Efficient Protocol for Improving the Development of Cryopreserved Embryonic Axes of Chestnut (Castanea sativa Mill.) by Encapsulation–Vitrification
Abstract
1. Introduction
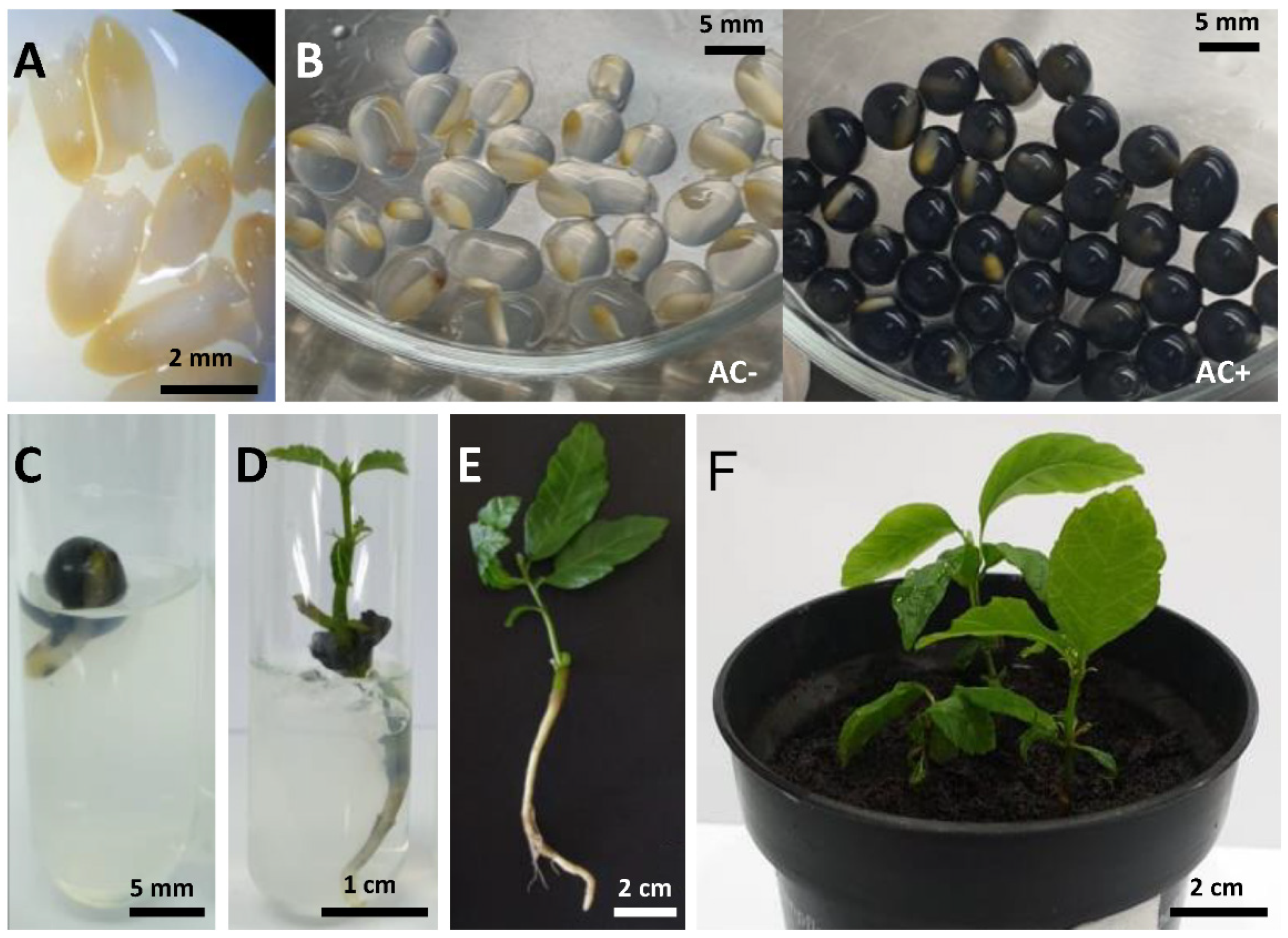
2. Results
Effect of LS and PVS2 Treatments on Survival and Regrowth of Encapsulated EAs
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Encapsulation
4.3. Encapsulation–Vitrification Technique for EA Cryopreservation
4.4. Data Collection and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dolukhanov, A. Rastitel’nost’Gruzii (Vegetation of Georgia); Metsniereba: Tbilisi, Georgia, 1989; Volume 1. (In Russian) [Google Scholar]
- Nakhutsrishvili, G. Forest vegetation of Georgia. In the Vegetation of Georgia (South Caucasus); Nakhutsrishvili, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 35–87. [Google Scholar]
- IUCN Red List of Threatened Species. Version 2020-3. Available online: https://www.iucnredlist.org (accessed on 18 December 2020).
- Barstow, M.; Khela, S. Castanea Sativa. The IUCN Red List of Threatened Species. 2018. Available online: https://dx.doi.org/10.2305/IUCN.UK.2018-1.RLTS.T202948A67740523.en (accessed on 18 December 2020).
- Tavadze, B.; Supatashvili, A.; Kapanadze, G.; Mamukashvili, T. Pathological status of chestnut stands in Tkibuli region (Georgia). Ann. For. 2012, 5, 21–32. [Google Scholar]
- Red List of Georgia. Edict of the President of Georgia #303 on Approval of the Red List of Georgia. Tbilisi. 2006. Available online: https://www.matsne.gov.ge/ka/document/view/97288?publication=0 (accessed on 18 December 2020).
- IUCN Red List Categories and Criteria 2012 Version 3.1, 2nd ed. Available online: https://www.iucn.org/content/iucn-red-list-categories-and-criteria-version-31-second-edition (accessed on 18 December 2020).
- Pence, V.C. Desiccation and survival of Aesculus, Castanea and Quercus embryo axis through cryopreservation. Cryobiology 1992, 29, 391–399. [Google Scholar] [CrossRef]
- Westengen, O.T.; Jeppson, S.; Guarino, L. Global Ex-Situ Crop Diversity Conservation and the Svalbard Global Seed Vault: Assessing the Current Status. PLoS ONE 2013, 8, e64146. [Google Scholar] [CrossRef] [PubMed]
- Lambardi, M.; De Carlo, A. Application of tissue culture to germplasm conservation of temperate broad-leaf trees. In Micropropagation of Woody Trees and Fruits; Jain, S.M., Ishii, K., Eds.; KluwerAcademic: Dordrecht, The Netherlands, 2003; pp. 815–840. [Google Scholar]
- Panis, B.; Lambardi, M. Status of cryopreservation technologies in plants (crops and forest trees). In The Role of Biotechnology in Exploring and Protecting Agricultural Genetic Resources; Ruane, J., Sonnino, A., Eds.; FAO: Rome, Italy, 2006; pp. 61–78. [Google Scholar]
- Panis, B. Sixty years of plant cryopreservation: From freezing hardy mulberry twigs to establishing reference crop collections for future generations. Acta Hortic. 2019, 1234, 1–8. [Google Scholar] [CrossRef]
- Janeiro, L.V.; Vieitez, A.M.; Ballester, A. Cold storage of the in vitro cultures of wild cherry, chestnut and oak. Ann. Sci. For. 1995, 52, 287–293. [Google Scholar] [CrossRef]
- Lambardi, M.; Benelli, C.; De Paoli, G.; Battistini, A. Biotechnologie per la conservazione del Castagno. In Proceedings of the Convegno Nazionale Castagno, Firenze, Italy, 25–27 October 2001; Bellini, E., Ed.; Università di Firenze: Marradi (Firenze), Italy, 2001; pp. 86–91. [Google Scholar]
- Corredoira, E.; Valladares, S.; Martinez, M.T.; Couselo, J.L.; San Jose, M.C.; Ballester, A.; Vieitez, A.M. Conservación de germoplasma en especies leñosas con técnicas de cultivo in vitro y almacenamiento en frío (Span). J. Rural Dev. 2011, 2, 15–24. [Google Scholar]
- Corredoira, E.; Martinez, M.T.; Cernadas, M.J.; San-Jose, M.C. Application of biotechnology in the conservation of the genus Castanea. Forests 2017, 8, 394. [Google Scholar] [CrossRef]
- Capuana, M.; Di Lonardo, S. In vitro conservation of chestnut (Castanea sativa) by slow growth. In Vitro Cell. Dev. Biol. Plant 2013, 49, 605–610. [Google Scholar] [CrossRef]
- Vidal, N.; Sanchez, C.; Jorquera, L.; Ballester, A.; Vieitez, A.M. Cryopreservation of chestnut by vitrification of in vitro-grown shoot tips. In Vitro Cell. Dev. Biol.-Plant 2005, 41, 63–68. [Google Scholar] [CrossRef]
- Jorquera, L.; Vidal, N.; Sánchez, C.; Vieitez, A.M. Optimizing conditions for successful plant regeneration from cryopreserved Castanea sativa shoot tips. Acta Hortic. 2005, 693, 511–518. [Google Scholar] [CrossRef]
- Corredoira, E.; San-Jose, M.C.; Ballester, A.; Vieitez, A.M. Cryopreservation of zygotic embryo axes and somatic embryos of European chestnut. CryoLetters 2004, 25, 33–42. [Google Scholar] [PubMed]
- Gaidamashvili, M.; Khurtsidze, E.; Benelli, K.; Lambardi, M. Development of an Efficient ‘One-Step Freezing’ Cryopreservation Protocol for a Georgian Provenance of Chestnut (Castanea sativa Mill.) Zygotic Embryos. Not. Bot. Hortic. Agrobo. 2019, 47, 1047–1054. [Google Scholar] [CrossRef]
- Holliday, C.; Merkle, S.A. Preservation of American chestnut germplasm by cryostorage of embryogenic cultures. J. Am. Chestnut Found. 2000, 14, 46–52. [Google Scholar]
- San Jose, M.C.; Jorquera, L.; Vidal, N.; Corredoira, E.; Sanchez, C. Cryopreservation of European chestnut germplasm. Acta Hortic. 2005, 693, 225–232. [Google Scholar] [CrossRef]
- Vieitez, A.M.; San Jose, M.C.; Corredoira, E. Cryopreservation of zygotic embryonic axes and somatic embryos of European chestnut. In Plant Embryo Cultures: Methods and Protocols, Methods in Molecular Biology; Thorpe, T.A., Yeung, E.C., Eds.; Springer: New York, NY, USA, 2011; Volume 710, pp. 201–213. [Google Scholar]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Fabre, J.; Dereuddre, J. Encapsulation-dehydration: A new approach to cryopreservation of Solanum shoot tips. CryoLetters 1990, 11, 413–426. [Google Scholar]
- Matsumoto, T.; Sakai, A.; Takahashi, C.; Yamada, K. Cryopreservation of in-vitro grown apical meristems of wasabi (Wasabia japonica) by encapsulation-vitrification method. CryoLetters 1995, 16, 189–196. [Google Scholar]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. CryoLetters 2007, 28, 151–172. [Google Scholar]
- Kulus, D. Application of synthetic seeds in propagation, storage, and preservation of Asteraceae plant species. In Synthetic Seeds Germplasm Regeneration, Preservation and Prospects; Faisal, A., Alatar, A., Eds.; Springer: Berlin, Germany, 2019; pp. 155–179. [Google Scholar]
- Sakai, A.; Hirai, D.; Niino, T. Development of PVS-Based Vitrification and Encapsulation–Vitrification Protocols. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 33–57. [Google Scholar]
- Niino, T.; Sakai, A. Cryopreservation of alginate-coated in vitro grown shoot tips of apple, pear and mulberry. Plant Sci. 1992, 87, 199–206. [Google Scholar] [CrossRef]
- Paul, H.; Daigny, G.; Sangwan-Norreel, B.S. Cryopreservation of apple (Malus domestica Borkh.) shoot tips following encapsulation-dehydration or encapsulation-vitrification. Plant Cell Rep. 2000, 19, 768–774. [Google Scholar] [CrossRef]
- Wang, Q.; Laamanen, J.; Uosukainen, M.; Valkonen, J.P.T. Cryopreservation of in vitro-grown shoot tips of raspberry (Rubus idaeus L.) by encapsulation–vitrification and encapsulation–dehydration. Plant Cell Rep. 2005, 24, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.C.; Mawassi, M.; Sahar, N.; Li, P.; Violeta, C.-T.; Gafny, R.; Sela, I.; Tanne, E.; Perl, A. Cryopreservation of grapevine (Vitis spp.) embryogenic cell suspensions by encapsulation–vitrification. Plant Cell Tissue Organ Cult. 2004, 77, 267–275. [Google Scholar] [CrossRef]
- Lambardi, M.; Halmagyi, A.; Benelli, C.; De Carlo, A. Seed cryopreservation for conservation of ancient Citrus germplasm. Adv Hortic Sci. 2007, 21, 198–202. [Google Scholar]
- Rai, M.K.; Asthana, P.; Singh, S.K.; Jaiswal, V.S.; Jaiswal, U. The encapsulation technology in fruit plants—A review. Biotechnol. Adv. 2009, 27, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Shahzad, A.; da Silva, J.A.T. Synseed technology—a complete synthesis. Biotechnol. Adv. 2013, 31, 186–207. [Google Scholar] [CrossRef] [PubMed]
- Benelli, C.; Micheli, M.; De Carlo, A. An improved encapsulation protocol for regrowth and conservation of four ornamental species. Acta Soc. Bot. Pol. 2017, 86, 3559. [Google Scholar] [CrossRef]
- Micheli, M.; Standardi, A.; da Silva, D.F. Encapsulation and Synthetic Seeds of Olive (Olea europaea L.): Experiences and Overview. In Synthetic Seeds—Germplasm Regeneration, Preservation and Prospects; Faisal, M., Alatar, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 347–361. [Google Scholar]
- Carlo, A.D.; Benelli, C.; Lambardi, M. Development of a shoot-tip vitrification protocol and comparison with encapsulation-based procedures for plum (Prunus domestica L.) cryopreservation. CryoLetters 2000, 21, 215–222. [Google Scholar]
- Kulus, D. Effect of bead composition, PVS type, and recovery medium in cryopreservation of bleeding heart ‘Valentine’—Preliminary Study. Agronomy 2020, 10, 891. [Google Scholar] [CrossRef]
- Kulus, D. Shoot Tip Cryopreservation of Lamprocapnos spectabilis (L.) fukuhara using different approaches and evaluation of stability on the molecular, biochemical, and plant architecture levels. Int. J. Mol. Sci. 2020, 21, 3901. [Google Scholar] [CrossRef]
- Kami, D. Cryopreservation of plant genetic resources. In Current Frontiers in Cryobiology; Katkov, I., Ed.; IntechOpen: London, UK, 2012; pp. 439–456. [Google Scholar]
- Kulus, D. Application of cryogenic technologies and somatic embryogenesis in the storage and protection of valuable genetic resources of ornamental plants. In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016. [Google Scholar]
- Halmagyi, A.; Deliu, C. Cryopreservation of carnation (Dianthus caryophyllus L.) shoot tips by encapsulation-vitrification. Sci. Hortic. 2007, 113, 300–306. [Google Scholar] [CrossRef]
- Kumar, M.B.A.; Vakeswaran, V.; Krishnasamy, V. Enhancement of synthetic seed conversion to seedlings in hybrid rice. Plant Cell Tissue Organ Cult. 2005, 81, 97–100. [Google Scholar] [CrossRef]
- Lulsdorf, M.M.; Tautorus, T.E.; Kikcio, S.I.; Bethune, T.D.; Dunstan, D.I. Germination of encapsulated embryos of interior spruce (Picea glauca engelmannii complex) and black spruce (Picea mariana Mill.). Plant Cell Rep. 1993, 12, 385–389. [Google Scholar] [CrossRef] [PubMed]
- George, E.F.; Sherrington, P.D. Plant Propagation by Tissue Culture—Handbook and Directory of Commercial Laboratories; Exegetics Ltd.: Eversley, UK, 1984. [Google Scholar]
- Lloyd, G.; McCown, B.H. Woody Plant Medium (WPM)—A mineral nutrient formulation for microculture of woody plant species. HortScience 1981, 16, 453. [Google Scholar]


| PVS2 (min) | Regrowth a (AC−) | Regrowth a (AC+) | ||
|---|---|---|---|---|
| Shoot Length (mm) | Root Length (mm) | Shoot Length (mm) | Root Length (mm) | |
| 0 | 4.0 b | 5.2 b | 5.0 c | 6.3 c |
| 30 | 9.2 a | 10.2 a | 14.5 a | 22.8 a |
| 60 | 6.5 b | 8.8 a | 8.7 b | 9.8 b |
| 90 | 5.9 b | 8.9 a | 8.3 b | 10.4 b |
| 120 | 4.9 b | 7.8 a | 7.9 b | 9.5 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaidamashvili, M.; Khurtsidze, E.; Kutchava, T.; Lambardi, M.; Benelli, C. Efficient Protocol for Improving the Development of Cryopreserved Embryonic Axes of Chestnut (Castanea sativa Mill.) by Encapsulation–Vitrification. Plants 2021, 10, 231. https://doi.org/10.3390/plants10020231
Gaidamashvili M, Khurtsidze E, Kutchava T, Lambardi M, Benelli C. Efficient Protocol for Improving the Development of Cryopreserved Embryonic Axes of Chestnut (Castanea sativa Mill.) by Encapsulation–Vitrification. Plants. 2021; 10(2):231. https://doi.org/10.3390/plants10020231
Chicago/Turabian StyleGaidamashvili, Mariam, Eka Khurtsidze, Tamari Kutchava, Maurizio Lambardi, and Carla Benelli. 2021. "Efficient Protocol for Improving the Development of Cryopreserved Embryonic Axes of Chestnut (Castanea sativa Mill.) by Encapsulation–Vitrification" Plants 10, no. 2: 231. https://doi.org/10.3390/plants10020231
APA StyleGaidamashvili, M., Khurtsidze, E., Kutchava, T., Lambardi, M., & Benelli, C. (2021). Efficient Protocol for Improving the Development of Cryopreserved Embryonic Axes of Chestnut (Castanea sativa Mill.) by Encapsulation–Vitrification. Plants, 10(2), 231. https://doi.org/10.3390/plants10020231









