Morphological and Chemical Evaluations of Leaf Surface on Particulate Matter2.5 (PM2.5) Removal in a Botanical Plant-Based Biofilter System
Abstract
:1. Introduction
2. Materials and Methods
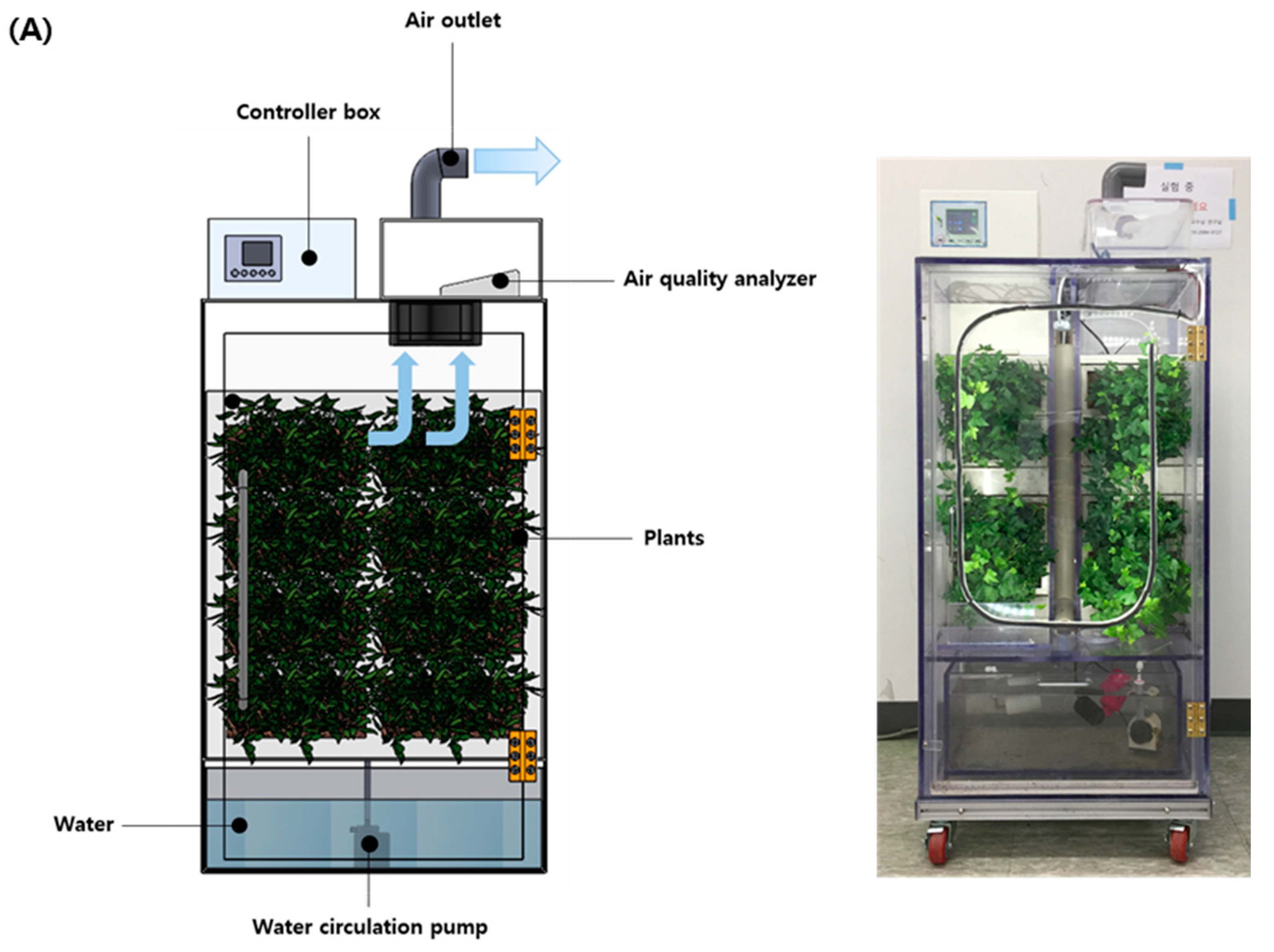
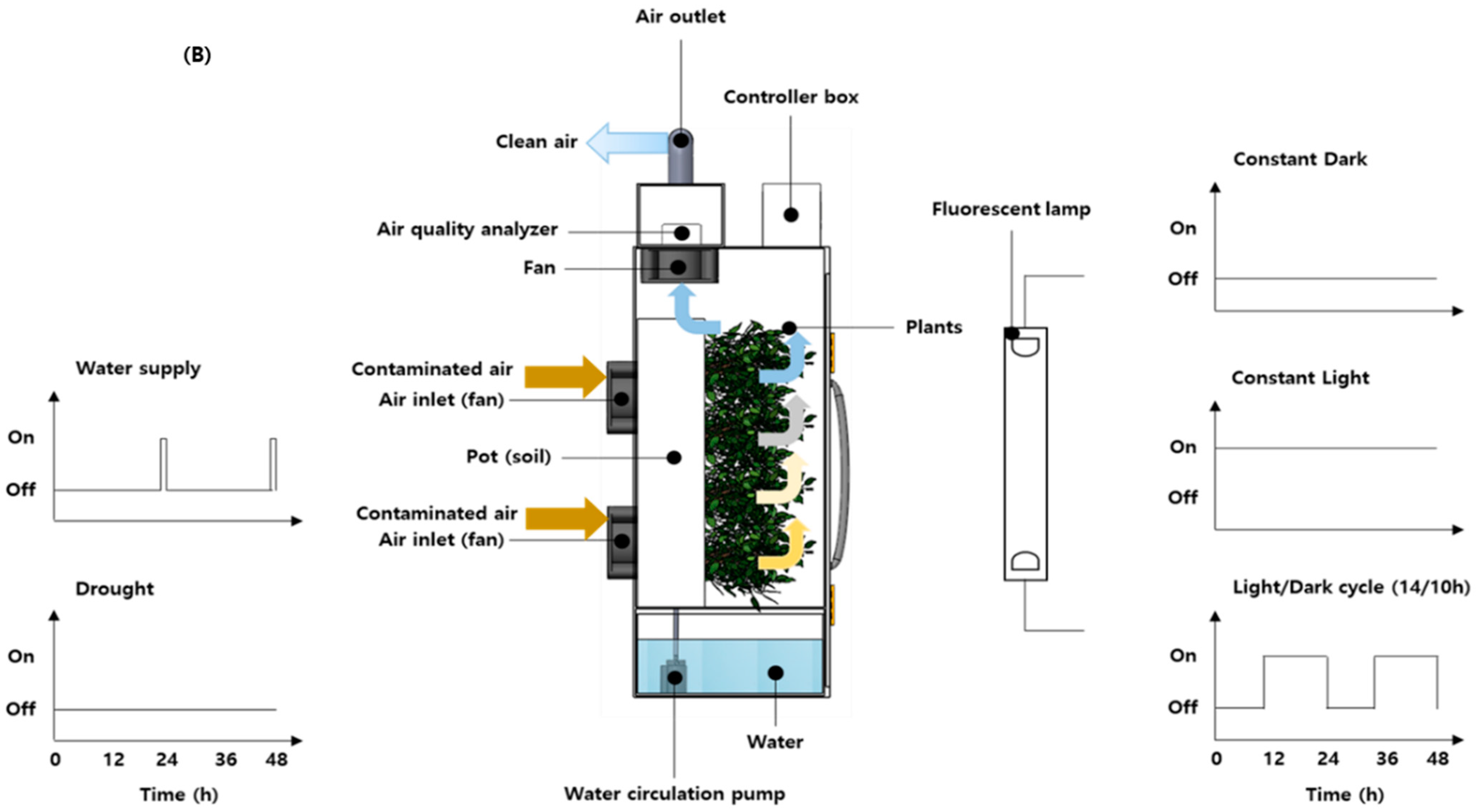
2.1. Experiment Design for the Biofilter System Containing Indoor Plants
2.2. Observation of A. japonica Leaf
2.3. Determination of Air Quality
2.4. Statistical Analysis
3. Results
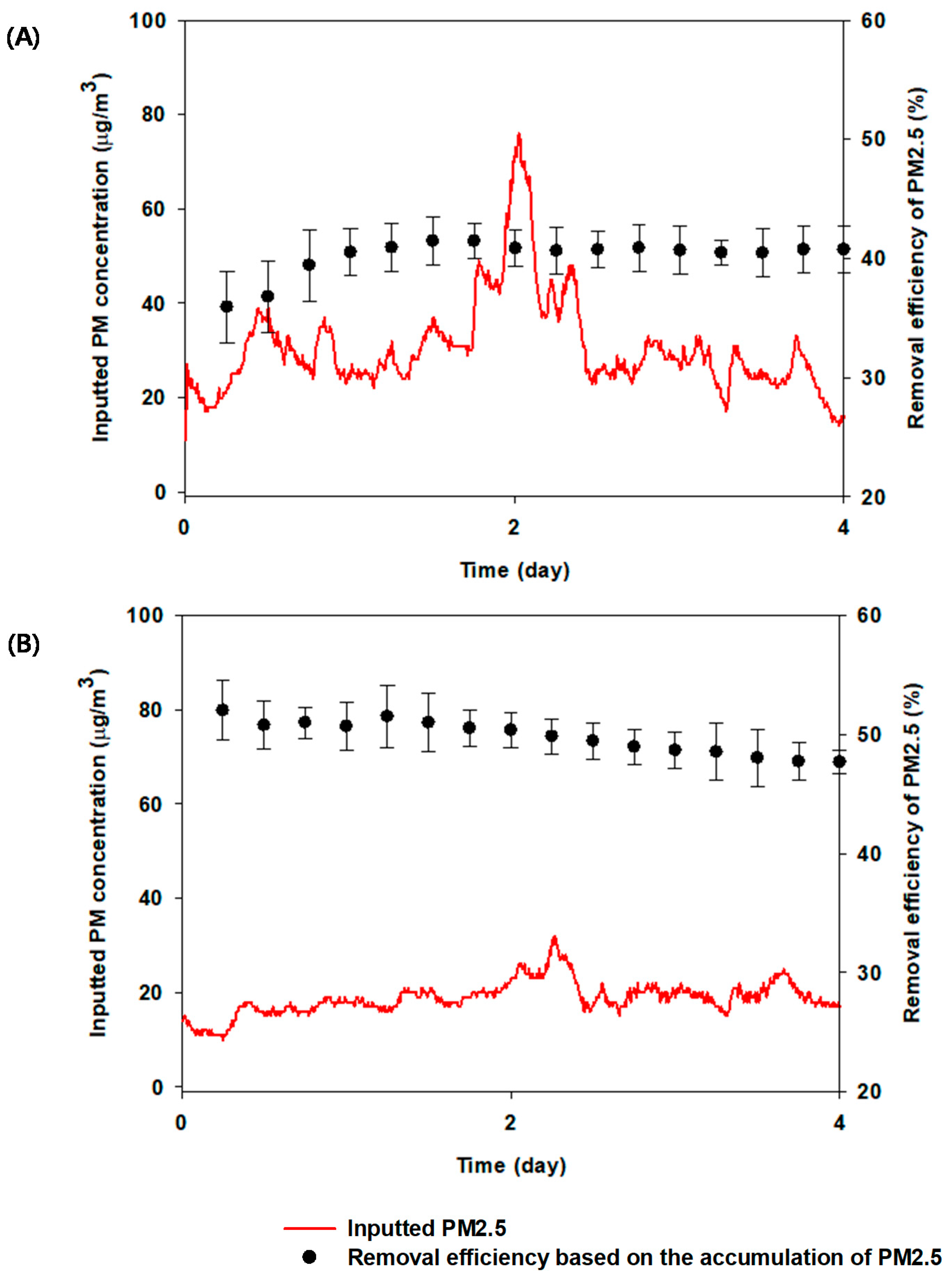
3.1. Evaluation of PM2.5 Capture and Air Quality Using Biofilter System in Parallel
3.2. Influences of Drought on PM2.5 Capture and Air Quality Using Biofilter System in Serial
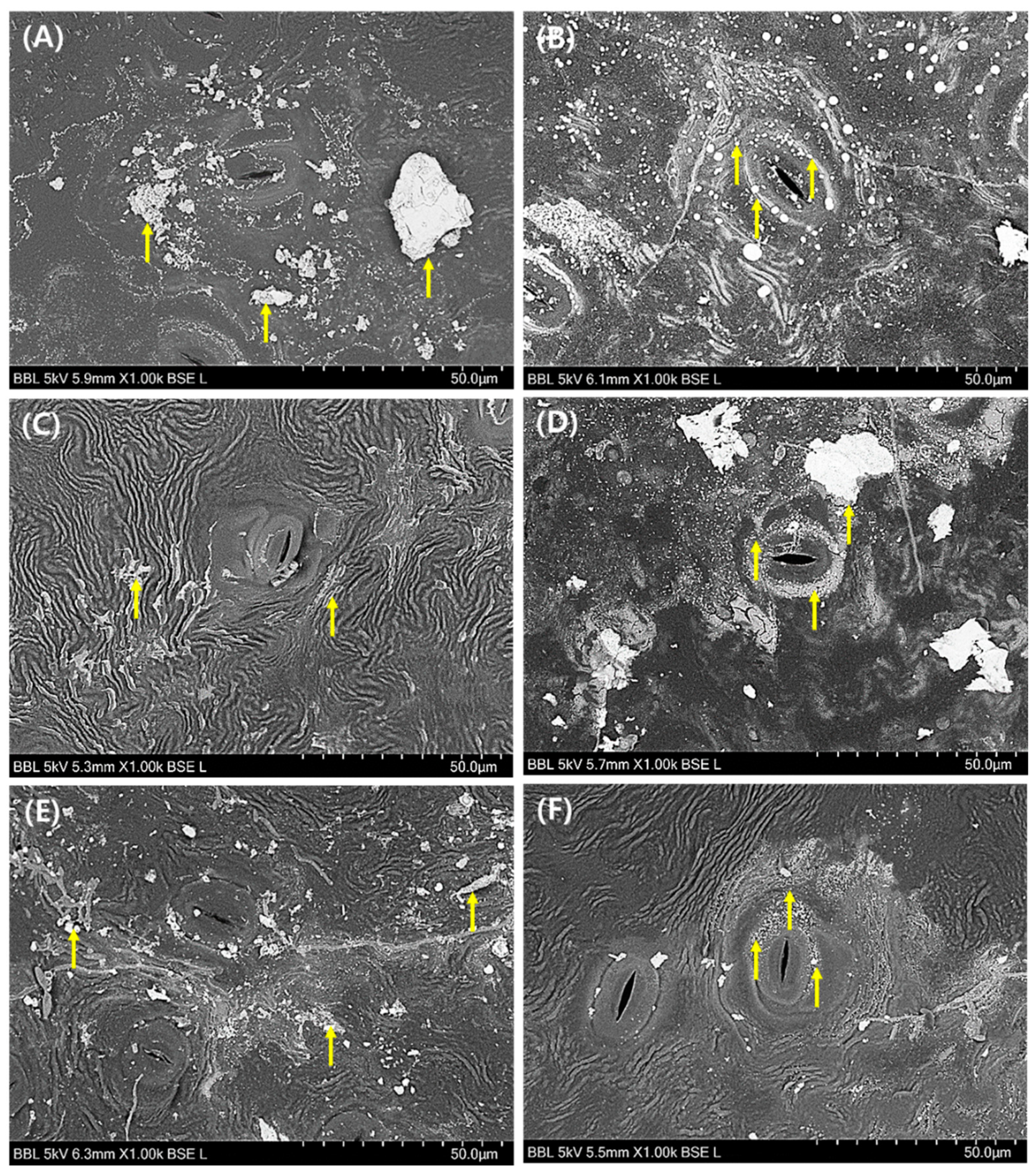
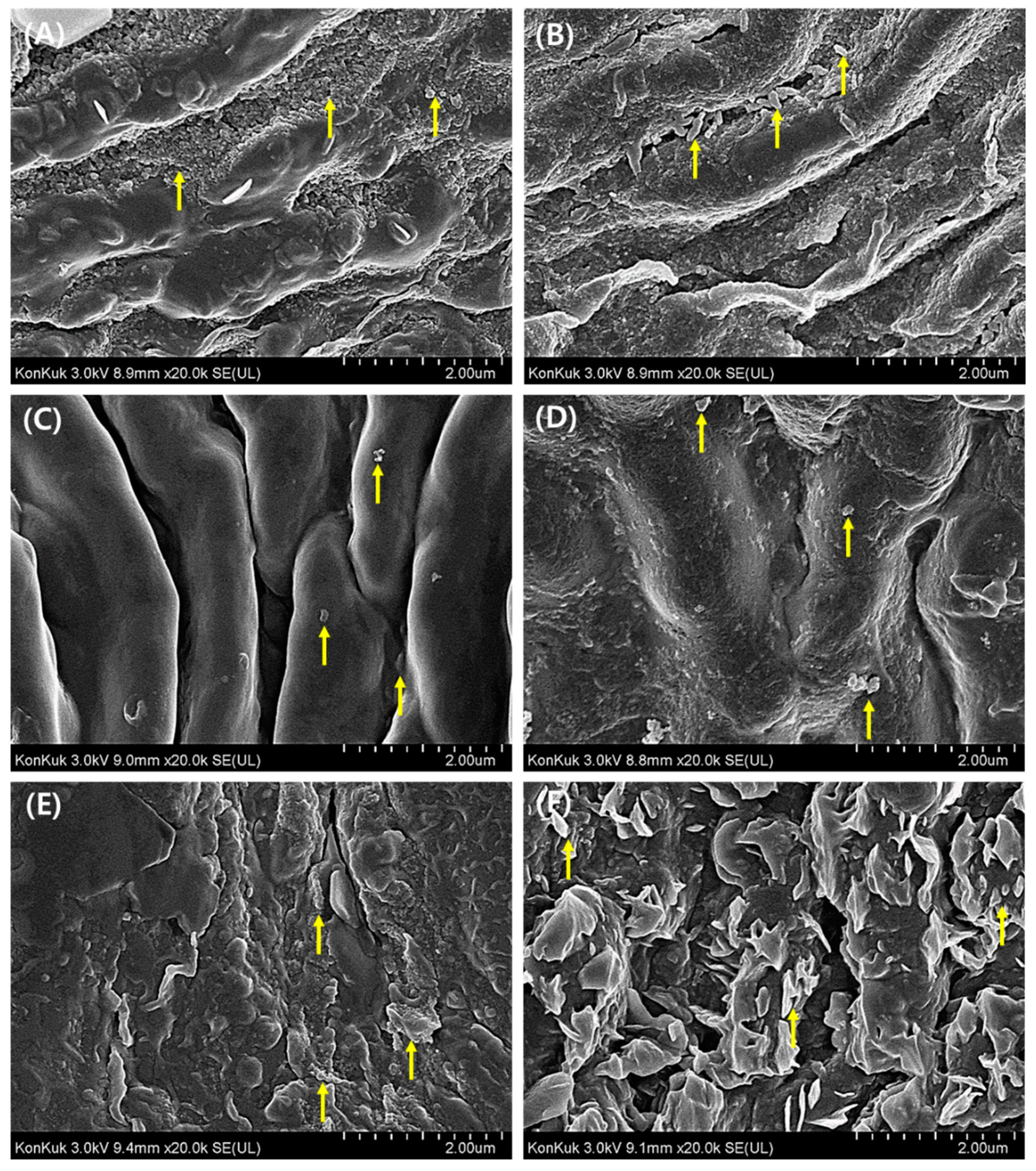
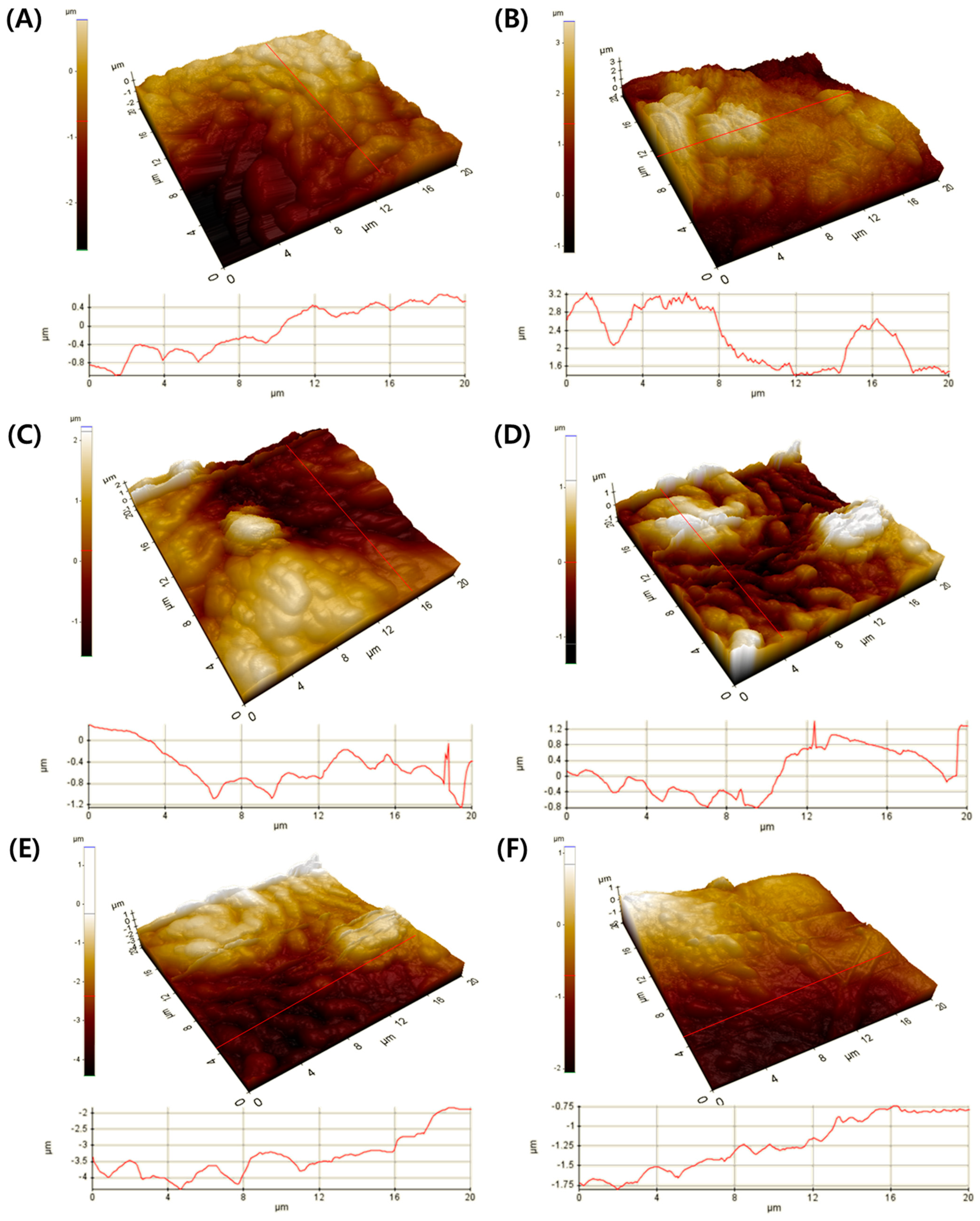
3.3. Morphological Evaluation of Leaf Surface on PM2.5 Removal
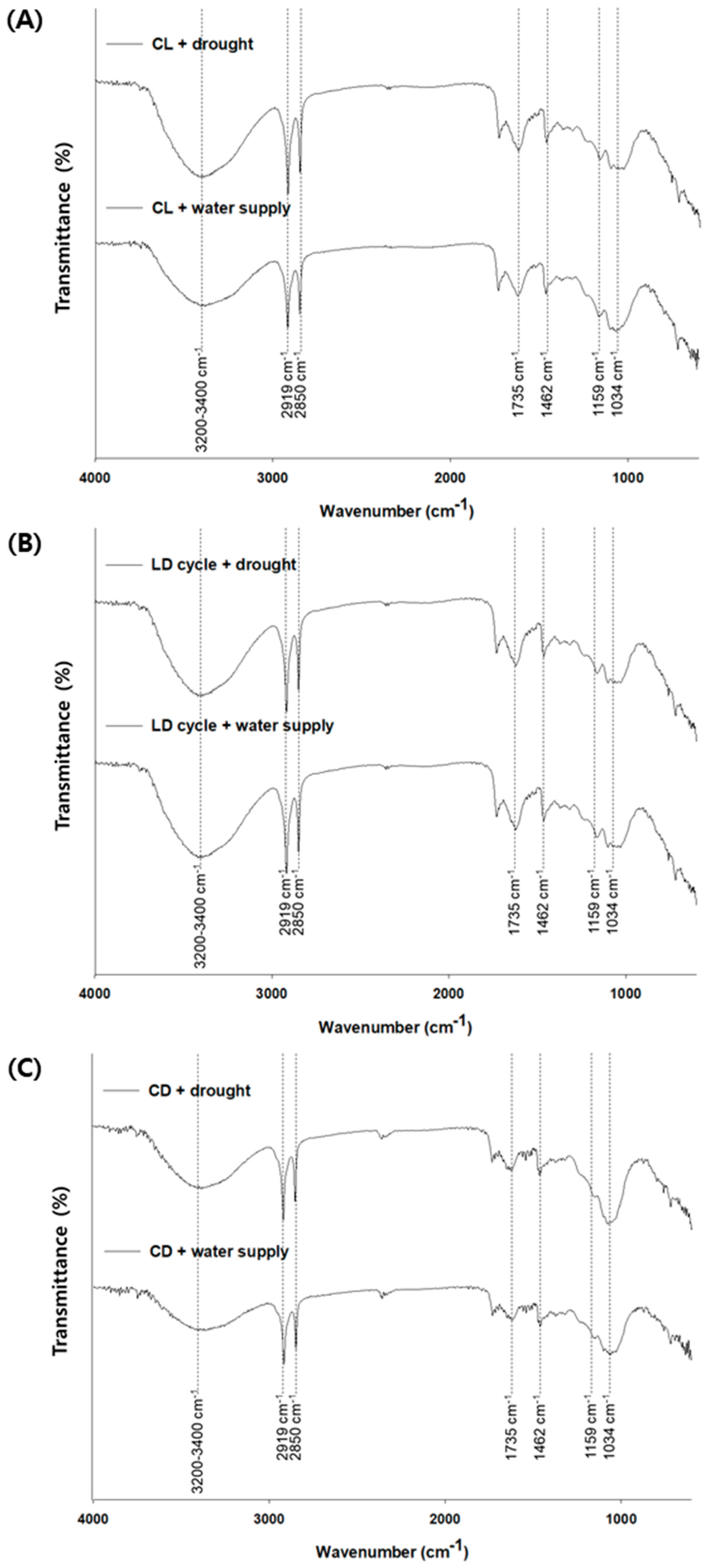
3.4. Chemical Evaluation of Leaf Surface for PM2.5 Removal
3.5. Comparison of PM2.5 Removal between H. helix and A. japonica
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Zhang, Z.; Bao, L.; Mo, L.; Yu, X.; Fan, D.; Lun, X. Influence of rainfall duration and intensity on particulate matter removal from plant leaves. Sci. Total Environ. 2017, 609, 11–16. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.J.; Lee, S.J. Efficient removal of indoor particulate matter using water microdroplets generated by a MHz-frequency ultrasonic atomizer. Build. Environ. 2020, 175, 106797. [Google Scholar] [CrossRef]
- Han, K.-T.; Ruan, L.-W. Effects of indoor plants on air quality: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 16019–16051. [Google Scholar] [CrossRef] [PubMed]
- Yeo, L.B. Psychological and physiological benefits of plants in the indoor environment: A mini and in-depth review. Int. J. Built Environ. Sustain. 2021, 8, 57–67. [Google Scholar] [CrossRef]
- Pettit, T.; Irga, P.; Abdo, P.; Torpy, F. Do the plants in functional green walls contribute to their ability to filter particulate matter? Build. Environ. 2017, 125, 299–307. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, W.; Mo, L.; Heal, M.R.; Xu, X.; Yu, X. Quantifying particulate matter accumulated on leaves by 17 species of urban trees in Beijing, China. Environ. Sci. Pollut. Res. 2018, 25, 12545–12556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, Z.; Chen, L.; McNulty, S. An investigation on the leaf accumulation-removal efficiency of atmospheric particulate matter for five urban plant species under different rainfall regimes. Atmos. Environ. 2019, 208, 123–132. [Google Scholar] [CrossRef]
- Shao, F.; Wang, L.; Sun, F.; Li, G.; Yu, L.; Wang, Y.; Zeng, X.; Yan, H.; Dong, L.; Bao, Z. Study on different particulate matter retention capacities of the leaf surfaces of eight common garden plants in Hangzhou, China. Sci. Total Environ. 2019, 652, 939–951. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, E.B.; Kim, J.E.; Song, H.J.; Choi, Y.K.; Kim, K.J.; Kumaran, R.S.; Lee, S.H.; Yang, Y.H.; Kim, H.J. Monitoring plant moisture content using an induction coil sensor. Bull. Korean Chem. Soc. 2019, 40, 1138–1141. [Google Scholar] [CrossRef]
- Dubey, P.; Sharma, P.; Kumar, V. Ftir and GC–MS spectral datasets of wax from Pinus roxburghii Sarg. needles biomass. Data Brief 2017, 15, 615–622. [Google Scholar] [CrossRef]
- Soffe, R.; Bernach, M.; Remus-Emsermann, M.N.; Nock, V. Replicating arabidopsis model leaf surfaces for phyllosphere microbiology. Sci. Rep. 2019, 9, 14420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, Z.; Bhushan, B. Surface characterization and adhesion and friction properties of hydrophobic leaf surfaces. Ultramicroscopy 2006, 106, 709–719. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by aba in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef]
- Burkhardt, J.; Peters, K.; Crossley, A. The presence of structural surface waxes on coniferousneedles affects the pattern of dry deposition of fine particles. J. Exp. Bot. 1995, 46, 823–831. [Google Scholar] [CrossRef]
- Treesubsuntorn, C.; Thiravetyan, P. Removal of benzene from indoor air by Dracaena sanderiana: Effect of wax and stomata. Atmos. Environ. 2012, 57, 317–321. [Google Scholar] [CrossRef]
- Treesubsuntorn, C.; Suksabye, P.; Weangjun, S.; Pawana, F.; Thiravetyan, P. Benzene adsorption by plant leaf materials: Effect of quantity and composition of wax. Water Air Soil Pollut. 2013, 224, 1736. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Gore, M.A.; Andrade-Sanchez, P.; French, A.N.; Hunsaker, D.J.; Salvucci, M.E. Decreased CO2 availability and inactivation of rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 2012, 83, 1–11. [Google Scholar] [CrossRef]
- Baitimirova, M.; Katkevics, J.; Baumane, L.; Bakis, E.; Viksna, A. Characterization of functional groups of airborne particulate matter. IOP Conf. Ser. Mater. Sci. Eng. 2013, 49, 012025. [Google Scholar] [CrossRef] [Green Version]
- Räsänen, J.V.; Yli-Pirilä, P.; Holopainen, T.; Joutsensaari, J.; Pasanen, P.; Kivimäenpää, M. Soil drought increases atmospheric fine particle capture efficiency of norway spruce. Boreal Environ. Res. 2012, 17, 21–30. [Google Scholar]
- Hong, J.; Peralta-Videa, J.R.; Rico, C.; Sahi, S.; Viveros, M.N.; Bartonjo, J.; Zhao, L.; Gardea-Torresdey, J.L. Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2014, 48, 4376–4385. [Google Scholar] [CrossRef] [PubMed]
- Schreck, E.; Foucault, Y.; Sarret, G.; Sobanska, S.; Cécillon, L.; Castrec-Rouelle, M.; Uzu, G.; Dumat, C. Metal and metalloid foliar uptake by various plant species exposed to atmospheric industrial fallout: Mechanisms involved for lead. Sci. Total Environ. 2012, 427, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, J.-Y.; Lee, J.-T.; Kim, K.-H.; Jung, K.; Bell, M.L. Characterization of fine particulate matter and associations between particulate chemical constituents and mortality in Seoul, Korea. Environ. Health Perspect. 2012, 120, 872–878. [Google Scholar] [CrossRef] [Green Version]
- Kwon, K.-J.; Park, B.-J. Particulate matter removal of indoor plants, Dieffenbachia amoena ‘marianne’ and Spathiphyllum spp. according to light intensity. J. Korean Inst. Landsc. Archit. 2018, 46, 62–68. [Google Scholar] [CrossRef]
- Cheng, Y.; He, D.; He, J.; Niu, G.; Gao, R. Effect of light/dark cycle on photosynthetic pathway switching and CO2 absorption in two dendrobium species. Front. Plant Sci. 2019, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Räsänen, J.V.; Holopainen, T.; Joutsensaari, J.; Pasanen, P.; Kivimäenpää, M. Particle capture efficiency of different-aged needles of Norway spruce under moderate and severe drought. Can. J. For. Res. 2014, 44, 831–835. [Google Scholar] [CrossRef] [Green Version]
- Turunen, M.; Huttunen, S. A review of the response of epicuticular wax of conifer needles to air pollution. J. Environ. Qual. 1990, 19, 35–45. [Google Scholar] [CrossRef]
- Popek, R.; Gawrońska, H.; Wrochna, M.; Gawroński, S.W.; Sæbø, A. Particulate matter on foliage of 13 woody species: Deposition on surfaces and phytostabilisation in waxes—A 3-year study. Int. J. Phytoremediation 2013, 15, 245–256. [Google Scholar] [CrossRef]

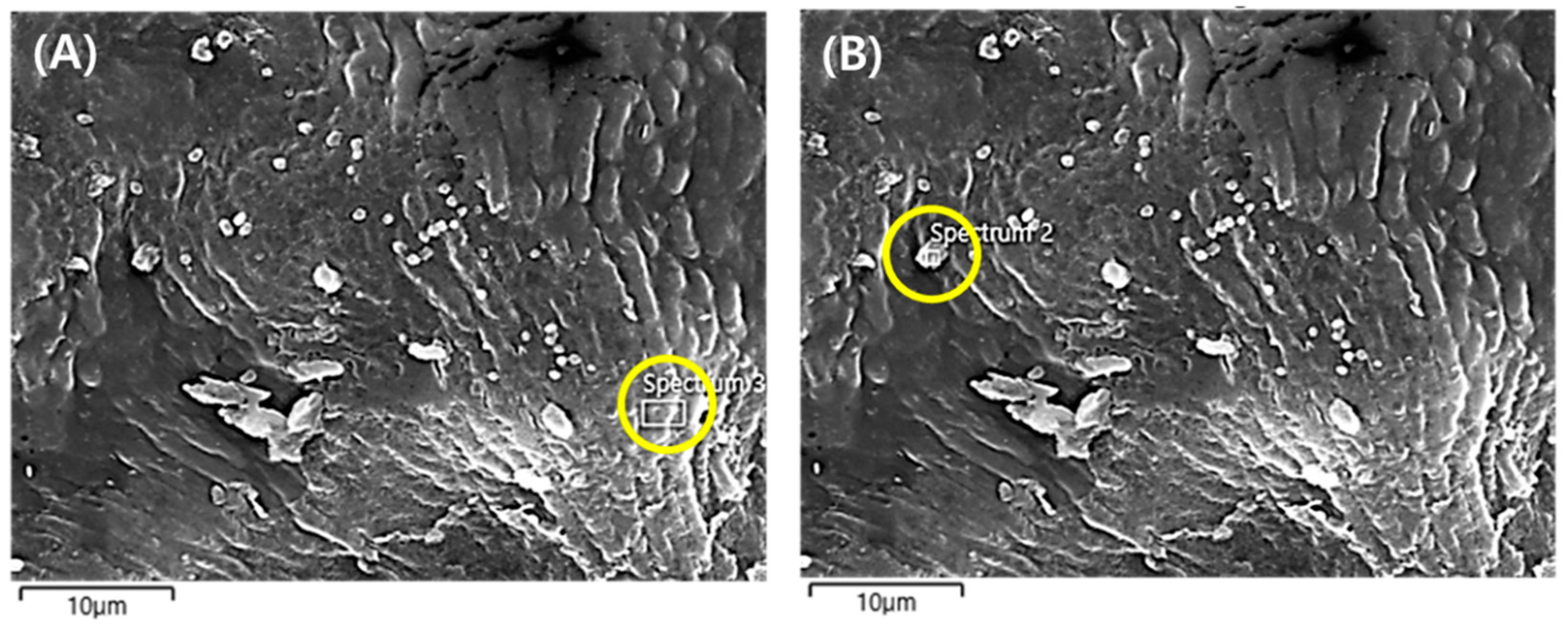
| C | O | Si | S | Cl | K | Ca | Pd | Pt | Total (wt%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Surface layer | 75.02 | 8.86 | 1.20 | - | 1.63 | 4.73 | 0.84 | 1.62 | 6.1 | 100 |
| Particle | 57.97 | 27.71 | 0.32 | 3.26 | 0.33 | 0.93 | 4.31 | 1.16 | 4.01 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-K.; Song, H.-J.; Jo, J.-W.; Bang, S.-W.; Park, B.-H.; Kim, H.-H.; Kim, K.-J.; Jeong, N.-R.; Kim, J.-H.; Kim, H.-J. Morphological and Chemical Evaluations of Leaf Surface on Particulate Matter2.5 (PM2.5) Removal in a Botanical Plant-Based Biofilter System. Plants 2021, 10, 2761. https://doi.org/10.3390/plants10122761
Choi Y-K, Song H-J, Jo J-W, Bang S-W, Park B-H, Kim H-H, Kim K-J, Jeong N-R, Kim J-H, Kim H-J. Morphological and Chemical Evaluations of Leaf Surface on Particulate Matter2.5 (PM2.5) Removal in a Botanical Plant-Based Biofilter System. Plants. 2021; 10(12):2761. https://doi.org/10.3390/plants10122761
Chicago/Turabian StyleChoi, Yong-Keun, Hak-Jin Song, Jeong-Wook Jo, Seong-Won Bang, Byung-Hoon Park, Ho-Hyun Kim, Kwang-Jin Kim, Na-Ra Jeong, Jeong-Hee Kim, and Hyung-Joo Kim. 2021. "Morphological and Chemical Evaluations of Leaf Surface on Particulate Matter2.5 (PM2.5) Removal in a Botanical Plant-Based Biofilter System" Plants 10, no. 12: 2761. https://doi.org/10.3390/plants10122761
APA StyleChoi, Y.-K., Song, H.-J., Jo, J.-W., Bang, S.-W., Park, B.-H., Kim, H.-H., Kim, K.-J., Jeong, N.-R., Kim, J.-H., & Kim, H.-J. (2021). Morphological and Chemical Evaluations of Leaf Surface on Particulate Matter2.5 (PM2.5) Removal in a Botanical Plant-Based Biofilter System. Plants, 10(12), 2761. https://doi.org/10.3390/plants10122761






