and a Non-Accumulator Species. Environ. Pollut. 2009, 157, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Golestanifard, A.; Puschenreiter, M.; Aryan, A.; Santner, J.; Wenzel, W.W. Metal Accumulation and Rhizosphere Characteristics of Noccaea rotundifolia ssp. cepaeifolia. Environ. Pollut. 2020, 266, 115088. [Google Scholar] [CrossRef]
- Keller, C.; Hammer, D.; Kayser, A.; Richner, W.; Brodbeck, M.; Sennhauser, M. Root Development and Heavy Metal Phytoextraction Efficiency: Comparison of Different Plant Species in the Field. Plant Soil 2003, 249, 67–81. [Google Scholar] [CrossRef]
- Kutschera, L.; Lichtenegger, E.; Sobotik, M. Wurzelatlas Mitteleuropäischer Grünlandpflanzen. G. Fischer-Verlag: Stuttgart, Germany, 1992; Volume 1. [Google Scholar]
- Gendre, D.; Czernic, P.; Conéjéro, G.; Pianelli, K.; Briat, J.-F.; Lebrun, M.; Mari, S. TcYSL3, a Member of the YSL Gene Family from the Hyper-Accumulator Thlaspi caerulescens, Encodes a Nicotianamine-Ni/Fe Transporter. Plant J. 2007, 49, 1–15. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? And What Makes Them so Interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of Metal and Metalloid Trace Elements: Facts and Fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Freitas, H.; Prasad, M.N.V.; Pratas, J. Analysis of Serpentinophytes from North–East of Portugal for Trace Metal Accumulation—Relevance to the Management of Mine Environment. Chemosphere 2004, 54, 1625–1642. [Google Scholar] [CrossRef] [PubMed]
- Ghaderian, S.M.; Mohtadi, A.; Rahiminejad, R.; Reeves, R.D.; Baker, A.J.M. Hyperaccumulation of Nickel by Two Alyssum Species from the Serpentine Soils of Iran. Plant Soil 2007, 293, 91–97. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J. Metal Accumulating Plants. Phytoremediation Toxic Met. Using Plants Clean Up environment; Ensley, R.I., Ed.; John Wiley Sons Inc.: New York, NY, USA, 2000. [Google Scholar]
- Roccotiello, E.; Serrano, H.C.; Mariotti, M.G.; Branquinho, C. Nickel Phytoremediation Potential of the Mediterranean Alyssoides utriculata (L.) Medik. Chemosphere 2015, 119, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Turgay, O.C.; Görmez, A.; Bilen, S. Isolation and Characterization of Metal Resistant-Tolerant Rhizosphere Bacteria from the Serpentine Soils in Turkey. Environ. Monit. Assess. 2012, 184, 515–526. [Google Scholar] [CrossRef]
- Sobczyk, M.K.; Smith, J.A.C.; Pollard, A.J.; Filatov, D.A. Evolution of Nickel Hyperaccumulation and Serpentine Adaptation in the Alyssum serpyllifolium Species Complex. Heredity 2017, 118, 31–41. [Google Scholar] [CrossRef]
- Rajakaruna, N.; Harris, T.B.; Alexander, E.B. Serpentine Geoecology of Eastern North America: A Review. Rhodora 2009, 111, 21–108. [Google Scholar] [CrossRef]
- Brady, K.U.; Kruckeberg, A.R.; Jr, H.D.B. Evolutionary Ecology of Plant Adaptation to Serpentine Soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Van der Ent, A.; Rajakaruna, N.; Boyd, R.; Echevarria, G.; Repin, R.; Williams, D. Global Research on Ultramafic (Serpentine) Ecosystems (8th International Conference on Serpentine Ecology in Sabah, Malaysia): A Summary and Synthesis. Aust. J. Bot. 2015, 63, 1–16. [Google Scholar] [CrossRef]
- Mengoni, A.; Schat, H.; Vangronsveld, J. Plants as Extreme Environments? Ni-Resistant Bacteria and Ni-Hyperaccumulators of Serpentine Flora. Plant Soil 2010, 331, 5–16. [Google Scholar] [CrossRef]
- Pollard, A.J.; Reeves, R.D.; Baker, A.J.M. Facultative Hyperaccumulation of Heavy Metals and Metalloids. Plant Sci. 2014, 217-218, 8–17. [Google Scholar] [CrossRef]
- Marsili, S.; Roccotiello, E.; Carbone, C.; Marescotti, P.; Cornara, L.; Mariotti, M.G. Plant Colonization on a Contaminated Serpentine Site. Northeast. Nat. 2009, 16, 297–308. [Google Scholar] [CrossRef]
- Global Hyperaccumulator Database. Available online: http://hyperaccumulators.smi.uq.edu.au/collection/ (accessed on 29 January 2021).
- Boyd, R.S. Plant Defense Using Toxic Inorganic Ions: Conceptual Models of the Defensive Enhancement and Joint Effects Hypotheses. Plant Sci. 2012, 195, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Selvi, F.; Carrari, E.; Colzi, I.; Coppi, A.; Gonnelli, C. Responses of Serpentine Plants to Pine Invasion: Vegetation Diversity and Nickel Accumulation in Species with Contrasting Adaptive Strategies. Sci. Total Environ. 2017, 595, 72–80. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R. Terrestrial Higher Plants Which Hyperaccumulate Metallic Elements. A Review of Their Distribution, Ecology and Phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Brooks, R.R. Serpentine and Its Vegetation: A Multidisciplinary Approach. Ecol. Phytogeogr. Physiol. Ser. 1987, 1, 52. [Google Scholar] [CrossRef]
- Chiarucci, A.; Baker, A.J.M. Advances in the Ecology of Serpentine Soils. Plant Soil 2007, 293, 1–2. [Google Scholar] [CrossRef]
- Pasquet, C.; Monna, F.; van Oort, F.; Gunkel-Grillon, P.; Laporte-Magoni, C.; Losno, R.; Chateau, C. Mobility of Ni, Co, and Mn in Ultramafic Mining Soils of New Caledonia, Assessed by Kinetic EDTA Extractions. Environ. Monit. Assess. 2018, 190, 638. [Google Scholar] [CrossRef]
- Raous, S.; Becquer, T.; Garnier, J.; de Martins, É.S.; Echevarria, G.; Sterckeman, T. Mobility of Metals in Nickel Mine Spoil Materials. Appl. Geochem. 2010, 25, 1746–1755. [Google Scholar] [CrossRef]
- Roccotiello, E.; Marescotti, P.; Di Piazza, S.; Cecchi, G.; Mariotti, M.G.; Zotti, M. Biodiversity in Metal-Contaminated Sites–Problem and Perspective—A Case Study. Significance 2015, 16, 26–28. [Google Scholar] [CrossRef]
- Sitko, K.; Rusinowski, S.; Kalaji, H.M.; Szopiński, M.; Małkowski, E. Photosynthetic Efficiency as Bioindicator of Environmental Pressure in A. halleri. Plant Physiol. 2017, 175, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, L.; Huang, X.; Zhou, Y.; Quan, Q.; Li, Y.; Zhu, X. Response of Photosynthesis to Different Concentrations of Heavy Metals in Davidia involucrata. PLoS ONE 2020, 15, e0228563. [Google Scholar] [CrossRef]
- Houri, T.; Khairallah, Y.; Zahab, A.A.; Osta, B.; Romanos, D.; Haddad, G. Heavy Metals Accumulation Effects on The Photosynthetic Performance of Geophytes in Mediterranean Reserve. J. King Saud Univ. Sci. 2020, 32, 874–880. [Google Scholar] [CrossRef]
- Chandra, R.; Kang, H. Mixed Heavy Metal Stress on Photosynthesis, Transpiration Rate, and Chlorophyll Content in Poplar Hybrids. For. Sci. Technol. 2016, 12, 55–61. [Google Scholar] [CrossRef]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Goltsev, V. Effects of Different Metals on Photosynthesis: Cadmium and Zinc Affect Chlorophyll Fluorescence in Durum Wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, A.G.; Apostolova, E.L.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis, I.-D.S.; Moustakas, M. Cadmium Toxicity in Salvia sclarea L.: An Integrative Response of Element Uptake, Oxidative Stress Markers, Leaf Structure and Photosynthesis. Ecotoxicol. Environ. Saf. 2021, 209, 111851. [Google Scholar] [CrossRef]
- Khator, K.; Saxena, I.; Shekhawat, G.S. Nitric Oxide Induced Cd Tolerance and Phytoremediation Potential of B. juncea by the Modulation of Antioxidant Defense System and ROS Detoxification. Biometals 2020. [Google Scholar] [CrossRef] [PubMed]
- Rosatto, S.; Roccotiello, E.; Di Piazza, S.; Cecchi, G.; Greco, G.; Zotti, M.; Vezzulli, L.; Mariotti, M. Rhizosphere Response to Nickel in a Facultative Hyperaccumulator. Chemosphere 2019. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Mari, S.; Gendre, D.; Pianelli, K.; Ouerdane, L.; Lobinski, R.; Briat, J.-F.; Lebrun, M.; Czernic, P. Root-to-Shoot Long-Distance Circulation of Nicotianamine and Nicotianamine-Nickel Chelates in the Metal Hyperaccumulator Thlaspi caerulescens. J. Exp. Bot. 2006, 57, 4111–4122. [Google Scholar] [CrossRef] [PubMed]
- Milner, M.J.; Kochian, L.V. Investigating Heavy-Metal Hyperaccumulation Using Thlaspi caerulescens as a Model System. Ann. Bot. 2008, 102, 3–13. [Google Scholar] [CrossRef]
- Roccotiello, E.; Zotti, M.; Mesiti, S.; Marescotti, P.; Carbone, C.; Cornara, L.; Mariotti, M.G. Biodiversity in Metal-Polluted Soils. Fresenius Environ. Bull. 2010, 19, 2420–2425. [Google Scholar]
- Whiting, S.N.; Leake, J.R.; McGrath, S.P.; Baker, A.J.M. Positive Responses to Zn and Cd by Roots of the Zn and Cd Hyperaccumulator Thlaspi caerulescens. New Phytol. 2000, 145, 199–210. [Google Scholar] [CrossRef]
- Seregin, I.V.; Erlikh, N.T.; Kozhevnikova, A.D. Nickel and Zinc Accumulation Capacities and Tolerance to These Metals in the Excluder Thlaspi arvense and the Hyperaccumulator Noccaea caerulescens. Russ. J. Plant Physiol. 2014, 61, 204–214. [Google Scholar] [CrossRef]
- Kidd, P.; Barceló, J.; Bernal, M.P.; Navari-Izzo, F.; Poschenrieder, C.; Shilev, S.; Clemente, R.; Monterroso, C. Trace Element Behaviour at the Root–Soil Interface: Implications in Phytoremediation. Environ. Exp. Bot. 2009, 67, 243–259. [Google Scholar] [CrossRef]
- Chaney, R.L.; Angle, J.S.; Broadhurst, C.L.; Peters, C.A.; Tappero, R.V.; Sparks, D.L. Improved Understanding of Hyperaccumulation Yields Commercial Phytoextraction and Phytomining Technologies. J. Environ. Qual. 2007, 36, 1429–1443. [Google Scholar] [CrossRef]
- Ryser, P.; Sauder, W.R. Effects of Heavy-Metal-Contaminated Soil on Growth, Phenology and Biomass Turnover of Hieracium piloselloides. Environ. Pollut. 2006, 140, 52–61. [Google Scholar] [CrossRef]
- Al Agely, A.; Sylvia, D.M.; Ma, L.Q. Mycorrhizae Increase Arsenic Uptake by the Hyperaccumulator Chinese Brake Fern ( Pteris vittata L.). J. Environ. Qual. 2005, 34, 2181–2186. [Google Scholar] [CrossRef]
- Trotta, A.; Falaschi, P.; Cornara, L.; Minganti, V.; Fusconi, A.; Drava, G.; Berta, G. Arbuscular Mycorrhizae Increase the Arsenic Translocation Factor in the As Hyperaccumulating Fern Pteris vittata L. Chemosphere 2006, 65, 74–81. [Google Scholar] [CrossRef]
- Nkrumah, P.N.; Echevarria, G.; Erskine, P.D.; van der Ent, A. Nickel Hyperaccumulation in Antidesma montissilam: From Herbarium Discovery to Collection in the Native Habitat. Ecol. Res. 2018, 1–11. [Google Scholar] [CrossRef]
- Mirouze, M.; Paszkowski, J. Epigenetic Contribution to Stress Adaptation in Plants. Curr. Opin. Plant Biol. 2011, 14, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Zhang, Y.; Xu, C.; Lin, X.; Zang, Q.; Zhuang, T.; Jiang, L.; von Wettstein, D.; Liu, B. Transgenerational Inheritance of Modified DNA Methylation Patterns and Enhanced Tolerance Induced by Heavy Metal Stress in Rice (Oryza sativa L.). PLoS ONE 2012, 7, e41143. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, L.; Song, J.; Cui, W.; Zhang, Y.; Jia, C.; Francis, D.; Rogers, H.J.; Sun, L.; Tai, P. Cadmium-Induced Genomic Instability in Arabidopsis: Molecular Toxicological Biomarkers for Early Diagnosis of Cadmium Stress. Chemosphere 2016, 150, 258–265. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Aloupi, M.; Kazakou, E.; Dimitrakopoulos, P.G. Intra-Specific Variation in Ni Tolerance, Accumulation and Translocation Patterns in the Ni-Hyperaccumulator Alyssum lesbiacum. Chemosphere 2014, 95, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Roccotiello, E.; Serrano, H.C.; Mariotti, M.G.; Branquinho, C. The Impact of Ni on the Physiology of a Mediterranean Ni-Hyperaccumulating Plant. Environ. Sci. Pollut. Res. 2016, 23, 12414–12422. [Google Scholar] [CrossRef]
- Pietrini, F.; Passatore, L.; Patti, V.; Francocci, F.; Giovannozzi, A.; Zacchini, M. Morpho-Physiological and Metal Accumulation Responses of Hemp Plants (Cannabis sativa L.) Grown on Soil from an Agro-Industrial Contaminated Area. Water 2019, 11, 808. [Google Scholar] [CrossRef]
- Wu, J.; Hu, J.; Wang, L.; Zhao, L.; Ma, F. Responses of Phragmites australis to Copper Stress: A Combined Analysis of Plant Morphology, Physiology and Proteomics. Plant Biol. (Stuttg) 2020. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 445–483. [Google Scholar]
- Živčák, M.; Olšovská, K.; Slamka, P.; Galambošová, J.; Rataj, V.; Shao, H.B.; Brestič, M. Application of Chlorophyll Fluorescence Performance Indices to Assess the Wheat Photosynthetic Functions Influenced by Nitrogen Deficiency. Plant Soil Environ. 2015, 60, 210–215. [Google Scholar] [CrossRef]
- Marchand, L.; Lamy, P.; Bert, V.; Quintela-Sabaris, C.; Mench, M. Potential of Ranunculus acris L. for Biomonitoring Trace Element Contamination of Riverbank Soils: Photosystem II Activity and Phenotypic Responses for Two Soil Series. Environ. Sci. Pollut. Res. 2016, 23, 3104–3119. [Google Scholar] [CrossRef] [PubMed]
- Quintela-Sabaris, C.; Marchand, L.; Kidd, P.S.; Friesl-Hanl, W.; Puschenreiter, M.; Kumpiene, J.; Mueller, I.; Neu, S.; Janssen, J.; Vangronsveld, J. Assessing Phytotoxicity of Trace Element-Contaminated Soils Phytomanaged with Gentle Remediation Options at Ten European Field Trials. Sci. Total Environ. 2017, 599, 1388–1398. [Google Scholar] [CrossRef]
- Visioli, G.; Vincenzi, S.; Marmiroli, M.; Marmiroli, N. Correlation between Phenotype and Proteome in the Ni Hyperaccumulator Noccaea caerulescens subsp. caerulescens. Environ. Exp. Bot. 2012, 77, 156–164. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular Mechanisms of Metal Hyperaccumulation in Plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Bakker, P.A.H.M.; Berendsen, R.L.; Doornbos, R.F.; Wintermans, P.C.A.; Pieterse, C.M.J. The Rhizosphere Revisited: Root Microbiomics. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chiarucci, A.; Fang, H.; Chen, M. An Interspecific Variation in Rhizosphere Effects on Soil Anti-Erodibility. Sci. Rep. 2020, 10, 2411. [Google Scholar] [CrossRef]
- Li, H.; Su, J.-Q.; Yang, X.-R.; Zhu, Y.-G. Distinct Rhizosphere Effect on Active and Total Bacterial Communities in Paddy Soils. Sci. Total Environ. 2019, 649, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M. Introduction: Some Consequences of Microbial Rhizosphere Competence for Plant and Soil. Rhizosphere 1990, 1–10. [Google Scholar]
- Sørensen, J. The Rhizosphere as a Habitat for Soil Microorganisms. Mod. Soil Microbiol. 1997, 21–45. [Google Scholar]
- Foster, R.C.; Rovira, A.D.; Cock, T.W. Ultrastructure of the Root-Soil Interface; American Phytopathological Society: Saint Paul, MN, USA, 1983. [Google Scholar]
- Xie, Y.; Fan, J.; Zhu, W.; Amombo, E.; Lou, Y.; Chen, L.; Fu, J. Effect of Heavy Metals Pollution on Soil Microbial Diversity and Bermudagrass Genetic Variation. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Liao, M.; Xie, X.M. Effect of Heavy Metals on Substrate Utilization Pattern, Biomass, and Activity of Microbial Communities in a Reclaimed Mining Wasteland of Red Soil Area. Ecotox. Environ. Saf. 2007, 66, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, D.; Li, J.; Yin, H.; Liu, H.; Liu, X.; Cheng, C.; Xiao, Y.; Liu, Z.; Yan, M. Response of Soil Microbial Communities and Microbial Interactions to Long-Term Heavy Metal Contamination. Environ. Pollut. 2017, 231, 908–917. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Wang, H.; Lin, Q.; Chen, X.; Chen, Y. The Influence of Soil Heavy Metals Pollution on Soil Microbial Biomass, Enzyme Activity, and Community Composition near a Copper Smelter. Ecotox. Environ. Saf. 2007, 67, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Azarbad, H.; Niklińska, M.; van Gestel, C.A.; van Straalen, N.M.; Röling, W.F.; Laskowski, R. Microbial Community Structure and Functioning along Metal Pollution Gradients. Environ. Toxicol. Chem. 2013, 32, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Meng, Q.; Luo, S.; Shen, J.; Chen, B.; Khan, K.Y.; Japenga, J.; Ma, X.; Yang, X.; Feng, Y. Enhanced Cd Extraction of Oilseed Rape (Brassica napus) by Plant Growth-Promoting Bacteria Isolated from Cd Hyperaccumulator Sedum Alfredii Hance. Int. J. Phytoremediat. 2017, 19, 281–289. [Google Scholar] [CrossRef]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The Role of Plant-Associated Bacteria in the Mobilization and Phytoextraction of Trace Elements in Contaminated Soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Vara Prasad, M.N.; Freitas, H.; Ae, N. Biotechnological Applications of Serpentine Soil Bacteria for Phytoremediation of Trace Metals. Crit. Rev. Biotechnol. 2009, 29, 120–130. [Google Scholar] [CrossRef]
- Khan, A.G. Role of Soil Microbes in the Rhizospheres of Plants Growing on Trace Metal Contaminated Soils in Phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Castro, C.; Prieto-Fernández, A.; Alvarez-Lopez, V.; Monterroso, C.; Cabello-Conejo, M.I.; Acea, M.J.; Kidd, P.S. Nickel Solubilizing Capacity and Characterization of Rhizobacteria Isolated from Hyperaccumulating and Non-Hyperaccumulating Subspecies of Alyssum serpyllifolium. Int. J. Phytoremediat. 2011, 13 (Suppl. 1), 229–244. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Sultana, F.; Islam, S. Plant Growth-Promoting Fungi (PGPF): Phytostimulation and Induced Systemic Resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2017; pp. 135–191. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F. Application and Mechanisms of Plant Growth Promoting Fungi (PGPF) for Phytostimulation. Org. Agric. 2020. [Google Scholar] [CrossRef]
- Thijs, S.; Langill, T.; Vangronsveld, J. Chapter Two—The Bacterial and Fungal Microbiota of Hyperaccumulator Plants: Small Organisms, Large Influence. In Advances in Botanical Research; Cuypers, A., Vangronsveld, J., Eds.; Phytoremediation: Oxford, UK; Elsevier Ltd.: Oxford, UK, 2017; Volume 83, pp. 43–86. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia. Edagricole: Bologna, Italy, 1982; 3 Vols. [Google Scholar]
- Assunção, A.G.L.; Bleeker, P.; ten Bookum, W.M.; Vooijs, R.; Schat, H. Intraspecific Variation of Metal Preference Patterns for Hyperaccumulation in Thlaspi caerulescens: Evidence from Binary Metal Exposures. Plant Soil 2008, 303, 289–299. [Google Scholar] [CrossRef]
- Macnair, M.R. The Hyperaccumulation of Metals by Plants. Adv. Bot. Res. 2003, 40, 63–105. [Google Scholar] [CrossRef]
- Fones, H.N.; Preston, G.M.; Smith, J.A.C. Variation in Defence Strategies in the Metal Hyperaccumulator Plant Noccaea caerulescens Is Indicative of Synergies and Trade-Offs between Forms of Defence. R. Soc. Open Sci. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Halimaa, P.; Blande, D.; Aarts, M.G.M.; Tuomainen, M.; Tervahauta, A.; Kärenlampi, S. Comparative Transcriptome Analysis of the Metal Hyperaccumulator Noccaea caerulescens. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Visioli, G.; Gullì, M.; Marmiroli, N. Noccaea caerulescens Populations Adapted to Grow in Metalliferous and Non-Metalliferous Soils: Ni Tolerance, Accumulation and Expression Analysis of Genes Involved in Metal Homeostasis. Environ. Exp. Bot. 2014, 105, 10–17. [Google Scholar] [CrossRef]
- Gonneau, C. Distribution, Écologie et Évolution de l’hyperaccumulation Des Éléments En Traces Par Noccaea caerulescens. Ph.D. Thesis, Université de Lorraine, Metz, France, 2014. [Google Scholar]
- Sterckeman, T.; Cazes, Y.; Gonneau, C.; Sirguey, C. Phenotyping 60 Populations of Noccaea caerulescens Provides a Broader Knowledge of Variation in Traits of Interest for Phytoextraction. Plant Soil 2017, 418, 523–540. [Google Scholar] [CrossRef]
- Agrawal, B.; Czymmek, K.J.; Sparks, D.L.; Bais, H.P. Transient Influx of Nickel in Root Mitochondria Modulates Organic Acid and Reactive Oxygen Species Production in Nickel Hyperaccumulator Alyssum Murale. J. Biol. Chem. 2013, 288, 7351–7362. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Chaney, R.L. Growth and Metal Accumulation of an Alyssum murale Nickel Hyperaccumulator Ecotype Co-Cropped with Alyssum montanum and Perennial Ryegrass in Serpentine Soil. Front. Plant Sci. 2016, 7, 451. [Google Scholar] [CrossRef] [PubMed]
- Kozhevnikova, A.D.; Seregin, I.V.; Erlikh, N.T.; Shevyreva, T.A.; Andreev, I.M.; Verweij, R.; Schat, H. Histidine-Mediated Xylem Loading of Zinc Is a Species-Wide Character in Noccaea caerulescens. New Phytol. 2014, 203, 508–519. [Google Scholar] [CrossRef]
- Arrigo, N.; de Harpe, M.L.; Litsios, G.; Zozomová-Lihová, J.; Španiel, S.; Marhold, K.; Barker, M.S.; Alvarez, N. Is Hybridization Driving the Evolution of Climatic Niche in Alyssum montanum. Am. J. Bot. 2016, 103, 1348–1357. [Google Scholar] [CrossRef]
- Rešetnik, I.; Satovic, Z.; Schneeweiss, G.M.; Liber, Z. Phylogenetic Relationships in Brassicaceae Tribe Alysseae Inferred from Nuclear Ribosomal and Chloroplast DNA Sequence Data. Mol. Phylogenet. Evol. 2013, 69, 772–786. [Google Scholar] [CrossRef]
- Li, Y.; Kong, Y.; Zhang, Z.; Yin, Y.; Liu, B.; Lv, G.; Wang, X. Phylogeny and Biogeography of Alyssum (Brassicaceae) Based on Nuclear Ribosomal ITS DNA Sequences. J. Genet. 2014, 93, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Jalas, J. Atlas Florae Europaeae Notes. 13. Suggestions on Alyssum and Lepidium (Cruciferae). Ann. Bot. Fennici 1996, 33, 283–284. [Google Scholar]
- Španiel, S.; Marhold, K.; Filová, B.; Zozomová-Lihová, J. Genetic and Morphological Variation in the Diploid–Polyploid Alyssum montanum in Central Europe: Taxonomic and Evolutionary Considerations. Plant Syst. Evol. 2011, 294, 1. [Google Scholar] [CrossRef]
- Španiel, S.; Marhold, K.; Passalacqua, N.G.; Zozomová-Lihová, J. Intricate Variation Patterns in the Diploid-Polyploid Complex of Alyssum montanum—A. repens (Brassicaceae) in the Apennine Peninsula: Evidence for Long-Term Persistence and Diversification. Am. J. Bot. 2011, 98, 1887–1904. [Google Scholar] [CrossRef]
- Zozomová-Lihová, J.; Marhold, K.; Španiel, S. Taxonomy and Evolutionary History of Alyssum montanum (Brassicaceae) and Related Taxa in Southwestern Europe and Morocco: Diversification Driven by Polyploidy, Geographic and Ecological Isolation. Taxon 2014, 63, 562–591. [Google Scholar] [CrossRef]
- Dorn, K.M.; Johnson, E.B.; Daniels, E.C.; Wyse, D.L.; Marks, M.D. Spring Flowering Habit in Field Pennycress (Thlaspi arvense) Has Arisen Multiple Independent Times. Plant Direct. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- McGinn, M.; Phippen, W.B.; Chopra, R.; Bansal, S.; Jarvis, B.A.; Phippen, M.E.; Dorn, K.M.; Esfahanian, M.; Nazarenus, T.J.; Cahoon, E.B.; et al. Molecular Tools Enabling Pennycress (Thlaspi arvense) as a Model Plant and Oilseed Cash Cover Crop. Plant Biotechnol. J. 2019, 17, 776–788. [Google Scholar] [CrossRef]
- Beilstein, M.A.; Al-Shehbaz, I.A.; Mathews, S.; Kellogg, E.A. Brassicaceae Phylogeny Inferred from Phytochrome A and NdhF Sequence Data: Tribes and Trichomes Revisited. Am. J. Bot. 2008, 95, 1307–1327. [Google Scholar] [CrossRef]
- Thomas, J.B.; Hampton, M.E.; Dorn, K.M.; David Marks, M.; Carter, C.J. The Pennycress (Thlaspi arvense L.) Nectary: Structural and Transcriptomic Characterization. BMC Plant Biol. 2017, 17, 201. [Google Scholar] [CrossRef]
- Warwick, S.I.; Francis, A.; Susko, D.J. The Biology of Canadian Weeds. 9. Thlaspi arvense L.(Updated). Can. J. Plant Sci. 2002, 82, 803–823. [Google Scholar] [CrossRef]
- Levy, A.; Gonzalez, I.S.; Mittelviefhaus, M.; Clingenpeel, S.; Paredes, S.H.; Miao, J.; Wang, K.; Devescovi, G.; Stillman, K.; Monteiro, F. Genomic Features of Bacterial Adaptation to Plants. Nat. Genet. 2018, 50, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Isbell, T. Thlaspi arvense (Pennycress) as a Biodiesel in a One Year-Two Crop Rotation with Soybean. In Association for the Advancement of Industrial Crops Conference; Agricultural Research Service U.S. Department of Agriculture: Washington, DC, USA, 2008; Volume 6. [Google Scholar]
- Eberle, C.A.; Thom, M.D.; Nemec, K.T.; Forcella, F.; Lundgren, J.G.; Gesch, R.W.; Riedell, W.E.; Papiernik, S.K.; Wagner, A.; Peterson, D.H. Using Pennycress, Camelina, and Canola Cash Cover Crops to Provision Pollinators. Ind. Crop. Prod. 2015, 75, 20–25. [Google Scholar] [CrossRef]
- Groeneveld, J.H.; Klein, A.-M. Pennycress-Corn Double-Cropping Increases Ground Beetle Diversity. Biomass Bioenerg. 2015, 77, 16–25. [Google Scholar] [CrossRef]
- Johnson, G.A.; Kantar, M.B.; Betts, K.J.; Wyse, D.L. Field Pennycress Production and Weed Control in a Double Crop System with Soybean in Minnesota. Agron. J. 2015, 107, 532–540. [Google Scholar] [CrossRef]
- Thom, M.D.; Forcella, F.; Eberle, C.A.; Matthees, H.L.; Weyers, S.L.; Gesch, R.W.; Ott, M.A.; Feyereisen, G.W.; Strock, J.S.; Wyse, D. Reduced-Nutrient Leachates in Cash Cover Crop-Soybean Systems. BioRxiv 2018, 254169. [Google Scholar] [CrossRef]
- Charlot, G. Colorimetric Determination of Elements, Principles and Methods: By G. Charlot; Elsevier: Amsterdam, The Netherlands, 1964. [Google Scholar]
- Küpper, H.; Lombi, E.; Zhao, F.-J.; Wieshammer, G.; McGrath, S.P. Cellular Compartmentation of Nickel in the Hyperaccumulators Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. J. Exp. Bot. 2001, 52, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, K.; Aggarwal, P.; Chakraborty, D.; Pradhan, S.; Narayan Garg, R.; Singh, R. Practical Manual on Measurement of Soil Physical Properties Practical; Division of Agricultural Physics, Indian Agricultural Research Institute: New Delhi, India, 2012. [Google Scholar]
- Baiyeri, K.P.; Mbah, B.N. Surface Sterilization and Duration of Seed Storage Influenced Emergence and Seedling Quality of African Breadfruit (Treculia africana Decne). Afr. J. Biotechnol. 2006, 5, 1393–1396. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Method 6200: Field Portable X-ray Fluorescence Spectrometry for the Determination of Elemental Concentrations in Soil and Sediment; Environmental Protection Agency: Washington, DC, USA.
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M. PSII Fluorescence Techniques for Measurement of Drought and High Temperature Stress Signal in Crop Plants: Protocols and Applications. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer India: New Delhi, India, 2013; pp. 87–131. ISBN 978-81-322-0807-5. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis in Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bosa, K.; Kościelniak, J.; Żuk-Gołaszewska, K. Effects of Salt Stress on Photosystem II Efficiency and CO2 Assimilation of Two Syrian Barley Landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Strasser, R.J. Experimental in Vivo Measurements of Light Emission in Plants: A Perspective Dedicated to David Walker. Photosynth. Res. 2012, 114, 69–96. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P. Frequently Asked Questions about in Vivo Chlorophyll Fluorescence: Practical Issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Furbank, R.T.; Caemmerer, S.; von Sheehy, J.; Edwards, G. C4 Rice: A Challenge for Plant Phenomics. Funct. Plant Biol. 2009, 36, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.M.; Melchinger, A.E.; Reif, J.C. Novel Throughput Phenotyping Platforms in Plant Genetic Studies. Trends Plant Sci. 2007, 12, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. 2011. ImageJ; US National Institutes of Health: Bethesda, MD, USA, 1997. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).