Abstract
Phosphorus (P) is an essential, non-renewable resource critical for crop productivity across the world. P is immobile in nature and, therefore, the identification of novel genotypes with efficient P uptake and utilization under a low P environment is extremely important. This study was designed to characterize eighty genotypes of different Lens species for shoot and root traits at two contrasting levels of P. A significant reduction in primary root length (PRL), total surface area (TSA), total root tips (TRT), root forks (RF), total dry weight (TDW), root dry weight (RDW) and shoot dry weight (SDW) in response to P deficiency was recorded. A principal component analysis revealed that the TDW, SDW and RDW were significantly correlated to P uptake and utilization efficiency in lentils. Based on total dry weight (TDW) under low P, L4727, EC718309, EC714238, PL-97, EC718348, DPL15, PL06 and EC718332 were found promising. The characterization of different Lens species revealed species-specific variations for the studied traits. Cultivated lentils exhibited higher P uptake and utilization efficiency as compared to the wild forms. The study, based on four different techniques, identified EC714238 as the most P use-efficient genotype. The genotypes identified in this study can be utilized for developing mapping populations and deciphering the genetics for breeding lentil varieties suited for low P environments.
1. Introduction
The lentil (Lens culinaris Medikus ssp. culinaris) is a self-pollinated legume species with the genome size of 4063 Mbp/1C [1]. It is an ancient crop and its domestication dates back to the Neolithic Agricultural Revolution in the eastern Mediterranean during the 8th and 7th millennia BC [2]. Thereafter, the crop disseminated to Central Asia, the Nile Valley and Europe during the period of Neolithic agriculture. The lentil was also part of the Harappan crop assemblage (2250 to 1750 BC) in the Indian subcontinent [3]. The crop is cultivated for its protein-rich seeds and valuable straw in North America, South Asia, and the Mediterranean region. The global lentil production was 5.73 mtons during 2019 and Canada was the leading lentil producer followed by India [4].
Phosphorus (P) is a non-renewable and essential nutrient with limited global reserves [5]. The use of phosphate fertilizers has increased more than four times in the past five decades and is expected to reach 22–27 mton/year by 2050 [6]. Being an essential element, P is involved in the synthesis of DNA, RNA and membrane proteins and lipids. It acts as a signaling molecule and is associated with cellular phosphorylation events [7]. Plants absorb P from the soil mainly in the form of soluble inorganic P. In soil, P availability is low due to its fixation with calcium in acidic soils and iron/aluminum in alkaline soils [8]. Therefore, to ensure high yield, farmers apply excessive P fertilizers. However, plants utilize only up to 30% of the applied P and the rest is fixed in the soil or may cause eutrophication [9]. Improved P uptake and utilization is critical to reduce the cost of cultivation. The improvement of P use efficiency (PUE) becomes more important in legumes as legumes require more external P for growth and development as compared to other crops [10].
Efforts have been made by breeders to improve P uptake and utilization efficiency in different crops by targeting specific traits [11,12]. Root traits such as root dry weight (RDW), total root length (TRL), total surface area (TSA), total root volume (TRV), total root tips (TRT) and fork number (FN) involved in P uptake are of higher significance in P-deficient soils [13,14,15]. The phenotyping of black gram genotypes for root traits suggested that RDW is a potential trait for improving P uptake [16], whereas TRL, TSA, TRV, TRT and FN and carboxylate exudation efficiency were important in green grams [17,18]. Shoot dry matter, total dry matter, and shoot P concentration were associated with high PUE in soybeans [19], whereas long root hairs and a high shoot to root ratio were positively correlated to PUE in Arabidopsis. Limited reports are available on the genotypic diversity for PUE in legumes [20,21]. The cultivated lentil, Lens culinaris ssp. Culinaris, is native to near East and Central Asia. Lens orientalis is the probable progenitor [22] of the lentil. Later, this genus was divided [23] into four species including seven species/subspecies: L. culinaris ssp. culinaris, ssp. orientalis (Boiss.) Ponert, ssp. tomentosus (Ladizinsky) Fergusan, Maxted, van Slageren and Robertson, and ssp. odemensis (Ladizinsky) Fergusan, Maxted, van Slageren and Robertson, and L. ervoides (Brign.) Grande, L. nigricans (M.Bieb.) Godron and L. lamottei (Czefr). The different wild Lens species are distributed in the Mediterranean region. The present study is the first attempt to characterize the Lens genotypes’ germplasm under sufficient and low P conditions for the identification of PUE efficient lines and the identification of potential root and shoot traits associated with P uptake and utilization efficiency. The experiments were conducted with the objective of characterizing 85 Lens genotypes for the phenotypic variation of morphological root and shoot traits under sufficient and low P conditions, as well as to identify suitable genotypes for PupE and PUtE and phosphorus use efficient genotype(s).
2. Results
2.1. Variation in Root and Shoot Traits under SP and LP
The studied genotypes revealed variation for the root and shoot traits under SP and LP conditions. Plant growth was adversely affected in all the genotypes under low P conditions. Shoot growth was higher in SP conditions, whereas root traits showed genotype-dependent variation in response to LP (Table 1). All the studied traits registered wide variation in genotypic values under SP and LP conditions (Table 1). Most of the traits showed reduced expression under LP conditions. The percent reduction was 5.91% in PRL, 17.70% in TSA, 2.56% in ARD, 9.61% in TRV, 24.07% in TRT, 38.57% in RF, 39.62% in SDW, 36.21% in RDW, 38.74% in TDW, and 69.23% in PupE. Only the TRL and RSR increased by 14.05% and 7.58%, respectively, under LP compared to the SP level. PupE reduced drastically under LP conditions, with percent change of mean at −69.23 percent. PUtiE varied from 30.53 to 97.50% with a mean of 63.10% and CV% of 14.30%. The coefficient of variation (CV%) was high for TRV, TRT, SDW, TDW, RSR, PupE and under P-deficient conditions, whereas PRL, TRL, TSA, ARD, RF and RDW showed higher variation under P-rich conditions. (Table 1).

Table 1.
Estimates of ranges and means, coefficient of variation (CV%), and percentage change in means for root and shoot traits under SP and LP in lentils.
2.2. Estimation of Genetic Variance and Broad-Sense Heritability
The genetic variance components for different Lens species were significant for the shoot and root traits except for the ARD under contrasting P conditions (Table 2). Genotypic variance for the ARD under SP, genotype and treatment interactions were non-significant. The broad-sense heritability range for different traits was from 0.27 to 0.87 and 0.56 to 0.86 under SP and LP conditions, respectively. High heritability was recorded for studied traits in both SP and LP conditions, except for ARD in the SP condition (very low 0.27).

Table 2.
Estimates of variance components and broad-sense heritability (H) for root and shoot traits under SP and LP conditions.
2.3. Genotypic Correlation among Root and Shoot Traits
The Pearson correlation analysis revealed the highly significant and positive correlations for most of the traits under SP (Table 3, upper diagonal) and LP (Table 3, lower diagonal). Significantly positive correlations were observed between TRL and PRL (r = 0.39), TRL and TSA (r = 0.91), TRL and TRV (r = 0.89), TRL and TRT (r = 0.75), TRL and RF (r = 0.82), TRL and RDW (r = 0.35) under LP.

Table 3.
Pearson correlation analysis for root and shoot traits under SP (upper diagonal) conditions and LP (lower diagonal) conditions.
TSA had a positive correlation with ARD, TRV, TRT, RF, RDW and PupE (r = 0.27, r = 0.91, r = 0.74, r = 0.81, r = 0.29 and r = 0.32, respectively), whereas TRV exhibited highly significant positive correlations with TRT, RF, SDW and RDW (r = 0.61, r = 0.76, r = 0.25, r = 0.32, respectively) under low P levels. TRT exhibited a positive correlation to RF, SDW, RDW, TDW and PupE (r = 0.61, r = 0.24, r = 0.29, r = 0.22 and r = 0.28, respectively) whereas RF exhibited a positive correlation with RDW (r = 0.37) under LP. Under SP conditions, TRL had a significantly positive correlation to TSA, TRV, TRT, and RF (r = 0.87, r = 0.84, r = 0.71 and r = 0.69, respectively) and TSA was positively correlated with TRV, TRT and RF (r = 0.88, r = 0.70, and r = 0.72, respectively). Some of the root traits exhibited negative correlation with SDW, RDW, TDW and RSR under both P regimes. The trait ARD was significantly correlated with PRL and TSA (r = 0.27 and r = 0.27, respectively) under low P environments and showed no correlation under sufficient P content. SDW was positively correlated to RDW and TDW (r = 0.67 and r = 0.94 at SP and r = 0.70 and r = 0.92 at LP). RDW was positively correlated with TDW (r = 0.84) at LP and at SP levels (r = 0.89). PupE had a significant and positive correlation with TRL, TSA, TRT, SDW, RDW and TDW (r = 0.37, r = 0.32, r = 0.28, r = 0.48, r = 0.41 and r = 50, respectively) whereas it was a non-significant correlation with PRL, ARD, TRV, and RSR under LP. PupE exhibited a significant correlation with TSA, TRV, RDW and TDW (r = 0.22, r = 0.24, r = 0.26 and r = 0.51, respectively) under SP.
2.4. Principal Component Analysis of Shoot and Root Traits
A principal component analysis (PCA) was carried out to identify the key traits contributing to responsiveness in lentils under P deficiency. PCA was analyzed via loading scores from the relative values of all traits under low P. The first two principal components explained 27.8% and 27.2% of the total variation for recorded traits under LP (Figure 1A). The biplot revealed that TRL, TRV, TRT, TSA and RF and their corelated traits such as RDW, SDW, and TDW are the key traits contributing to total variation. These root and shoot traits exhibited a significant correlation with PutiE and PupE. A scree plot between eigen values of factors and principal components depicted the maximum variation that resulted from PC1 to PC4. The eigen values from PC1 to PC2 are 3.61, 3.53, 1.46 and 1.16, respectively, whereas the percentage of explained variances from PC1 to PC2 are 27.8%, 27.2%, 11.3% and 8.9%, respectively (Figure 1B).
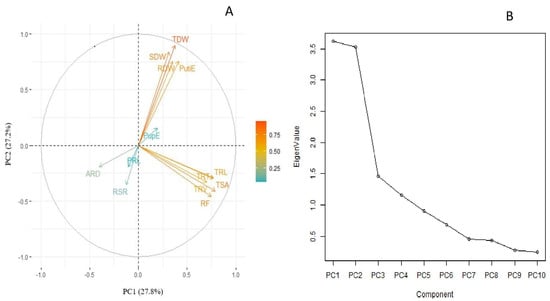
Figure 1.
(A) Biplot and (B) scree plot using relative values of tested root and shoot traits of lentil germplasm lines under LP conditions. The arrow represents different root and shoot traits, whereas its length corresponds to the contribution of each trait for total variation. PRL, primary root length (cm); TRL, total root length (cm); TSA, total surface area (cm3); ARD, average root diameter (mm); TRV, total root volume (cm3); TRT, total root tips; RF, root fork; SDW, shoot dry weight (mg); RDW, root dry weight (mg); TDW, total dry weight (mg); RSR, root to shoot ratio (mg mg−1); PupE, P uptake efficiency (mg plant−1); PutiE, P utilization efficiency (%).
2.5. Genetic Variation in Lens sp.
The genetic variation recorded for the studied traits in six different Lens species is presented in Figure 2. The highest PRL was recorded for L. orientalis under SP conditions. PRL recorded an increase in LP conditions in comparison to SP conditions for L. culinaris and L. ervoides. In the rest of the species, a relatively higher PRL was recorded under SP conditions (Figure 2A). A high TRL was recorded in L. culinaris under both LP and SP conditions, as compared to other Lens species (Figure 2B). Under LP conditions, higher TRL values were recorded for L. ervoides, L. lamottei and L. nigricans as compared to SP conditions in these species. The highest TSA was recorded for L. culinaris in both LP and SP conditions (Figure 2C). A high TSA in LP conditions was recorded in L. ervoides, L. odemensis, L. lamottei and L. nigricans as compared to SP conditions. A higher ARD was recorded for L.nigricans, L. lamottie and L. culinaris. The ARD increased under LP conditions as compared to SP conditions for L. culinaris (Figure 2D). L.culinaris exhibited a higher TRV in both LP and SP conditions, in comparison to other species. A high TRV was recorded for L. nigricans and L. orientalis in LP conditions as compared to SP conditions (Figure 2E). Maximum TRT values were recorded for L. culinaris. A higher TRT was recorded in LP conditions for L. nigricans and L. lamotte (Figure 2F). The RF increased in LP conditions over SP conditions for L. ervoides, L. odemensis, L. lamottei and L. nigricans (Figure 2G). Among the studied species L. culinaris exhibited a higher RF. The highest SDW, RDW and TDW were recorded for L. nigricans (Figure 2H–J). The SDW, RDW and TDW in LP conditions were lower than the SDW, RDW and TDW in SP conditions for all the studied species. The RSR values were higher in LP conditions in comparison to SP conditions in all studied species except L. nigricans (Figure 2K). The highest P uptake efficiency in LP conditions was recorded for L. nigricans, followed by L.ervoides (Figure 2L). The highest P utilization efficiency was recorded for L. culinaris, and an almost similar P utilization efficiency was recorded for L. lamottei and L. nigricans (Figure 2M).
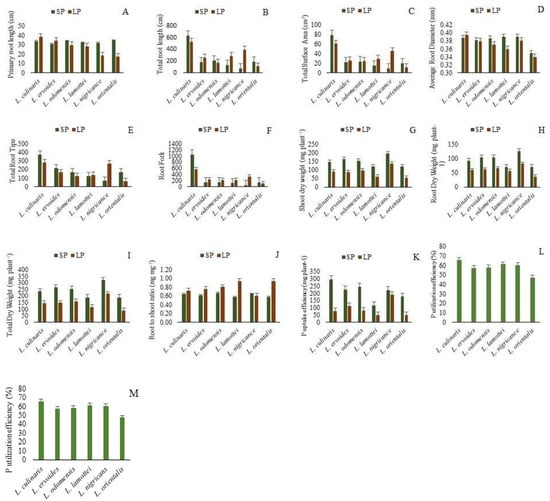
Figure 2.
Performance of Lens sp. under sufficient phosphorus (SP) and low phosphorus (LP). (A) Primary root length (cm), (B) total root length (cm), (C) total surface area (cm3), (D) average root diameter (mm), (E) total root volume (cm3) (F) total root tips, (G) root fork, (H) shoot dry weight (mg plant−1), (I) root dry weight (mg plant−1), (J) total dry weight (mg plant−1), (K) root to shoot ratio (mg mg−1), (L) P uptake efficiency (mg plant−1), (M) P utilization efficiency (%); SP, sufficient P; LP, low P.
2.6. Identification of Promising Genotypes for PupE and PutiE
Based on the TDW under low P levels, the top 10% of genotypes were identified as promising towards P deficiency (Table 4). The eight identified genotypes (L4727, EC718309, EC714238, PL-97, EC718348, DPL15, PL06 and EC718332) also had the top 10% for SDW, RDW, PupE and PUtiE. Among the eight identified genotypes, some of the common genotypes have shown better performance for SDW, RDW, PupE and PutiE. The genotypes EC718309, EC714238, EC718348 and PL06 belonged in the top 10% for TDW, SDW, and RDW. The genotypes EC718309 and EC718348 belonged in the top 10% for TDW, SDW, RDW and PupE. The genotypes PL06 and EC718332 belonged in the top 10% for TDW, SDW and PutiE. However, L4727 was in the top 10% for shoot dry weight and total dry weight, whereas DPL-15 belonged to the top 10% for traits TDW and RDW. The genotype EC718332 belonged in the top 10% for TDW, SDW, PupE and PutiE.

Table 4.
Selected lentil genotypes belonging to the top (8) 10% for TDW and SDW, along with the same genotypes also being in the top 10% for RDW, PupE and PUtiE.
PupE was highest for PL-97 (427 mg plant−1), followed by L4727 (229 mg plant−1), EC714238 (223 mg plant−1), EC718348 (195 mg plant−1), EC718309 (186 mg plant−1), PL06 (177 mg plant−1) and EC718332 (127 mg plant−1) under SP. PupE increased in some of the selected genotypes under LP compared to SP levels and was highest for EC718309 (295 mg plant−1), followed by EC718348 (273 mg plant−1), EC718332 (175 mg plant−1) and PL06 (115 mg plant−1) (Figure 3A). PUtiE was the highest for PL06 (94%), followed by EC718332 (93%), PL-97 (89%), EC718309 (83%), L4727 (73%), EC714238 (61%), EC718348 (59%) and DPL15 (51%) (Figure 3B). The results showed that these genotypes were highly efficient in uptake as well as the utilization of P under a limited P environment.
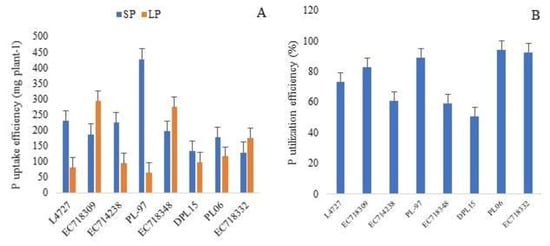
Figure 3.
Performance of the top 10% of lentil genotypes selected based on total dry weight (A), P uptake efficiency (mg plant−1) (B), P utilization efficiency (%); SP, sufficient phosphorus; LP, low phosphorus.
Genotype, treatment and their interactions were significant for TRL, TSA, TRT, TRV and RF in the selected genotypes at a probability level 0.01 under two P levels (Table 5). As compared to SP levels, TRL, TRT, TSA, TRV and RF increased in L4727, EC718309, EC714238, DPL-15, PL06 and EC718332 in response to P deficiency, whereas they decreased non-significantly in the rest of the genotypes. PL-97 showed increased TRL, TRT, and RF but a reduction in TSA and TRV under LP. The genotype EC718332 exhibited reduction in all the root traits under LP (Figure 4).

Table 5.
Analysis of variance (ANOVA) for tested root traits in identified genotypes under two P levels.
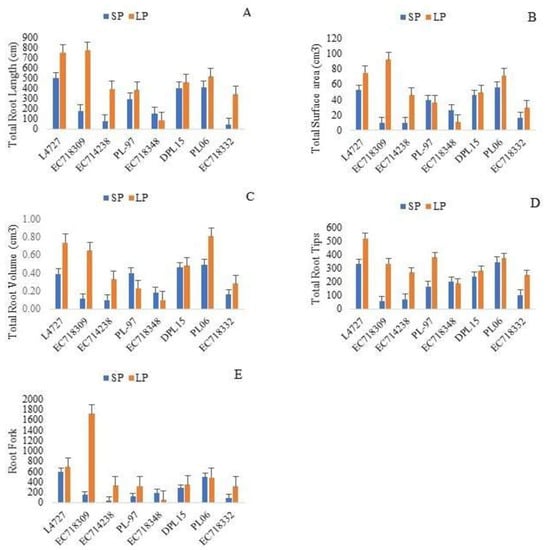
Figure 4.
(A) Total root length (cm), (B) total surface area (cm3), (C) total root volume (cm3), (D) total root tips, (E) root fork in selected lentil genotypes.
The mean value of the studied traits of five genotypes selected based on PupE and PutiE under LP are presented in Table 6.

Table 6.
Mean value of the studied traits of five genotypes selected based on PupE and PutiE under LP.
A principal component analysis (PCA) was carried out with the studied traits for PupE and PutiE under LP conditions. The first two principal components of the biplots explained 61.9% and 91.7% of the total variation, respectively (Figure 5A,B). The study of the PutiE biplot revealed that TRT, TRV, TRL, RF and TSA exhibited a positive correlation with PupE (Figure 5A). The biplot for PutiE revealed that RDW was positively correlated to PutiE. However, the TDW exhibited a meager contribution towards PutiE (Figure 5B).
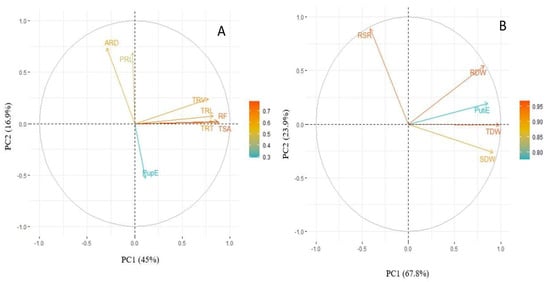
Figure 5.
(A) Biplot PupE and (B) biplot PutiE using the relative values of tested root and shoot traits of lentil germplasm lines under LP conditions. The arrow represents different root and shoot traits, whereas its length corresponds to the contribution of each trait to the total variation. PRL, primary root length (cm); TRL, total root length (cm); TSA, total surface area (cm3); ARD, average root diameter (mm); TRV, total root volume (cm3); TRT, total root tips; RF, root fork; SDW, shoot dry weight (mg); RDW, root dry weight (mg); TDW, total dry weight (mg); RSR, root to shoot ratio (mg mg−1); PupE, P uptake efficiency (mg plant−1); PutiE, P utilization efficiency (%).
2.7. Categorization of Lens Genotypes for PUE
Technique 1: The scoring of traits SDW, RDW, TDW, RSR, P percent, PupE, and PutiE under both the LP and SP revealed substantial variation among the 85 genotypes investigated (Table S2). The population was characterized for PUE based on the population mean and standard deviation of the studied traits. Under LP conditions, the genotype EC 714,238 scored highest (19 out of 20), followed by PL 06, WBL 81, SEHORE 74–3, MC 6, and EC 718,332 (18 out of 20). Under HP circumstances, the genotypes PL 05, EC 714238, and EC 718,339 scored highest (18 out of 20), followed by HUL 57, IPL 406, IC 268238, and L 4602 (17 out of 20). JL 1, IPL 406, L 4610, IG 129560, L 4650, EC 718464, EC 718312, EC 718281, and EC 718,282 (12 out of 20) in LP conditions and genotype L 4650 (11 out of 20) in HP conditions had the lowest scores among the 85 genotypes. By combining the scores at both P levels, EC 714,238 (37 out of 40) and EC 718,339 (34 out of 40) scored the highest for overall performance among the genotypes investigated. L 4717 and L 4650 had the lowest score (23 out of 30), indicating that they performed poorly for PUE among the genotypes.
Technique 2: The genotypes were categorized into four groups based on TDM and PutiEin for both HP and LP regimes (Figure 6A,B). The genotypes PL06, DPL62, WBL-81, L4147, BM4, HUL-57 and EC718243 under LP conditions and genotypes L4727, EC718330 and EC718276 under HP conditions were classified in the ER group. Furthermore, the genotypes L4650, I-G-Y-50 and MC6 were grouped in the INR category under HP conditions. By contrast, under LP conditions, the genotypes IPL7103, EC 718464, KBL-104, EC 718,306 and EC 718,355 were categorized in the INR group. Interestingly, the genotypes PL-97, EC714238 and EC718339 were found in the ER group under both P conditions, whereas the genotypes EC718292, L4610, JL-1, EC 718351, EC718409 and EC718282 were categorized in the INR group under both P conditions. The genotypes IPL81, DPL15, EC718348 and EC718295 were categorized in the ENR group under both P conditions. The genotypes IC321808, JL-7, ILWL-118, L4649, P43120, IG134340, LH84-8, L4618, PL02 and L4076 were categorized in the IR group under both P conditions.
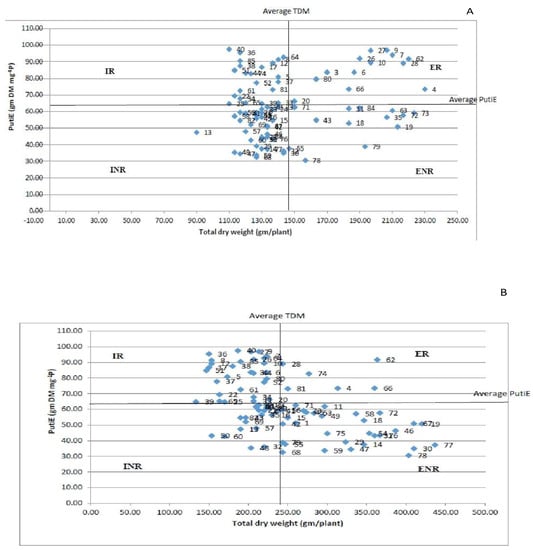
Figure 6.
(A) Grouping of genotypes into four classes (IR, ER, ENR and INR) under HP conditions; (B) grouping of genotypes into four classes (IR, ER, ENR and INR) under LP conditions.
Technique 3: Under both P conditions, the categorization based on this technique results in the analysis of modest changes among genotypes. The HDM-HP group included the genotypes EC718309, EC718332, and EC718339 under LP conditions and PL-05, IPL7103, and IPL-406 under HP conditions (Figure 7A,B). Under LP conditions exclusively, the genotype PRECOZ was classified as LDM-LP. Under both circumstances, the genotype P43120 was classified as LDM-MP. In both HP and LP regimes, the genotypes EC718347, L4618, IC208352, and KBL-104 were classified as MDM-MP. Under both P circumstances, the majority of the genotypes were classified as MDM-MP. Under LP conditions, no genotypes were classified as LDM-HP, whereas under HP conditions, no genotypes were classified as LDM-LP. Surprisingly, genotypes EC718309 and PL-05 with a high TDM and PupE were classified as HDM-HP under both LP and HP conditions, whereas genotype EC718276 with a high TDM and PupE was classified as MDM-HP under HP conditions.
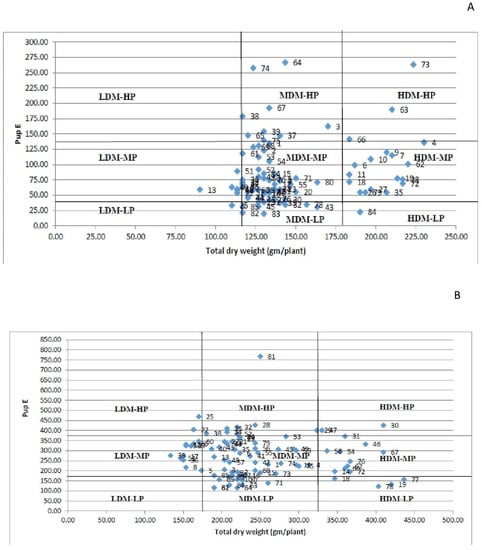
Figure 7.
(A) Grouping of genotypes into nine classes under HP conditions; (B) grouping of genotypes into nine classes under LP conditions.
Technique 4: This non-graphical technique is based on the STS score tabulated based on seven P deficiency tolerance indices of 85 genotypes. Among the studied genotypes, the highest STS score was recorded by DPL15 (110.46) followed by EC 714,238 (87.86), whereas the lowest was recorded by P 43,120 (−60.47) followed by IG69568 (−58.16) (Table S3).
3. Discussion
The present study was designed to evaluate a panel of eighty-five diverse Lens genotypes for root and shoot traits at the seedling stage at contrasting P levels under controlled environments. The Lens genotypes were characterized for root and shoot traits in response to different P levels for the identification of superior Lens genotypes with significant P use. The most promising approach for developing a P use-efficient cultivar is by improving the key traits involved in the P uptake and utilization in the plant [24,25] Root architecture is instrumental to nutrient and water uptake, which has been poorly studied in lentils [26]. Significant phenotypic and genetic variations, moderate to high levels of heritability, and significant correlations among different traits were recorded at different P levels (Table 1, Table 2 and Table 3). This can be attributed to the diversity among and within the studied species for phosphorus uptake and utilization efficiency. These findings are in agreement with those reported in Brassica [27], common beans [28], maize [29] and green grams [18] under nutrient stress [18,30,31,32]. Significant genotypic variation was reported for root and shoot traits in rice [33,34], wheat [30], and mung beans [18] and for P use traits in mung beans [18]. Limited efforts have been made to study genetic diversity for phosphorus uptake and utilization efficiency in legumes.
A significant reduction in seedling growth traits (TDW, SDW, RDW) along with different root traits (PRL, TSA, ARD, TRV, TRT and RF) was recorded in response to P deficiency (Table 1). PRL, TSA, RV and root branching are important components of root architecture and play important roles in determining the rate of nutrient uptake. Although genotype-dependent variation was evident for different traits, most of the genotypes registered a significant reduction in response to P deficiency. In mung beans, P-efficient genotypes exhibited a better RSA, TRV and carbon exudation efficiency [17]. In rice, the RDW and RSR increased with a reduction in the SDW [26]. Root architectural plasticity was correlated to the RDW, root length density, and lateral roots in response to low P [35].
The Pearson correlation coefficient explained a highly significant correlation among most of the traits at the seedling stage under different P levels (Table 3). We noted significant correlation between the TRL and TSV, TRV, TRT and RF in lentils. Similarly, the TRL was positively correlated with the TSA and TRV in maize [36], with the TRT and RF in mung beans [18], and the SDW, RDW, and TDW under both SP and LP conditions. In the present study, the TDW was positively correlated with the RDW and SDW and negatively correlated to the RSR under low P levels (Table 3). The ARD showed a non-significant correlation to most of the traits in the present study as observed in chickpeas [37]. In contrast to our findings, the ARD was highly significant and negatively correlated with the TRT and RF under LP in mung beans and can be used as an important trait to differentiate nutrient availability [18]. A significant correlation among the root traits TRT, TRL, RSA, TRV, and ARD was reported in maize at the seedling stage [38].
The PCA identified the most contributing traits responsible for total variation as the TRL, TSA, TRT, RF, SDW, RDW, TDW, PutiE and PupE under LP (Figure 1). The present results are in accordance with previous studies, where P stress alters the TRL, SDW, TSA and TRT in mung beans [18] and the TRL and TSA in common beans [28]. We identified promising genotypes based on the TDW under P-deficient conditions (Table 4). The selected superior genotypes belonged to the top 10% for SDW, but few differed in ranking for TRL, RDW and SDW under SP and LP, which can be due to variation in the root, shoot traits and genotype x treatment interaction [39,40,41]. We observed that the SDW was positively correlated with the RDW and TDW under both P conditions. The RDW was positively correlated with the SDW under stress conditions in maize [42], whereas the TRL and RDW were significantly correlated with root traits under P and N stress conditions in maize [15,43]. It was suggested that the TDW, RDW, and SDW are potential phenotypic traits for the selection of lentil genotypes under P efficiency. The evaluation of soybean germplasm revealed moderate to higher values of heritability for most of the root and shoot traits [44,45]. Traits such as the TRL, TSA, TRV, TRT, RDW, and SDW contributed most to the genetic diversity and significantly correlated with P accumulation under SP and LP conditions [46,47]. This result was similar to the results of previous studies, which revealed that the traits TRL, TSA, RDW, and SDW are the major traits for selection of genotypes in maize under different stress conditions [36,42].
In the present study, we observed that genotypes with a high TDW and SDW under low P were also promising for PupE and PutiE (Table 4). PUtiE was also significantly correlated with TDW under different P regimes in rice [48]. Though the genotypes which had higher TDW along with RDW showed better performance for physiological P use. It can be attributed to the correlation between RDW and different root traits, which resulted in efficient P uptake in these genotypes. The increased root length was responsible for higher P uptake from low P supply in barley and sugar beet roots [49,50] whereas the TRT, RSA, TRL and root hair length were important in P uptake in other studies [51,52]. In rice, the genotypic variation in P uptake was due to the RDW and RSA under P deficiency [53]. The potential use of L. ervoidis, L. nigricans and L. culinaris ssp. odemensis for drought tolerance [54], and L. culinaris ssp. orientalis for cold tolerance [55] and salinity tolerance [56] has been investigated. The utility of wild Lens species for phosphorus uptake and utilization requires an investigation with a large number of genotypes of each species/subspecies. In this study a limited number of genotypes of wild Lens were investigated. The variations were recorded for different studied traits at LP and SP conditions in this study.
According to the classification approach reported earlier [33,57], lentil genotypes were classified into four categories based on P efficiency and responsiveness: ER, ENR, IR, and INR. Under both P circumstances, the most efficient genotypes, EC714238 and EC718339, were classified as ER. Under HP conditions, the genotypes EC 718,330 and EC 7188276, which were in the ER category under LP, were moved to the IR category. This emphasizes the significance of categorization at both high and low P levels. The genotypes classified as ER were well suited to soils with variable levels of P. The genotypes in the ENR group, on the other hand, could be successfully cultivated in P-depleted soils. The IR genotypes could be employed in a crossbreeding program to incorporate P-responsive characteristics. The genotypes of the INR category, on the other hand, have no part in the PUE enhancement program [58]. This method of categorization allows for the identification of genotypes suitable for a variety of growing conditions and P levels [59]. This strategy, however, is primarily dependent on the population mean. As a result, the difference between efficient and inefficient, responsive and nonresponsive kinds is relatively thin [58]. The genotypes EC 718,276 and PL 97 in the LP condition and NDL-1 and EC 718,287 in the HP condition, for example, were at the borderline between efficient and inefficient groups. As a result, genotypes with a minor deviation from the population mean are difficult to categorize as efficient or inefficient, or responsive or nonresponsive. As a result, this technique is ineffective for investigating and categorizing genotypes on a wide scale [57].
The genotypes were classified into nine groups (LDM-HP, LDM-MP, LDM-LP, MDM-HP, MDM-MP, MDM-LP, HDM-HP, HDM-MP, and HDM-LP) using a graph with the TDM and TPU on the x and y axes, respectively, in both HP and LP conditions [60]. By generating nine groups, this categorization method may discern tiny changes between genotypes [59]. This method, on the other hand, is more suited to categorizing genotypes at low P levels [57]. The efficient genotype EC714238 with high TPU and TDM was clustered in HDM-MP under both HP and LP conditions in this investigation. HDM-HP genotypes are efficient in P uptake, and their use for biomass production suggests the genotype’s ability to produce greater biomass under a variety of P regimes [30]. L 4717 was classified as MDM-MP in both LP and HP situations, whereas L 4650 was classified as LDM-MP in LP and MDM-MP in HP. Both P absorption and use in biomass production are inefficient in LDM-LP genotypes. The genotypes in the LDM-MP group are good at absorbing P but not so good at being used for biomass production.
As a result, the three-way categorization of genotypes (low, medium, and high) allows for the discovery of significant differences between the high and low groups while also providing the most room for medium type genotypes. Such differences explain genotype adaptation across a wide range of P regimes and provide the genetic foundation for PUE enhancement in breeding strategies. The categorization of genotypes using stress tolerance indices calculated from genotype dry mass under control and stress situations was reported earlier [32,61]. The P deficiency tolerance indices of all genotypes were computed using TDM under both HP and LP conditions in the current investigation. The susceptibility indices SSI, TI, and SI have a negative association with yield/biomass and are used to distinguish between stress-tolerant and susceptible genotypes [62], whereas the tolerance indices MPI, GMPI, and STI have a positive connection with yield/biomass and can be used to select genotypes with high average yield/biomass and stress tolerance [63].
The studied Lens genotypes exhibited significant genetic variability and heritability for different root and shoot traits at two contrasting levels of phosphorus. Most of the recorded parameters revealed a remarkable reduction in P deficient conditions. Wild Lens species exhibited low PupE and PutiE in comparison to cultivated species (Figure 2L,M). Genotypes EC718309, EC718348, EC718332 and PL06 were better in P uptake as well as in P utilization compared to other genotypes. The identified genotypes can be utilized for the development of mapping population for the identification of QTLs responsible for P uptake and utilization efficiency. The characterization of the studied genotypes using different techniques identified the genotype EC714238 as having the highest STS score, indicating that it was highly phosphorus use efficient under LP conditions. The genotype can be utilized in breeding programs and in genetic studies.
4. Materials and Methods
4.1. Plant Materials and Plant Growth Conditions
A diverse set of eighty-five Lens genotypes, including twenty-six cultivated varieties, twenty-eight advanced breeding lines and thirty-one wild species accessions, were used for studying root and shoot traits under sufficient phosphorus (SP) and low phosphorus (LP) conditions (Table S1). The seeds of wild Lens species were provided by ICARDA, Aleppo, Syria. The characterization of eighty-five Lens genotypes was carried out under hydroponic conditions at two levels of phosphorus, consisting of sufficient P (SP) and low P (LP). The experiment was conducted under a greenhouse at the National Initiative on Climate Resilient Agriculture (NICRA), a controlled environment facility of the Indian Agricultural Research Institute, New Delhi, India in 2019 (winter season). The growth conditions maintained throughout the study period were day and night temperatures of 25 and 16 °C, respectively, a photoperiod of 12 h, and the relative humidity at 85%. The seeds were surface sterilized with 0.1% (w/v) HgCl2 for 3 min followed by double distilled water rinsing. The seeds of wild Lens species were scarified, wrapped in germination paper and kept in the dark for the germination. After the emergence of cotyledonary leaves, 8–10-day-old seedlings of uniform size were transferred to the Hoagland solution. The basal macronutrient solution consisted of 1 mM MgSO4, 0.92 mM K2SO4, 0.75 mM CaCl2.2H2O, 0.04 mM Fe-EDTA, 5 mM Urea, and a micronutrient combination of 2.4 μM H3BO3, 0.9 μM MnSO4, 0.6 μM ZnSO4, 0.62 μM CuSO4, and 0.6 μM Na2MoO4 [64]. Plastic trays (30 × 45 × 15 cm) with a capacity of 10 L basal nutrients were used for hydroponics. The 2” thick thermocol sheet was used to support the seedlings, which had holes at a distance of 5 × 5 cm to maintain row-to-row and plant-to-plant spacing. Fifteen genotypes with three replications were raised per container. The aquarium air pump was used to maintain air circulation in the medium. The nutrient solution was replaced on alternate days to avoid any microbial contamination. Using 1 M KOH or 1 M HCL, the pH of the nutrient solution was maintained at 6.0. The N:P ratio in hydroponic solution was 16N:1P in the SP condition and 1666 N:1P in the LP condition. The study was conducted with a series of P concentrations to select the sufficient and low P levels. The analysis of the observations on chlorophyll content, biomass and visual symptoms led to the selection of the sufficient (300 μM) and low (3 μM) P concentrations, and the phosphorous was supplemented in the form of KH2PO4.
4.2. Trait Measurements
One-month-old seedlings were harvested to study the root and shoot traits raised under SP and LP conditions. The roots were separated from the shoots and were scanned using an Epson Perfect V700 Pro scanner (Seiko Epson, Suwa, Japan). The grayscale images in TIFF format were studied with WinRHIZO Pro 2016a software. The root system was spread in an acrylic tray, avoiding overlapping among them. The broken root segments were manually separated during the root scanning. Based on an image analysis of the root architecture, the traits measured were primary root length (PRL, cm), total root length (TRL, cm), total surface area (TSA, cm2), average root diameter (ARD, cm), total root volume (TRV, cm3), total root tips (TRT, cm), and root fork (RF). The shoots and roots of the plants were oven dried at 65 °C for 48 h to obtain the desired shoot dry weight (SDW, mg plant−1), root dry weight (RDW, mg plant−1), total dry weight (TDW, mg plant−1), and root to shoot ratio (RSR, mg mg−1).
4.3. Estimation of P Concentration
For the P estimation, 0.1 gm grounded samples of the studied genotypes were digested with a 10 mL di-acid mixture (HNO3: HClO4, 9:4) and a volume that was made up to 50 mL. It was filtered through Whatman No. 42 filter paper. The samples were run on an inductively coupled plasma optical emission spectrometer (ICP-OES; model 5110, Agilent Technologies (Santa Clara, CA, USA) which was calibrated using the standard for measuring the absorbance of the blue-colored phosphomolybdate complex at 660 nm [65]. The results of four replications were averaged and the P concentration was expressed as mg g−1 dry weight. The P uptake efficiency (PupE) and P utilization efficiency (PutiE) were calculated using the following formulas [60,66]:
PupE (mg plant−1) = P concentration (mg mg−1) × dry matter (mg plant−1)
PutiE (%) = TDW (LP)/TDW (SP) × 100
4.4. Estimation of Phosphorus Use Efficiency
The PUE was measured using four techniques. Technique 1 [67,68], the studied genotypes were classified (based on population mean and standard deviation) as efficient (>µ + SD), medium (µ + SD to µ − SD) and inefficient (<µ − SD). Technique 2 [33,34], classified Lens genotypes in four groups: efficient responsive (ER), efficient non-responsive (ENR), inefficient and non-responsive (INR) and inefficient but responsive (IR). Technique 3 [30], grouped Lens genotypes by plotting TPU and TDM on the y-axis and x-axis, respectively, in nine groups: high dry mass-high P (HDM-HP), high dry mass-medium P (HDM-MP), high dry mass-low P (HDM-LP), medium dry mass-high P (MDM-HP), medium dry mass-medium P (MDM-MP), medium-dry mass-low P (MDM-LP), low dry mass–high P (LDM-HP), low dry mass-medium P (LDM-MP) and low dry mass-low P (LDM-LP). Technique 4 [31,32], utilized stress tolerance score for characterizing the genotypes for PUE
where stress susceptibility index (SSI) = (1 − T/C)/(1 − xT/xC), mean productivity index (MPI) = (C + T)/2, geometric mean productivity index (GMPI) = √C × T√C, harmonic mean index (HMI) = 2 (C × T)/(C + T), stress tolerance index (STI) = (C × T)/(xC)2, tolerance index (TI) = C − T and stress index (SI) = T/ where C represents the total dry mass under control conditions and T represents the total dry mass under treatment conditions. xC and xT represent the average total dry mass (TDM) of all studied genotypes under SP (control) and LP (treatment) conditions, respectively.
Stress tolerance score (STC) = SSI + MPI + GMPI + HMI + STI + TI + SI
4.5. Statistical Analysis
The STAR (Statistical Tool for Agricultural Research) 2.1.0 software was used to estimate the coefficient of variation, variance components to predict genotypic values and the Pearson correlation coefficients under SP and LP conditions [69].
The percent change in response to P stress was calculated as follows: {[(SP − LP)/SP] × 100}. The broad-sense heritability was estimated based on expected mean squares for each trait under both SP and LP and with combined analysis as follows [70]:
and
where σ2G, σ2GE and σ2 e are genotypic variance, variance due to genotype × P level interaction, and error variance, respectively; ‘r’ is the replication.
Broad-sense heritability H= σ2G/(σ2 G+σ2 e/r)
Combined heritability Hcom = σ2G/(σ2G+(σ2GE)/e) + (σ2e)/re))
All the measured traits were subjected to principal component analysis (PCA) to identify the common trends of the multidimensional data sets. The principal component analysis (PCA) was performed for different root and shoot traits by using relative values under LP with the help of an R software package “FactoMineR” to detect the most contributing traits [71].
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants10122711/s1. Table S1: Lens genotypes characterized for traits related to phosphorus use efficiency; Table S2: Classification of genotypes into efficient (E), medium (M) and inefficient (I) types based on five traits recorded under normal and low phosphorus conditions. Table S3: Phosphorus deficiency tolerance indices were calculated for 85 genotypes grown under high and low phosphorus conditions.
Author Contributions
Conceptualization, V.R., H.K.D. and G.P.M.; methodology, V.R., H.K.D., R.P., M.S.A., A.S. and K.T.; formal analysis, V.R. and R.B.; resources, H.K.D., G.P.M., S.K., R.P. and R.B.; data curation, V.R. and R.B.; writing—original draft preparation, V.R., D.K. and R.B.; writing—review and editing, H.K.D., G.P.M. and S.K.; supervision, H.K.D. and G.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported and funded by the Indian Council of Agricultural Research (ICAR) and the CGIAR Program on Grain Legumes and Dryland Agriculture (GLDC) funded by CGIAR fund donors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
The authors are thankful to the Director, ICAR-Indian Agricultural Research Institute, New Delhi, India and the Director, ICAR-National Bureau of Plant Genetic Resources, New Delhi, India for providing the resources and facilities to carry out the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arumuganathan, K.; Earle, E.D. Nuclear DNA content of some important plant species. Plant Mol. Biol. Rep. 1991, 9, 208–218. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Fuller, Q.D.; Harvey, L.E. The archaeobotany of Indian pulses: Identification, processing and evidence for cultivation. Environ. Archaeol. 2006, 11, 219–246. [Google Scholar] [CrossRef]
- FAOstat. Statistics Database of the Food and Agriculture Organization of the United Nations. 2021. Available online: http://www.fao.org/statistics/databases/en/ (accessed on 27 July 2021).
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; Lopez-Arredondo, D.; Wissuwa, M.; Rouached, H. Improving phosphorus use efficiency: A complex trait with emerging opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Mogollón, J.M.; Beusen, A.H.W.; Van Grinsven, H.J.M.; Westhoek, H.; Bouwman, A.F. Future agricultural phosphorus demand according to the shared socioeconomic pathways. Glob. Environ. Chang. 2018, 50, 149–163. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Zhang, H.; Chu, S.; Zhang, X.; Yin, D.; Yu, D.; Zhang, D. A genetic relationship between phosphorus efficiency and photosynthetic traits in soybean as revealed by QTL analysis using a high-density genetic map. Front. Plant Sci. 2016, 7, 924. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Jez, J.M.; Lee, S.G.; Sherp, A.M. The next green movement: Plant biology for the environment and sustainability. Science 2016, 353, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Kidd, D.R.; Ryan, M.H.; Haling, R.E.; Lambers, H.; Sandral, G.A.; Yang, Z.; Simpson, R.J. Rhizosphere carboxylates and morphological root traits in pasture legumes and grasses. Plant Soil 2015, 402, 77–89. [Google Scholar] [CrossRef]
- Hammond, J.; Broadley, M.; White, P.; King, G.; Bowen, H.; Hayden, R.; Meacham, M.; Mead, A.; Overs, T.; Spracklen, W.; et al. Shoot yield drives phosphorus use efficiency in Brassica oleracea and correlates with root architecture traits. J. Exp. Bot. 2009, 60, 1953–1968. [Google Scholar] [CrossRef]
- Rose, T.J.; Wissuwa, M. Rethinking internal phosphorus utilization efficiency (PUE): A new approach is needed to improve PUE in grain crops. Adv. Agron. 2012, 116, 185–217. [Google Scholar]
- Sarkar, B.C.; Karmoker, J. Effects of phosphorus deficiency on the root growth of lentil seedlings grown in rhizobox. Bangladesh J. Bot. 2009, 38, 215–218. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, H.; Geng, L.; Kan, G.; Cui, S.; Meng, Q.; Gai, J.; Yu, D. Detection of quantitative trait loci for phosphorus deficiency tolerance at soybean seedling stage. Euphytica 2009, 167, 313–322. [Google Scholar] [CrossRef]
- Van de Wiel, C.C.; van der Linden, C.G.; Scholten, O.E. Improving phosphorus use efficiency in agriculture: Opportunities for breeding. Euphytica 2016, 207, 1–22. [Google Scholar] [CrossRef]
- Jakkeral, S.A.; Kajjidoni, S.T.; Koti, R.V. Genotypic variation for root traits to phosphorus deficiency in blackgram (Vigna mungo L. Hepper). Karnataka J. Agric. Sci. 2009, 22, 946–950. [Google Scholar]
- Pandey, R.; Meena, S.K.; Krishnapriya, V.; Ahmad, A.; Kishora, N. Root carboxylate exudation capacity under phosphorus stress does not improve grain yield in green gram. Plant Cell Rep. 2014, 33, 919–928. [Google Scholar] [CrossRef]
- Reddy, V.; Aski, M.; Mishra, G.; Dikshit, H.K.; Singh, A.; Pandey, R.; Singh, M.P.; Gayacharan; Ramtekey, V.; Rai, N.; et al. Genetic variation for root architectural traits in response to phosphorus deficiency in mungbean at the seedling stage. PLoS ONE 2020, 15, e0221008. [Google Scholar] [CrossRef] [PubMed]
- Furlani, A.M.C.; Furlani, R.R.; Tanaka, R.T.; Mascarenhas, H.A.A.; Delgado, M.D.P. Variability of soybean germplasm in relation to phosphorus uptake and use efficiency. Sci. Agric. 2002, 59, 529–536. [Google Scholar] [CrossRef][Green Version]
- Pang, J.; Zhao, H.; Bansal, R.; Bohuon, E.; Lambers, H.; Ryan, M.H.; Siddique, K.H. Leaf transpiration plays a role in phosphorus acquisition among a large set of chickpea genotypes. Plant Cell Environ. 2018, 41, 2069–2079. [Google Scholar] [CrossRef]
- Pang, J.; Ryan, M.H.; Tibbett, M.; Cawthray, G.R.; Siddique, K.H.M.; Bolland, M.D.A.; Denton, M.D.; Lambers, H. Variation in morphological and physiological parameters in herbaceous perennial legumes in response to phosphorus supply. Plant Soil 2010, 331, 241–255. [Google Scholar] [CrossRef]
- Ladizinsky, G. The origin of lentil and its wild genepool. Euphytica 1979, 28, 179–187. [Google Scholar] [CrossRef]
- Ferguson, M.E.; Maxted, N.; Slangeren, H.V.; Robertson, L.D. A reassessment of the taxonomy of Lens Mill. (Leguminosae, Papilionoidae, Vicieae). Bot. J. Lin. Soc. 2000, 133, 41–59. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A.; Santos, A.B. Phosphorus Uptake and Use Efficiency in Field Crops. J. Plant Nutr. 2013, 36, 2013–2022. [Google Scholar] [CrossRef]
- Faez, A.; Adam, P.; David, J. Root Architecture and Genetic Variations Associated with Phosphorus Uptake in Rice. Int. J. Appl. Agric. Sci. 2015, 1, 1. [Google Scholar] [CrossRef][Green Version]
- Gorim, Y.L.; Vanderberg, A. Root Traits, Nodulation and Root Distribution in Soil for Five Wild Lentil Species and Lens culinaris (Medik.) Grown under Well-Watered Conditions. Front. Plant Sci. 2017, 8, 1632. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Oki, Y.; Adachi, T.; Murata, Y.; Khan, M.H. Relative phosphorus utilization efficiency, growth response, and phosphorus uptake kinetics of brassica cultivars under a phosphorus stress environment. Commun. Soil Sci. Plant Anal. 2007, 38, 1061–1085. [Google Scholar] [CrossRef]
- Silva, D.A.; Esteves, J.A.; Gonçalves, J.G.; Azevedo, C.V.; Ribeiro, T.; Chiorato, A.F.; Carbonell, S.A. Evaluation of common bean genotypes for phosphorus use efficiency in eutrophic Oxisol. Bragantia 2016, 75, 152–163. [Google Scholar] [CrossRef]
- Wang, Q.J.; Yuan, Y.; Liao, Z.; Jiang, Y.; Wang, Q.; Zhang, L.; Lu, Y. Genome-Wide Association Study of 13 Traits in Maize Seedlings under Low Phosphorus Stress. Plant Genome 2019, 12, 190039. [Google Scholar] [CrossRef]
- Gill, H.; Singh, A.; Sethi, S.; Behl, R. Phosphorus uptake and use efficiency in different varieties of bread wheat (Triticum aestivum L.). Arch. Agron. Soil Sci. 2004, 50, 563–572. [Google Scholar] [CrossRef]
- Negarestani, M.; Tohidi-Nejad, E.; Khajoei-Nejad, G.; Nakhoda, B.; Mohammadi-Nejad, G. Comparison of Different Multivariate Statistical Methods for Screening the Drought Tolerant Genotypes of Pearl Millet (Pennisetum americanum L.) and Sorghum (Sorghum bicolor L.). Agronomy 2019, 9, 645. [Google Scholar] [CrossRef]
- Grzesiak, S.; Hordyńska, N.; Szczyrek, P.; Grzesiak, M.T.; Noga, A.; Szechyńska-Hebda, M. Variation among wheat (Triticumaestivum L.) genotypes in response to the drought stress: I–selection approaches. J. Plant Interact. 2019, 14, 30–44. [Google Scholar] [CrossRef]
- Fageria, N.K. Workshop on Adaption of Plants to Soil Stresses. Screening Crop Genotypes for Mineral Stresses; University of Nebraska: Lincoln, NE, USA, 1993; pp. 142–162. [Google Scholar]
- Kosar, H.S.; Gill, M.A.; Aziz, T.; Tahir, M.A. Relative phosphorus utilization efficiency of wheat genotypes in hydroponics. Pak. J. Agric. Sci. 2003, 40, 28–32. [Google Scholar]
- Sandhu, N.; Raman, K.; Torres, R.; Audebert, A.; Dardou, A.; Kumar, A.; Henry, A. Rice Root Architectural Plasticity Traits and Genetic Regions for Adaptability to Variable Cultivation and Stress Conditions. Plant Physiol. 2016, 171, 2562–2576. [Google Scholar] [CrossRef]
- Li, P.; Chen, F.; Cai, H.; Liu, J.; Pan, Q.; Liu, Z.; Yuan, L. genetic relationship between nitrogen use efficiency and seedling root traits in maize as revealed by QTL analysis. J. Exp. Bot. 2015, 66, 3175–3188. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Bansal, R.; Zhao, H.; Bohuon, E.; Lambers, H.; Ryan, M.H.; Siddique, K.H. The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol. 2018, 219, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Zhang, W.; Liu, Y.; Gao, Y.; Wang, X.; Yan, J.; Yang, X.; Li, J. Genetic analysis of seedling root traits reveals the association of root trait with other agronomic traits in maize. BMC Plant Biol. 2018, 18, 171. [Google Scholar] [CrossRef]
- Narang, R.A.; Bruene, A.; Altmann, T. Analysis of Phosphate Acquisition Efficiency in Different Arabidopsis Accessions. Plant Physiol. 2000, 124, 1786–1799. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.J.; Rose, M.T.; Pariasca-Tanaka, J.; Heuer, S.; Wissuwa, M. The frustration with utilization: Why have improvements in internal phosphorus utilization efficiency in crops remained so elusive? Front. Plant Sci. 2011, 2, 73. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, S.; Chakraborty, A.S.; Yelne, R.; Kavishetty, V.; Biswas, T.; Bhattacharyya, S. Phosphate acquisition efficiency and phosphate starvation tolerance locus (PSTOL1) in rice. J. Genet. 2014, 93, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.; Caixeta, D.; Rezende, W.; Schuster, A.; Azevedo, C.; e Silva, F.F.; DeLima, R. Genotypic variation and relationships among traits for root morphology in a panel of tropical maize inbred lines under contrasting nitrogen levels. Euphytica 2019, 215, 51. [Google Scholar] [CrossRef]
- Abdel Ghani, A.; Kumar, B.; Reyes-Matamoros, J.; Gonzalez-Portilla, P.; Jansen, C.; Martin, J.; Lee, M.; Lubberstedt, T. Genotypic variation and relationships between seedling and adult plant traits in maize (Zea mays L.) inbred lines grown under contrasting nitrogen levels. Euphytica 2012, 189, 123–133. [Google Scholar] [CrossRef]
- Krishnapriya, V.; Pandey, R. Root exudation index: Screening organic acid exudation and phosphorus acquisition efficiency in soybean genotypes. Crop Pasture Sci. 2016, 67, 1096. [Google Scholar] [CrossRef]
- Ao, J.; Fu, J.; Tian, J.; Yan, X.; Liao, H. Genetic variability for root morph-architecture traits and root growth dynamics as related to phosphorus efficiency in soybean. Funct. Plant Biol. 2010, 37, 304. [Google Scholar] [CrossRef]
- Aziz, T.; Ahmed, I.; Farooq, M.; Maqsood, M.A.; Sabir, M. Variation in phosphorus efficiency among Brassica cultivars I: Internal utilization and phosphorus remobilization. J. Plant Nutr. 2011, 34, 2006–2017. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, L.; Du, M.; Zhang, Y.; Zhang, Y. Localized and Moderate Phosphorus Application Improves Plant Growth and Phosphorus Accumulation in Rosa multiflora Thunb. ex Murr. via Efficient Root System Development. Forests 2020, 11, 570. [Google Scholar] [CrossRef]
- Wissuwa, M.; Kondo, K.; Fukuda, T.; Mori, A.; Rose, M.T.; Pariasca-Tanaka, J.; Kretzschmar, T.; Haefele, S.M.; Rose, T.J. Unmasking novel loci for internal phosphorus utilization efficiency in rice germplasm through genome-wide association analysis. PLoS ONE 2015, 10, e0124215. [Google Scholar] [CrossRef]
- Steingrobe, B.; Schmid, H.; Claassen, N. Root production and root mortality of winter barley and its implication with regard to phosphate acquisition. Plant Soil 2001, 237, 239–248. [Google Scholar] [CrossRef]
- Steingrobe, B. Root renewal of sugar beet as a mechanism of P uptake efficiency. J. Plant Nutr. Soil Sci. 2001, 164, 533–539. [Google Scholar] [CrossRef]
- Carvalho, P.; Foulkes, M.J. Roots root and Uptake of Water and Nutrients roots uptake of water and nutrients. In Sustainable Food Production; Springer: New York, NY, USA, 2013. [Google Scholar]
- Ghimire, B.; Hulbert, S.H.; Steber, C.M.; Garland-Campbell, K.; Sanguinet, K.A. Characterization of root traits for improvement of spring wheat in the Pacific Northwest. Agron. J. 2020, 112, 228–240. [Google Scholar] [CrossRef]
- Mori, A.; Fukuda, T.; Vejchasarn, P.; Nestler, J.; Pariasca-Tanaka, J.; Wissuwa, M. The role of root size versus root efficiency in phosphorus acquisition in rice. J. Exp. Bot. 2016, 67, 1179–1189. [Google Scholar] [CrossRef]
- Hamdi, A.; Erskine, W. Reaction of wild species of the genus Lens to drought. Euphytica 1996, 91, 173–179. [Google Scholar] [CrossRef]
- Hamdi, A.; Küsmenoĝlu, I.; Erskine, W. Sources of winter hardiness in wild lentil. Genet. Resour. Crop Evol. 1996, 43, 63–67. [Google Scholar] [CrossRef]
- Singh, D.; Singh, C.K.; Kumari, S.; Tomar, R.S.S.; Karwa, S.; Singh, R.; Singh, R.B.; Sarkar, S.K.; Pal, M. Discerning morpho-anatomical, physiological and molecular multiformity in cultivated and wild genotypes of lentil with reconciliation to salinity stress. PLoS ONE 2017, 12, e0190462. [Google Scholar] [CrossRef]
- Bilal, H.M.; Aziz, T.; Maqsood, M.A.; Farooq, M.; Yan, G. Categorization of wheat genotypes for phosphorus efficiency. PLoS ONE 2018, 13, e0205471. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Aslam, M.; Shah, J.A.; Depar, N.; Memon, M.Y. Relative growth response of hydroponically grown wheat genotypes to deficient and adequate phosphorus levels. Pak. J. Agric. Agric. Eng. Vet. Sci. 2016, 32, 169–181. [Google Scholar]
- Irfan, M.; Abbas, M.; Shah, J.A.; Akram, M.A.; Depar, N.; Memon, M.Y. Categorization and Identification of Brassica Genotypes for Phosphorus Utilization Efficiency. Int. J. Agric. Biol. 2020, 23, 227–234. [Google Scholar] [CrossRef]
- Irfan, M.; Aziz, T.; Maqsood, M.A.; Bilal, H.M.; Siddique, K.H.M.; Xu, M. Phosphorus (P) use efficiency in rice is linked to tissue-specific biomass and P allocation patterns. Sci. Rep. 2020, 10, 4278. [Google Scholar] [CrossRef] [PubMed]
- Thiry, A.A.; Chavez Dulanto, P.N.; Reynolds, M.P.; Davies, W.J. How can we improve crop genotypes to increase stress resilience and productivity in a future climate? A new crop screening method based on productivity and resistance to abiotic stress. J. Exp. Bot. 2016, 67, 5593–5603. [Google Scholar] [CrossRef]
- Sareen, S.; Tyagi, B.S.; Sharma, I. Response estimation of wheat synthetic lines to terminal heat stress using stress indices. J. Agric. Sci. 2012, 4, 97–104. [Google Scholar] [CrossRef][Green Version]
- Khodarahmpour, Z.; Choukan, R.; Bihamta, M.R.; Hervan, E.M. Determination of the best heat stress tolerance indices in maize (Zea mays L.) inbred lines and hybrids under Khuzestan Province conditions. J. Agric. Sci. Technol. 2011, 13, 111–121. [Google Scholar]
- Sivasakthi, K.; Tharanya, M.; Kholová, J.; Wangari Muriuki, R.; Thirunalasundari, T.; Vadez, V. Chickpea genotypes contrasting for vigor and canopy conductance also differ in their dependence on different water transport pathways. Front. Plant Sci. 2017, 8, 1663. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Neto, A.P.; Favarin, J.L.; Hammond, J.P.; Tezotto, T.; Couto, H.T. Analysis of Phosphorus Use Efficiency Traits in Coffea Genotypes Reveals Coffea arabica and Coffeacanephora Have Contrasting Phosphorus Uptake and Utilization Efficiencies. Front. Plant Sci. 2016, 7, 408. [Google Scholar] [CrossRef]
- Osborne, L.D.; Rengel, Z. Screening cereals for genotypic variation in efficiency of phosphorus uptake and utilisation. Aust. J. Agric. Res. 2002, 53, 295–303. [Google Scholar] [CrossRef]
- Aziz, T.; Finnegan, P.M.; Lambers, H.; Jost, R. Organ-Specific phosphorus-allocation patterns and transcript profiles linked to phosphorus efficiency in two contrasting wheat genotypes. Plant Cell Environ. 2014, 37, 943–960. [Google Scholar] [CrossRef] [PubMed]
- Gulles, A.A.; Bartolome, V.I.; Morantte, R.I.Z.A.; Nora, L.A. Randomization and analysis of data using STAR (Statistical Tool for Agricultural Research). Philipp. J. Crop Sci. 2014, 39, 137. [Google Scholar]
- Hallauer, A.R.; Miranda Filho, J.B.; Carena, M.J. Quantitative Genetics in Maize Breeding; Springer: New York, NY, USA, 2010. [Google Scholar]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).