Comparison of Plant Morphology, Yield and Nutritional Quality of Fagopyrum esculentum and Fagopyrum tataricum Grown under Field Conditions in Belgium
Abstract
1. Introduction
2. Results
2.1. Plant Morphology
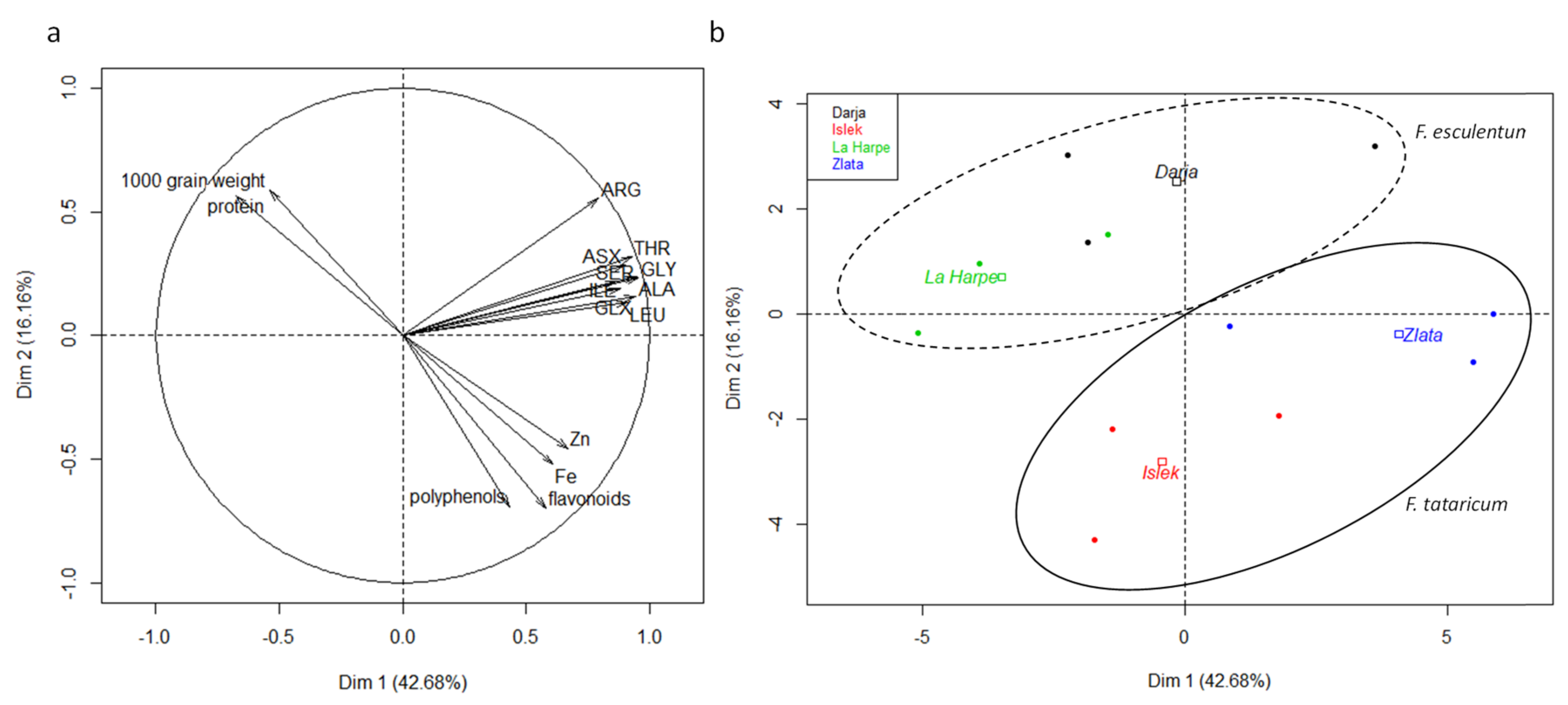
2.2. Nutritional Qualities
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Plant Description
4.3. Nutritional Qualities of the Seeds
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, C.G. Buckwheat: Fagopyrum esculentum Moench; Promoting the Conservation and Use of Underutilized and Neglected Crops, 19; International Plant Genetic Resources Institute: Rome, Italy, 1997; pp. 1–95. [Google Scholar]
- Christa, K.; Soral-Śmietana, M. Buckwheat Grains and Buckwheat Products—Nutritional and Prophylactic Value of Their Components—A Review. Czech J. Food Sci. 2008, 26, 153–162. [Google Scholar] [CrossRef]
- Small, E. 54. Buckwheat—The World’s Most Biodiversity-Friendly Crop? Biodiversity 2017, 18, 108–123. [Google Scholar] [CrossRef]
- Jacquemart, A.-L.; Cawoy, V.; Kinet, J.-M.; Ledent, J.-F.; Quinet, M. Is Buckwheat (Fagopyrum esculentum Moench) Still a Valuable Crop Today? Eur. J. Plant Sci. Biotechnol. 2012, 6, 1–10. [Google Scholar]
- Murai, M.; Ohnishi, O. Population Genetics of Cultivated Common Buckwheat, Fagopyrum esculentum Moench. X. Diffusion Routes Revealed by RAPD Markers. Genes Genet. Syst. 1996, 71, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Yasui, Y. Gene Flow Signature in the S-Allele Region of Cultivated Buckwheat. BMC Plant Biol. 2019, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; He, M.; Fan, Y.; Zhao, H.; Gao, B.; Yang, K.; Li, F.; Tang, Y.; Gao, Q.; Lin, T. Resequencing of Global Tartary Buckwheat Accessions Reveals Multiple Domestication Events and Key Loci Associated with Agronomic Traits. Genome Biol. 2020, 22, 23. [Google Scholar]
- Bekkering, C.S.; Tian, L. Thinking Outside of the Cereal Box: Breeding Underutilized (Pseudo)Cereals for Improved Human Nutrition. Front. Genet. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Koval, D.; Plocková, M.; Kyselka, J.; Skřivan, P.; Sluková, M.; Horáčková, Š. Buckwheat Secondary Metabolites: Potential Antifungal Agents. J. Agric. Food Chem. 2020, 68, 11631–11643. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal Grains: Nutritional Value, Health Benefits and Current Applications for the Development of Gluten-Free Foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Rahman, H.; Thushar, S.; Singh, R.K. Healthy and Resilient Cereals and Pseudo-Cereals for Marginal Agriculture: Molecular Advances for Improving Nutrient Bioavailability. Front. Genet. 2020, 11. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 22 December 2020).
- Aubert, L.; Konrádová, D.; Kebbas, S.; Barris, S.; Quinet, M. Comparison of High Temperature Resistance in Two Buckwheat Species Fagopyrum esculentum and Fagopyrum tataricum. J. Plant Physiol. 2020, 251, 153222. [Google Scholar] [CrossRef] [PubMed]
- Aubert, L.; Konrádová, D.; Barris, S.; Quinet, M. Different Drought Resistance Mechanisms between Two Buckwheat Species Fagopyrum esculentum and Fagopyrum tataricum. Physiol. Plant. 2020, 13248. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.; Gresta, F.; Sperlinga, E.; Ruberto, G. Effect of Sowing Time and Soil Water Content on Grain Yield and Phenolic Profile of Four Buckwheat (Fagopyrum esculentum Moench.) Varieties in a Mediterranean Environment. J. Food Compos. Anal. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Schulte auf’m Erley, G.; Kaul, H.-P.; Kruse, M.; Aufhammer, W. Yield and Nitrogen Utilization Efficiency of the Pseudocereals Amaranth, Quinoa, and Buckwheat under Differing Nitrogen Fertilization. Eur. J. Agron. 2005, 22, 95–100. [Google Scholar] [CrossRef]
- Strahm, S.; Füglistaller, D.; Lädrach, C.; Enggist, A.; Thuet, A.; Luginbühl, C.; Ramseier, H.; Hiltbrunner, J. Growing Buckwheat in Switzerland: New Varieties for an Old Niche Crop. Agrar. Schweiz 2019, 10, 198–205. [Google Scholar]
- Kreft, I.; Zhou, M.; Golob, A.; Germ, M.; Likar, M.; Dziedzic, K.; Luthar, Z. Breeding Buckwheat for Nutritional Quality. Breed Sci. 2020, 70, 67–73. [Google Scholar] [CrossRef]
- Ahmed, A.; Khalid, N.; Ahmad, A.; Abbasi, N.A.; Latif, M.S.Z.; Randhawa, M.A. Phytochemicals and Biofunctional Properties of Buckwheat: A Review. J. Agric. Sci. 2014, 152, 349–369. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive Value of Pseudocereals and Their Increasing Use as Functional Gluten-Free Ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Zhu, F. Chemical Composition and Health Effects of Tartary Buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from Garden: Bioactive Compounds of Buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef]
- Eggum, B.O.; Kreft, I.; Javornik, B. Chemical Composition and Protein Quality of Buckwheat (Fagopyrum esculentum Moench). Plant Food Hum. Nutr. 1980, 30, 175–179. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a Functional Food and Its Effects on Health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.Ö.; Ayhan, N.Y.; Meriç, Ç.S. Buckwheat: A Useful Food and Its Effects on Human Health. Curr. Nutr. Food Sci. 2020, 16, 29–34. [Google Scholar] [CrossRef]
- Bellaloui, N.; McClure, A.M.; Mengistu, A.; Abbas, H.K. The Influence of Agricultural Practices, the Environment, and Cultivar Differences on Soybean Seed Protein, Oil, Sugars, and Amino Acids. Plants 2020, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Podolska, G.; Górecka, D.; Russel, H.; Dziedzic, K.; Boguszewska, E. Abiotic Stress Affects the Yield and Nutrients of Buckwheat Grains. Zemdirbyste 2019, 106, 233–240. [Google Scholar] [CrossRef]
- Halbrecq, B.; Romedenne, P.; Ledent, J.F. Evolution of Flowering, Ripening and Seed Set in Buckwheat (Fagopyrum esculentum Moench): Quantitative Analysis. Eur. J. Agron. 2005, 3, 209–224. [Google Scholar] [CrossRef]
- Bavec, F.; Pusnik, S.; Rajcan, I. Yield Performance of Two Buckwheat Genotypes Grown as a Full-Season and Stubble-Crop. Rostl. Vyrob. 2002, 48, 351–355. [Google Scholar] [CrossRef]
- Golob, A.; Stibilj, V.; Kreft, I.; Germ, M. The Feasibility of Using Tartary Buckwheat as a Se-Containing Food Material. J. Chem. 2015, 2015, 246042. [Google Scholar] [CrossRef]
- Luthar, Z. New varieties of buckwheat and antioxidant content. In Proceedings of the Novi Izzivi v Agronomiji 2019, Laško, Slovenia, 31 January–1 February 2019; pp. 118–124. [Google Scholar]
- Cawoy, V.; Ledent, J.-F.; Kinet, J.-M.; Jacquemart, A.-L. Floral Biology of Common Buckwheat (Fagopyrum esculentum Moench). Eur. J. Plant Sci. Biotechnol. 2009, 3, 1–9. [Google Scholar]
- Wu, L.-Y.; Wang, B.; Schoen, D.J.; Huang, S.-Q. Transitions from Distyly to Homostyly Are Associated with Floral Evolution in the Buckwheat Genus (Fagopyrum). Am. J. Bot. 2017, 104, 1232–1240. [Google Scholar] [CrossRef]
- Popović, V.; Sikora, V.; Berenji, J.; Filipović, V.; Dolijanović, Ž.; Ikanović, J.; Dončić, D. Analysis of Buckwheat Production in the World and Serbia. Econ. Agric. 2014, 61, 1–10. [Google Scholar] [CrossRef]
- Brunori, A.; Brunori, A.; Baviello, G.; Marconi, E.; Colonna, M.; Ricci, M. The Yield of Five Buckwheat (Fagopyrum esculentum Moench) Varieties Grown in Central and Southern Italy. Fagopyrum 2005, 22, 98–102. [Google Scholar]
- Gavric, T.; Cadro, S.; Gadzo, D.; Djikic, M.; Bezdrob, M.; Jovovic, Z.; Jurkovic, J.; Hamidovic, S. Influence of Meteorological Parameters on the Yield and Chemical Composition of Common Buckwheat (Fagopyrum esculentum Moench). Agric. Cult. For. 2018, 64. [Google Scholar] [CrossRef]
- Qin, P.; Wang, Q.; Shan, F.; Hou, Z.; Ren, G. Nutritional Composition and Flavonoids Content of Flour from Different Buckwheat Cultivars. Int. J. Food Sci. Technol. 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Ge, R.H.; Wang, H. Nutrient Components and Bioactive Compounds in Tartary Buckwheat Bran and Flour as Affected by Thermal Processing. Int. J. Food Prop. 2020, 23, 127–137. [Google Scholar] [CrossRef]
- Pongrac, P.; Vogel-Mikuš, K.; Jeromel, L.; Vavpetič, P.; Pelicon, P.; Kaulich, B.; Gianoncelli, A.; Eichert, D.; Regvar, M.; Kreft, I. Spatially Resolved Distributions of the Mineral Elements in the Grain of Tartary Buckwheat (Fagopyrum tataricum). Food Res. Int. 2013, 54, 125–131. [Google Scholar] [CrossRef]
- IPGRI. Descriptors for Buckwheat (Fagopyrum Spp.); International Plant Genetics Resources: Rome, Italy, 1994; ISBN 978-92-9043-221-0. [Google Scholar]
- Kjeldahl, J. Neue Methode Zur Bestimmung Des Stickstoffs in Organischen Korpern. Z. Fur. Anal. Chem. 1883, 366–382. [Google Scholar] [CrossRef]
- Meussen, B.J.; van Zeeland, A.N.T.; Bruins, M.E.; Sanders, J.P.M. A Fast and Accurate UPLC Method for Analysis of Proteinogenic Amino Acids. Food Anal. Methods 2014, 7, 1047–1055. [Google Scholar] [CrossRef]
| F. esculentum | F. tataricum | ||||
|---|---|---|---|---|---|
| Parameter | n | Darja | La Harpe | Islek | Zlata |
| Vegetative Growth | |||||
| Plant height (cm) | 10 | 137.6 ± 10.2 a | 98 ± 13.1 b | 126 ± 16.8 a | 139.1 ± 18.6 a |
| Number of leaves | 10 | 58.7 ± 27.2 a | 30.5 ± 5.6 b | 36.4 ± 25.9 ab | 50.7 ± 33.3 ab |
| Number of branches | 10 | 5.2 ± 0.8 b | 4.4 ± 1 b | 6.7 ± 1 a | 5.5 ± 1.3 ab |
| Number of nodes | 10 | 15 ± 2.3 b | 10.8 ± 3.4 c | 20.1 ± 2.3 a | 20.4 ± 3.4 a |
| Stem dry weight (g) | 5 | 7.1 ± 2.7 a | 6.5 ± 1.9 a | 4.8 ± 1.4 a | 5.3 ± 1.4 a |
| Leaves dry weight (g) | 5 | 0.9 ± 0.3 a | 1.2 ± 0.4 a | 0.3 ± 0.2 a | 0.8 ± 0.8 a |
| Reproductive Growth | |||||
| Node of the first inflorescence | 10 | 6.3 ± 0.8 bc | 5.5 ± 1.2 c | 7.9 ± 1 a | 7.2 ± 0.8 ab |
| Number of inflorescences | 10 | 69.1 ± 18 ab | 45.2 ± 21.1 b | 89 ± 40.5 ab | 107.6 ± 63.3 a |
| Inflorescence dry weight (g) | 5 | 1.6 ± 0.8 b | 18.1 ± 5.2 a | 8.4 ± 3 ab | 6.2 ± 1.5 ab |
| Flowers per inflorescence | 10 | 73.7 ± 20.2 b | 108.5 ± 27 a | 29.3 ± 5.5 c | 31.1 ± 5.8 c |
| Viable seeds per inflorescence | 10 | 7.5 ± 2.2 c | 35.8 ± 10.4 a | 11.5 ± 3.1 bc | 17 ± 3.9 b |
| Aborted seeds per inflorescence | 10 | 3.3 ± 2.6 b | 15 ± 10.2 a | 4.6 ± 2.7 b | 3.4 ± 2 b |
| Seed set (%) | 10 | 10.8 ± 3.3 c | 33.3 ± 5.4 b | 38.8 ± 4.5 b | 51.5 ± 5 a |
| Thousand-grain weight (g) | 5 | 27.5 ± 0.4 b | 36.1 ± 4.7 a | 19.3 ± 0.7 c | 21.4 ± 0.8 bc |
| F. esculentum | F. tataricum | ||||
|---|---|---|---|---|---|
| Parameter | n | Darja | La Harpe | Islek | Zlata |
| Protein content (%) | 3 | 14.6 ± 1.3 ab | 16.1 ± 1.2a | 12.8 ± 0.1b | 12.8 ± 0.9 b |
| Amino acid concentrations (mg/g FW) | |||||
| Asparagine 1 | 3 | 7.87 ± 0.37 a | 7.03 ± 0.51 a | 7.47 ± 0.50 a | 8.17 ± 0.32 a |
| Cysteine | 3 | 2.00 ± 0.61 a | 1.97 ± 0.15 a | 2.10 ± 0.10 a | 2.40 ± 0.26 a |
| Glutamine 2 | 3 | 18.41 ± 1.82 a | 16.62 ± 1.36 a | 17.52 ± 1.20 a | 20.18 ± 0.90 a |
| Serine | 3 | 4.88 ± 0.36 a | 4.67 ± 0.14 a | 4.77 ± 0.13 a | 4.95 ± 0.31 a |
| Histidine 3 | 3 | 2.22 ± 0.16 a | 2.17 ± 0.14 a | 2.05 ± 0.10 a | 2.17 ± 0.15 a |
| Glycine | 3 | 6.90 ± 0.49 a | 6.37 ± 0.33 a | 6.60 ± 0.30 a | 7.23 ± 0.42 a |
| Threonine 3 | 3 | 4.42 ± 0.33 a | 4.00 ± 0.28 a | 4.13 ± 0.19 a | 4.58 ± 0.50 a |
| Arginine | 3 | 10.37 ± 0.90 a | 9.48 ± 0.36 a | 9.25 ± 0.31 a | 10.48 ± 0.50 a |
| Methionine 3,4 | 3 | 1.60 ± 0.46 ab | 1.47 ± 0.42 ab | 0.90 ± 0.35 b | 2.03 ± 0.23 a |
| Alanine | 3 | 5.22 ± 0.36 ab | 4.82 ± 0.20 b | 5.02 ± 0.24 ab | 5.53 ± 0.25 a |
| Tyrosine | 3 | 1.77 ± 0.12 b | 1.87 ± 0.23 b | 2.07 ± 0.15 a | 2.00 ± 0.10 a |
| Valine 3 | 3 | 3.20 ± 0.26 a | 2.92 ± 0.38 a | 3.45 ± 0.13 a | 3.18 ± 0.16 a |
| Phenylalanine 3 | 3.8 ± 0.21 a | 3.5 ± 0.21 a | ND | ND | |
| Isoleucine 3 | 3 | 2.68 ± 0.17 a | 2.55 ± 0.73 a | 2.63 ± 0.10 a | 2.83 ± 0.13 a |
| Leucine 3 | 3 | 5.85 ± 0.35 ab | 5.37 ± 0.37 b | 5.72 ± 0.32 ab | 6.4 ± 0.33 a |
| Lysine 3 | 3 | 2.87 ± 0.24 a | 3.00 ± 0.40 a | 2.83 ± 0.16 a | 3.80 ± 0.99 a |
| Proline | 3 | 2.30 ± 0.26 a | 1.73 ± 0.15 a | 1.93 ± 0.11 a | 2.40 ± 0.66 a |
| F. esculentum | F. tataricum | ||||
|---|---|---|---|---|---|
| Mineral | n | Darja | La Harpe | Islek | Zlata |
| K (mg/g DW) | 5 | 3.72 ± 0.48 a | 4.58 ± 0.24 a | 4.16 ± 0.13 a | 4.37 ± 0.90 a |
| Na (mg/g DW) | 5 | 1.86 ± 0.65 ab | 1.10 ± 0.32 b | 2.54 ± 1.08 a | 2.36 ± 0.60 ab |
| Ca (mg/g DW) | 5 | 0.16 ± 0.09 a | 0.22 ± 0.07 a | 0.21 ± 0.04 a | 0.11 ± 0.04 a |
| Mg (mg/g DW) | 5 | 1.22 ± 0.17 b | 1.50 ± 0.12 a | 1.17 ± 0.07 b | 1.13 ± 0.08 b |
| Cu (µg/g DW) | 5 | 6.57 ± 1.74 a | 5.82 ± 0.95 a | 6.75 ± 0.60 a | 5.88 ± 1.38 a |
| Fe (µg/g DW)3 | 5 | 19.90 ± 2.33 b | 25.94 ± 3.05 ab | 30.51 ± 4.21 ab | 34.66 ± 5.01 a |
| Zn (µg/g DW) | 5 | 18.14 ± 3.06 b | 21.17 ± 2.80 ab | 22.85 ± 1.88 ab | 26.17 ± 4.80 a |
| F. esculentum | F. tataricum | ||||
|---|---|---|---|---|---|
| Antioxidant | n | Darja | La Harpe | Islek | Zlata |
| Flavonoids (mg/g FW) | 3 | 0.10 ± 0.03 b | 0.02 ± 0.01 b | 3,44 ± 103 a | 3,79 ± 105 a |
| Polyphenols (mg/g FW) | 3 | 3.00 ± 0.13 b | 3.25 ± 0.17 ab | 4.48 ± 1.07 ab | 4.90 ± 0.34 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubert, L.; Decamps, C.; Jacquemin, G.; Quinet, M. Comparison of Plant Morphology, Yield and Nutritional Quality of Fagopyrum esculentum and Fagopyrum tataricum Grown under Field Conditions in Belgium. Plants 2021, 10, 258. https://doi.org/10.3390/plants10020258
Aubert L, Decamps C, Jacquemin G, Quinet M. Comparison of Plant Morphology, Yield and Nutritional Quality of Fagopyrum esculentum and Fagopyrum tataricum Grown under Field Conditions in Belgium. Plants. 2021; 10(2):258. https://doi.org/10.3390/plants10020258
Chicago/Turabian StyleAubert, Lauranne, Christian Decamps, Guillaume Jacquemin, and Muriel Quinet. 2021. "Comparison of Plant Morphology, Yield and Nutritional Quality of Fagopyrum esculentum and Fagopyrum tataricum Grown under Field Conditions in Belgium" Plants 10, no. 2: 258. https://doi.org/10.3390/plants10020258
APA StyleAubert, L., Decamps, C., Jacquemin, G., & Quinet, M. (2021). Comparison of Plant Morphology, Yield and Nutritional Quality of Fagopyrum esculentum and Fagopyrum tataricum Grown under Field Conditions in Belgium. Plants, 10(2), 258. https://doi.org/10.3390/plants10020258






