Rapid Light-Response Curve of Chlorophyll Fluorescence in Terrestrial Plants: Relationship to CO2 Exchange among Five Woody and Four Fern Species Adapted to Different Light and Water Regimes
Abstract
1. Introduction
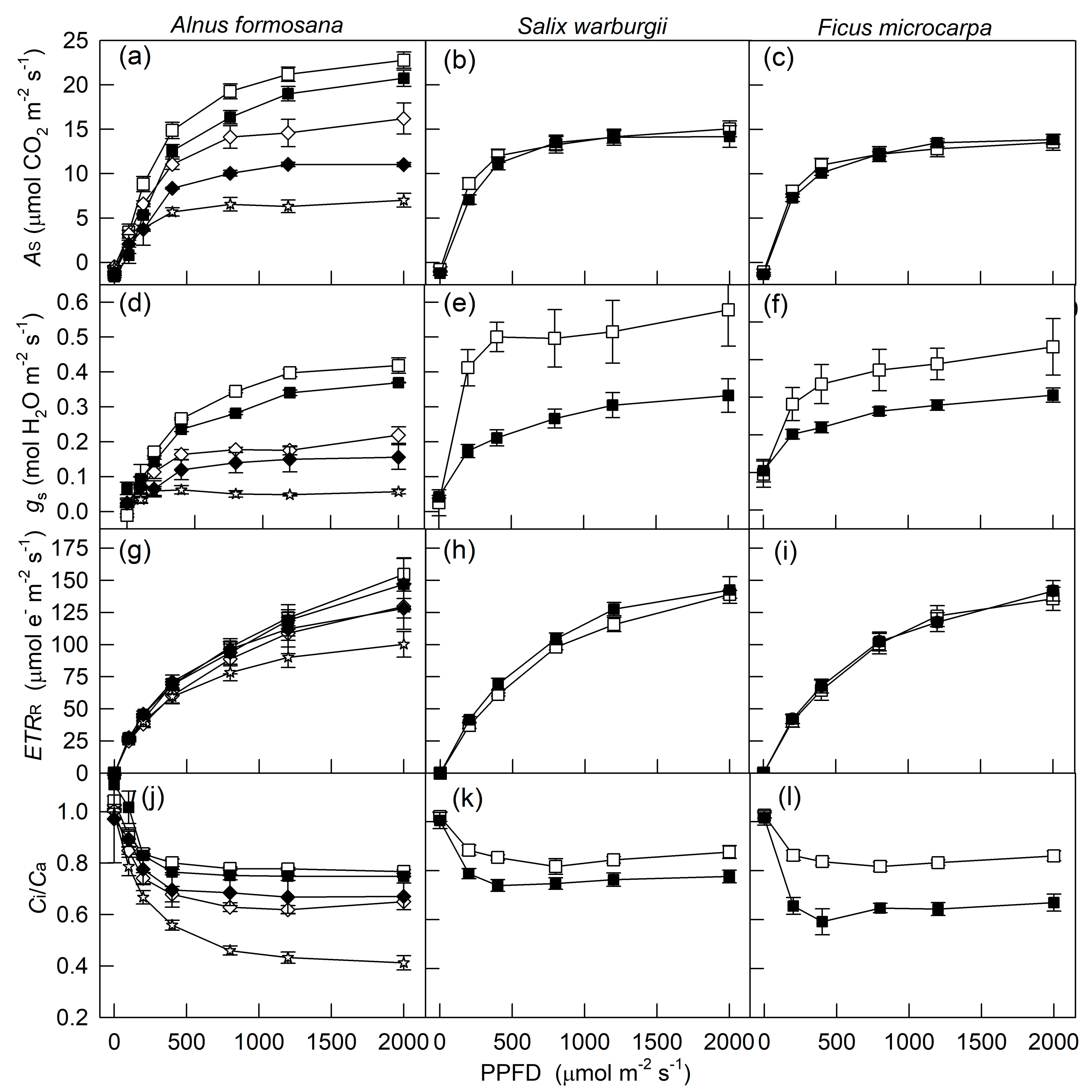
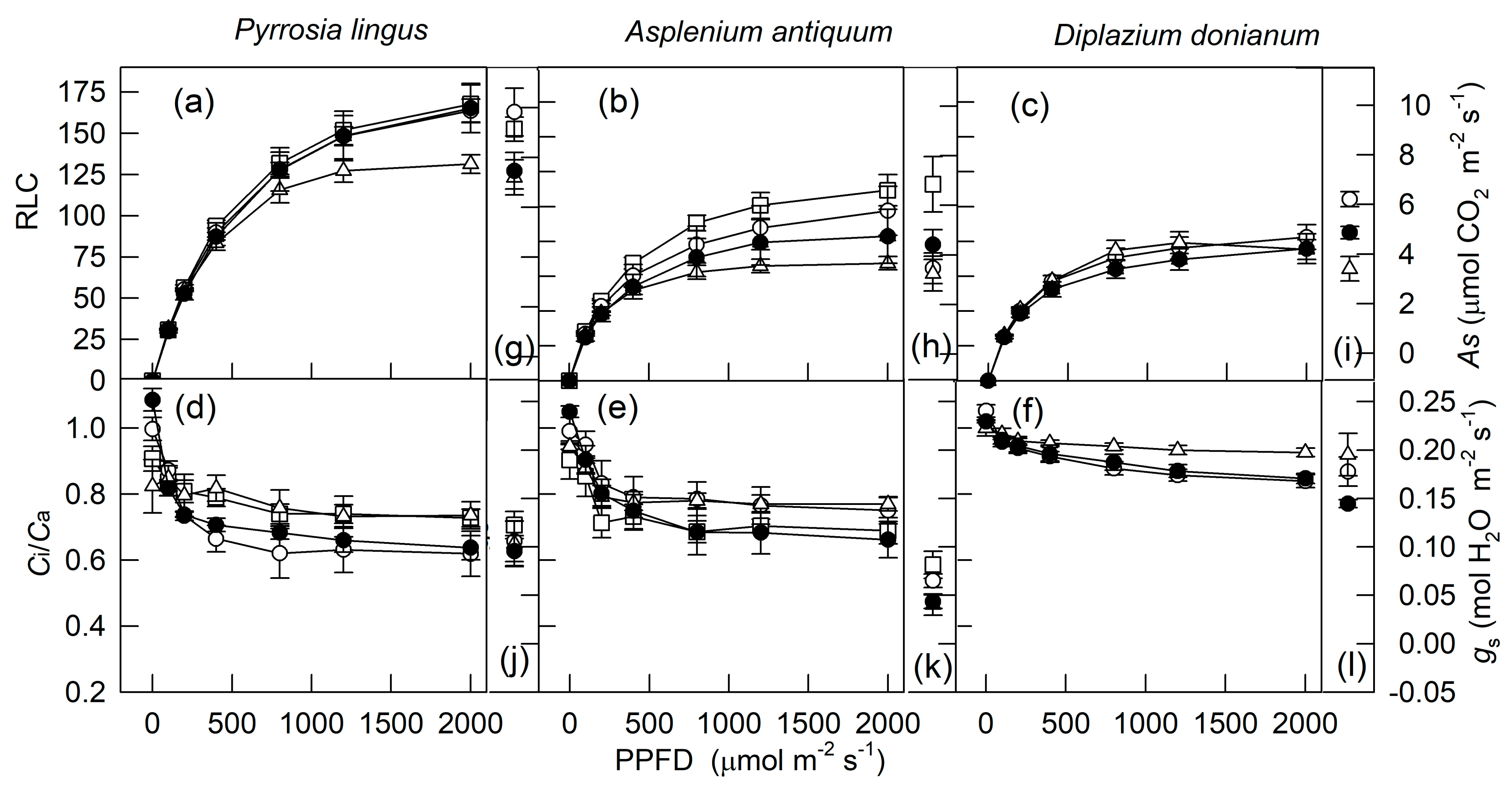
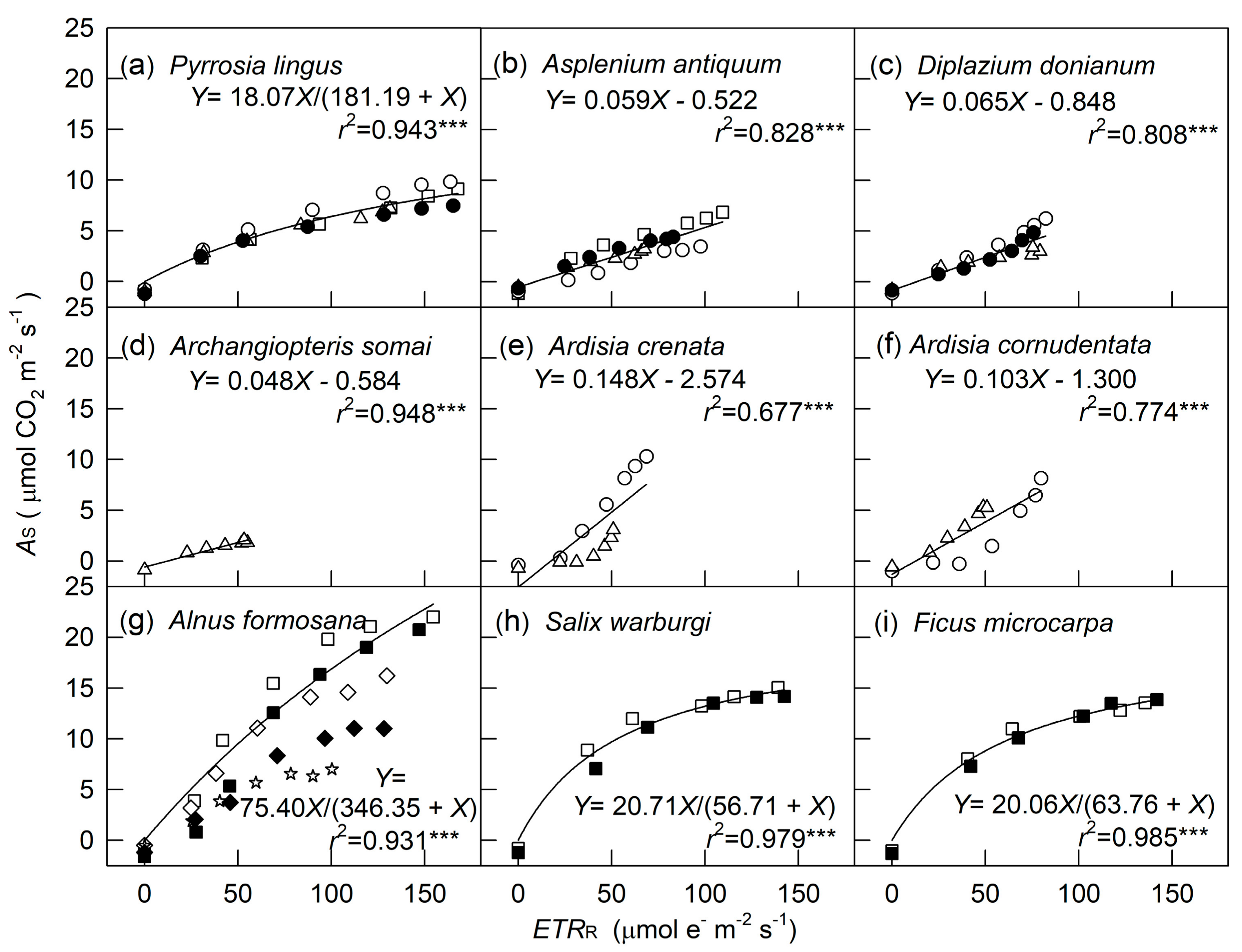
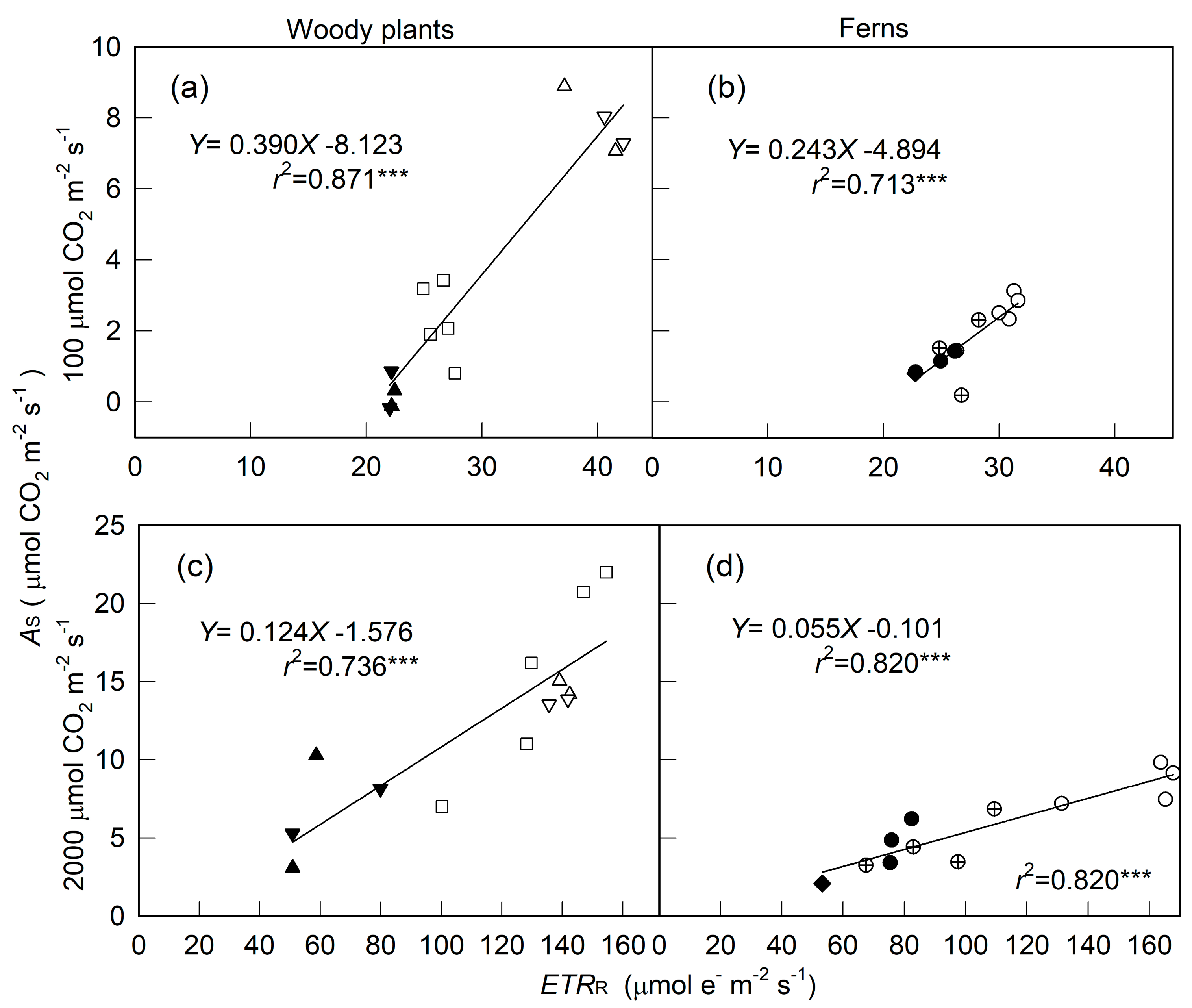
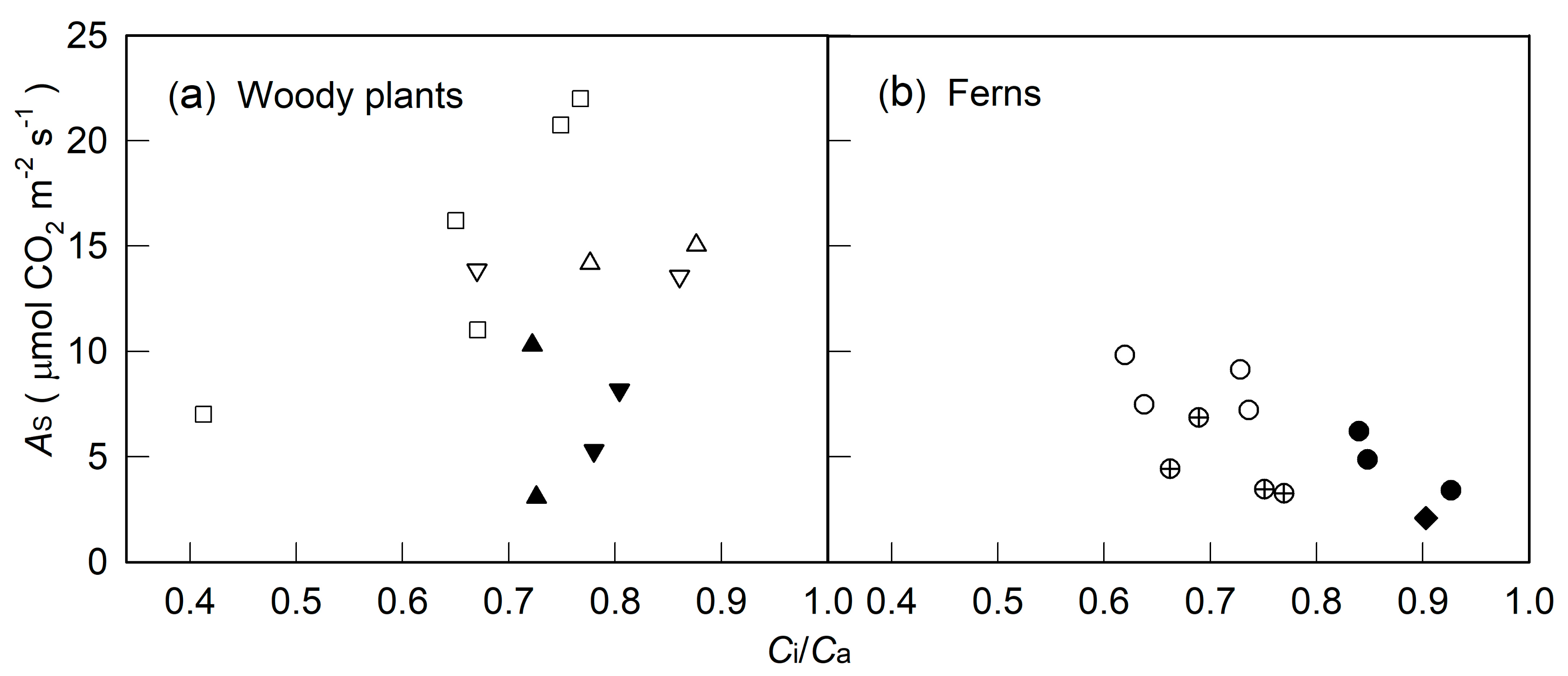
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Measurements
4.3. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Berry, J.A.; Downton, W.J.S. Environmental regulation of photosynthesis. In Photosynthesis; Govindjee, Ed.; Academic Press: London, UK, 1982; Volume 2, pp. 263–343. [Google Scholar]
- Hölscher, D.; Leuschner, C.; Bohman, K.; Hagemeier, M.; Juhrbandt, J.; Tjitrosemito, S. Leaf gas exchange of trees in old-growth and young secondary forest stands in Sulawesi, Indonesia. Trees 2006, 20, 278–285. [Google Scholar] [CrossRef]
- Wong, S.L.; Chen, C.W.; Huang, H.W.; Weng, J.H. Using combined measurements of gas exchange and chlorophyll fluorescence to investigate the photosynthetic light responses of plant species adapted to different light regimes. Photosynthetica 2012, 50, 206–214. [Google Scholar] [CrossRef]
- Coe, R.A.; Lin, H.C. Light-Response Curves in Land Plants. In Photosynthesis: Methods and Protocols; Covshoff, S., Ed.; Springer Science + Business Media: New York, NY, USA, 2018; pp. 83–93. [Google Scholar]
- Rascher, U.; Liebig, M.; Lüttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2000, 23, 1397–1405. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Roháček, K.; Barták, M. Technique of the modulated chlorophyll fluorescence: Basic concepts, useful parameters, and some applications. Photosynthetica 1999, 37, 339–363. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Krall, J.P.; Edwards, G.E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 1992, 86, 180–187. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W., III; Barker, D.H.; Logan, B.A.; Bowlong, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Kato, M.C.; Hikosaka, K.; Hirotsu, N.; Makino, A.; Hirose, T. The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in photosystem II. Plant Cell Physiol. 2003, 44, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.W., III; Zarter, C.R.; Ebbert, V.; Demmig-Adams, B. Photoprotective strategies of overwintering evergreens. BioScience 2004, 54, 41–49. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W., III. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Peterson, R.B. Regulation of electron transport in photosystems I and II in C3, C3-C4, and C4 species of Panicum in response to changing irradiance and O2 levels. Plant Physiol. 1994, 105, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Phys. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C.; Okamura, M. Cyclic electron flow within PSII protects PSII from its photoinhibition in thylakoid membranes from Spinach chloroplasts. Plant Cell Physiol. 2003, 44, 457–462. [Google Scholar] [CrossRef]
- Cheng, L.; Fuchigami, L.H.; Breen, P.J. The relationship between photosystem II efficiency and quantum yield for CO2 assimilation is not affected by nitrogen content in apple leaves. J. Exp. Bot. 2001, 52, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, E.; Bravo, L.A.; Corcuera, L.J.; Johnson, G.N. Is electron transport to oxygen an important mechanism in photoprotection? Contrasting responses from Antarctic vascular plants. Physiol. Plant. 2007, 130, 185–194. [Google Scholar] [CrossRef]
- Ripley, B.S.; Gilbert, M.E.; Ibrahim, D.G.; Osborne, C.P. Drought constraints on C4 photosynthesis: Stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata. J. Exp. Bot. 2007, 58, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Cornic, G.; Briantais, J.M. Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 1991, 183, 178–184. [Google Scholar] [CrossRef]
- Oberhuber, W.; Edwards, G.E. Temperature dependence of the linkage of quantum yield of photosystem II to CO2 fixation in C4 and C3 plants. Plant Physiol. 1993, 101, 507–512. [Google Scholar] [CrossRef]
- White, A.J.; Critchley, C. Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res. 1999, 59, 63–72. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Pleban, J.R.; Guadagno, C.R.; Mackay, D.S.; Weinig, C.; Ewers, B.E. Rapid chlorophyll a fluorescence light response curves mechanistically inform photosynthesis modeling. Plant Physiol. 2020, 183, 602–619. [Google Scholar] [CrossRef]
- Longstaff, B.J.; Kildea, T.; Runcie, J.W.; Cheshire, A.; Dennison, W.C.; Hurd, C.; Kana, T.; Raven, J.A.; Larkum, A.W.D. An in situ study of photosynthetic oxygen exchange and electron transport rate in the marine macroalga Ulva lactuca (Chlorophyta). Photosynth. Res. 2002, 74, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Carr, H.; Björk, M. A methodological comparison of photosynthetic oxygen evolution and estimated electron transport rate in tropical Ulva (Chlorophyceae) species under different light and inorganic carbon conditions. J. Phycol. 2003, 39, 1125–1131. [Google Scholar] [CrossRef]
- Lesser, M.P.; Slattery, M.; Stat, M.; Ojimi, M.; Gates, R.D.; Grottoli, A. Photoacclimatization by the coral Montastraea cavernosa in the mesophotic zone: Light, food, and genetics. Ecology 2010, 91, 990–1003. [Google Scholar] [CrossRef]
- Suggett, D.J.; Moore, C.M.; Geider, R.J. Estimating aquatic productivity from active fluorescence measurements. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Suggett, D.J., Borowitzka, M.A., Prášil, O., Eds.; Springer Science + Business Media B.V.: Dordrecht, The Netherland, 2011; pp. 103–127. [Google Scholar]
- Serôdio, J.; Vieira, S.; Cruz, S.; Coelho, H. Rapid light-response curves of chlorophyll fluorescencein microalgae: Relationship to steady-state light curves and non-photochemical quenching in benthic diatom-dominated assemblages. Photosynth. Res. 2006, 90, 29–43. [Google Scholar] [CrossRef]
- Cruz, S.; Serôdio, J. Relationship of rapid light curves of variable fluorescence to photoacclimation and non-photochemical quenching in a benthic diatom. Aquat. Bot. 2008, 88, 256–264. [Google Scholar] [CrossRef]
- Yarnold, J.; Ross, I.L.; Hankamer, B. Photoacclimation and productivity of Chlamydomonas reinhardtii grown in fluctuating light regimes which simulate outdoor algal culture conditions. Algal Res. 2016, 13, 182–194. [Google Scholar] [CrossRef]
- Houliez, E.; Lefebvre, S.; Lizon, F.; Schmitt, F.G. Rapid light curves (RLC) or non-sequential steady-state light curves (N-SSLC): Which fluorescence-based light response curve methodology robustly characterizes phytoplankton photosynthetic activity and acclimation status? Mar. Biol. 2017, 164, 175. [Google Scholar] [CrossRef]
- Liu, N.; Lin, Z.F.; Guan, L.L.; Lin, G.Z.; Peng, C.L. Light acclimation and HSO3− damage on photosynthetic apparatus of three subtropical forest species. Ecotoxicology 2009, 18, 929–938. [Google Scholar] [CrossRef]
- Liang, K.M.; Lin, Z.F.; Ren, H.; Liu, N.; Zhang, Q.M.; Wang, J.; Wang, Z.F.; Guan, L.L. Characteristics of sun- and shade-adapted populations of an endangered plant Primulina tabacum Hance. Photosynthetica 2010, 48, 494–506. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hort. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Sma-Air, S.; Ritchie, R.J. Photosynthesis in a Vanda sp orchid with photosynthetic roots. J. Plant Physiol. 2020, 251, 153187. [Google Scholar] [CrossRef] [PubMed]
- Waldhoff, D.; Furch, B.; Junk, W.J. Fluorescence parameters, chlorophyll concentration, and anatomical features as indicators for flood adaptation of an abundant tree species in Central Amazonia: Symmeria paniculata. Environ. Exp. Bot. 2002, 48, 225–235. [Google Scholar] [CrossRef]
- Li, Q.M.; Liu, B.; Wu, Y.; Zou, Z.R. Interactive effects of drought stresses and elevated CO2 concentration on photochemistry efficiency of cucumber seedlings. J. Integr. Plant Biol. 2008, 50, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Azhar, A.; Makihara, D.; Naito, H.; Asano, K.; Takagi, M.; Unoki, S.; Tomita, R.; Abbas, B.; Ehara, H. Sago palm (Metroxylon sagu Rottb.) response to drought condition in terms of leaf gas exchange and chlorophyll a fluorescence. Plant Prod. Sci. 2020, 24, 1794914. [Google Scholar] [CrossRef]
- Zheng, L.; Steppe, K.; Labeke, M.V. Spectral quality of monochromatic LED affects photosynthetic acclimation to high-intensity sunlight of Chrysanthemum and Spathiphyllum. Physiol. Plant. 2020, 169, 10–26. [Google Scholar] [CrossRef]
- Quinnell, R.; Howell, D.; Ritchie, R.J. Photosynthesis of an epiphytic resurrection fern Davallia angustata (Wall. ex Hook. & Grev.). Aust. J Bot. 2017, 65, 348–356. [Google Scholar]
- Vavasseur, A.; Raghavendra, A.S. Guard cell metabolism and CO2 sensing. New Phytol. 2005, 165, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, Y.Q.; Liu, Y.F.; Shi, P.L. Simulation of the stomatal conductance of winter wheat in response to light temperature and CO2 changes. Ann. Bot. 2004, 93, 435–441. [Google Scholar] [CrossRef]
- Huang, J.; Boerner, R.E.J.; Rebbeck, J. Ecophysiological responses of two herbaceous species to prescribed burning, alone or in combination with overstory thinning. Am. J. Bot. 2007, 94, 755–763. [Google Scholar] [CrossRef]
- Brodribb, T. Dynamics of changing intercellular CO2 concentration (ci) during drought and determination of minimum functional ci. Plant Physiol. 1996, 111, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Larcher, W. Physiological Plant Ecology, 3rd ed.; Springer: Berlin, Germany, 1995; pp. 44–46, 74–264. [Google Scholar]
- Weng, J.H.; Chien, C.T.; Chen, C.W.; Lai, X.M. Effects of osmotic and high light stresses on PSII efficiency of attached and detached leaves of three tree species adapted to different water regimes. Photosynthetica 2011, 49, 555–563. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.M. Evolution of the Stomatal Regulation of Plant Water Content. Plant Physiol. 2017, 174, 639–649. [Google Scholar] [CrossRef]
- Haworth, M.; Elliott-Kingston, C.; McElwain, J.C. Stomatal control as a driver of plant evolution. J. Exp. Bot. 2011, 62, 2419–2423. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Chen, C.W.; Huang, H.W.; Weng, J.H. Using combined measurements for comparison of light induction of stomatal conductance, electron transport rate and CO2 fixation in woody and fern species adapted to different light regimes. Tree Physiol. 2012, 32, 535–544. [Google Scholar] [CrossRef]
- Allen, M.T.; Pearcy, R.W. Stomatal behavior and photosynthetic performance under dynamic light regimes in a seasonally dry tropical rain forest. Oecologia 2000, 122, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Herlory, O.; Richard, P.; Blanchard, G. Methodology of light response curves: Application of chlorophyll fluorescence to microphytobenthic biofilms. Mar. Biol. 2007, 153, 91–101. [Google Scholar] [CrossRef]
- Gulías, J.; Flexas, J.; Abadía, A.; Medrano, H. Photosynthetic responses to water deficit in six Mediterranean sclerophyll species: Possible factors explaining the declining distribution of Rhamnus ludovici-salvatoris, an endemic Balearic species. Tree Physiol. 2002, 22, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Toma, T.; Marjenah, M. Limitation of leaf carbon gain by stomatal and photochemical processes in the top canopy of Macaranga conifera, a tropical pioneer tree. Tree Physiol. 1999, 19, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.M. Nitrite photoreduction in vivo is inhibited by oxygen. Plant Physiol. 1990, 92, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol. 2004, 136, 2443–2450. [Google Scholar] [CrossRef]
- Liao, T.S.; Weng, J.H. Ecophysiological characteristics of Taiwan alder (Alnus formosana Makino) adapted to the subtropical region. Tree Physiol. 2002, 22, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.-Y.; Wong, S.-L.; Weng, J.-H. Rapid Light-Response Curve of Chlorophyll Fluorescence in Terrestrial Plants: Relationship to CO2 Exchange among Five Woody and Four Fern Species Adapted to Different Light and Water Regimes. Plants 2021, 10, 445. https://doi.org/10.3390/plants10030445
Huang M-Y, Wong S-L, Weng J-H. Rapid Light-Response Curve of Chlorophyll Fluorescence in Terrestrial Plants: Relationship to CO2 Exchange among Five Woody and Four Fern Species Adapted to Different Light and Water Regimes. Plants. 2021; 10(3):445. https://doi.org/10.3390/plants10030445
Chicago/Turabian StyleHuang, Meng-Yuan, Shau-Lian Wong, and Jen-Hsien Weng. 2021. "Rapid Light-Response Curve of Chlorophyll Fluorescence in Terrestrial Plants: Relationship to CO2 Exchange among Five Woody and Four Fern Species Adapted to Different Light and Water Regimes" Plants 10, no. 3: 445. https://doi.org/10.3390/plants10030445
APA StyleHuang, M.-Y., Wong, S.-L., & Weng, J.-H. (2021). Rapid Light-Response Curve of Chlorophyll Fluorescence in Terrestrial Plants: Relationship to CO2 Exchange among Five Woody and Four Fern Species Adapted to Different Light and Water Regimes. Plants, 10(3), 445. https://doi.org/10.3390/plants10030445






