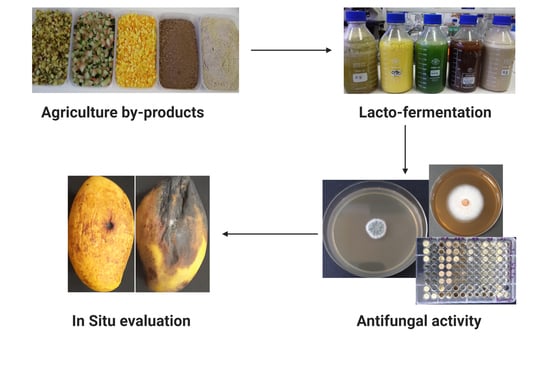
Effects of Lacto-Fermented Agricultural By-Products as a Natural Disinfectant against Post-Harvest Diseases of Mango (Mangifera indica L.)
Abstract
1. Introduction
2. Results
2.1. Antifungal Activity
2.2. pH Value and Peptide Concentration
2.3. Number of LAB in the Fermentation Mixtures
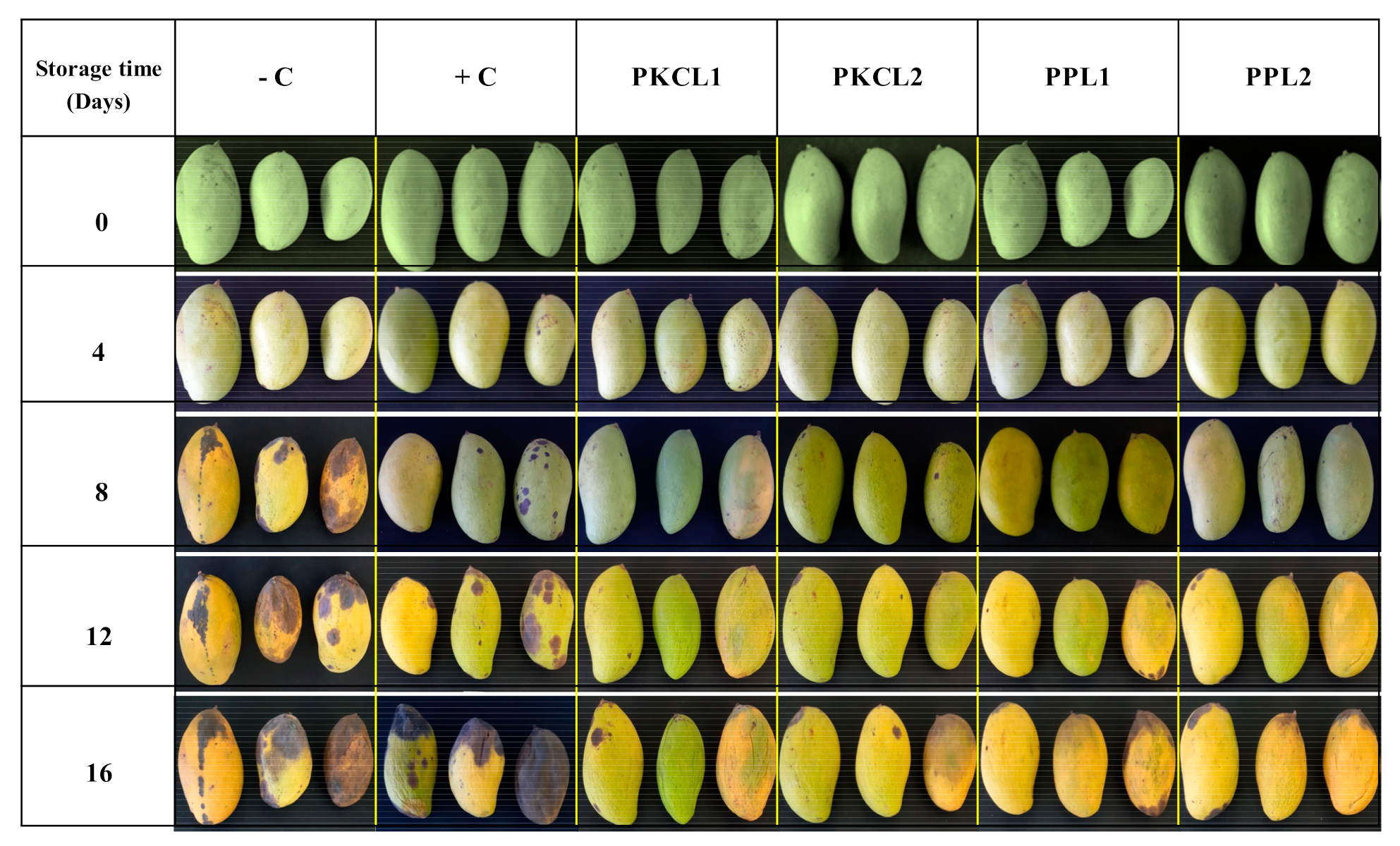
2.4. Disease Incidence
2.5. Disease Severity Index
2.6. Total Conidial Concentration
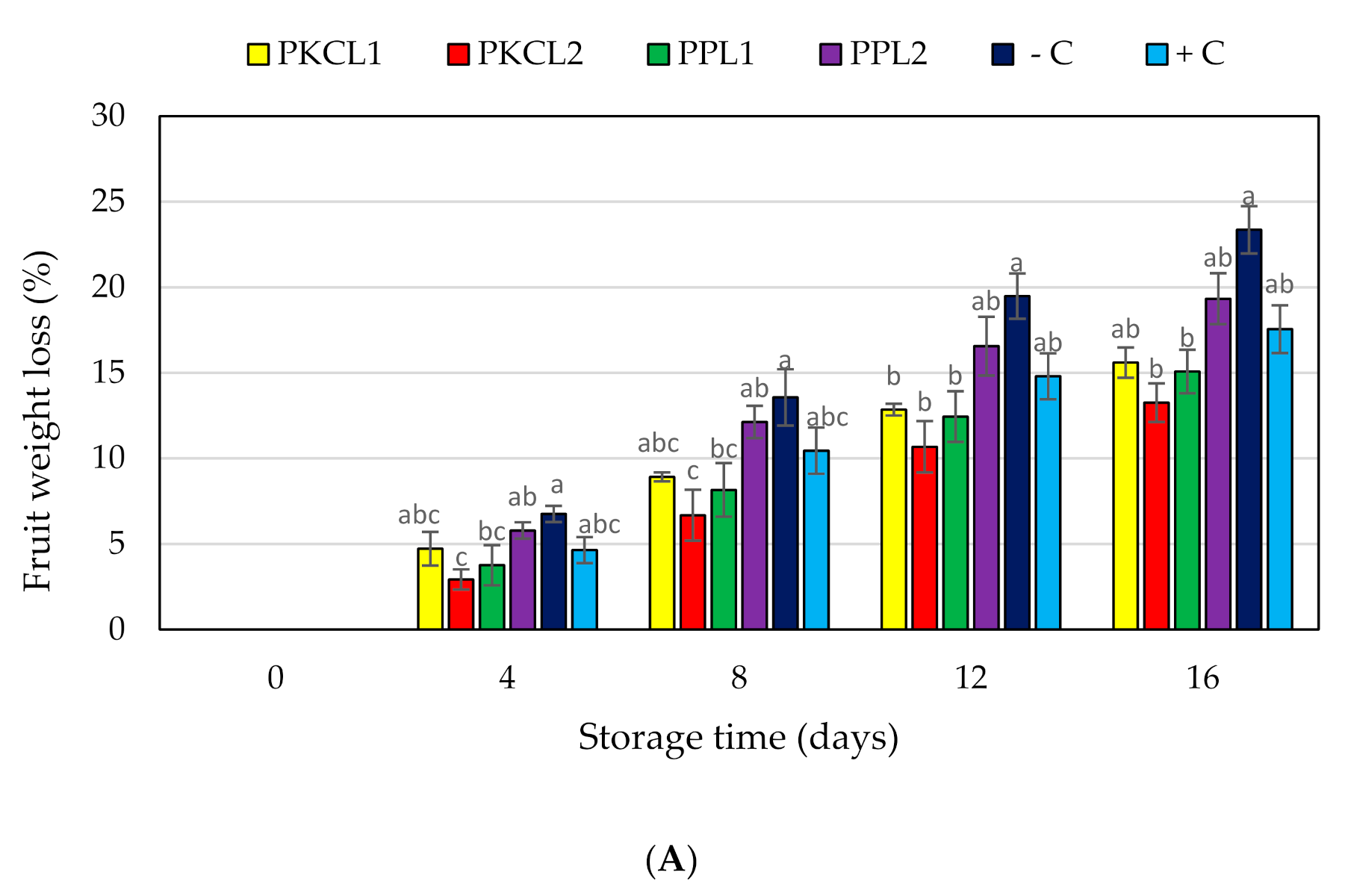
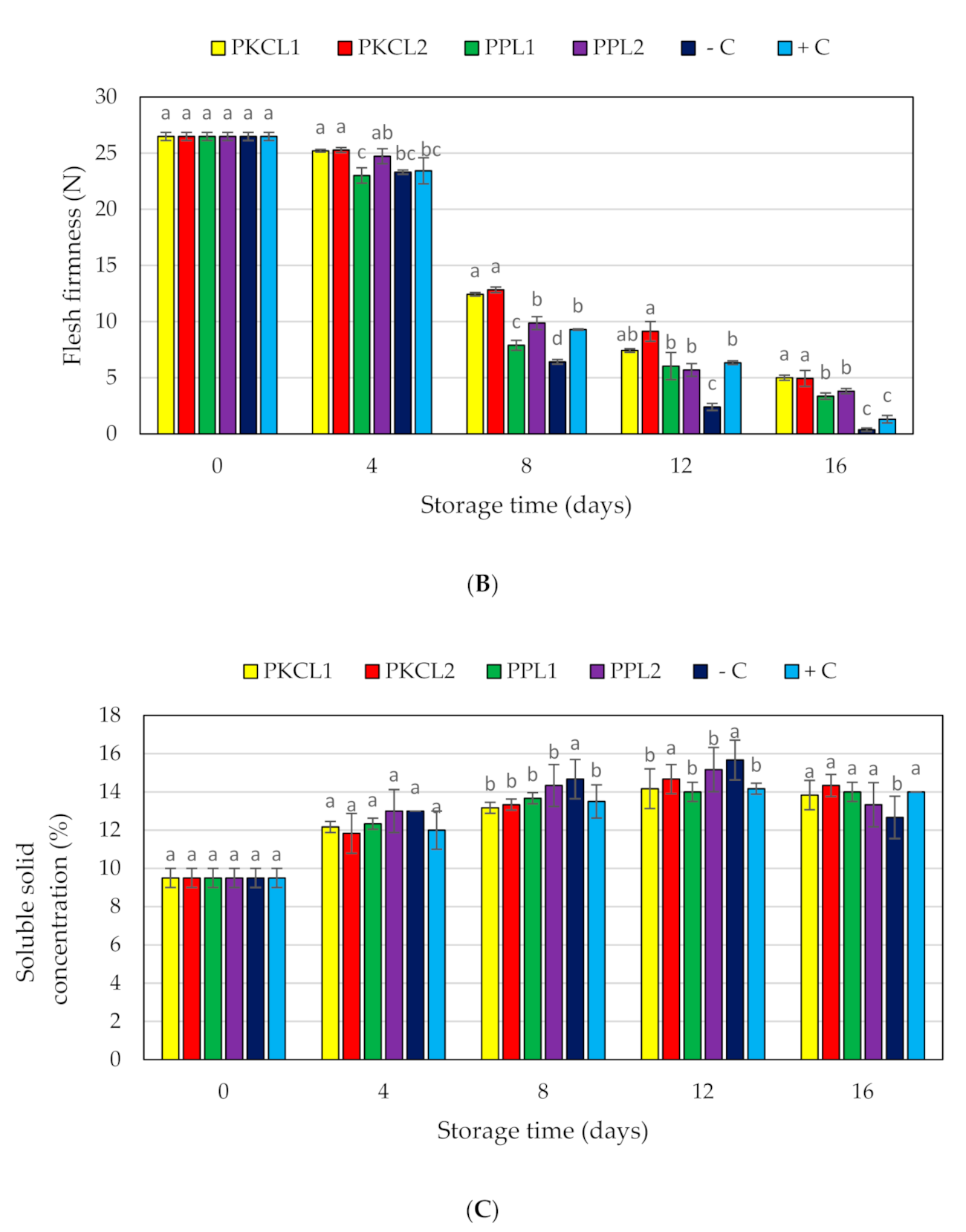
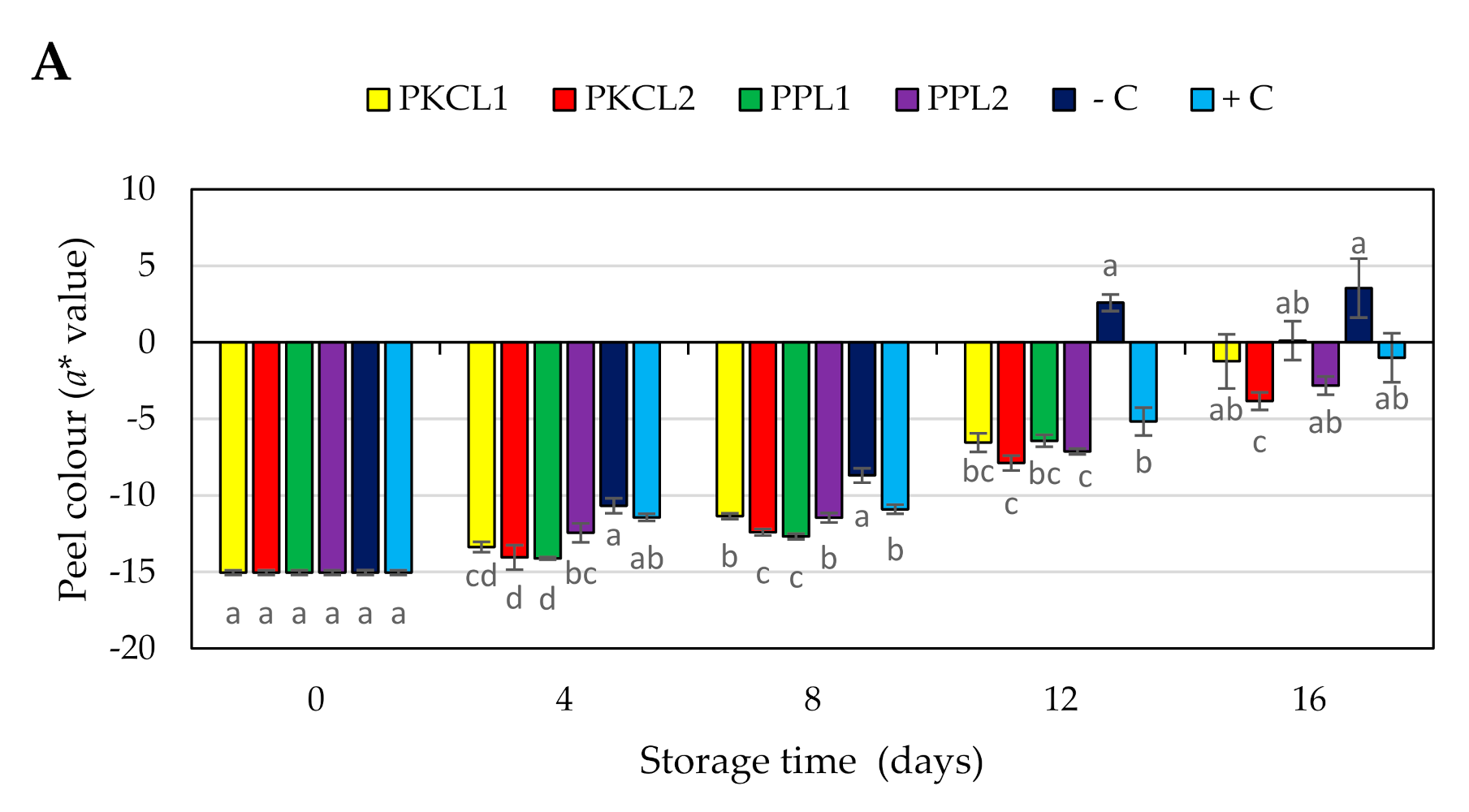
2.7. Quality Parameters for Mango
2.7.1. Fruit Weight Loss
2.7.2. Firmness
2.7.3. Total Soluble Solids
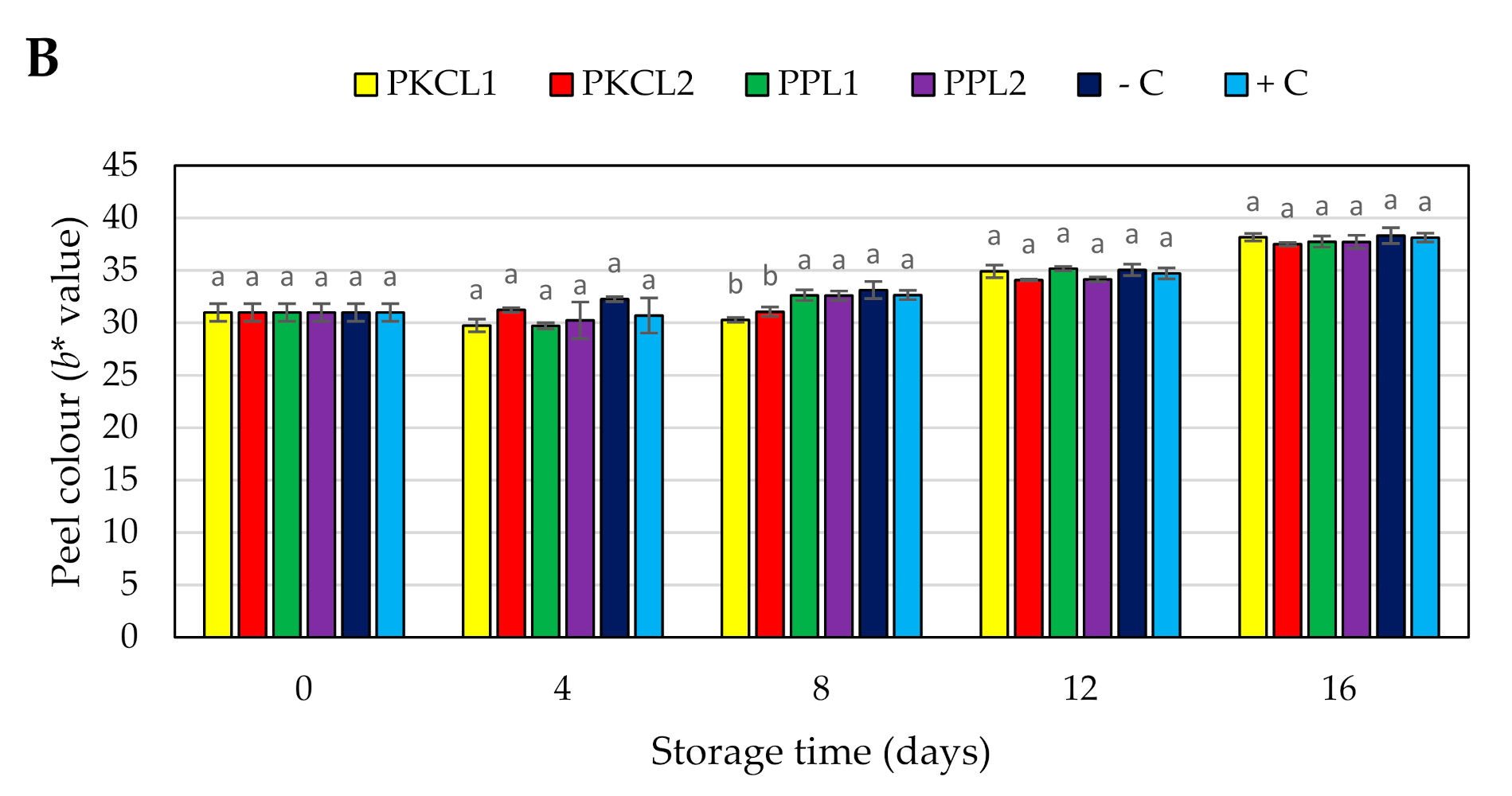
2.7.4. Peel Colour
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Culture Conditions
4.2. Substrates Preparation and Lacto-Fermentation Process
4.3. Counting of LAB in the Fermentation Mixtures
4.4. Antifungal Assays
4.4.1. Antifungal Assay in Liquid System
4.4.2. Antifungal Assay in a Solid System
4.5. In-Situ Evaluation
4.6. Disease Incidence
4.7. Disease Severity Index
- DSI = disease severity index
- Σ (a+ b+ c...) = summation score of diseased fruit
- N = total number of fruit in sample
- Z = the score of the highest diseased sample
4.8. Specific Conidial Concentration
4.9. Total Peptide Concentration
4.10. pH Value
4.11. Quality Parameters of Mango
4.11.1. Fruit Firmness
4.11.2. Total Soluble Solids
4.11.3. Weight Loss
4.11.4. Peel Colour
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brecht, J.K.; Siddiq, M.; Sidhu, J.S. (Eds.) Handbook of Mango Fruit: Production. In Postharvest Science, Processing Technology and Nutrition; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Prusky, D.; Alkan, N.; Mengiste, T.; Fluhr, R. Quiescent and necrotrophic lifestyle choice during postharvest disease development. Annu. Rev. Phytopathol. 2013, 51, 155–176. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Costa, V.S.; Michereff, S.J.; Martins, R.B.; Gava, C.A.T.; Mizubuti, E.S.G.; Câmara, M.P.S. Species of Botryosphaeriaceae associated on mango in Brazil. Eur. J. Plant Pathol. 2010, 127, 509–519. [Google Scholar] [CrossRef]
- Sivakumar, D.; Jiang, Y.; Yahia, E.M. Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 2011, 44, 1254–1263. [Google Scholar] [CrossRef]
- Palou, L. Postharvest treatments with GRAS salts to control fresh fruit decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef]
- Onaran, A.; Yanar, Y. In vivo and in vitro antifungal activities of five plant extracts against various plant pathogens. Egypt J. Biol. Pest Control 2016, 26, 405. Available online: https://www.researchgate.net/publication/305810724_In_vivo_and_In_vitro_Antifungal_Activities_of_Five_Plant_Extracts_Against_Various_Plant_Pathogens (accessed on 6 January 2021).
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Romanazzi, G.; Lichter, A.; Gabler, F.M.; Smilanick, J.L. Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2012, 63, 141–147. [Google Scholar] [CrossRef]
- Marín, A.; Plotto, A.; Atarés, L.; Chiralt, A. Lactic acid bacteria incorporated into edible coatings to control fungal growth and maintain postharvest quality of grapes. HortScience 2019, 54, 337–343. [Google Scholar] [CrossRef]
- Barrios-Roblero, C.; Rosas-Quijano, R.; Salvador-Figueroa, M.; Gálvez-López, D.; Vázquez-Ovando, A. Antifungal lactic acid bacteria isolated from beverages with activity against Colletotrichum gloeosporioides. Food Biosci. 2019, 29, 47–54. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Hassan, Z.; Bakar, F.A.; Algboory, H.L.; Saari, N. Novel antifungal peptides produced by Leuconostoc mesenteroides DU15 effectively inhibit growth of Aspergillus niger. J. Food Sci. 2015, 80, 1026–1030. [Google Scholar] [CrossRef]
- Ström, K.; Sjögren, J.; Broberg, A.; Schnürer, J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo (L-Phe-L-Pro) and cyclo (L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 2002, 68, 4322–4327. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, J.; Schnürer, J. Lactobacillus coryniformis subsp. Coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 2001, 67, 1–5. [Google Scholar] [CrossRef] [PubMed]
- El-Mabrok, A.S.W.; Hassan, Z.; Mokhtar, A.M.; Hussain, K.M.A.; Kahar, F.K.S.B.A. Screening of lactic acid bacteria as biocontrol against (Collectotrichum capsici) on chilli bangi. Res. J. Appl. Sci. 2012, 7, 446–473. [Google Scholar] [CrossRef]
- Trias, R.; Bañeras, L.; Montesinos, E.; Badosa, E. Lactic acid bacteria from fresh fruit and vegetables as biocontrol agents of phytopathogenic bacteria and fungi. Int. J. Microbiol. 2008, 11, 231. [Google Scholar] [CrossRef]
- Sathe, S.J.; Nawani, N.N.; Dhakephalkar, P.K.; Kapadnis, B.P. Antifungal lactic acid bacteria with potential to prolong shelf-life of fresh vegetables. J. Appl. Microbiol. 2007, 103, 2622–2628. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, Y.; Wang, J.; Zhang, H.; Qi, W. Production and characterization of antifungal compounds produced by Lactobacillus plantarum IMAU10014. PLoS ONE 2012, 7, e29452. [Google Scholar] [CrossRef]
- Shalini, R.; Gupta, D.K. Utilization of pomace from apple processing industries: A review. J. Food Sci. Tech. 2010, 47, 365–371. [Google Scholar] [CrossRef]
- Rodríguez Couto, S. Exploitation of biological wastes for the production of value-added products under solid-state fermentation conditions. Biotechnol. J. Healthc. Nutr. Technol. 2008, 3, 859–870. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Algboory, H.L.; Kadum, H.; Mohammed, N.K.; Saari, N.; Hassan, Z.; Hussin, A.S.M. Antifungal activity determination for the peptides generated by Lactobacillus plantarum TE10 against Aspergillus flavus in maize seeds. Food Control 2020, 109, 106898. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef]
- Ryan, L.A.; Zannini, E.; Dal Bello, F.; Pawlowska, A.; Koehler, P.; Arendt, E.K. Lactobacillus amylovorus DSM 19280 as a novel food-grade antifungal agent for bakery products. Int. J. Food Microbiol. 2011, 146, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.P.; Morgan, S.; Hill, C. Preservation and fermentation: Past, present and future. Int. J. Food Microbiol. 2002, 79, 3–16. [Google Scholar] [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Lind, H.; Jonsson, H.; Schnürer, J. Antifungal effect of dairy propionibacteria- contribution of organic acids. Int. J. Food Microbiol. 2005, 98, 157–165. [Google Scholar] [CrossRef]
- Gerez, C.L.; Carbajo, M.S.; Rollán, G.; Torres Leal, G.; Font de Valdez, G. Inhibition of citrus fungal pathogens by using lactic acid bacteria. J. Food Sci. 2010, 75, M354–M359. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Chang, H.C. Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. Int. J. Food Microbiol. 2010, 139, 56–63. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, M.; Dong, S.; Xu, J.; Song, H.; Zhao, Y. Effect of an antifungal peptide from oyster enzymatic hydrolysates for control of gray mold (Botrytis cinerea) on harvested strawberries. Postharvest Boil. Technol. 2007, 46, 95–98. [Google Scholar] [CrossRef]
- Guillén, G.; López Caballero, M.E.; Alemán, A.; López de Lacey, A.; Giménez, B.; Montero García, P. Antioxidant and Antimicrobial Peptide Fractions from Squid and Tuna Skin Gelatin; Sea By-Products as Real Material: New Ways of Application; Instituto del Frío (CSIC): Madrid, Spain, 2010; ISBN 978-81-7895-485-1. [Google Scholar]
- Ricci, A.; Bernini, V.; Maoloni, A.; Cirlini, M.; Galaverna, G.; Neviani, E.; Lazzi, C. Vegetable By-Product Lacto-Fermentation as a New Source of Antimicrobial Compounds. Microorganisms 2019, 7, 607. [Google Scholar] [CrossRef]
- Ghazvini, R.D.; Kouhsari, E.; Zibafar, E.; Hashemi, S.J.; Amini, A.; Niknejad, F. Antifungal activity and aflatoxin degradation of Bifidobacterium bifidum and Lactobacillus fermentum against toxigenic Aspergillus parasiticus. Open Microbiol. J. 2016, 10, 197. [Google Scholar] [CrossRef]
- Ogunbanwo, S.T.; Adebayo, A.A.; Ayodele, M.A.; Okanlawon, B.M.; Edema, M.O. Effects of lactic acid bacteria and Saccharomyces cerevisae co-cultures used as starters on the nutritional contents and shelf life of cassava-wheat bread. J. Appl. Biosci. 2018, 12, 612–622. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Saavedra, J.I.Q.; Chiralt, A. Antilisterial and physical properties of biopolymer films containing lactic acid bacteria. Food Control 2014, 35, 200–206. [Google Scholar] [CrossRef]
- Arulrajah, B.; Muhialdin, B.J.; Zarei, M.; Hasan, H.; Saari, N. Lacto-fermented Kenaf (Hibiscus cannabinus L.) seed protein as a source of bioactive peptides and their applications as natural preservatives. Food Control 2020, 110, 106969. [Google Scholar] [CrossRef]
- Svanström, Å.; Boveri, S.; Boström, E.; Melin, P. The lactic acid bacteria metabolite phenyllactic acid inhibits both radial growth and sporulation of filamentous fungi. BMC Res. Notes 2013, 6, 464. [Google Scholar] [CrossRef] [PubMed]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria- Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Gerez, C.L.; Torres, M.J.; De Valdez, G.F.; Rollán, G. Control of spoilage fungi by lactic acid bacteria. Biol. Control 2013, 64, 231–237. [Google Scholar] [CrossRef]
- Kwak, M.K.; Liu, R.; Kim, M.K.; Moon, D.; Kim, A.H.; Song, S.H.; Kang, S.O. Cyclic dipeptides from lactic acid bacteria inhibit the proliferation of pathogenic fungi. J. Microbiol. 2014, 52, 64–70. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Deng, L.; Ming, J.; Yao, S.; Zeng, K. Control of sour rot in citrus fruit by three insect antimicrobial peptides. Postharvest Biol. Technol. 2019, 149, 200–208. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Pastor, C.; Vargas, M.; Chiralt, A.; González-Martínez, C.; Cháfer, M. Effect of hydroxypropylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biol. Technol. 2011, 60, 57–63. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Quality of cold-stored strawberries as affected by chitosan–oleic acid edible coatings. Postharvest Boil. Technol. 2006, 41, 164–171. [Google Scholar] [CrossRef]
- Pasquali, R.C.; Taurozzi, M.P.; Bregni, C. Some considerations about the hydrophilic–lipophilic balance system. Int. J. Pharm. 2008, 356, 44–51. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Phase transitions in starch based films containing fatty acids. Effect on water sorption and mechanical behaviour. Food Hydrocoll. 2013, 30, 408–418. [Google Scholar] [CrossRef]
- Marín, A.; Atarés, L.; Cháfer, M.; Chiralt, A. Properties of biopolymer dispersions and films used as carriers of the biocontrol agent Candida sake CPA-1. LWT-Food Sci. Technol. 2017, 79, 60–69. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Algboory, H.L.; Mohammed, N.K.; Kadum, H.; Hussin, A.S.; Saari, N.; Hassan, Z. Discovery and development of novel anti-fungal peptides against food spoiling fungi. Curr. Drug Discov. Technol. 2020, 17, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Tian, M.S.; Prakash, S.; Elgar, H.J.; Young, H.; Burmeister, D.M.; Ross, G.S. Responses of strawberry fruit to 1-methylcyclopropene (1-MCP) and ethylene. Plant Growth Regul. 2000, 32, 83–90. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Ku, V.V.V. Use of 1-MCP to extend the time to ripen of green tomatoes and postharvest life of ripe tomatoes. Postharvest Biol. Technol. 2002, 26, 85–90. [Google Scholar] [CrossRef]
- Harris, D.R.; Seberry, J.A.; Wills, R.B.H.; Spohr, L.J. Effect of fruit maturity on efficiency of 1-methylcyclopropene to delay the ripening of bananas. Postharvest Boil. Technol. 2000, 20, 303–308. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K.; Sánchez-González, L.; Muneret, L.; Jeandel, C.; Hamdi, M.; Desobry, S. Alginate coatings containing grapefruit essential oil or grapefruit seed extract for grapes preservation. Int. J. Food Sci. Technol. 2014, 49, 952–959. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest technology of horticultural crops. University of California. Agric. Nat. Resour. 2002, 3311, 535. [Google Scholar]
- Wade, N.L.; Kavanagh, E.E.; Hockley, D.G.; Brady, C.J. Relationship between softening and the polyuronides in ripening banana fruit. J. Sci. Food Agric. 1992, 60, 61–68. [Google Scholar] [CrossRef]
- Marin-Rodriguez, M.C.; Smith, D.L.; Manning, K.; Orchard, J.; Seymour, G.B. Pectate lyase gene expression and enzyme activity in ripening banana fruit. Plant Mol. Biol. 2003, 51, 851–857. [Google Scholar] [CrossRef]
- Johnston, J.W.; Gunaseelan, K.; Pidakala, P.; Wang, M.; Schaffer, R.J. Co- ordination of early and late ripening events in apples is regulated through differential sensitivities to ethylene. J. Exp. Bot. 2009, 60, 2689–2699. [Google Scholar] [CrossRef] [PubMed]
- Anthon, G.E.; Sekine, Y.; Watanabe, N.; Barrett, D.M. Thermal inactivation of pectin methylesterase, polygalacturonase, and peroxidase in tomato juice. J. Agric. Food Chem. 2002, 50, 6153–6159. [Google Scholar] [CrossRef] [PubMed]
- Raviyan, P.; Zhang, Z.; Feng, H. Ultrasonication for tomato pectinmethylesterase inactivation: Effect of cavitation intensity and temperature on inactivation. J. Food Eng. 2005, 70, 189–196. [Google Scholar] [CrossRef]
- Terefe, N.S.; Gamage, M.; Vilkhu, K.; Simons, L.; Mawson, R.; Versteeg, C. The kinetics of inactivation of pectin methylesterase and polygalacturonase in tomato juice by thermosonication. Food Chem. 2009, 117, 20–27. [Google Scholar] [CrossRef]
- Juge, N. Plant protein inhibitors of cell wall degrading enzymes. Trends Plant Sci. 2006, 11, 359–367. [Google Scholar] [CrossRef]
- Cordenunsi, B.R.; Lajolo, F.M. Starch breakdown during banana ripening: Sucrose synthase and sucrose phosphate synthase. J. Agric. Food Chem. 1995, 43, 347–351. [Google Scholar] [CrossRef]
- Hofman, P.J.; Jobin-Decor, M.; Meiburg, G.F.; Macnish, A.J.; Joyce, D.D.C. Ripening and quality responses of avocado, custard apple, mango and papaya fruit to 1-methylcyclopropene. Aust. J. Exp. Agric. 2001, 41, 567–572. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Terras, F.R.; Cammue, B.P.; Vanderleyden, J. An automated quantitative assay for fungal growth inhibition. FEMS Microbiol. Lett. 1990, 69, 55–59. [Google Scholar] [CrossRef]
- Abril, M.; Curry, K.J.; Smith, B.J.; Wedge, D.E. Improved micro assays used to test natural product-based and conventional fungicides on plant pathogenic fungi. Plant Dis. 2008, 92, 106–112. [Google Scholar] [CrossRef][Green Version]
- Erukainure, O.L.; Oke, O.V.; Daramola, A.O.; Adenekan, S.O.; Umanhonlen, E.E. Improvement of the biochemical properties of watermelon rinds subjected to Saccharomyces cerevisae solid media fermentation. Pak. J. Nutr. 2010, 9, 806–809. [Google Scholar] [CrossRef]
- Wehr, H.M.; Frank, J.F. (Eds.) Standard Methods for the Examination of Dairy Products; American Public Health Association: Washington, DC, USA, 2004; pp. 327–404. [CrossRef]
- Lavermicocca, P.; Valerio, F.; Visconti, A. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl. Environ. Microbiol. 2003, 69, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.S.; Abu Ghdeib, S.I. Antifungal activity of plant extracts against dermatophytes. Mycoses 1999, 42, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Thokchom, R.; Mandal, G. Effect of postharvest treatments on physical characteristics of mango. Int. J. Pharmacogn. Phytochem. 2019, 8, 2239–2243. [Google Scholar]
- Xu, D.; Qin, H.; Ren, D. Prolonged preservation of tangerine fruits using chitosan/montmorillonite composite coating. Postharvest Biol. Technol. 2018, 143, 50–57. [Google Scholar] [CrossRef]
- Akhtar, K.P.; Alam, S.S. Assessment keys for some important diseases of mango. Pak. J. Biol. Sci. 2002, 5, 246–250. [Google Scholar] [CrossRef]
- Campos-M, M.; Campos-C, R. Applications of quartering method in soils and foods. Int. J. Eng. Res. Appl. 2017, 7, 35–39. [Google Scholar] [CrossRef]
- Goodno, C.C.; Swaisgood, H.E.; Catignani, G.L. A fluorimetric assay for available lysine in proteins. Anal. Biochem. 1981, 115, 203–211. [Google Scholar] [CrossRef]

| Factors | Growth Inhibition (%) | ||||
|---|---|---|---|---|---|
| Colletotrichum | Botryodiplodia | Aspergillus | Aspergillus | Aspergillus | |
| Gloeosporioides | Theobromae | Niger | Flavus | Variecolor | |
| Liquid System | |||||
| LAB strains (L) | |||||
| Control | 4.30 d | 4.71 c | 1.48 c | 4.07 c | 4.74 d |
| L. plantarum ATCC8014 | 49.55 b | 46.26 a | 2.26 b,c | 47.02 a,b | 34.54 b |
| L. fermentum ATCC9338 | 62.84 a | 50.46 a | 11.79 a | 44.17 b | 40.57 a |
| L. casei ATCC33 | 38.91 c | 43.70 a | 2.96 b,c | 50.95 a,b | 38.34 a,b |
| L. plantarum CF3 | 38.99 c | 33.23 b | 8.28 a,b | 44.02 b | 42.01 a |
| L. plantarum Tempe37 | 31.64 c | 28.67 b | 11.81 a | 53.80 a | 17.00 c |
| LSD at α 0.05 | 9.35 | 8.95 | 8.63 | 8.9 | 4.87 |
| Substrates (S) | |||||
| WR | 28.06 b | 32.08 b | 3.29 b | 37.85 b | 18.79 d |
| OP | 24.66 b | 20.19 c | 5.44 a,b | 32.46 b | 25.11 c |
| PP | 51.61 a | 49.44 a | 10.93 a | 45.80 a | 34.51 a,b |
| PKC | 56.34 a | 50.55 a | 12.53 a | 50.94 a | 31.45 b |
| RB | 27.86 b | 20.27 c | 12.15 a | 36.33 b | 37.79 a |
| LSD at α 0.05 | 8.16 | 7.8 | 7.53 | 7.77 | 4.25 |
| Interaction | |||||
| L × S | ** | ** | ** | ** | ** |
| Solid System | |||||
| LAB strains (L) | |||||
| Control | 6.15 f | 7.14 e | 2.11 d | 5.93 f | 6.08 d |
| L. plantarum ATCC8014 | 61.49 a | 63.32 a | 9.89 b | 40.31 c | 11.53 c |
| L. fermentum ATCC9338 | 58.79 b | 47.68 b | 20.49 a | 31.15 d | 36.62 a |
| L. casei ATCC33 | 46.76 c | 40.24 c | 7.09 b | 55.53 b | 28.82 b |
| L. plantarum CF3 | 50.87 b | 34.90 d | 19.18 a | 34.08 d | 43.31 a |
| L. plantarum Tempe37 | 35.05 d | 49.25 b | 19.47 a | 63.32 a | 7.07 c |
| LSD at α 0.05 | 3.38 | 3.57 | 3.17 | 3.39 | 4.89 |
| Substrates (S) | |||||
| WR | 35.06 c | 53.34 b | 13.26 b | 56.19 b | 13.05 c |
| OP | 32.17 c | 40.02 c | 34.58 a | 29.98 d | 33.08 a |
| PP | 68.21 a | 52.09 b | 7.73 d | 62.90 a | 24.53 b |
| PKC | 66.61 a | 63.17 a | 11.70 b,c | 49.89 c | 16.92 c |
| RB | 50.39 b | 26.77 d | 8.87 c,d | 25.42 e | 39.78 a |
| LSD at α 0.05 | 3.38 | 3.59 | 3.17 | 3.39 | 6.89 |
| Interaction | |||||
| L × S | ** | ** | ** | ** | ** |
| Factors | pH | Peptide Concentration (µg mL−1) |
|---|---|---|
| LAB strains (L) | ||
| L. plantarum ATCC8014 | 3.62 e | 113.32 b |
| L. fermentum ATCC9338 | 3.63 e | 117.64 a |
| L. casei ATCC33 | 3.73 d | 97.21 c |
| L. plantarum CF3 | 3.85 c | 93.08 d |
| L. plantarum Tempe37 | 4.02 b | 81.19 e |
| Sterile substrates | 4.88 a | 12.75 f |
| LSD at α 0.05 | 0.07 | 2.48 |
| Substrates (S) | ||
| WR | 3.85 b | 72.93 c |
| OP | 4.26 a | 66.49 d |
| PP | 3.63 d | 91.43 b |
| PKC | 3.77 c | 107.02 a |
| RB | 4.25 a | 91.45 b |
| LSD at α 0.05 | 0.06 | 2.16 |
| Interaction | ||
| L × S | ** | ** |
| Factors | LAB Counts (log 10 CFU mL−1) |
|---|---|
| LAB strains (L) | |
| L. plantarum ATCC8014 | 5.90 a |
| L. fermentum ATCC9338 | 5.90 a |
| L. casei ATCC33 | 5.89 a |
| L. plantarum CF3 | 5.91 a |
| L. plantarum Tempe37 | 5.90 a |
| LSD at α 0.05 | 0.021 |
| Substrates (S) | |
| MRS broth -control | 6.03 a |
| PKC | 5.98 b |
| PP | 5.96 b |
| OP | 5.71 e |
| WR | 5.92 c |
| RB | 5.81 d |
| LSD at α 0.05 | 0.023 |
| Fermentation times (T) | |
| 0 h | 4.54 d |
| 24 h | 5.83 c |
| 48 h | 6.05 b |
| 72 h | 7.19 a |
| LSD at α 0.05 | 0.018 |
| Interaction | |
| L × S | ns |
| L × T | ns |
| S × T | ** |
| L × S × T | ns |
| Treatments | Total Conidial Concentration (log10 CFU mL−1) | Disease Severity Index (%) |
|---|---|---|
| PKCL1 | 5.41 ± 0.06 c | 13.3 ± 7.6 c |
| PKCL2 | 5.32 ± 0.03 d | 20.0 ± 17.3 b,c |
| PPL1 | 5.59 ± 0.02 b | 31.7 ± 25.6 a,b,c |
| PPL2 | 5.54 ± 0.02 b | 33.3 ± 10.4 a,b,c |
| C− | 6.74 ± 0.01 a | 80.0 ± 19.1 a |
| C+ | 5.54 ± 0.02 b | 70.0 ± 26.4 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranjith, F.H.; Muhialdin, B.J.; Yusof, N.L.; Mohammed, N.K.; Miskandar, M.H.; Hussin, A.S.M. Effects of Lacto-Fermented Agricultural By-Products as a Natural Disinfectant against Post-Harvest Diseases of Mango (Mangifera indica L.). Plants 2021, 10, 285. https://doi.org/10.3390/plants10020285
Ranjith FH, Muhialdin BJ, Yusof NL, Mohammed NK, Miskandar MH, Hussin ASM. Effects of Lacto-Fermented Agricultural By-Products as a Natural Disinfectant against Post-Harvest Diseases of Mango (Mangifera indica L.). Plants. 2021; 10(2):285. https://doi.org/10.3390/plants10020285
Chicago/Turabian StyleRanjith, Fernando H., Belal J. Muhialdin, Noor L. Yusof, Nameer K. Mohammed, Muhammad H. Miskandar, and Anis Shobirin Meor Hussin. 2021. "Effects of Lacto-Fermented Agricultural By-Products as a Natural Disinfectant against Post-Harvest Diseases of Mango (Mangifera indica L.)" Plants 10, no. 2: 285. https://doi.org/10.3390/plants10020285
APA StyleRanjith, F. H., Muhialdin, B. J., Yusof, N. L., Mohammed, N. K., Miskandar, M. H., & Hussin, A. S. M. (2021). Effects of Lacto-Fermented Agricultural By-Products as a Natural Disinfectant against Post-Harvest Diseases of Mango (Mangifera indica L.). Plants, 10(2), 285. https://doi.org/10.3390/plants10020285