Effect of Herbicides on the Management of the Invasive Weed Solanum rostratum Dunal (Solanaceae)
Abstract
1. Introduction
2. Results
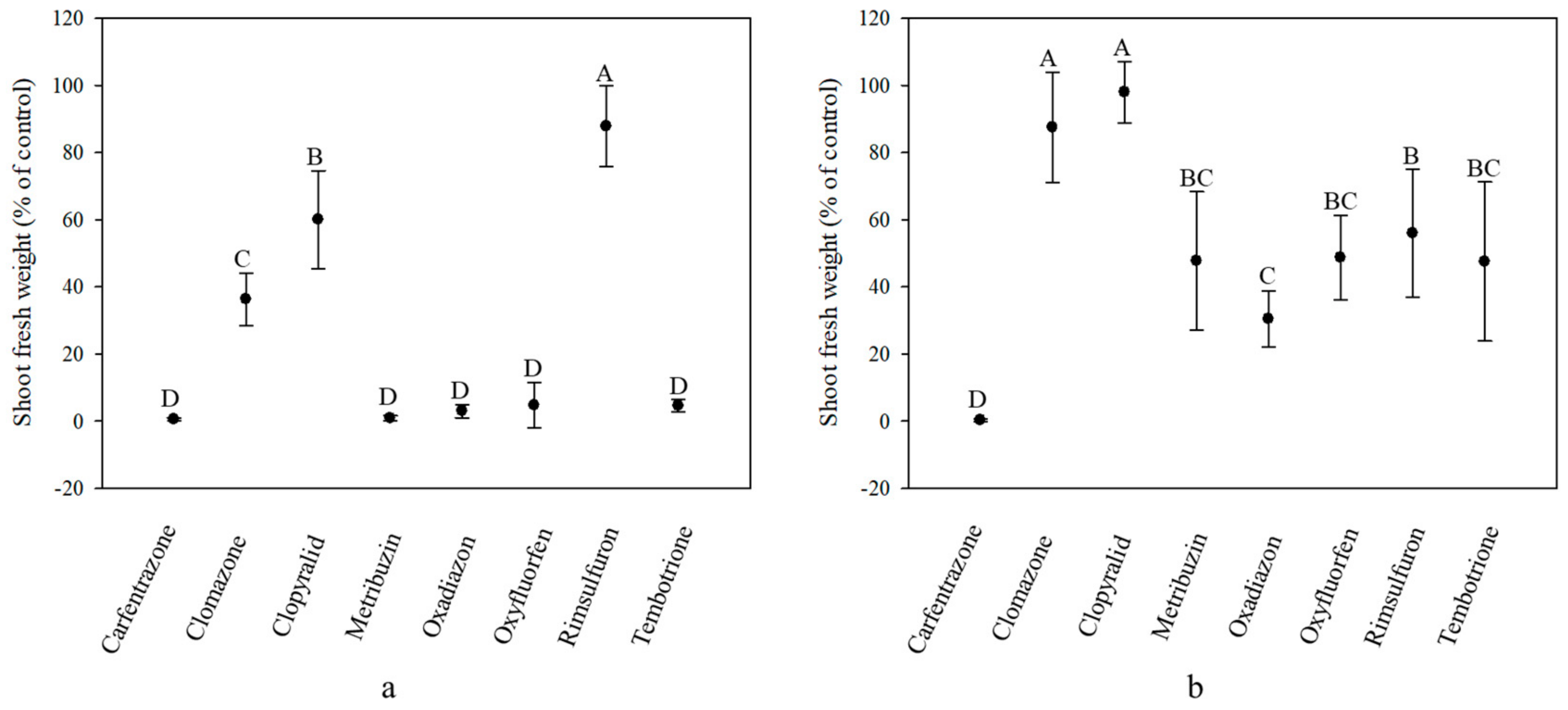
2.1. Herbicide Response of Ginegar (GO) Plants Treated at Different Growth Stages
2.2. Herbicide Response of Givat Yoav (GY) Plants Treated at Different Growth Stages
2.3. Herbicide Effect on Plants Treated at Different Growth Stage
2.4. Variation in Herbicide Response among Populations
2.5. Synergism of the Surfactant with PPO Inhibitors
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Herbicides Application
4.3. Surfactant Effect
4.4. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frodin, D.G. History and concepts of big plant genera. Taxon 2004, 53, 753–776. [Google Scholar] [CrossRef]
- Knapp, S.; Vorontsova, M.S.; Särkinen, T. Dichotomous keys to the species of Solanum L. (Solanaceae) in continental Africa, Madagascar (incl. the Indian Ocean islands), Macaronesia and the Cape Verde Islands. PhytoKeys 2019, 127, 39–76. [Google Scholar] [CrossRef]
- Bryson, C.T.; Reddy, K.N.; Byrd, J.D. Growth, development, and morphological differences among native and nonnative prickly nightshades (Solanum spp.) of the southeastern United States. Invasive Plant Sci. Manag. 2012, 5, 341–352. [Google Scholar] [CrossRef]
- Danin, A. Flora of Israel Online. Available online: http://www.flora.huji.ac.il (accessed on 14 January 2021).
- Bassett, I.J.; Munro, D.B. The biology of Canadian weeds. 78. Solunum carolinense L. and Solanum rostratum Dunal. Can. J. Plant Sci. 1986, 66, 977–991. [Google Scholar] [CrossRef]
- Zhao, J.; Solís-Montero, L.; Lou, A.; Vallejo-Marín, M. Population structure and genetic diversity of native and invasive populations of Solanum rostratum (Solanaceae). PLoS ONE 2013, 8, e79807. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Alessandrini, A.; Arrigoni, P.V.; Banfi, E.; Bernardo, L.; Bovio, M.; Brundu, G.; Cagiotti, M.R.; Camarda, I.; Carli, E.; et al. Inventory of the non-native flora of Italy. Plant Biosyst. 2009, 143, 386–430. [Google Scholar] [CrossRef]
- Rushing, D.W.; Murray, D.S.; Verhalen, L.M. Verhalen. Weed interference with cotton (Gossypium hirsutum). I. Buffalobur (Solanum rostratum). Weed Sci. 1985, 33, 810–814. [Google Scholar] [CrossRef]
- Lyon, D.J.; Kniss, A.; Miller, S.D. Carfentrazone improves broadleaf weed control in proso and foxtail millets. Weed Technol. 2007, 21, 84–87. [Google Scholar] [CrossRef]
- Salas, R.A.; Burgos, N.R.; Tranel, P.J.; Singh, S.; Glasgow, L.; Scott, R.C.; Nichols, R.L. Resistance to PPO-inhibiting herbicide in Palmer amaranth from Arkansas. Pest Manag. Sci. 2016, 72, 864–869. [Google Scholar] [CrossRef]
- Rangani, G.; Salas-Perez, R.A.; Aponte, R.A.; Knapp, M.; Craig, I.R.; Mietzner, T.; Langaro, A.C.; Noguera, M.M.; Porri, A.; Roma-Burgos, N. A novel single-site mutation in the catalytic domain of protoporphyrinogen oxidase ix (PPO) confers resistance to PPO-inhibiting herbicides. Front. Plant Sci. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Jensen, P.K. Tolerance to foliage-applied herbicides in combining peas: Effect of growth stage, cultivar type and herbicide. Crop Prot. 1993, 12, 214–218. [Google Scholar] [CrossRef]
- Falk, J.S.; Shoup, D.E.; Al-Khatib, K.; Peterson, D.E. Protox-resistant common waterhemp (Amaranthus rudis) response to herbicides applied at different growth stages. Weed Sci. 2006, 54, 793–799. [Google Scholar] [CrossRef]
- Hess, F.D.; Foy, C.L. Interaction of surfactants with plant cuticles. Weed Tech. 2000, 14, 807–813. [Google Scholar] [CrossRef]
- Matzrafi, M.; Herrmann, I.; Nansen, C.; Kliper, T.; Zait, Y.; Ignat, T.; Siso, D.; Rubin, B.; Karnieli, A.; Eizenberg, H. Hyperspectral technologies for assessing seed germination and trifloxysulfuron-methyl response in Amaranthus palmeri (palmer amaranth). Front. Plant Sci. 2017, 8, 474. [Google Scholar] [CrossRef]
- Iwakami, S.; Uchino, A.; Kataoka, Y.; Shibaike, H.; Watanabe, H.; Inamura, T. Cytochrome P450 genes induced by bispyribac-sodium treatment in a multiple-herbicide-resistant biotype of Echinochloa phyllopogon. Pest Manag. Sci. 2014, 70, 549–558. [Google Scholar] [CrossRef]
- Riechers, D.E.; Wax, L.M.; Liebl, R.A.; Bush, D.R. Surfactant-increased glyphosate uptake into plasma membrane vesicles isolated from common lambsquarters leaves. Plant Physiol. 1994, 105, 1419–1425. [Google Scholar] [CrossRef]
- Kirkwood, R.C.; Hetherington, R.; Reynolds, T.L.; Marshall, G. Absorption, localisation, translocation and activity of glyphosate in barnyardgrass (Echinochloa crus-galli (L) Beauv): Influence of herbicide and surfactant concentration. Pest Manag. Sci. 2000, 56, 359–367. [Google Scholar] [CrossRef]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Koch, H.J.; Trimpler, K.; Jacobs, A.; Stockfisch, N. Crop rotational effects on yield formation in current sugar beet production—Results from a farm survey and field trials. Front. Plant Sci. 2018, 9, 231. [Google Scholar] [CrossRef]
- Davis, V.M.; Gibson, K.D.; Mock, V.A.; Johnson, W.G. In-field and soil-related factors that affect the presence and prediction of glyphosate-resistant horseweed (Conyza canadensis) populations collected from indiana soybean fields. Weed Sci. 2009, 57, 281–289. [Google Scholar] [CrossRef]
- Doucet, C.; Weaver, S.E.; Hamill, A.S.; Zhang, J. Separating the effects of crop rotation from weed management on weed density and diversity. Weed Sci. 1999, 47, 729–735. [Google Scholar] [CrossRef]
- Rubin, B.; Matzrafi, M. Weed management in israel-challenges and approaches. In Weed Science in the Asian-Pacific Region; Indian Society of Weed Science: Jabalpur, India, 2015; pp. 253–270. [Google Scholar]
- Shalimu, D.; Qiu, J.; Tan, D.; Baskin, C.C.; Baskin, J.M. Seed biology of the invasive species buffalobur (Solanum rostratum) in Northwest China. Weed Sci. 2012, 60, 219–224. [Google Scholar] [CrossRef]


| Mean Shoot Fresh Weight (% of Control) | ||||
|---|---|---|---|---|
| Population a | Herbicide b | 4–5 cm c | 8–9 cm | p-Value |
| GY | Carfentrazone-ethyl | 0.57 | 0.32 | 0.2567 |
| Clomazone | 36.34 | 87.56 | <0.0001 | |
| Clopyralid | 60.09 | 98.00 | <0.0001 | |
| Metribuzin | 0.89 | 47.75 | <0.0001 | |
| Oxadiazon | 3.01 | 30.44 | <0.0001 | |
| Oxyfluorfen | 4.77 | 48.75 | <0.0001 | |
| Rimsulfuron | 87.84 | 56.00 | 0.0008 | |
| Tembotrione | 4.61 | 47.56 | <0.0001 | |
| GO | Carfentrazone-ethyl | 0.22 | 0.19 | 0.8502 |
| Clomazone | 79.52 | 103.34 | 0.0084 | |
| Clopyralid | 42.54 | 88.89 | <0.0001 | |
| Metribuzin | 6.42 | 75.93 | <0.0001 | |
| Oxadiazon | 5.65 | 39.69 | <0.0001 | |
| Oxyfluorfen | 2.76 | 33.13 | <0.0001 | |
| Rimsulfuron | 93.83 | 110.08 | 0.0134 | |
| Tembotrione | 10.76 | 75.31 | <0.0001 | |
| Treatment a | Mean (SE) b | Lower 95% | Upper 95% | Survival (%) |
|---|---|---|---|---|
| 0.5% surfactant | 103.40 (7.30) a | 84.65 | 122.16 | 100% |
| Control | 102.14 (5.51) a | 89.67 | 114.61 | 100% |
| 0.25% surfactant | 94.04 (9.04) ab | 71.92 | 116.16 | 100% |
| 1% surfactant | 65.50 (4.89) b | 53.52 | 77.48 | 100% |
| Oxadiazon + 1% surfactant | 28.00 (8.01) c | 9.87 | 46.13 | 70% |
| Oxadiazon + 0.25% surfactant | 26.18 (6.41) c | 11.69 | 40.68 | 80% |
| Oxifluorfen | 25.54 (9.73) c | 2.54 | 48.54 | 50% |
| Oxadiazon | 19.38 (7.26) c | 2.94 | 35.81 | 50% |
| Oxadiazon + 0.5% surfactant | 9.84 (3.54) c | 1.84 | 17.85 | 40% |
| Oxifluorfen + 0.5% surfactant | 6.57 (4.42) c | −3.63 | 16.77 | 30% |
| Oxifluorfen + 0.25% surfactant | 4.10 (4.08) c | −5.14 | 13.34 | 20% |
| Oxifluorfen + 1% surfactant | 3.38 (3.36) c | −4.37 | 11.13 | 20% |
| Common Name | Trade Name | MOA a | Manufacturer | Rate (g ai ha−1) |
|---|---|---|---|---|
| Oxyfluorfen | Galigan® | PPO | ADAMA-Agan | 352.5 |
| Carfentrazone-ethyl | Spotlight® | PPO | FMC | 0.9 |
| Oxadiazon | Ronstar® | PPO | Bayer | 875 |
| Clopyralid | Lontrel® | Auxinic herbicide | Corteva | 50 |
| Clomazone | Comand® | Carotenoid biosynthesis | FMC | 540 |
| Tembotrione | Laudis® | HPPD | Bayer | 99 |
| Metribuzin | Sencor® | PSII | Bayer | 175 |
| Rimsulfuron | Titus® | ALS | Corteva | 25 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Nassar, J.; Matzrafi, M. Effect of Herbicides on the Management of the Invasive Weed Solanum rostratum Dunal (Solanaceae). Plants 2021, 10, 284. https://doi.org/10.3390/plants10020284
Abu-Nassar J, Matzrafi M. Effect of Herbicides on the Management of the Invasive Weed Solanum rostratum Dunal (Solanaceae). Plants. 2021; 10(2):284. https://doi.org/10.3390/plants10020284
Chicago/Turabian StyleAbu-Nassar, Jackline, and Maor Matzrafi. 2021. "Effect of Herbicides on the Management of the Invasive Weed Solanum rostratum Dunal (Solanaceae)" Plants 10, no. 2: 284. https://doi.org/10.3390/plants10020284
APA StyleAbu-Nassar, J., & Matzrafi, M. (2021). Effect of Herbicides on the Management of the Invasive Weed Solanum rostratum Dunal (Solanaceae). Plants, 10(2), 284. https://doi.org/10.3390/plants10020284






