Involvement of Polyamine Metabolism in the Response of Medicago truncatula Genotypes to Salt Stress
Abstract
1. Introduction
2. Results
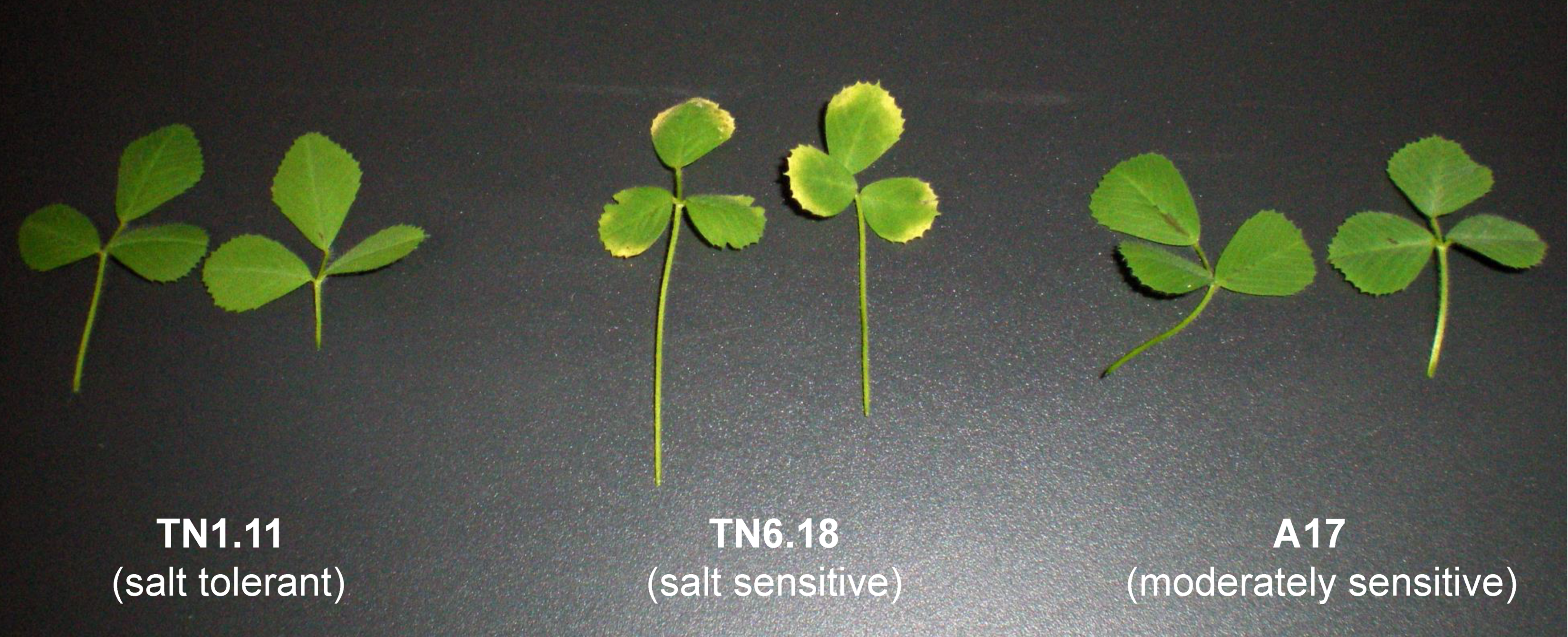
2.1. Phenotypic Alterations Resulting from Salt Stress in Medicago Truncatula Genotypes
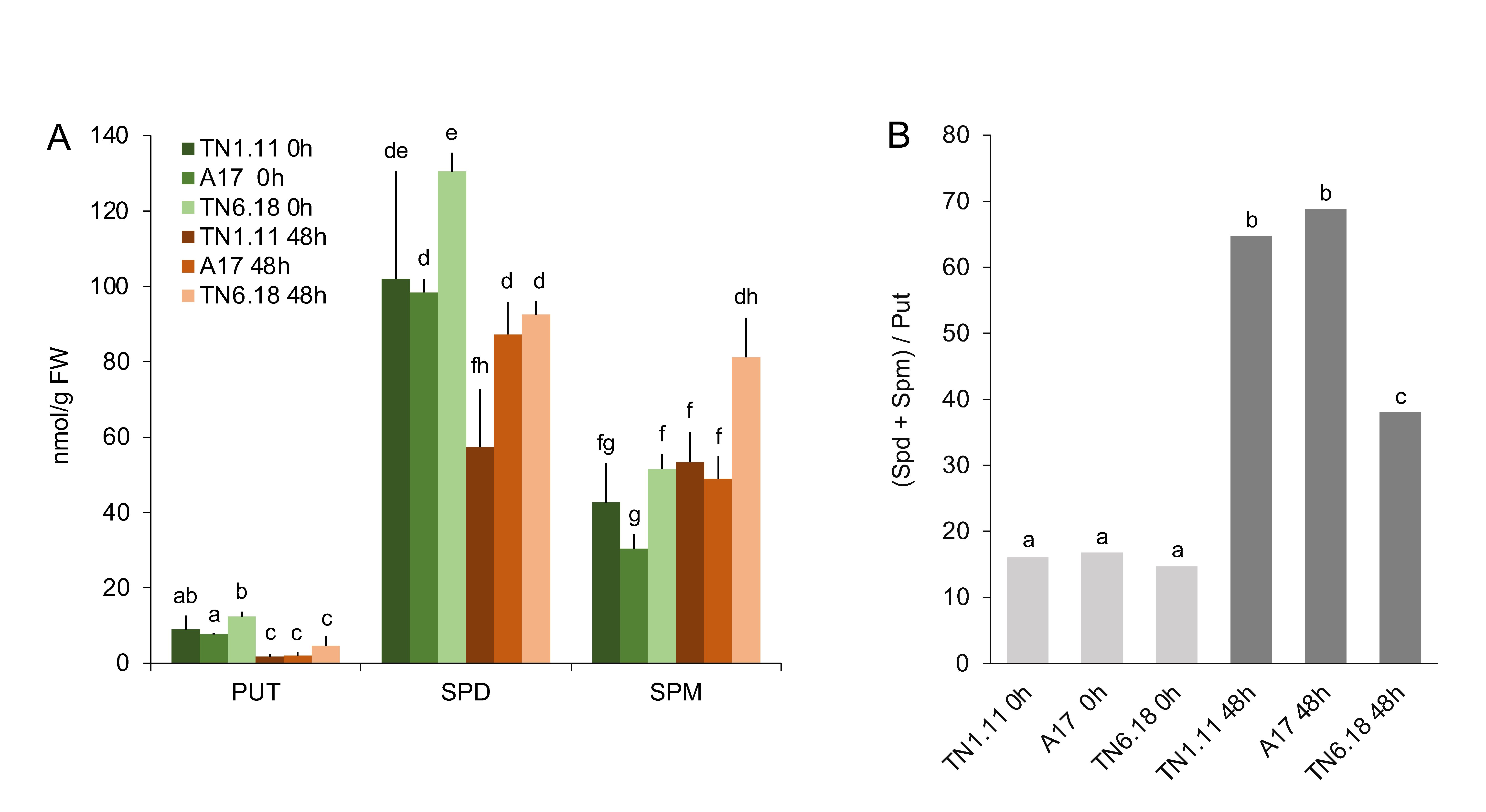
2.2. Polycationic Forms of Polyamines are Regulated in a Genotype-Specific Manner
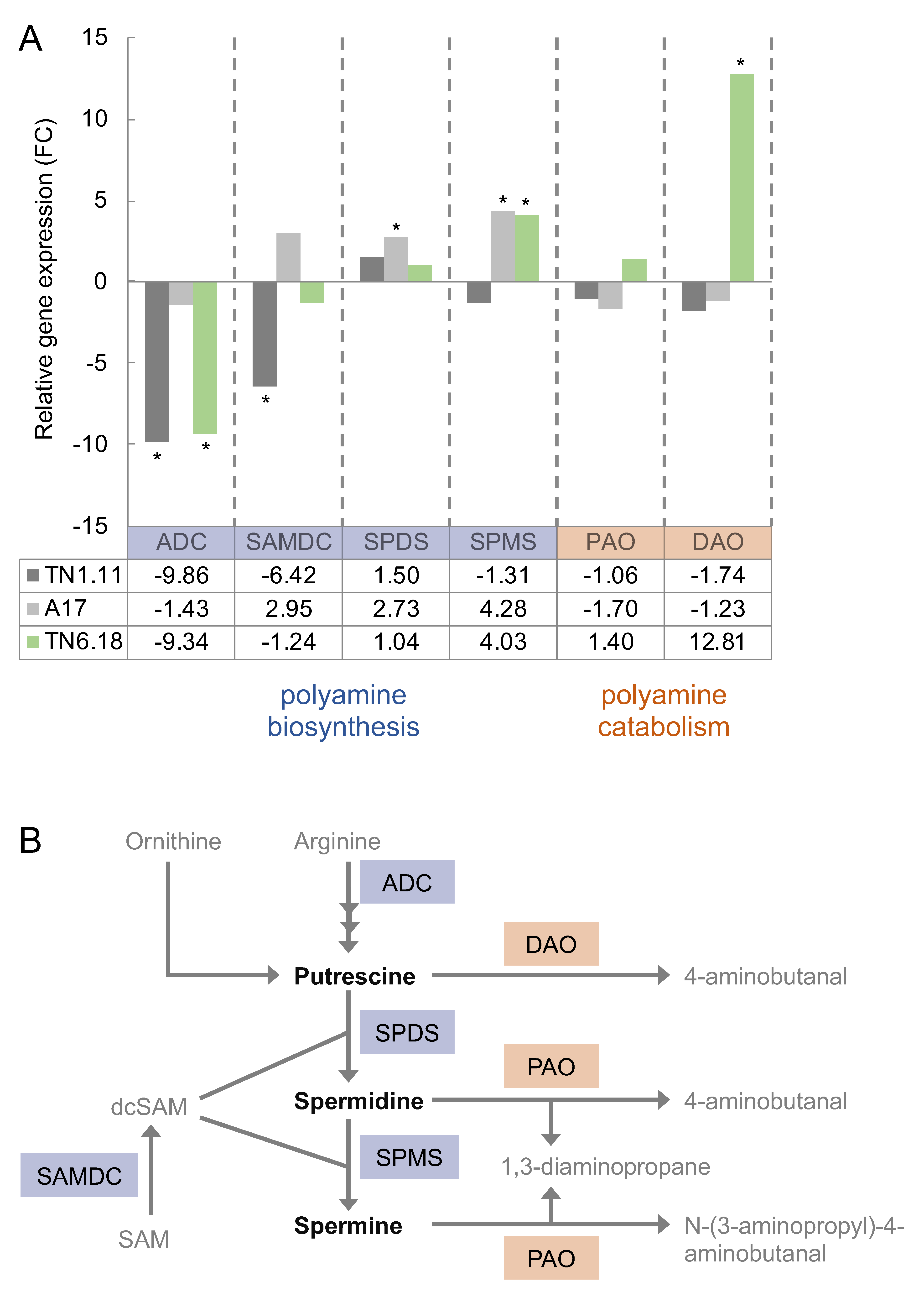
2.3. Transcript Levels Highlight a Distinct Regulation in the Sensitive Medicago Genotype in Response to Salt Stress Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Salt Stress Conditions
4.2. Analysis of Free PAs by HPLC
4.3. RNA Isolation, cDNA Synthesis and Real-Time RT-qPCR Assay
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World. Safeguarding against Economic Slowdowns and Downturns; FAO: Rome, Italy, 2019; p. 239. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Sequera-Mutiozabal, M.; Antoniou, C.; Tiburcio, A.F.; Alcázar, R.; Fotopoulos, V. Polyamines: Emerging hubs promoting drought and salt stress tolerance in plants. Curr. Mol. Biol. Rep. 2017, 3, 28–36. [Google Scholar] [CrossRef]
- Galston, A.W.; Sawhney, R.K. Polyamines in plant physiology. Plant Physiol. 1990, 94, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef]
- Richards, F.J.; Coleman, R.G. Occurrence of putrescine in potassium-deficient barley. Nature 1952, 170, 460. [Google Scholar] [CrossRef]
- Mo, H.; Pua, E.C. Up-regulation of arginine decarboxylase gene expression and accumulation of polyamines in mustard (Brassica juncea) in response to stress. Physiol. Plant. 2002, 114, 439–449. [Google Scholar] [CrossRef]
- Tanou, G.; Ziogas, V.; Belghazi, M.; Christou, A.; Filippou, P.; Job, D.; Fotopoulos, V.; Molassiotis, A. Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant Cell Environ. 2014, 37, 864–885. [Google Scholar] [CrossRef]
- Zarza, X.; Atanasov, K.E.; Marco, F.; Arbona, V.; Carrasco, P.; Kopka, J.; Fotopoulos, V.; Munnik, T.; Gómez-Cadenas, A.; Tiburcio, A.F.; et al. Polyamine Oxidase 5 loss-of-function mutations in Arabidopsis thaliana trigger metabolic and transcriptional reprogramming and promote salt stress tolerance. Plant Cell Environ. 2017, 40, 527–542. [Google Scholar] [CrossRef]
- Lopez, M.; Herrera-Cervera, J.A.; Iribarne, C.; Tejera, N.A.; Lluch, C. Growth and nitrogen fixation in Lotus japonicus and Medicago truncatula under NaCl stress: Nodule carbon metabolism. J. Plant Physiol. 2008, 165, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Aranjuelo, I.; Arrese-Igor, C.; Molero, G. Nodule performance within a changing environmental context. J. Plant Physiol. 2014, 171, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Wu, J.; Cona, A.; Tavladoraki, P.; Angelini, R.; Roubelakis-Angelakis, K.A. The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J. Exp. Bot. 2012, 63, 5003–5015. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, A.F.; Wollenweber, B.; Zilberstein, A.; Koncz, C. Abiotic stress tolerance. Plant Sci. 2012, 182, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Das, S.P.; Mandal, C.; Gupta, S.; Das, K.; Dey, N.; Adak, M.K. Variations of antioxidative responses in two rice cultivars with polyamine treatment under salinity stress. Physiol. Mol. Biol. Plants 2012, 18, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Mhadhbi, H.; Fotopoulos, V.; Mylona, P.V.; Jebara, M.; Aouani, M.E.; Polidoros, A.N. Role of antioxidant gene–enzyme responses in Medicago truncatula genotypes with different degrees of sensitivity to high salinity. Physiol. Plant. 2011, 141, 201–214. [Google Scholar] [CrossRef]
- Mhadhbi, H.; Fotopoulos, V.; Mylona, P.V.; Jebara, M.; Aouani, M.E.; Polidoros, A.N. Alternative oxidase (Aox1) gene expression in roots of Medicago truncatula is a genotype-specific component of salt stress tolerance. J. Plant Physiol. 2013, 170, 111–114. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Jiménez-Bremont, J.F.; Ruiz, O.A.; Rodríguez-Kessler, M. Modulation of spermidine and spermine levels in maize seedlings subjected to long-term salt stress. Plant Physiol. Biochem. 2007, 45, 812–821. [Google Scholar] [CrossRef]
- Basu, R.; Ghosh, B. Polyamines in various rice (Oryza sativa L.) genotypes with respect to sodium chloride salinity. Physiol. Plant. 1991, 82, 575–581. [Google Scholar] [CrossRef]
- Zapata, P.J.; Serrano, M.; Pretel, M.T.; Amoros, A.; Botella, M.A. Polyamines and ethylene changes during germination of different plant species under salinity. Plant Sci. 2004, 167, 781–788. [Google Scholar] [CrossRef]
- Roy, P.; Niyogi, K.; SenGupta, D.N.; Ghosh, B. Spermidine treatment to rice seedlings recovers salinity stress-induced damage of plasma membrane and PM-bound H+-ATPase in salt-tolerant and salt-sensitive rice cultivars. Plant Sci. 2005, 168, 583–591. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Takahashi, Y.; Berberich, T.; Imai, A.; Miyazaki, A.; Takahashi, T.; Michael, A.; Kusano, T. The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett. 2006, 580, 6783–6788. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.C.; Lopez-Cobollo, R.; Alcázar, R.; Zarza, X.; Koncz, C.; Altabella, T.; Salinas, J.; Tiburcio, A.F.; Ferrando, A. Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiol. 2008, 148, 1094–1105. [Google Scholar] [CrossRef]
- Tassoni, A.; Franceschetti, M.; Bagni, N. Polyamines and salt stress response and tolerance in Arabidopsis thaliana flowers. Plant Physiol. Biochem. 2008, 46, 607–613. [Google Scholar] [CrossRef]
- Aziz, A.; Martin-Tanguy, J.; Larher, F. Stress-induced changes in polyamine and tyramine levels can regulate proline accumulation in tomato leaf discs treated with sodium chloride. Physiol. Plant. 1998, 104, 195–202. [Google Scholar] [CrossRef]
- Marcé, M.; Brown, D.S.; Capell, T.; Figueras, X.; Tiburcio, A.F. Rapid high-performance liquid chromatographic method for quantitation of polyamines as their dansyl derivatives: Application to plant and animal tissues. J. Chromatogr. B Biomed. Appl. 1995, 666, 329–333. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expressions software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, 2002–2036. [Google Scholar] [CrossRef]
- Filippou, P.; Antoniou, C.; Fotopoulos, V. The nitric oxide donor sodium nitroprusside regulates polyamine and proline metabolism in leaves of Medicago truncatula plants. Free Rad. Biol. Med. 2013, 56, 172–183. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniou, C.; Zarza, X.; Gohari, G.; Panahirad, S.; Filippou, P.; Tiburcio, A.F.; Fotopoulos, V. Involvement of Polyamine Metabolism in the Response of Medicago truncatula Genotypes to Salt Stress. Plants 2021, 10, 269. https://doi.org/10.3390/plants10020269
Antoniou C, Zarza X, Gohari G, Panahirad S, Filippou P, Tiburcio AF, Fotopoulos V. Involvement of Polyamine Metabolism in the Response of Medicago truncatula Genotypes to Salt Stress. Plants. 2021; 10(2):269. https://doi.org/10.3390/plants10020269
Chicago/Turabian StyleAntoniou, Chrystalla, Xavier Zarza, Gholamreza Gohari, Sima Panahirad, Panagiota Filippou, Antonio F. Tiburcio, and Vasileios Fotopoulos. 2021. "Involvement of Polyamine Metabolism in the Response of Medicago truncatula Genotypes to Salt Stress" Plants 10, no. 2: 269. https://doi.org/10.3390/plants10020269
APA StyleAntoniou, C., Zarza, X., Gohari, G., Panahirad, S., Filippou, P., Tiburcio, A. F., & Fotopoulos, V. (2021). Involvement of Polyamine Metabolism in the Response of Medicago truncatula Genotypes to Salt Stress. Plants, 10(2), 269. https://doi.org/10.3390/plants10020269









