Evolution of Plant Na+-P-Type ATPases: From Saline Environments to Land Colonization
Abstract
1. Introduction
2. Results
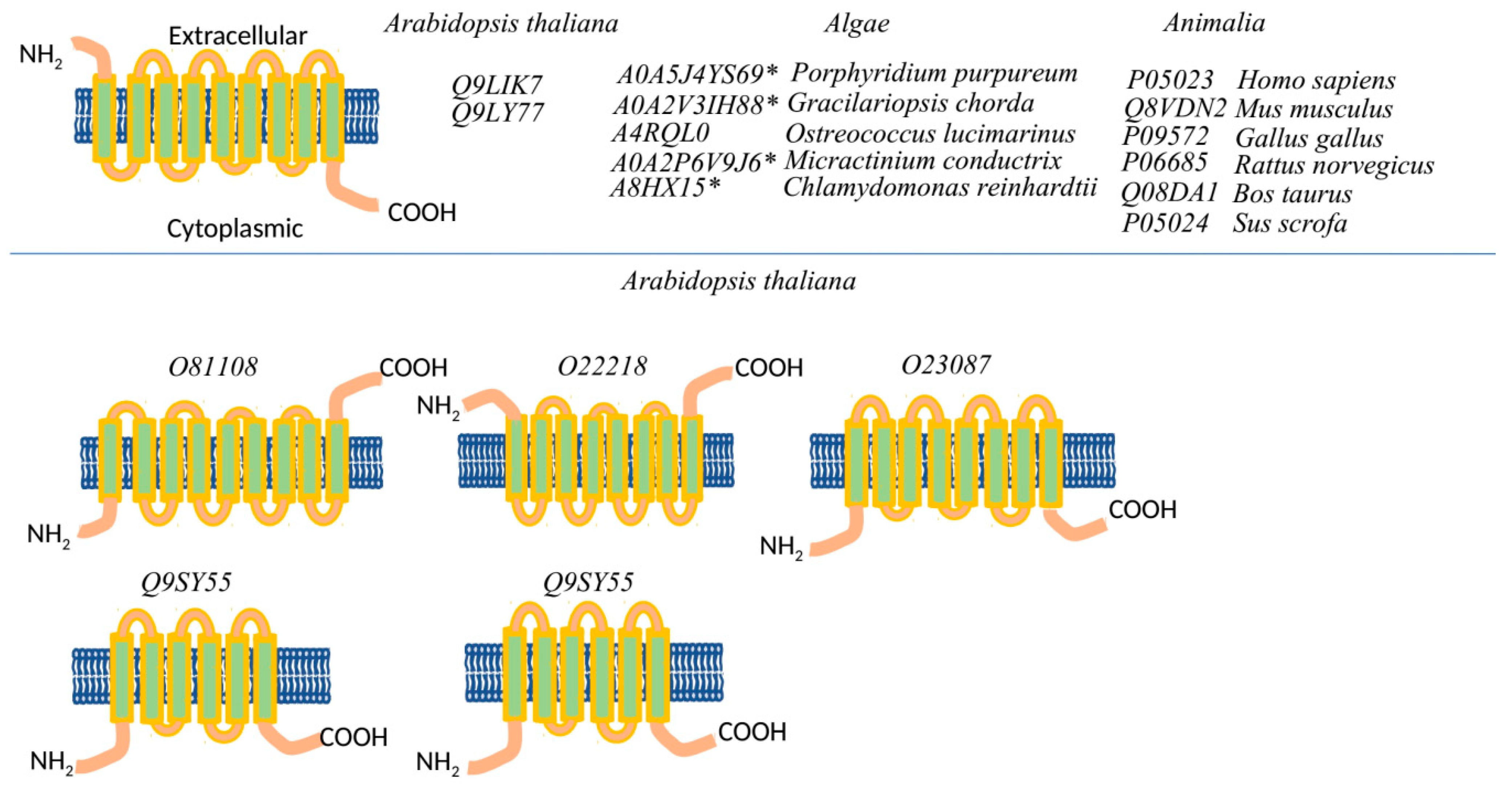
2.1. Domain Organization of the Plants P-ATPases, Comparison to Animals
2.1.1. Comparison of the Ca2+ and Na+/K+ Binding Sites
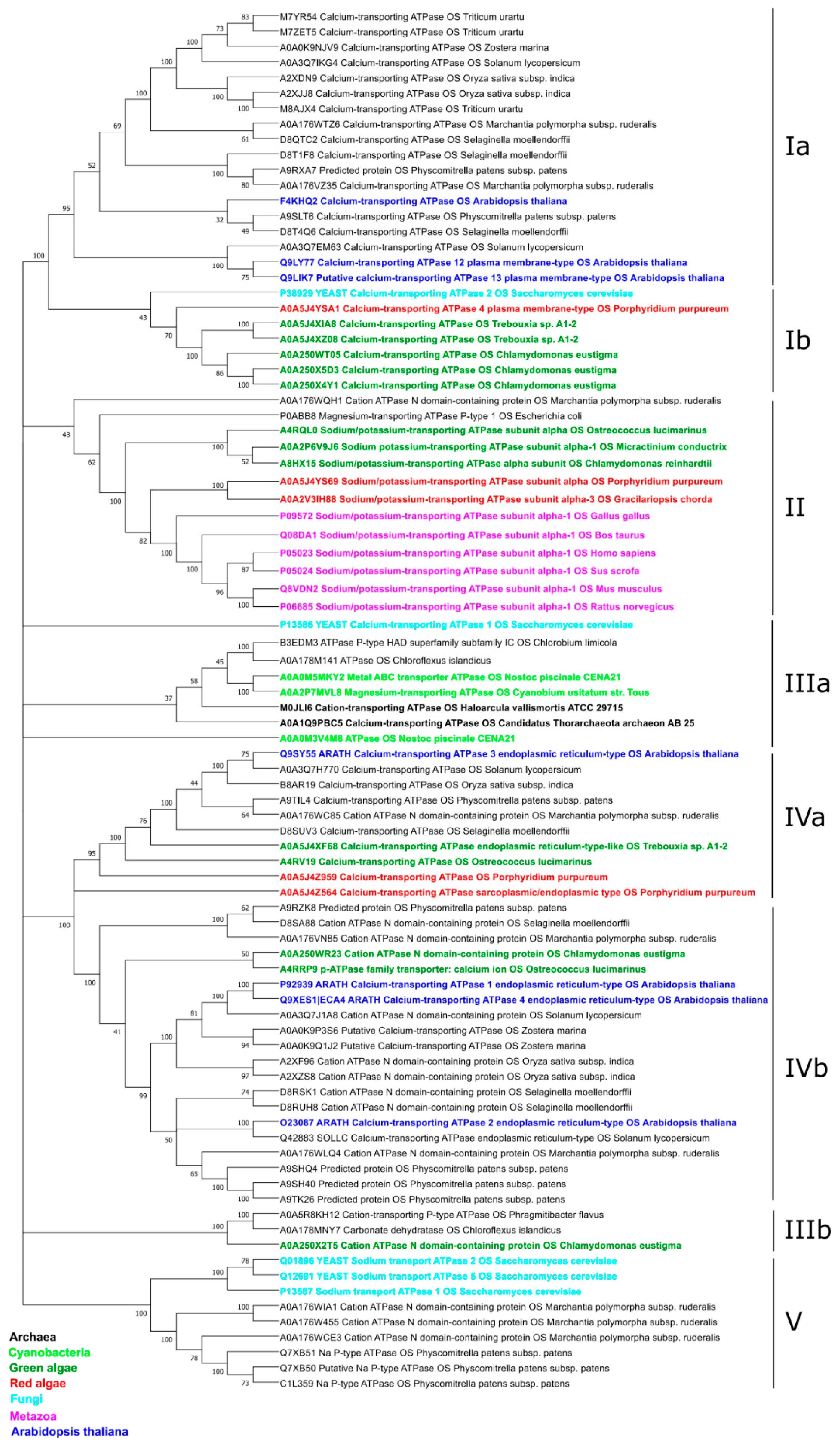
2.1.2. Phylogeny of the α-Subunit
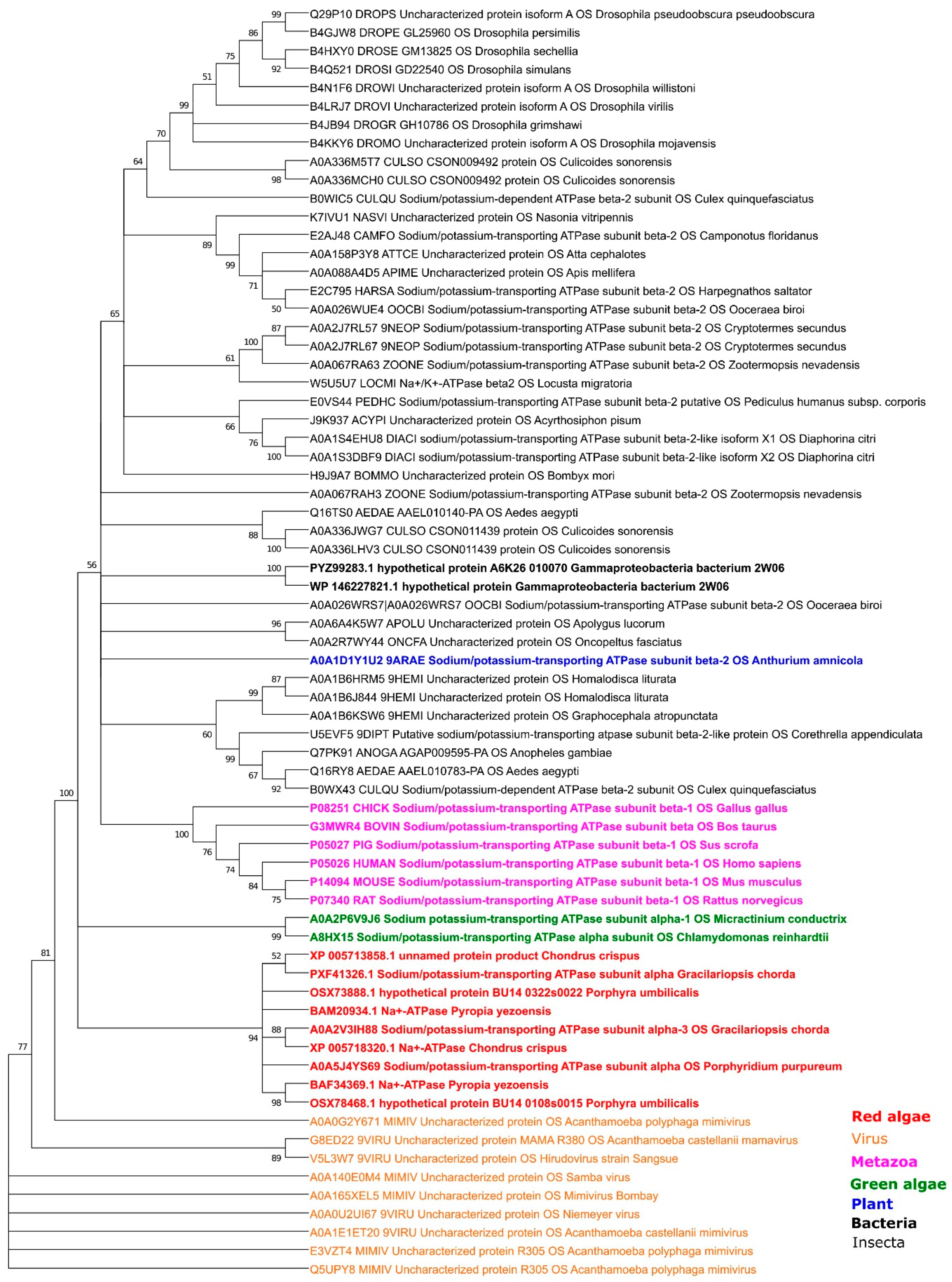
2.1.3. Phylogeny of the β-Subunit
2.1.4. Comparison of the Binding-Sites
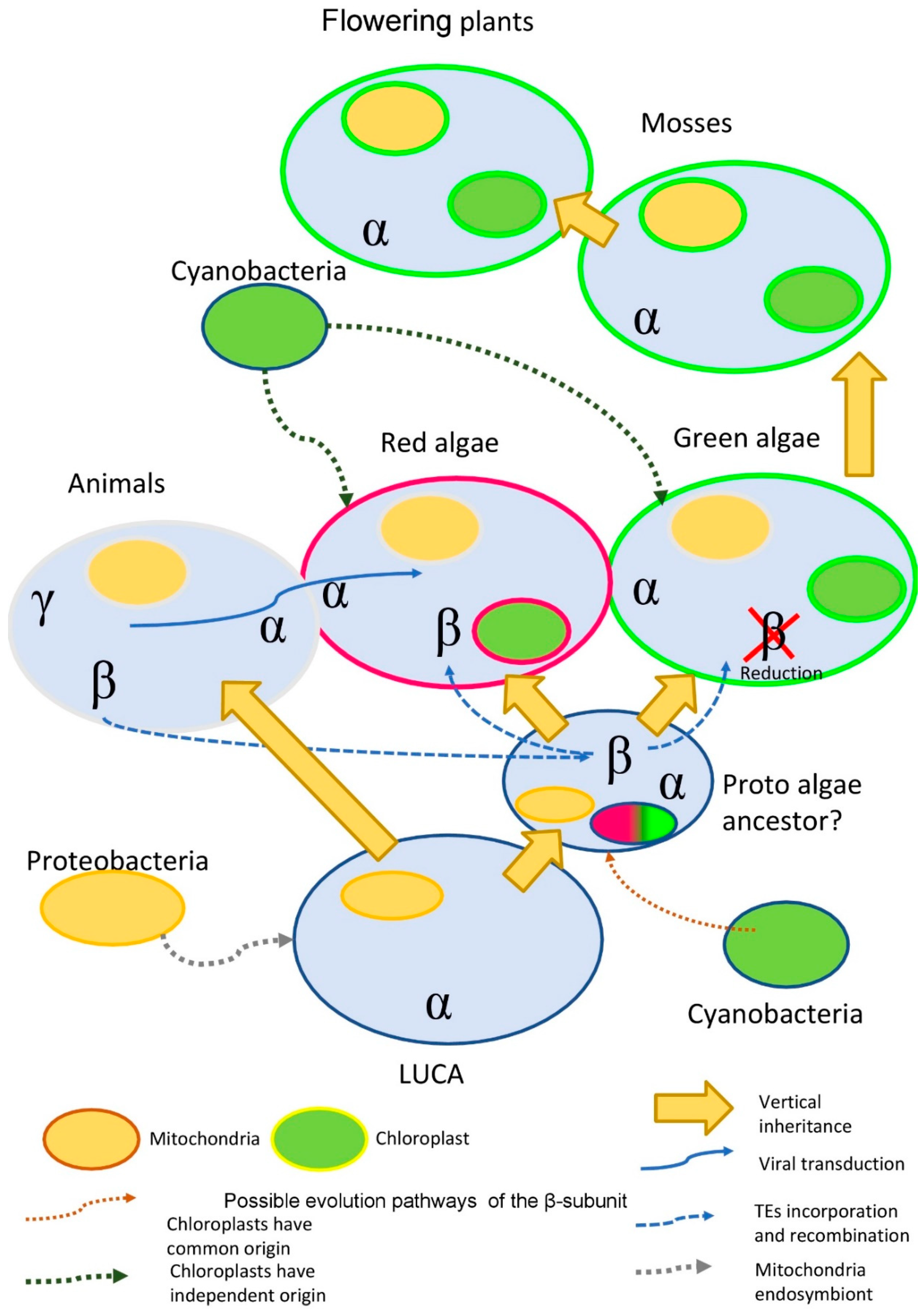
3. Discussion
- (1)
- Ancient transduction. We found nine β-subunit-like proteins in several amoeba viruses (Mimivirus genus). While amoeba is the current target for the virus, it is possible, that earlier in evolution virus was able to facilitate the transfer of some genetic material to the algae. Interestingly, this idea is not a novelty for red algae. It was shown that the Ectocarpus siliculosusvirus (EsV-1) pandemic for the marine brown algae, E. siliculosus, encodes a protein similar to the histidine kinase. Histidine kinase is a crucial part of the two-component signaling pathway, common for bacteria, fungi, and algae. In plants, histidine kinases participate in the signaling pathway of the plant hormone cytokinin, which regulates multiple vital processes [70].
- (2)
- The acquisition of the β-subunit-like protein with transposable elements TEs. It is known, that red algae have passed two phases of large-scale genome reduction [71] and have incorporated multiple TEs in their genome with the subsequent recombination of the TEs, which could provide an evolutionary advantage [72].
- (3)
- The preservation of genes after horizontal and endosymbiotic gene transfer (HGT and EGT). Previous research data suggest that red algae stand between prokaryotes and green algae, acting as a mediator in horizontal and endosymbiotic gene transfer [73]. The study of the red alga Porphyridium purpureum genome suggests that red algae have saved many genes that green algae lost during evolution [74].
4. Materials and Methods
4.1. Sequences Retrieval
4.2. Domain Organization and Active Sites
4.3. Multiple Sequence Alignments and Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- FAO. 2020. Available online: http://www.fao.org/global-soil-partnership/areas-of-work/soil-salinity/en/ (accessed on 7 October 2020).
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.; Rubio, F. High-affinity potassium and sodium transport systems in plants. J. Exp. Bot. 2006, 57, 1149–1160. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Ahmad, I.; Patishtan, J. Regulation of Na+ fluxes in plants. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Volkov, V. Salinity tolerance in plants. Quantitative approach to ion transport starting from halophytes and stepping to genetic and protein engineering for manipulating ion fluxes. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Martínez, V.; Benito, B.; Rubio, F. Comparison between Arabidopsis and Rice for Main Pathways of K+ and Na+ Uptake by Roots. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Dabravolski, S.A.; Pan, T.; Shabala, S. Phylogenetic Diversity and Physiological Roles of Plant Monovalent Cation/H+ Antiporters. Front. Plant Sci. 2020, 11, 573564. [Google Scholar] [CrossRef]
- Pedersen, J.T.; Palmgren, M. Why do plants lack sodium pumps and would they benefit from having one? Funct. Plant Biol. 2017, 44, 473. [Google Scholar] [CrossRef]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Shi, H.; Quintero, F.J.; Pardo, J.M.; Zhu, J.-K. The Putative Plasma Membrane Na+/H+ Antiporter SOS1 Controls Long-Distance Na+ Transport in Plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef]
- Shi, H.; Zhu, J.-K. Regulation of expression of the vacuolar Na+/H+ antiporter gene AtNHX1 by salt stress and abscisic acid. Plant Mol. Biol. 2002, 50, 543–550. [Google Scholar] [CrossRef]
- Martínez-Atienza, J.; Jiang, X.; Garciadeblas, B.; Mendoza, I.; Zhu, J.-K.; Pardo, J.M.; Quintero, F.J. Conservation of the Salt Overly Sensitive Pathway in Rice. Plant Physiol. 2007, 143, 1001–1012. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, Z.-Z.; Zhou, X.-F.; Yin, H.-B.; Li, X.; Xin, X.-F.; Hong, X.-H.; Zhu, J.-K.; Gong, Z. Overexpression of SOS (Salt Overly Sensitive) Genes Increases Salt Tolerance in Transgenic Arabidopsis. Mol. Plant 2009, 2, 22–31. [Google Scholar] [CrossRef]
- Feki, K.; Quintero, F.J.; Khoudi, H.; Leidi, E.O.; Masmoudi, K.; Pardo, J.M.; Brini, F. A constitutively active form of a durum wheat Na+/H+ antiporter SOS1 confers high salt tolerance to transgenic Arabidopsis. Plant Cell Rep. 2014, 33, 277–288. [Google Scholar] [CrossRef]
- Yadav, N.; Shukla, P.; Jha, A.; Agarwal, P.K.; Jha, B. The SbSOS1 gene from the extreme halophyte Salicornia brachiata enhances Na+ loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Biol. 2012, 12, 188. [Google Scholar] [CrossRef]
- Møller, J.V.; Juul, B.; le Maire, M. Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim. Biophys. Acta BBA Rev. Biomembr. 1996, 1286, 1–51. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Harper, J.F. Pumping with plant P-type ATPases. J. Exp. Bot. 1999, 50, 883–893. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Axelsen, K.B. Evolution of P-type ATPases. Biochim. Biophys. Acta BBA Bioenerg. 1998, 1365, 37–45. [Google Scholar] [CrossRef]
- Pedersen, C.N.S.; Axelsen, K.B.; Harper, J.F.; Palmgren, M.G. Evolution of Plant P-Type ATPases. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, L.R.; López-Marqués, R.L.; McDowell, S.C.; Okkeri, J.; Licht, D.; Schulz, A.; Pomorski, T.; Harper, J.F.; Palmgren, M.G. The Arabidopsis P4 -ATPase ALA3 Localizes to the Golgi and Requires a β-Subunit to Function in Lipid Translocation and Secretory Vesicle Formation. Plant Cell 2008, 20, 658–676. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.Ø.; Linnanto, J.; Frigaard, N.-U.; Chr Nielsen, N.; Miller, M. A model of the protein—pigment baseplate complex in chlorosomes of photosynthetic green bacteria. Photosynth. Res. 2010, 104, 233–243. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.J.; Sim, S.J.; Ordureau, A.; Wei, L.; Harper, J.W.; Shao, S.; Park, E. The endoplasmic reticulum P5A-ATPase is a transmembrane helix dislocase. Science 2020, 369, eabc5809. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Pedersen, B.P.; Morth, J.P.; Nissen, P. Structure determination using poorly diffracting membrane-protein crystals: The H+-ATPase and Na+, K+-ATPase case history. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 309–313. [Google Scholar] [CrossRef]
- Garciadeblas, B.; Benito, B.; Rodríguez-Navarro, A. Plant cells express several stress calcium ATPase but apparently no sodium ATPase. Plant Soil 2001, 235, 181–192. [Google Scholar] [CrossRef]
- Benito, B.; Rodriguez-Navarro, A. Molecular cloning and characterization of a sodium-pump ATPase of the moss Physcomitrella patens. Plant J. 2003, 36, 382–389. [Google Scholar] [CrossRef]
- Kumari, J.; Rathore, M.S. Na+/K+-ATPase a Primary Membrane Transporter: An Overview and Recent Advances with Special Reference to Algae. J. Membr. Biol. 2020, 253, 191–204. [Google Scholar] [CrossRef]
- Pardo, J.M.; Cubero, B.; Leidi, E.O.; Quintero, F.J. Alkali cation exchangers: Roles in cellular homeostasis and stress tolerance. J. Exp. Bot. 2006, 57, 1181–1199. [Google Scholar] [CrossRef] [PubMed]
- Lunde, C.; Drew, D.P.; Jacobs, A.K.; Tester, M. Exclusion of Na+ via Sodium ATPase (PpENA1) Ensures Normal Growth of Physcomitrella patens under Moderate Salt Stress. Plant Physiol. 2007, 144, 1786–1796. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prista, C.; Almagro, A.; Loureiro-Dias, M.C.; Ramos, J. Physiological basis for the high salt tolerance of Debaryomyces hansenii. Appl. Environ. Microbiol. 1997, 63, 4005–4009. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Ford, K.; Kretschmer, J.; Tester, M. Rice plants expressing the moss sodium pumping ATPase PpENA1 maintain greater biomass production under salt stress: A moss ATPase expressed in rice. Plant Biotechnol. J. 2011, 9, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Gao, X.; Li, W.; Zhao, J.; Zhao, Y.; Zhang, H. Overexpression of ENA1 from yeast increases salt tolerance in Arabidopsis. J. Plant Biol. 2008, 51, 159–165. [Google Scholar] [CrossRef]
- Nakayama, H.; Yoshida, K.; Shinmyo, A. Yeast plasma membrane Ena1p ATPase alters alkali-cation homeostasis and confers increased salt tolerance in tobacco cultured cells. Biotechnol. Bioeng. 2004, 85, 776–789. [Google Scholar] [CrossRef]
- Balnokin, Y.V.; Popova, L.G. The ATP-driven Na+-pump in the plasma membrane of the marine unicellular alga, Platymonas viridis. FEBS Lett. 1994, 343, 61–64. [Google Scholar] [CrossRef]
- Balnokin, Y.V.; Popova, L.G.; Andreev, I.M. Electrogenicity of the Na+-ATPase from the marine microalga Tetraselmis (Platymonas) viridis and associated H+ countertransport. FEBS Lett. 1999, 462, 402–406. [Google Scholar] [CrossRef]
- Gimmler, H.; Weis, U.; Weis, C.; Kugel, H.; Treffny, B. Dunaliella acidophila (Kalina) Masyuk-an alga with a positive membrane potential. New Phytol. 1989, 113, 175–184. [Google Scholar] [CrossRef]
- Shono, M.; Hara, Y.; Wada, M.; Fujii, T. A Sodium Pump in the Plasma Membrane of the Marine Alga Heterosigma akashiwo. Plant Cell Physiol. 1996, 37, 385–388. [Google Scholar] [CrossRef][Green Version]
- Shono, M.; Wada, M.; Hara, Y.; Fujii, T. Molecular cloning of Na+-ATPase cDNA from a marine alga, Heterosigma akashiwo. Biochim. Biophys. Acta BBA Biomembr. 2001, 1511, 193–199. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic Engineering of Algae for Enhanced Biofuel Production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Popova, L.G.; Belyaev, D.V.; Shuvalov, A.V.; Yurchenko, A.A.; Matalin, D.A.; Khramov, D.E.; Orlova, Y.V.; Balnokin, Y.V. In silico Analyses of Transcriptomes of the Marine Green Microalga Dunaliella tertiolecta: Identification of Sequences Encoding P-type ATPases. Mol. Biol. 2018, 52, 520–531. [Google Scholar] [CrossRef]
- Asamizu, E.; Nakajima, M.; Kitade, Y.; Saga, N.; Nakamura, Y.; Tabata, S. Comparison of RNA expression profiles between the two generations of Porphyra yezoensis (Rhodophyta), based on expressed sequence tag frequency analysis. J. Phycol. 2003, 39, 923–930. [Google Scholar] [CrossRef]
- Haro, R.; Bañuelos, M.A.; Senn, M.E.; Barrero-Gil, J.; Rodríguez-Navarro, A. HKT1 Mediates Sodium Uniport in Roots. Pitfalls in the Expression of HKT1 in Yeast. Plant Physiol. 2005, 139, 1495–1506. [Google Scholar] [CrossRef]
- Kishimoto, M.; Shimajiri, Y.; Oshima, A.; Hase, A.; Mikami, K.; Akama, K. Functional expression of an animal type-Na+-ATPase gene from a marine red seaweed Porphyra yezoensis increases salinity tolerance in rice plants. Plant Biotechnol. 2013, 30, 417–422. [Google Scholar] [CrossRef]
- Morth, J.P.; Pedersen, B.P.; Buch-Pedersen, M.J.; Andersen, J.P.; Vilsen, B.; Palmgren, M.G.; Nissen, P. A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell Biol. 2011, 12, 60–70. [Google Scholar] [CrossRef]
- Peterson, G.L.; Hokin, L.E. Improved purification of brine-shrimp (Artemia saline) (Na+, K+)-activated adenosine triphosphatase and amino-acid and carbohydrate analyses of the isolated subunits. Biochem. J. 1980, 192, 107–118. [Google Scholar] [CrossRef]
- Mercer, R.W. Structure of the Na,K-ATPase. Int. Rev. Cytol. 1993, 137, 139–168. [Google Scholar]
- Morth, J.P.; Pedersen, B.P.; Toustrup-Jensen, M.S.; Sørensen, T.L.-M.; Petersen, J.; Andersen, J.P.; Vilsen, B.; Nissen, P. Crystal structure of the sodium—Potassium pump. Nature 2007, 450, 1043–1049. [Google Scholar] [CrossRef]
- Béguin, P.; Nagashima, K.; Gonoi, T.; Shibasaki, T.; Takahashi, K.; Kashima, Y.; Ozaki, N.; Geering, K.; Iwanaga, T.; Seino, S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature 2001, 411, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Vagin, O.; Tokhtaeva, E.; Sachs, G. The Role of the β 1 Subunit of the Na,K-ATPase and Its Glycosylation in Cell-Cell Adhesion. J. Biol. Chem. 2006, 281, 39573–39587. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bhatla, S.C. A novel fluorescence imaging approach to monitor salt stress-induced modulation of ouabain-sensitive ATPase activity in sunflower seedling roots. Physiol. Plant. 2014, 150, 540–549. [Google Scholar] [CrossRef]
- Keisham, M.; Mukherjee, S.; Bhatla, S. Mechanisms of Sodium Transport in Plants—Progresses and Challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, C.; Nakasako, M.; Nomura, H.; Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 2000, 405, 647–655. [Google Scholar] [CrossRef]
- Nyblom, M.; Poulsen, H.; Gourdon, P.; Reinhard, L.; Andersson, M.; Lindahl, E.; Fedosova, N.; Nissen, P. Crystal Structure of Na+, K+-ATPase in the Na+-Bound State. Science 2013, 342, 123–127. [Google Scholar] [CrossRef]
- Dempski, R.E.; Hartung, K.; Friedrich, T.; Bamberg, E. Fluorometric Measurements of Intermolecular Distances between the α- and β-Subunits of the Na+/K+-ATPase. J. Biol. Chem. 2006, 281, 36338–36346. [Google Scholar] [CrossRef]
- Toyoshima, C.; Iwasawa, S.; Ogawa, H.; Hirata, A.; Tsueda, J.; Inesi, G. Crystal structures of the calcium pump and sarcolipin in the Mg2+-bound E1 state. Nature 2013, 495, 260–264. [Google Scholar] [CrossRef]
- Dabravolski, S.A. The Resurgence of Dirigent Story: Time for a Bacterial Chapter. Curr. Microbiol. 2020, 77, 517–521. [Google Scholar] [CrossRef]
- de Vries, J.; de Vries, S.; Slamovits, C.H.; Rose, L.E.; Archibald, J.M. How Embryophytic is the Biosynthesis of Phenylpropanoids and their Derivatives in Streptophyte Algae? Plant Cell Physiol. 2017, 58, 934–945. [Google Scholar] [CrossRef]
- Martone, P.T.; Estevez, J.M.; Lu, F.; Ruel, K.; Denny, M.W.; Somerville, C.; Ralph, J. Discovery of Lignin in Seaweed Reveals Convergent Evolution of Cell-Wall Architecture. Curr. Biol. 2009, 19, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, I.; Pettolino, F.A.; Bacic, A.; Ralph, J.; Lu, F.; O’Neill, M.A.; Fei, Z.; Rose, J.K.C.; Domozych, D.S.; Willats, W.G.T. The charophycean green algae provide insights into the early origins of plant cell walls: Cell-wall evolution and the Charophycean green algae. Plant J. 2011, 68, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Pulido, P.A.; Novoa-Aponte, L.; Villamil, N.; Soto, C.Y. The DosR dormancy regulator of Mycobacterium tuberculosis stimulates the Na (+)/K (+) and Ca (2+) ATPase activities in plasma membrane vesicles of mycobacteria. Curr. Microbiol. 2014, 69, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Torres, C.; Novoa-Aponte, L.; Soto, C.Y. Pma1 is an alkali/alkaline earth metal cation ATPase that preferentially transports Na+ and K+ across the Mycobacterium smegmatis plasma membrane. Microbiol. Res. 2015, 176, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Narrregaard, A.; Vilsen, B.; Andersen, J.P. Chimeric Ca2+-ATPase/Na+,K+-ATPase molecules. Their phosphoenzyme intermediates and sensitivity to Ca2+ and thapsigargin. FEBS Let. 1993, 336, 248–264. [Google Scholar] [CrossRef]
- Geisler, M.; Frangne, N.; Gomès, E.; Martinoia, E.; Palmgren, M.G. The ACA4 Gene of Arabidopsis Encodes a Vacuolar Membrane Calcium Pump That Improves Salt Tolerance in Yeast. Plant Physiol. 2000, 124, 1814–1827. [Google Scholar] [CrossRef]
- Anil, V.S.; Rajkumar, P.; Kumar, P.; Mathew, M.K. A Plant Ca2+ Pump, ACA2, Relieves Salt Hypersensitivity in Yeast: Modulation of Cytosolic Calcium Signature and Activation of Adaptive Na+ Homeostasis. J. Biol. Chem. 2008, 283, 3497–3506. [Google Scholar] [CrossRef]
- Rui, H.; Artigas, P.; Roux, B. The selectivity of the Na+/K+-pump is controlled by binding site protonation and self-correcting occlusion. eLife 2016, 5, e16616. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Qiu, H.; Price, D.C.; Yang, E.C.; Yoon, H.S.; Bhattacharya, D. Evidence of ancient genome reduction in red algae (Rhodophyta). J. Phycol. 2015, 51, 624–636. [Google Scholar] [CrossRef]
- Collen, J.; Porcel, B.; Carre, W.; Ball, S.G.; Chaparro, C.; Tonon, T.; Barbeyron, T.; Michel, G.; Noel, B.; Valentin, K.; et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc. Natl. Acad. Sci. USA 2013, 110, 5247–5252. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.X.; Yang, E.C.; Banerjee, T.; Yoon, H.S.; Martone, P.T.; Estevez, J.M.; Bhattacharya, D. Red and Green Algal Monophyly and Extensive Gene Sharing Found in a Rich Repertoire of Red Algal Genes. Curr. Biol. 2011, 21, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Price, D.C.; Chan, C.X.; Qiu, H.; Rose, N.; Ball, S.; Weber, A.P.M.; Cecilia Arias, M.; Henrissat, B.; Coutinho, P.M.; et al. Genome of the red alga Porphyridium purpureum. Nat. Commun. 2013, 4, 1941. [Google Scholar] [CrossRef] [PubMed]
- Shiryev, S.A.; Papadopoulos, J.S.; Schaffer, A.A.; Agarwala, R. Improved BLAST searches using longer words for protein seeding. Bioinformatics 2007, 23, 2949–2951. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.-Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019, 47, D464–D474. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Buchan, D.W.A.; Jones, D.T. The PSIPRED Protein Analysis Workbench: 20 years on. Nucleic Acids Res. 2019, 47, W402–W407. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Whelan, S.; Goldman, N. A General Empirical Model of Protein Evolution Derived from Multiple Protein Families Using a Maximum-Likelihood Approach. Mol. Biol. Evol. 2001, 18, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Le, S.Q.; Gascuel, O. An Improved General Amino Acid Replacement Matrix. Mol. Biol. Evol. 2008, 25, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. CABIOS 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Isayenkov, S.V. Genetic sources for the development of salt tolerance in crops. Plant Growth Regul. 2019, 89, 1–17. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabravolski, S.A.; Isayenkov, S.V. Evolution of Plant Na+-P-Type ATPases: From Saline Environments to Land Colonization. Plants 2021, 10, 221. https://doi.org/10.3390/plants10020221
Dabravolski SA, Isayenkov SV. Evolution of Plant Na+-P-Type ATPases: From Saline Environments to Land Colonization. Plants. 2021; 10(2):221. https://doi.org/10.3390/plants10020221
Chicago/Turabian StyleDabravolski, Siarhei A., and Stanislav V. Isayenkov. 2021. "Evolution of Plant Na+-P-Type ATPases: From Saline Environments to Land Colonization" Plants 10, no. 2: 221. https://doi.org/10.3390/plants10020221
APA StyleDabravolski, S. A., & Isayenkov, S. V. (2021). Evolution of Plant Na+-P-Type ATPases: From Saline Environments to Land Colonization. Plants, 10(2), 221. https://doi.org/10.3390/plants10020221






