RETRACTED: Antimicrobial and Wound Healing Potential of a New Chemotype from Piper cubeba L. Essential Oil and In Silico Study on S. aureus tyrosyl-tRNA Synthetase Protein
Abstract
1. Introduction
2. Results
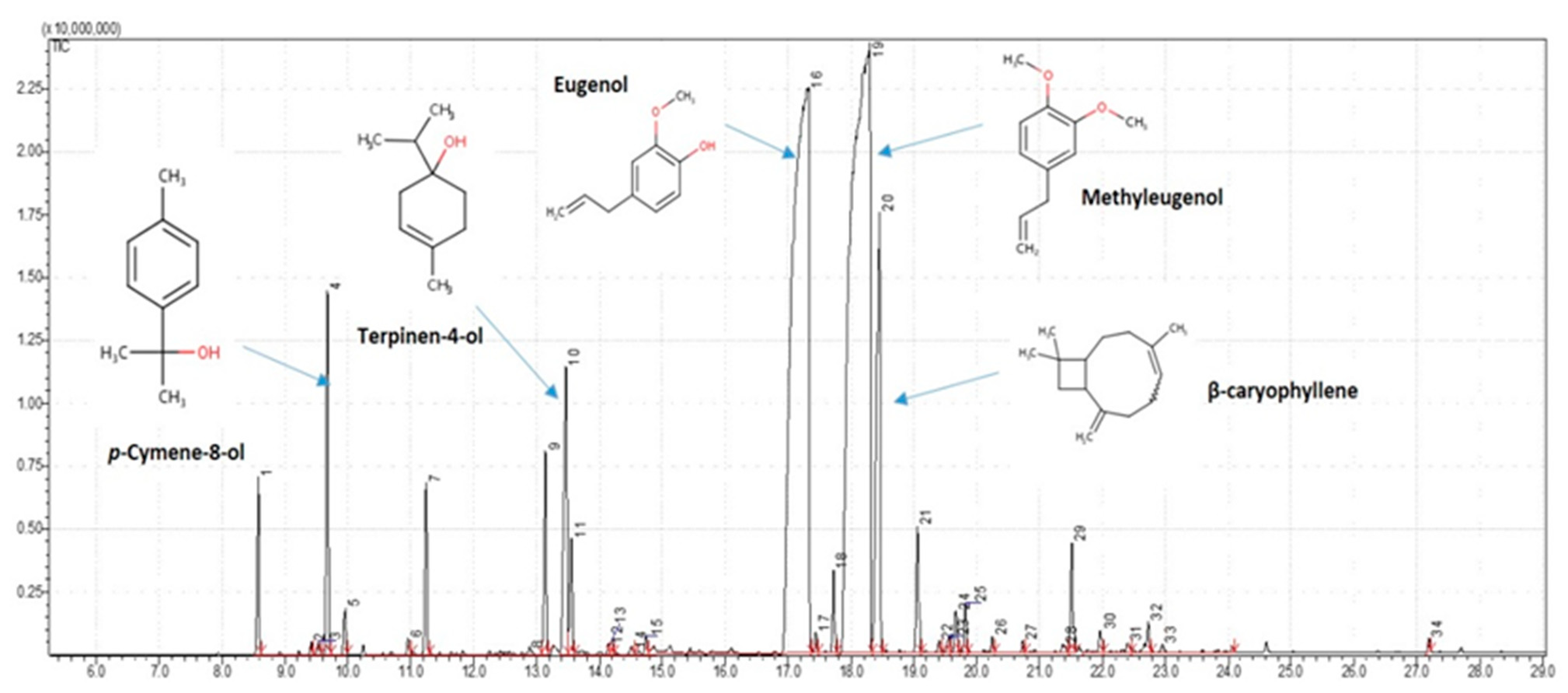
2.1. Phytochemical Descriptions
2.2. Biological Properties
2.2.1. Antimicrobial Activity
2.2.2. Wound Healing Activity
General Characteristics of Animals
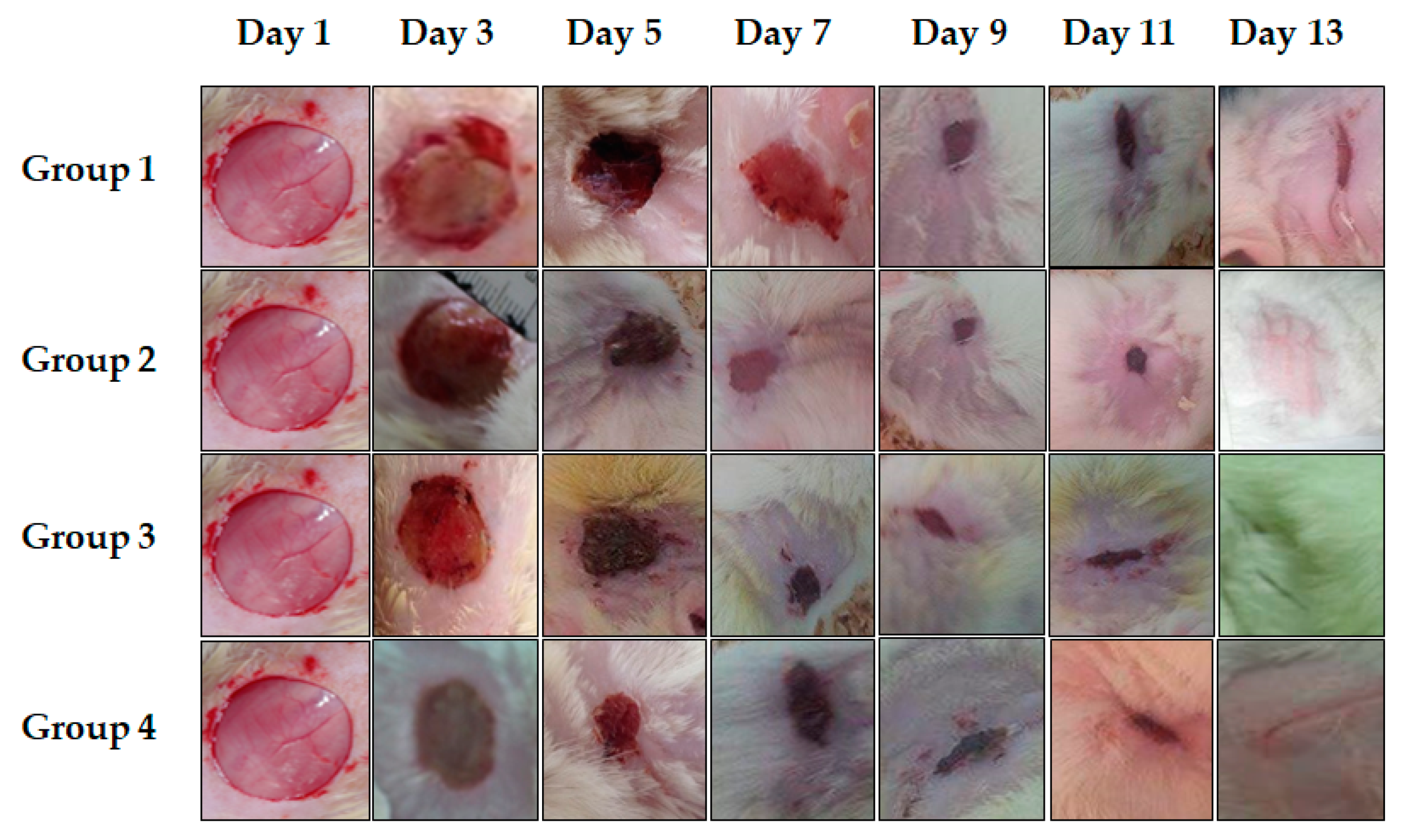
Qualitative Study: Chromatic Assessment of Wounds
Quantitative Study: Evaluation of Percentages of the Contraction of Wounds
Histological Study
Malonedialdehyde (MDA) Level
Effect of Treatment on Superoxide Dismutases (SODs) Activity
2.3. Molecular Docking Study
2.3.1. Binding Energies vs. PCEO Compounds
2.3.2. Receptor–Ligands Interaction Analysis
3. Discussion
3.1. Antimicrobial Activity
3.2. Wound Healing Activity
3.3. Molecular Docking Study vs. Antimicrobial Effect
4. Materials and Methods
4.1. Used Products
4.2. Plant Material and Extraction of Essential Oil
4.3. Chemical Analysis GC/MS
4.4. Antimicrobial Activity
4.4.1. Microbial Strains Used
4.4.2. Determination of MIC, MBC and MFC
4.5. Healing Activity
4.5.1. Used Animals, Experimental Groups and Mechanical Wound Induction
4.5.2. Oxidative Stress
4.5.3. Histopathological Study
4.6. Molecular Docking Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sen, C.K.; Roy, S. Redox signals in wound healing. Biochim. Biophys. Acta 2008, 1780, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS modulating technologies for augmentation of the healing process. Int. Wound J. 2015, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed]
- Andre-Levigne, D.; Modarressi, A.; Pepper, M.S.; Pittet-Cuenod, B. Reactive oxygen species and nox enzymes are emerging as key players in cutaneous wound repair. Int. J. Mol. Sci. 2017, 18, 2149. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef]
- Bryan, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wohlert, S.; Hunt, J.A. Reactive oxygen species (ROS) a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur. Cells Mater. 2012, 24, 249–265. [Google Scholar] [CrossRef]
- Shenoy, R.R.; Sudheendra, A.T.; Nayak, P.G.; Paul, P.; Kutty, N.G.; Rao, C.M. Normal and delayed wound healing is improved by sesamol, an active constituent of Sesamumindicum (L.) in albino rats. J. Ethnopharmacol. 2011, 133, 608–612. [Google Scholar] [CrossRef]
- Felhi, S.; Hajlaoui, H.; Ncir, M.; Bakari, S.; Ktari, N.; Saoudi, M.; Gharsallah, N.; Kadri, A. Nutritional, phytochemical and antioxidant evaluation and FT-IR analysis of freeze dried extracts of Ecballium elaterium fruit juice from three localities. Food Sci. Technol. 2016, 36, 646–655. [Google Scholar] [CrossRef]
- Bakari, S.; Daoud, A.; Felhi, S.; Smaoui, S.; Gharsallah, N.; Kadri, A. Proximate analysis, mineral composition, phytochemical contents, antioxidant and antimicrobial activities and GC-MS investigation of various solvent extracts of cactus cladode. Food Sci. Technol. 2017, 27, 286–293. [Google Scholar] [CrossRef]
- Snoussi, M.; Noumi, E.; Punchappady-Devasya, R.; Trabelsi, N.; Kanekar, S.; Nazarro, F.; Fratianni, F.; Flamini, G.; De Feo, V.; Al-sieni, A. Antioxidant properties and anti-quorum sensing potential of Carum copticum essential oil and phenolics against Chromobacterium violaceum. J. Food Sci. Technol. 2018, 55, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Mseddi, K.; Alimi, F.; Noumi, E.; Veettil, V.N.; Deshpande, S.; Adnan, M.; Hamdi, A.; Elkahoui, S.; Alghamdi, A.; Kadri, A.; et al. Thymus musilii Velen. as a promising source of potent bioactive compounds with its pharmacological properties: In vitro and in silico analysis. Arab. J. Chem. 2020, 13, 6782–6801. [Google Scholar] [CrossRef]
- Bakari, S.; Hajlaoui, H.; Daoud, A.; Mighri, H.; Ross-Garcia, J.M.; Gharsallah, N.; Kadri, A. Phytochemicals, antioxidant and antimicrobial potentials and LC-MS analysis of hydroalcoholic extracts of leaves and flowers of Erodium glaucophyllum collected from Tunisian Sahara. Food Sci. Biotechnol. 2016, 38, 310–317. [Google Scholar] [CrossRef]
- Felhi, S.; Baccouch, N.; Ben Salah, H.; Smaoui, S.; Allouche, N.; Gharsallah, N.; Kadri, A. Nutritional constituents, phytochemical profiles, in vitro antioxidant and antimicrobial properties and gas chromatography-mass spectrometry (GC-MS) analysis of various solvent extracts from grape seeds (Vitis vinifera L.). Food Sci. Biotechnol. 2016, 25, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Ben Mefteh, F.; Daoud, A.; Bouket, A.C.; Thissera, B.; Kadri, Y.; Cherif-Silini, H.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Oszako, T.; et al. Date Palm Trees Root-Derived Endophytes as Fungal Cell Factories for Diverse Bioactive Metabolites. Int. J. Mol. Sci. 2018, 19, 1986. [Google Scholar] [CrossRef]
- Gad-Elkareem, M.A.M.; Abdelgadir, E.H.; Badawy, O.M.; Kadri, A. Potential antidiabetic effect of ethanolic and aqueous-ethanolic extracts of Ricinus communis leaves on streptozotocin-induced diabetes in rats. PeerJ 2019, 7, e6441. [Google Scholar] [CrossRef]
- Snoussi, M.; Dehmani, A.; Noumi, E.; Flamini, G.; Papetti, A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. Strains. Microb. Pathog. 2016, 90, 13–21. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Merghni, A.; Nazzaro, F.; Quindós, G.; Akdamar, G.; Mastouri, M.; Al-Sieni, A.; Ceylan, O. Phytochemical composition, anti-biofilm and anti-quorum sensing potential of fruit, stem and leaves of Salvadora persica L. methanolic extracts. Microb. Pathog. 2017, 109, 169–176. [Google Scholar] [CrossRef]
- Noumi, A.; Merghni, A.; Alreshidi, M.M.; Haddad, O.; Akmadar, G.; De Martino, L.; Mastouri, M.; Ceylan, O.; Snoussi, M.; Al-sieni, A.; et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules 2018, 23, 2672. [Google Scholar] [CrossRef]
- Muscar’a, M.N.; McKnight, W.; Asfaha, S.; Wallace, J.L. Wound collagen deposition in rats: Effects of an NO-NSAID and a selective COX-2 inhibitor. Br. J. Pharmacol. 2000, 129, 681–686. [Google Scholar] [CrossRef]
- Tsala, D.E.; Amadou, D.; Habtemariam, S. Natural wound healing and bioactive natural products. Phytopharmacology 2013, 4, 532–560. [Google Scholar]
- Silva, S.M.M.D.; Costa, C.R.R.; Gelfuso, G.M.; Guerra, E.N.S.; Nóbrega, Y.K.M.; Gomes, S.M.; Pic-Taylor, A.; Fonseca-Bazzo, Y.M.; Silveira, D.; Magalhães, P.O. Wound Healing Effect of Essential Oil Extracted from Eugenia dysenterica DC (Myrtaceae) Leaves. Molecules 2018, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.A.A.; Al-Said, M.S.; Raish, M.; Khaled, J.M.; Alharbi, N.S.; Alatar, A.; Ahmad, A.; Alsohaibani, M.; Al-Yahya, M.; Rafatullah, S. Chemical composition, anti-inflammatory and antioxidant activities of the essential oil of Piper cubeba L. Rom. Biotechnol. Lett. 2017, 22, 12366–12376. [Google Scholar]
- Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies. Plants 2020, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Oliviera, J.D.; Alves, C.C.F.; Miranda, M.L.D.; Martins, C.H.G.; Silva, T.S.; Ambrosio, M.A.L.V.; Alves, J.M.; Silva, J.P. Rendimento, composição química e atividades antimicrobiana e antioxidante do óleo essencial de folhas de Campomanesia adamantium submetidas a diferentes métodos de secagem. Rev. Bras. Plantas Med. 2016, 18, 502–510. [Google Scholar] [CrossRef]
- Chamandi, G.; Olama, Z.; Holail, H. Antimicrobial effect of Propolis from different geographic origins in Lebanon. Int. J. Curr. Microbiol. 2015, 4, 328–342. [Google Scholar]
- Hyldgaard, M.; Mygind, T.; Rikke, L.M. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 1–24. [Google Scholar] [CrossRef]
- Fajemiroye, J.O.; Galdino, M.P.; De Paula, M.A.J.; Rocha, F.F.; Akanmu, M.A.; Vanderlinde, A.F.; Zjawiony, K.J.; Costa, E.A. Anxiolytic and antidepressant like effects of natural food flavour (E)-methyl isoeugenol. Food Funct. 2011, 5, 1819–1828. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Lopez, M.K.N.; Hadisurya, M.; Cornwall, R.G. Antimicrobial Investigation and Structure activity analysis of natural eugenol derivatives against several oral bacteria. J. Pharm. Biol. 2019, 5, 1. [Google Scholar]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, S.K.; Kirubanandan, S.; Sripriya, R.; Sehgal, P.K. Triphala promotes healing of infected full-thickness dermal wound. J. Surg. Res. 2008, 144, 94–101. [Google Scholar]
- Nayak, B.S.; Anderson, M.; Pereire, P. Evaluation of wound healing potential of Catharanthus roseus leaf extract in rats. Fitoterpia 2007, 78, 540–544. [Google Scholar] [CrossRef]
- Ortonne, J.P.; Clevy, J.P. Physiology of cutaneous cicatrization. Rev. Prat. 1994, 44, 1733–1737. [Google Scholar]
- Corsi, R.C.C.; Pirana, S.; Muraco, F.A.E.; Jorge, D. Cicatrizaçäo das feridas; revisäo da literature. Rev. Bras. Cir. Cardiovasc. 1994, 84, 17–24. [Google Scholar]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Tarameshloo, M.; Norouzian, M.; Zarein-Dolab, S.; Dadpay, M.; Mohsenifar, J.; Gazor, R. Aloe vera gel and thyroid hormone cream may improve wound healing in Wistar rats. Nat. Cell Biol. 2012, 45, 170–177. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Nayagam, A.A.J.; Natarajan, R. Wound healing potentials of herbal ointment containing Calendula officinalis Linn. on the alteration of immunological markers and biochemical parameters in excision wounded animals. Clin. Phytosci. 2020, 7, 77. [Google Scholar] [CrossRef]
- Meenakshi, S.; Ragavan, G.; Nath, V.; Ajay Kumar, S.R.; Shanta, M. Antimicrobial, wound healing and antioxidant activity of Plagiochasma appendiculatum. J. Ethanopharmacol. 2006, 1, 67–72. [Google Scholar]
- Shanmugam, K.R.; Mallikarjuna, K.; Kesireddy, N.; Sathyavelu, K. Reddy Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2011, 49, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Hfaiedh, M.; Brahmi, D.; Zourgui, L. Protective Role of Cactus Cladodes Extract on Sodium Dichromate-Induced Testicular Injury and Oxidative Stress in Rats. Biol. Trace Elem. Res. 2014, 159, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Musalmah, M.; Fairuz, A.H.; Gapor, M.T.; Ngah, W.Z.W. Effect of vitamin E on plasma malondialdehyde, antioxidant enzyme levels and the rates of wound closures during wound healing in normal and diabetic rats. Asia Pac. J. Clin. Nutr. 2002, 11, S448–S451. [Google Scholar] [CrossRef] [PubMed]
- Getie, M.; Gebre-Mariam, T.; Rietz, R.; Neubert, R.H. Evaluation of the release profiles of flavonoids from topical formulations of the crude extract of the leaves of Dodonea viscosa (Sapindaceae). Die Pharm. 2002, 57, 320–322. [Google Scholar]
- Jafarian, A.; Zolfaghari, B.; Parnianifard, M. The effects of methanolic, chloroform, and ethylacetate extracts of the Cucurbita pepo L. on the delay type hypersensitivity and antibody production. Res. Pharm. Sci. 2012, 7, 217–224. [Google Scholar]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef]
- Zito, P.; Sajeva, M.; Bruno, M.; Rosselli, S.; Maggio, A.; Senatore, F. Essential oils composition of two Sicilian cultivars of Opuntia ficus-indica (L.) Mill. (Cactaceae) fruits (prickly pear). Nat. Prod. Res. 2013, 27, 1305–1314. [Google Scholar] [CrossRef]
- Bezerra, D.A.C.; Rodrigues, F.F.G.; Costa, J.G.M.; Pereira, A.V.; Sousa, E.O.; Rodrigues, O.G. Abordagem fitoquímica, composição bromatológica e atividade antibacteriana de Mimosa tenuiflora (Wild) Poiret E Piptadenia stipulacea (Benth). Biol. Sci. 2011, 33, 99–106. [Google Scholar] [CrossRef]
- Beg, M.A.; Ansari, S.; Athar, F. Molecular docking studies of Calotropis gigantea phytoconstituents against Staphylococcus aureus tyrosyl-tRNA synthetase protein. J. Bacteriol. Mycol. 2020, 8, 78–91. [Google Scholar]
- Fernandes, F.H.; Guterres, Z.R.; Violante, I.M.P.; Lopes, T.F.S.; Garcez, W.S.; Garcez, F.R. Evaluation of mutagenic and antimicrobial properties of brown propolis essential oil from the Brazilian Cerrado biome. Toxicol. Rep. 2015, 2, 1482–1488. [Google Scholar] [CrossRef]
- Bougatsos, C.; Ngassapa, O.; Runyoro, D.K.; Chinou, I.B. Chemical composition and in vitro antimicrobial activity of the essential oils of two Helichrysum species from Tanzania. Z. Naturforsch. 2004, 59, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.M.F.; Morais, S.A.L.; Vieira, R.B.K.; Napolitano, D.R.; Guzman, V.B.; Moraes, T.S.; Cunha, L.C.S.; Martins, C.H.G.; Chang, R.; Oliveira, A.; et al. Chemical composition, cytotoxic, and antibacterial activity of the essential oil from Eugenia calycina Cambess. leaves against oral bacteria. Ind. Crops Prod. 2015, 65, 71–78. [Google Scholar] [CrossRef]
- Tan, N.; Satana, D.; Sem, B.; Tan, E.; Altan, H.B.; Demirci, B.; Uzun, M. Antimycobacterial and antifungal activities of selected four Salvia species. Rec. Nat. Prod. 2016, 10, 593–603. [Google Scholar]
- Hilmer, S.N.; Tran, K.; Rubie, P.; Wright, J.; Gnjidic, D.; Mitchell, S.J.; Matthews, S. Gentamicin pharmacokinetics in old age and frailty. Br. J. Clin. Pharmacol. 2011, 71, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Brehm-Stecher, B.F.; Johnson, E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 2003, 47, 3357–3360. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.F.; Costa, J.G.; Coutinho, H.D. Enhancement of the antibiotic activity of gentamicin by volatile compounds of Zanthoxylum articulatum. Indian J. Med. Res. 2010, 131, 833–835. [Google Scholar]
- Skaltsa, H.D.; Demetzos, C.; Lazari, D.; Sokovic, M. Essential oil analysis and antimicrobial activity of eight Stachys species from Greece. Phytochemistry 2003, 64, 743–752. [Google Scholar] [CrossRef]
- Pérez-López, A.; Cirio, A.T.; Rivas-Galindo, V.M.; Salazar, A.R.; Torres, N.W. Activity against Streptococcus pneumoniae of the Essential Oil and δ-Cadinene Isolated from Schinus molle Fruit. J. Essent. Oil Res. 2011, 23, 25–28. [Google Scholar] [CrossRef]
- Yang, D.; Michel, L.; Chaumont, J.P.; Millet-Clerc, J. Use of caryophyllene oxide as an antifungal agent in an in vitro experimental model of onychomycosis. Mycopathologia 1999, 148, 79–82. [Google Scholar] [CrossRef]
- Guillen, M.D.; Cabo, N.; Burillo, J. Characterisation of the essential oils of some cultivated aromatic plants of industrial interest. J. Sci. Food Agric. 1996, 70, 359–363. [Google Scholar] [CrossRef]
- Moreira, R.R.D.; Martins, G.Z.; Botelho, V.T.; Santos, L.E.; Cavaleiro, C.; Salgueiro, L.; Andrade, G.; Martins, C.H.G. Composition and activity against oral pathogens of the essential oil Melampodium divaricatum (Rich.) DC. Chem. Biodivers. 2014, 11, 438–444. [Google Scholar] [CrossRef]
- Silva, E.A.J.; Estevam, E.B.B.; Silva, T.S.; Nicolella, H.D.; Furtado, R.A.; Alves, C.C.F.; Souchie, E.L.; Martins, C.H.G.; Tavares, D.C.; Barbosa, L.C.A.; et al. Antibacterial and antiproliferative activities of the fresh leaf essential oil of Psidium guajava L. (Myrtaceae). Braz. J. Biol. 2019, 79, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Ghannay, S.; Bakari, S.; Ghabi, A.; Kadri, A.; Msaddek, M.; Aouadi, K. Stereoselective synthesis of enantiopure N-substituted pyrrolidin-2,5-dione derivatives by 1,3-dipolar cycloaddition and assessment of their in vitro antioxidant and antibacterial activities. Bioorg. Med. Chem. Lett. 2017, 27, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Ghannay, S.; Kadri, A.; Aouadi, K. Synthesis, in vitro antimicrobial assessment, and computational investigation of pharmacokinetic and bioactivity properties of novel trifluoromethylated compounds using in silico ADME and toxicity prediction tools. Monatsh. Chem. 2020, 151, 267–280. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for ligand-receptor docking. Curr. Protoc. Bioinform. 2008, 24, 8–14. [Google Scholar] [CrossRef]
- Pedretti, A.; Villa, L.; Vistoli, G. VEGA—An open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J. Comput. Aided Mol. Des. 2004, 18, 167–173. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Dassault Systemes BIOVIA. BIOVIA Discovery Studio Visualizer; v16.1.0.15350; Dassault Systemes: San Diego, CA, USA, 2015. [Google Scholar]





| Microorganisms Tested | Inhibition Zones Diameter (mm) | PCEO | |||
|---|---|---|---|---|---|
| PCEO | Chloramphenicol | MIC mg/mL | MBC mg/mL | MBC/MIC Ratio | |
| Gram-positive bacteria | |||||
| Bacillus cereus (JN 934390) | 15.0 ± 0.6 a | 26.0 ± 1.0 b | 3.12 | 12.5 | 4 |
| Bacillus subtilis (JN 934392) | 16.0 ± 0.7 a | 24.0 ± 0.0 b | 6.25 | 12.5 | 2 |
| Staphylococcus aureus (ATCC 6538) | 19.5 ± 1.0 b | 16.5 ± 0.5 a | 1.56 | 3.12 | 2 |
| Listeria monocytogenes (ATCC 19115) | 19.0 ± 0.5 b | 12.0 ± 2.0 a | 1.56 | 3.12 | 2 |
| Micrococcus luteus (NCIMB 8166) | 16.0 ± 0.8 a | 20.0 ± 0.0 b | 1.56 | 6.25 | 4 |
| Gram-negative bacteria | |||||
| Klebsiella pneumoniae (ATCC 10031) | 13.0 ± 0.8 a | 22.0 ± 1.0 b | 3.12 | 6.25 | 2 |
| Salmonella enterica (ATCC 43972) | 23.0 ± 0.8 b | 16.0 ± 0.0 a | 12.5 | 25 | 2 |
| Salmonella typhimurium (ATCC 19430) | 13.5 ± 1.5 a | 17.0 ± 1.0 b | 6.25 | 12.5 | 2 |
| Escherichia coli (ATCC 25922) | 21.0 ± 0.8 a | 23.5 ± 0.5 b | 3.12 | 6.25 | 2 |
| Fungal strains | PCEO | Cycloheximide | MIC | MFC | MFC/MIC Ratio |
| Pythium catenulatum (AY598675) | 13.0 ± 0.5 a | 17.5 ± 1.5 b | 6.25 | 25 | 4 |
| Fusarium oxysporum (AB586994) | 17.0 ± 0.1 a | 20.0 ± 2.0 b | 3.12 | 12.5 | 4 |
| Fusarium phyllophilum (AB587006) | 0.0 ± 0.0 a | 14.5 ± 0.5 b | - | - | - |
| Fusarium sp. (JX391934) | 15.0 ± 0.8 a | 18.0 ± 1.5 b | 3.12 | 12.5 | 4 |
| Test Groups | Weights (g) | |
|---|---|---|
| Before Treatment | After Treatment (Day 13) | |
| Group 1: control rats treated with physiological saline | 188.4 ± 9.6 aA | 207.0 ± 8.8 bA |
| Group 2: rats treated with cream without PCEO | 189.6 ± 10.4 aA | 205.7 ± 8.0 bA |
| Group 3: rats treated with PCEO | 188.8 ± 13.5 aA | 226.2 ± 7.9 bC |
| Group 4: rats treated with the reference “Cicaflora®” | 187.3 ± 9.5 aA | 215.0 ± 5.8 bB |
| Day | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 13 |
|---|---|---|---|---|---|---|---|
| Group 1 | 3.70 ± 0.48 eC | 2.69 ± 0.15 eAB | 2.21 ± 0.26 dA | 1.46 ± 0.25 cA | 0.46 ± 0.08 bAB | 0.26 ± 0.08 abA | 0.19 ± 0.04 ab |
| Group 2 | 3.11 ± 0.84 fA | 2.63 ± 0.52 eAB | 2.15 ± 0.80 dA | 1.45 ± 0.28 cA | 0.37 ± 0.14 bA | 0.25 ± 0.06 abA | 0.17 ± 0.04 Ab |
| Group 3 | 3.43 ± 0.64 cB | 2.55 ± 0.36 cA | 2.59 ± 0.38 cb | 2.44 ± 0.98 cC | 0.60 ± 0.14 bB | 0.22 ± 0.05 abA | 0.11 ± 0.02 aA |
| Group 4 | 3.70 ± 0.37 fC | 2.80 ± 0.39 eB | 2.52 ± 0.40 Db | 1.80 ± 0.41 cB | 0.48 ± 0.12b AB | 0.34 ± 0.20 aB | 0.28 ± 0.18 aC |
| With Wounds | Without Wounds | ||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group A | |
| Normal | Cream (−) PCEO | Cream (+) PCEO | Reference | Not Treated | |
| MDA | 1.67 ± 0.56 d | 1.19 ± 0.41 c | 0.78 ± 0.18 b | 0.61 ± 0.24 b | 0.43 ± 0.28 a |
| With Wounds | Without Wounds | ||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group A | |
| Normal | Cream (−) PCEO | Cream (+) PCEO | Reference | Not Treated | |
| SOD | 80.64 ± 9.01 a | 121.37 ± 21.77 b | 148.58 ± 44.13 c | 144.67 ± 38.87 c | 165.87 ± 8.99 d |
| Compounds | Interacting Residues Receptor vs. Targets 1JIJ | Binding Energy (kcal/mol) |
|---|---|---|
| Methyleugenol (Main compound 1) | van der Waals: Gly38, Asp40, Leu70, Thr75, Asp80, Asn124, Tyr170, Gln174, Gly 192, Gly193, Gln196. H-bond: Asp177 (3.77). C-H bond: Pro45 (3.45). Alkyl: Cys37 (4.75) | −5.8 |
| Eugenol (Main compound 2) | van der Waals: Tyr36, Gly38, Asp40, Gly72, Thr75, Asn124, Tyr170, Gln174, Asp177, Gln190, Val191, Gly192, Gln196, Ile200. Alkyl/Pi-Alkyl: Cys37 (4.39), Leu70 (4.84) | −6.3 |
| δ-cadinene | van der Waals: Gly38, Asp40, Asp80, Tyr170, Gln174, Thr75, Gln190, Gly192, Gly193, Gln196, Ile200. Alkyl/Pi-Alkyl: Tyr36 (4.63), Cys37 (4.65), Ala39 (4.35), Leu70 (3.99) (5.18) | 7.1 |
| β-caryophyllene oxide | van der Waals: Gly38, Asp40, Thr42, His47, Thr75, Asp80, Ser82, Lys84, Arg88, Tyr170, Gln174, Asp195, Gln196. H-bond: His50 (2.19). Alkyl/Pi-Alkyl: Ala39 (5.12), His50 (4.78) | −6.5 |
| Viridiflorol | van der Waals: Tyr36, Cys37, Gly38, Ala39, Asp40, Gly72, Thr75, Asp80, Asn124, Tyr170, Gln174, Asp177, Gln190, Val191, Glu192, Gln196, Ile200. Alkyl: Leu70 (4.71) (5.33) | −7.2 |
| Spathulenol | van der Waals: Cys37, Ala39, Gly38, Asp40, Thr75, Asp80, Asn124, Asp177, Gln190, Gly 192, Gln196, Asn199, Ile200. C-H bond: Gln174 (1.81). Alkyl/Pi-Alkyl: Tyr36 (4.88) (5.35), Leu70 (4.24) (5.28), Tyr 170 (5.31) | −6.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri, A. RETRACTED: Antimicrobial and Wound Healing Potential of a New Chemotype from Piper cubeba L. Essential Oil and In Silico Study on S. aureus tyrosyl-tRNA Synthetase Protein. Plants 2021, 10, 205. https://doi.org/10.3390/plants10020205
Alminderej F, Bakari S, Almundarij TI, Snoussi M, Aouadi K, Kadri A. RETRACTED: Antimicrobial and Wound Healing Potential of a New Chemotype from Piper cubeba L. Essential Oil and In Silico Study on S. aureus tyrosyl-tRNA Synthetase Protein. Plants. 2021; 10(2):205. https://doi.org/10.3390/plants10020205
Chicago/Turabian StyleAlminderej, Fahad, Sana Bakari, Tariq I. Almundarij, Mejdi Snoussi, Kaïss Aouadi, and Adel Kadri. 2021. "RETRACTED: Antimicrobial and Wound Healing Potential of a New Chemotype from Piper cubeba L. Essential Oil and In Silico Study on S. aureus tyrosyl-tRNA Synthetase Protein" Plants 10, no. 2: 205. https://doi.org/10.3390/plants10020205
APA StyleAlminderej, F., Bakari, S., Almundarij, T. I., Snoussi, M., Aouadi, K., & Kadri, A. (2021). RETRACTED: Antimicrobial and Wound Healing Potential of a New Chemotype from Piper cubeba L. Essential Oil and In Silico Study on S. aureus tyrosyl-tRNA Synthetase Protein. Plants, 10(2), 205. https://doi.org/10.3390/plants10020205





