Pre-Anthesis Cytokinin Applications Increase Table Grape Berry Firmness by Modulating Cell Wall Polysaccharides
Abstract
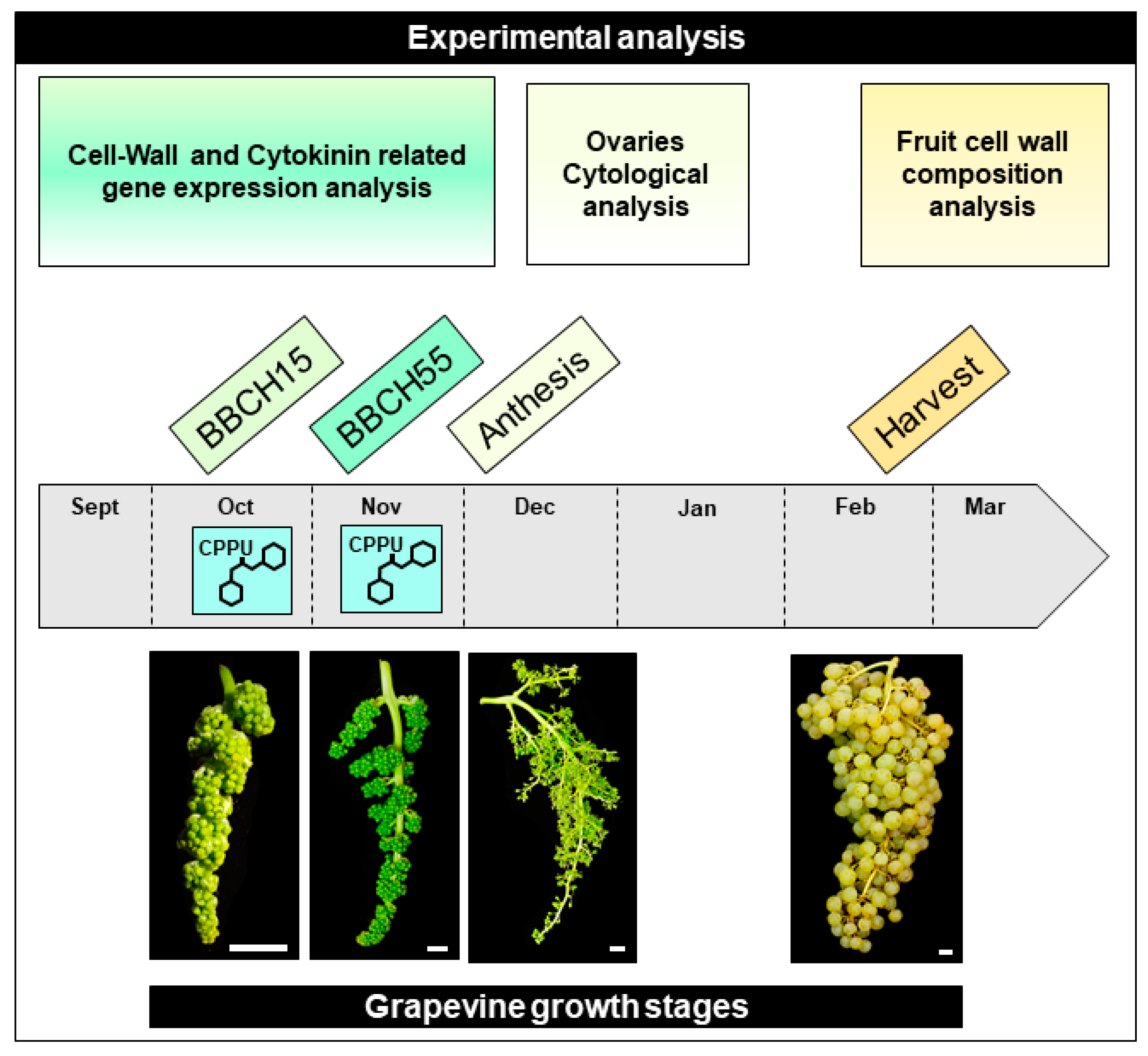
:1. Introduction
2. Results
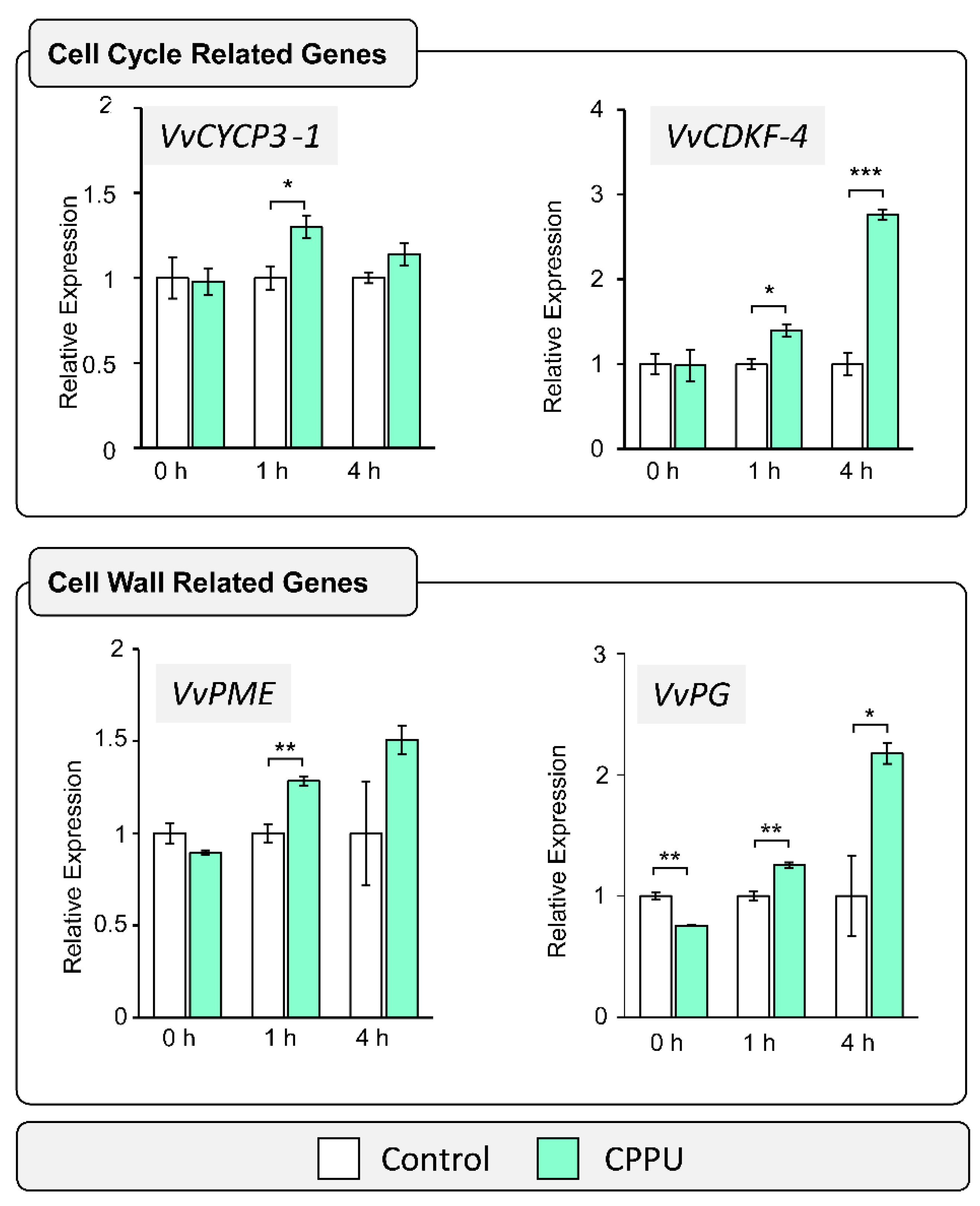
2.1. Cell Wall and Cell Cycle-Related Gene Expression Analysis in Response to CPPU Applications by RT-qPCR
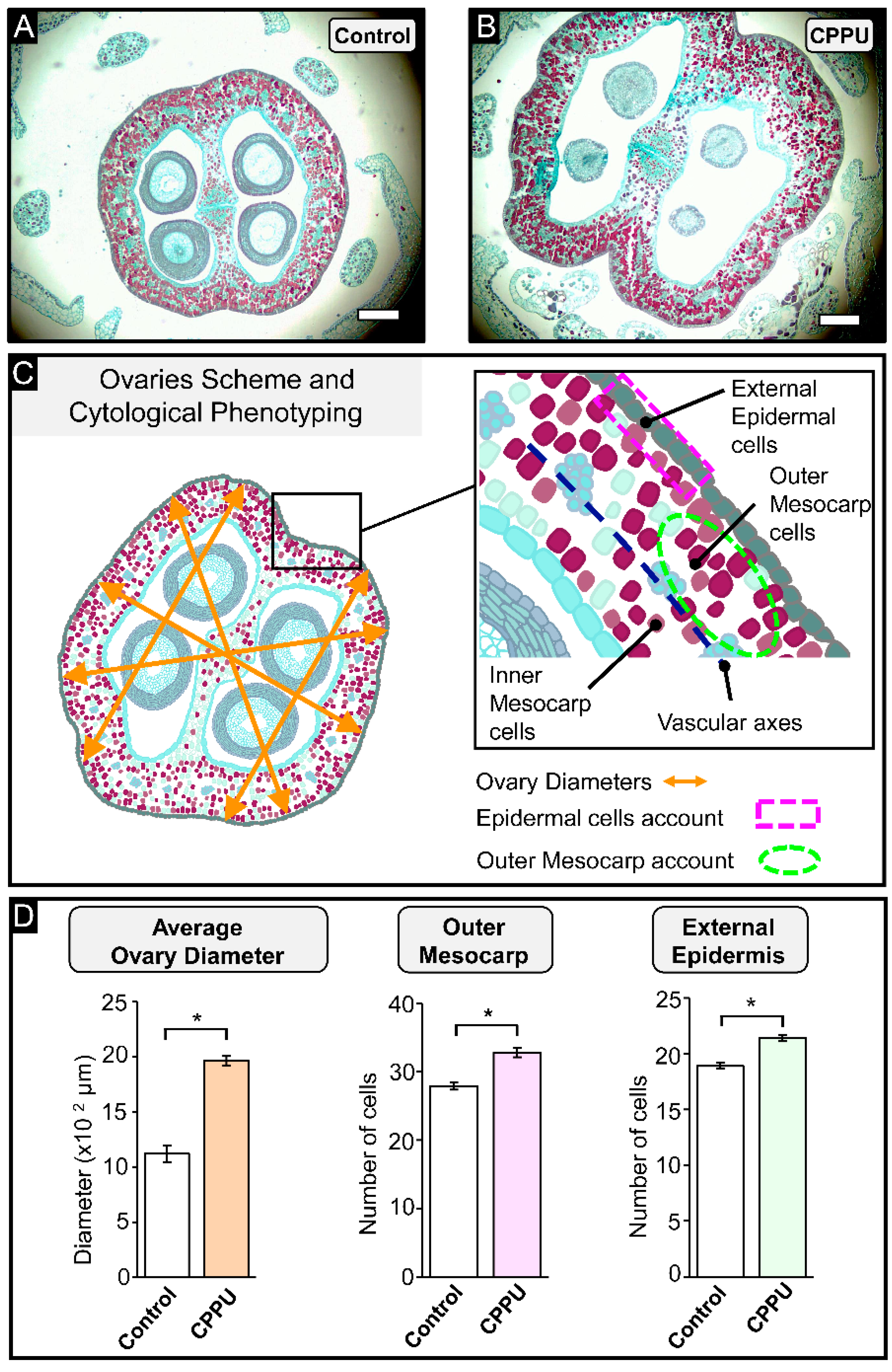
2.2. Cell Count and Inflorescence Size Analysis at Pre-Anthesis Stage
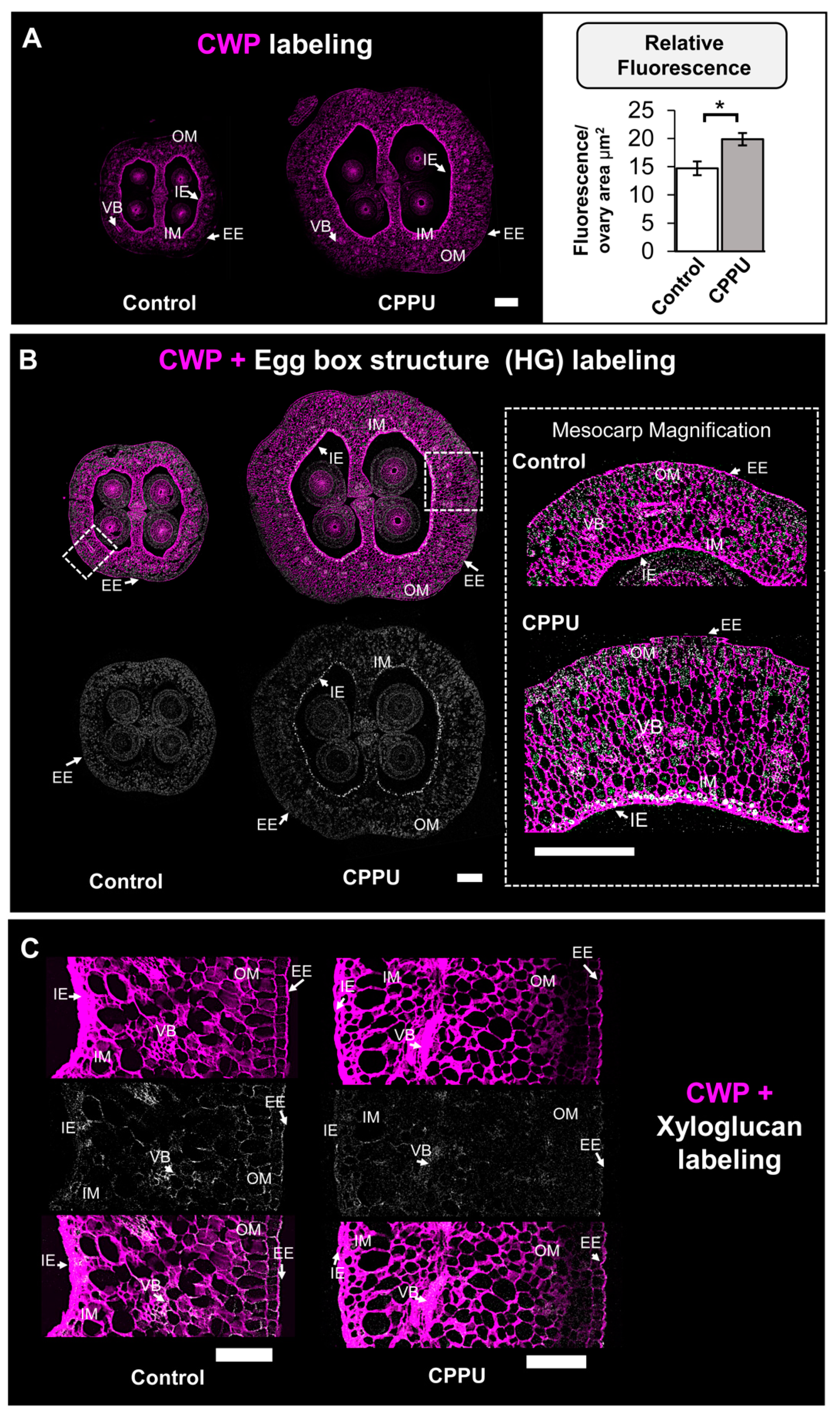
2.3. Immunohistochemical Analysis of CELL Wall Structure from CPPU-Treated Inflorescences
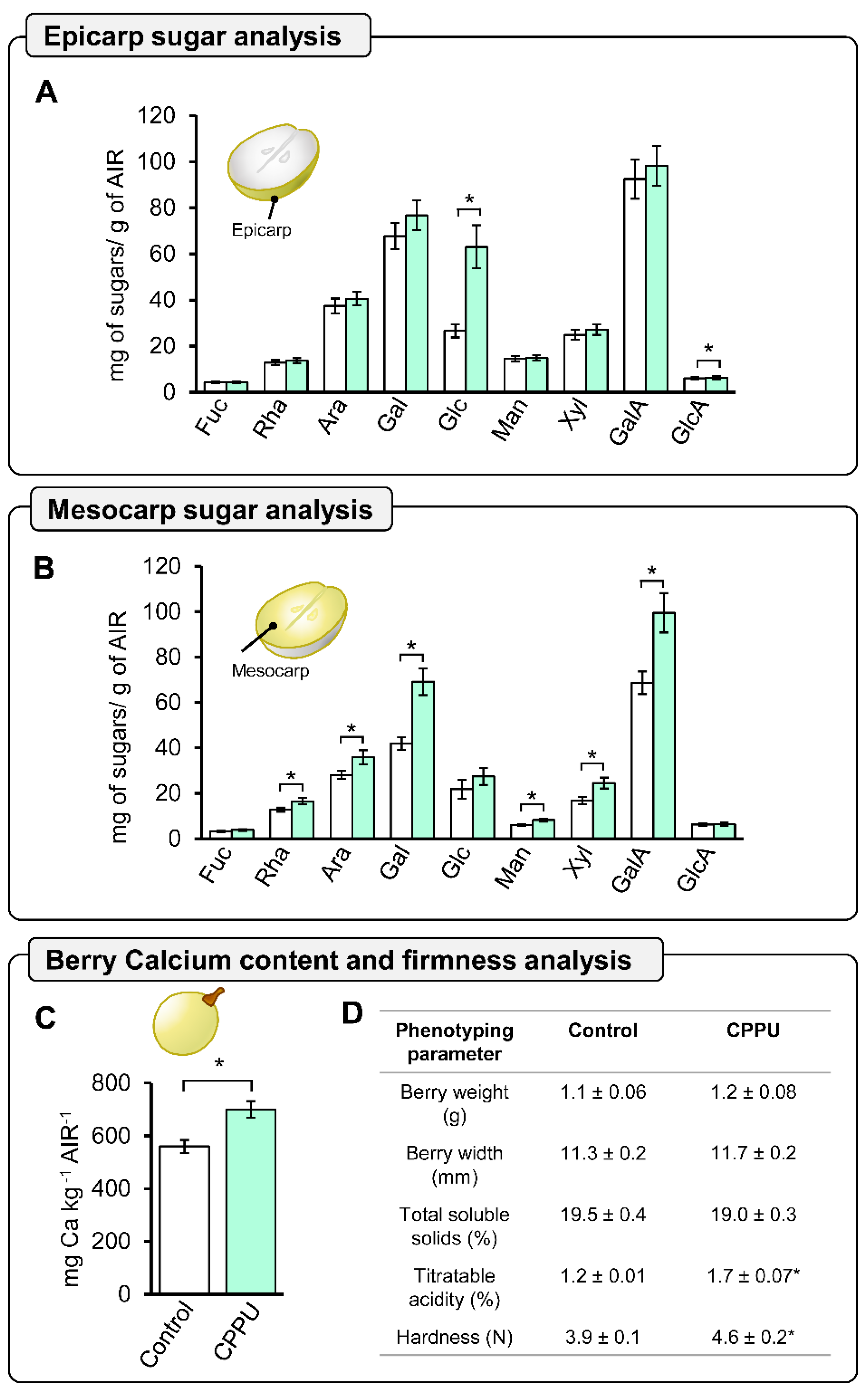
2.4. Global Cell Wall Non-Cellulosic Monosaccharide Composition Analysis of CPPU-Treated Grape Berries at Harvest by HPAEC-PAD
2.5. Phenotype and Firmness Properties of CPPU-Treated Table Grapes
3. Discussion
3.1. Cell Wall and Cell Cycle-Related Transcript Expressions Are Differentially Accumulated in Response to Early CPPU Applications
3.2. CPPU Treatment Increased Cell Count and Inflorescence Size at Anthesis Stage
3.3. CPPU Treatment Induces Changes in Ovary Cell Wall Content
3.4. Early CPPU Treatment Changes Cell Wall Composition and Promotes an Increase in Berry Firmness at Harvest
4. Materials and Methods
4.1. Plant Material and CPPU Treatments
4.2. RNA Extraction and cDNA Synthesis
4.3. qRT-PCR and Selection of Candidate Genes
4.4. Optical Microscopy and Immunofluorescence
4.4.1. Inflorescence Fixation and Section
4.4.2. Light Microscopy—Histochemical Staining
4.4.3. Confocal Laser Scanning Microscopy—Immunofluorescence
4.4.4. Quantification of Immunofluorescence Signal
4.5. Analysis of Cell Wall Monosaccharide Composition
4.6. Calcium Quantification
4.7. Harvest Berry Phenotypic Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Agriculture Organization Corporate Statistical Database (FAOSTAT). 2019. Available online: http://www.fao.org/faostat/en/ (accessed on 10 July 2021).
- Balic, I.; Ejsmentewicz, T.; Sanhueza, D.; Silva, C.; Peredo, T.; Olmedo, P.; Barros, M.; Verdonk, J.C.; Paredes, R.; Meneses, C.; et al. Biochemical and physiological study of the firmness of table grape berries. Postharvest Biol. Technol. 2014, 93, 15–23. [Google Scholar] [CrossRef]
- Han, D.H.; Lee, C.H. The effects of GA3, CPPU and ABA applications on the quality of kyoho (Vitis vinifera L. X Labrusca L.) grape. Acta Hortic. 2004, 653, 193–197. [Google Scholar] [CrossRef]
- Hardie, W.J.; O’Brien, T.P.; Jaudzems, V.G. Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust. J. Grape Wine Res. 1996, 2, 97–142. [Google Scholar] [CrossRef]
- Koprna, R.; De Diego, N.; Dundálková, L.; Spíchal, L. Use of cytokinins as agrochemicals. Bioorg. Med. Chem. 2016, 24, 484–492. [Google Scholar] [CrossRef]
- Ugare, B.; Banerjee, K.; Ramteke, S.D.; Pradhan, S.; Oulkar, D.P.; Utture, S.C.; Adsule, P.G. Dissipation kinetics of forchlorfenuron, 6-benzyl aminopurine, gibberellic acid and ethephon residues in table grapes (Vitis vinifera). Food Chem. 2013, 141, 4208–4214. [Google Scholar] [CrossRef]
- Coombe, B.G. The Development of Fleshy Fruits. Annu. Rev. Plant Physiol. 1976, 27, 207–228. [Google Scholar] [CrossRef]
- Coombe, B.G.; McCarthy, M.G. Dynamics of grape berry growth and physiology of ripening. Aust. J. Grape Wine Res. 2000, 6, 131–135. [Google Scholar] [CrossRef]
- Royo, C.; Torres-Pérez, R.; Mauri, N.; Diestro, N.; Cabezas, J.A.; Marchal, C.; Lacombe, T.; Ibáñez, J.; Tornel, M.; Carreño, J.; et al. The Major Origin of Seedless Grapes Is Associated with a Missense Mutation in the MADS-Box Gene VviAGL11. Plant Physiol. 2018, 177, 1234–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabadal, T.J.; Bukovac, M.J. Effect of CPPU on fruit development of selected seedless and seeded grape cultivars. HortScience 2006, 41, 154–157. [Google Scholar] [CrossRef]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014, 65, 4561–4575. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, A.G.; Wardle, D.A.; Zurowski, C.; Looney, N.E. Phenylureas CPPU and Thidiazuron Affect Yield Components, Fruit Composition, and Storage Potential of Four Seedless Grape Selections. J. Am. Soc. Hortic. Sci. 1992, 117, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Rolle, L.; Siret, R.; Segade, S.R.; Maury, C.; Gerbi, V.; Jourjon, F. Instrumental Texture Analysis Parameters as Markers of Table-Grape and Winegrape Quality: A Review. Am. J. Enol. Vitic. 2012, 63, 11–28. [Google Scholar] [CrossRef] [Green Version]
- Curry, E.A.; Greene, D.W. CPPU Influences Fruit Quality, Fruit Set, Return Bloom, and Preharvest Drop of Apples. HortScience 1993, 28, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Qian, C.; Ren, N.; Wang, J.; Xu, Q.; Chen, X.; Qi, X. Effects of exogenous application of CPPU, NAA and GA4+7 on parthenocarpy and fruit quality in cucumber (Cucumis sativus L.). Food Chem. 2018, 243, 410–413. [Google Scholar] [CrossRef]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Jáuregui-Riquelme, F.; Kremer-Morales, M.S.; Alcalde, J.A.; Pérez-Donoso, A.G. Pre-anthesis CPPU Treatment Modifies Quality and Susceptibility to Post-harvest Berry Cracking of Vitis vinifera cv. ‘Thompson Seedless.’ J. Plant Growth Regul. 2017, 36, 413–423. [Google Scholar] [CrossRef]
- Dello Ioio, R.; Linhares, F.S.; Sabatini, S. Emerging role of cytokinin as a regulator of cellular differentiation. Curr. Opin. Plant Biol. 2008, 11, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, C.; Rubio-Somoza, I.; Sibout, R.; Persson, S. Phytohormones and the cell wall in Arabidopsis during seedling growth. Trends Plant Sci. 2010, 15, 291–301. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Levesque-Tremblay, G.; Pelloux, J.; Braybrook, S.A.; Müller, K. Tuning of pectin methylesterification: Consequences for cell wall biomechanics and development. Planta 2015, 242, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Chea, S.; Yu, D.J.; Park, J.; Oh, H.D.; Chung, S.W.; Lee, H.J. Fruit softening correlates with enzymatic and compositional changes in fruit cell wall during ripening in ‘Bluecrop’ highbush blueberries. Sci. Hortic. (Amst.) 2019, 245, 163–170. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Vincken, J.P.; Schols, H.A.; Oomen, R.J.F.J.; McCann, M.C.; Ulvskov, P.; Voragen, A.G.J.; Visser, R.G.F. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol. 2003, 132, 1781–1789. [Google Scholar] [CrossRef] [Green Version]
- Olmedo, P.; Zepeda, B.; Rojas, B.; Silva-Sanzana, C.; Delgado-Rioseco, J.; Fernández, K.; Balic, I.; Arriagada, C.; Moreno, A.A.; Defilippi, B.G.; et al. Cell wall calcium and hemicellulose have a role in the fruit firmness during storage of blueberry (Vaccinium spp.). Plants 2021, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Espinoza, C.; Di Genova, A.; Correa, J.; Silva, R.; Maass, A.; González-Agüero, M.; Orellana, A.; Hinrichsen, P. Transcriptome profiling of grapevine seedless segregants during berry development reveals candidate genes associated with berry weight. BMC Plant Biol. 2016, 16, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Pozo, J.C.; Lopez-Matas, M.A.; Ramirez-Parra, E.; Gutierrez, C. Hormonal control of the plant cell cycle. Physiol. Plant. 2005, 123, 173–183. [Google Scholar] [CrossRef]
- Vidal, S.; Williams, P.; O’Neill, M.A.; Pellerin, P. Polysaccharides from grape berry cell walls. Part I: Tissue distribution and structural characterization of the pectic polysaccharides. Carbohydr. Polym. 2001, 45, 315–323. [Google Scholar] [CrossRef]
- Ma, Y.; Sawhney, V.K.; Steeves, T.A. Staining of paraffin-embedded plant material in safranin and fast green without prior removal of the paraffin. Can. J. Bot. 1993, 71, 996–999. [Google Scholar] [CrossRef]
- Doco, T.; Williams, P.; Pauly, M.; O’Neill, M.A.; Pellerin, P. Polysaccharides from grape berry cell walls. Part II. Structural characterization of the xyloglucan polysaccharides. Carbohydr. Polym. 2003, 53, 253–261. [Google Scholar] [CrossRef]
- Liners, F.; Letesson, J.-J.; Didembourg, C.; Van Cutsem, P. Monoclonal Antibodies against Pectin: Recognition of a Conformation Induced by Calcium. Plant Physiol. 1989, 91, 1419–1424. [Google Scholar] [CrossRef]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Chiabrando, V.; Giacalone, G.; Rolle, L. Mechanical behaviour and quality traits of highbush blueberry during postharvest storage. J. Sci. Food Agric. 2009, 89, 989–992. [Google Scholar] [CrossRef]
- Fu, F.Q.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Spatio-temporal changes in cell division, endoreduplication and expression of cell cycle-related genes in pollinated and plant growth substances-treated ovaries of cucumber. Plant Biol. 2010, 12, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Dewitte, W.; Scofield, S.; Alcasabas, A.A.; Maughan, S.C.; Menges, M.; Braun, N.; Collins, C.; Nieuwland, J.; Prinsen, E.; Sundaresan, V.; et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc. Natl. Acad. Sci. USA 2007, 104, 14537–14542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Fatima, F.; Haider, M.S.; Shazadee, H.; Liu, Z.; Zheng, T.; Fang, J. Genome-wide identification and expression profiling of the polygalacturonase (PG) and pectin methylesterase (PME) genes in grapevine (Vitis vinifera L.). Int. J. Mol. Sci. 2019, 20, 3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gigli-Bisceglia, N.; Engelsdorf, T.; Strnad, M.; Vaahtera, L.; Khan, G.A.; Yamoune, A.; Alipanah, L.; Novák, O.; Persson, S.; Hejatko, J.; et al. Cell wall integrity modulates Arabidopsis thaliana cell cycle gene expression in a cytokinin- and nitrate reductase-dependent manner. Development 2018, 145, dev166678. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, L.; Pradal, M.; Lopez, G.; Berud, F.; Romieu, C.; Torregrosa, L. Berry size variability in Vitis vinifera L. Vitis–J. Grapevine Res. 2006, 45, 53–55. [Google Scholar]
- Scorza, R.; May, L.G.; Purnell, B.; Upchurch, B. Differences in Number and Area of Mesocarp Cells between Small- and Large-fruited Peach Cultivars. J. Am. Soc. Hortic. Sci. 2019, 116, 861–864. [Google Scholar] [CrossRef]
- Fernandez, L.; Romieu, C.; Moing, A.; Bouquet, A.; Maucourt, M.; Thomas, M.R.; Torregrosa, L. The grapevine fleshless berry mutation. A unique genotype to investigate differences between fleshy and nonfleshy fruit. Plant Physiol. 2006, 140, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Montecchiarini, M.L.; Silva-Sanzana, C.; Valderramo, L.; Alemano, S.; Gollán, A.; Rivadeneira, M.F.; Bello, F.; Vázquez, D.; Blanco-Herrera, F.; Podestá, F.E.; et al. Biochemical differences in the skin of two blueberries (Vaccinium corymbosum) varieties with contrasting firmness: Implication of ions, metabolites and cell wall related proteins in two developmental stages. Plant Physiol. Biochem. 2021, 162, 483–495. [Google Scholar] [CrossRef]
- Saez-Aguayo, S.; Parra-Rojas, J.P.; Sepúlveda-Orellana, P.; Celiz-Balboa, J.; Arenas-Morales, V.; Sallé, C.; Salinas-Grenet, H.; Largo-Gosens, A.; North, H.M.; Ralet, M.C.; et al. Transport of UDP-Rhamnose by URGT2, URGT4, and URGT6 Modulates Rhamnogalacturonan-I Length. Plant Physiol. 2021, 185, 914–933. [Google Scholar] [CrossRef]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galbraith, D.W. Microfluorimetric quantitation of cellulose biosynthesis by plant protoplasts using Calcofluor White. Physiol. Plant. 1981, 53, 111–116. [Google Scholar] [CrossRef]
- Anderson, C.T.; Carroll, A.; Akhmetova, L.; Somerville, C. Real-Time Imaging of Cellulose Reorientation during Cell Wall Expansion in Arabidopsis Roots. Plant Physiol. 2010, 152, 787–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amelung, W.; Cheshire, M.V.; Guggenberger, G. Determination of neutral and acidic sugars in soil by capillary gas-liquid chromatography after trifluoroacetic acid hydrolysis. Soil Biol. Biochem. 1996, 28, 1631–1639. [Google Scholar] [CrossRef]
- Anees, M.; Gao, L.; Umer, M.J.; Yuan, P.; Zhu, H.; Lu, X.; He, N.; Gong, C.; Kaseb, M.O.; Zhao, S.; et al. Identification of Key Gene Networks Associated with Cell Wall Components Leading to Flesh Firmness in Watermelon. Front. Plant Sci. 2021, 12, 1164. [Google Scholar] [CrossRef]
- Li, T.; Yun, Z.; Wu, Q.; Qu, H.; Duan, X.; Jiang, Y. Combination of Transcriptomic, Proteomic, and Metabolomic Analysis Reveals the Ripening Mechanism of Banana Pulp. Biomolecules 2019, 9, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uluisik, S.; Chapman, N.H.; Smith, R.; Poole, M.; Adams, G.; Gillis, R.B.; Besong, T.M.D.; Sheldon, J.; Stiegelmeyer, S.; Perez, L.; et al. Genetic improvement of tomato by targeted control of fruit softening. Nat. Biotechnol. 2016, 34, 950–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, N.M.; McCann, M.C.; Roberts, K. Pectin modification in cell walls of ripening tomatoes occurs in distinct domains. Plant Physiol. 1997, 114, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Liang, J.; Zhu, X.; Xiao, K.; Li, T.; Hu, J. Auxin- and cytokinin-induced berries set in grapevine partly rely on enhanced gibberellin biosynthesis. Tree Genet. Genomes 2016, 12, 41. [Google Scholar] [CrossRef]
- Cruz-Castillo, J.G.; Baldicchi, A.; Frioni, T.; Marocchi, F.; Moscatello, S.; Proietti, S.; Battistelli, A.; Famiani, F. Pre-anthesis CPPU low dosage application increases “Hayward” kiwifruit weight without affecting the other qualitative and nutritional characteristics. Food Chem. 2014, 158, 224–228. [Google Scholar] [CrossRef]
- Pang, M.; McD Stewart, J.; Zhang, J. A mini-scale hot borate method for the isolation of total RNA from a large number of cotton tissue samples. Afr. J. Biotechnol. 2011, 10, 15430–15437. [Google Scholar] [CrossRef]
- Balic, I.; Vizoso, P.; Nilo-Poyanco, R.; Sanhueza, D.; Olmedo, P.; Sepúlveda, P.; Arriagada, C.; Defilippi, B.G.; Meneses, C.; Campos-Vargas, R. Transcriptome analysis during ripening of table grape berry cv. Thompson Seedless. PLoS ONE 2018, 13, e0190087. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Lekanne Deprez, R.H.; Moorman, A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Silva-Sanzana, C.; Celiz-Balboa, J.; Garzo, E.; Marcus, S.E.; Parra-Rojas, J.P.; Rojas, B.; Olmedo, P.; Rubilar, M.A.; Rios, I.; Chorbadjian, R.A.; et al. Pectin methylesterases modulate plant homogalacturonan status in defenses against the aphid Myzus persicae. Plant Cell 2019, 31, 1913–1929. [Google Scholar] [CrossRef] [Green Version]
- Zepeda, B.; Olmedo, P.; Ejsmentewicz, T.; Sepúlveda, P.; Balic, I.; Balladares, C.; Delgado-Rioseco, J.; Fuentealba, C.; Moreno, A.A.; Defilippi, B.G.; et al. Cell wall and metabolite composition of berries of Vitis vinifera (L.) cv. Thompson Seedless with different firmness. Food Chem. 2018, 268, 492–497. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, B.; Suárez-Vega, F.; Saez-Aguayo, S.; Olmedo, P.; Zepeda, B.; Delgado-Rioseco, J.; Defilippi, B.G.; Pedreschi, R.; Meneses, C.; Pérez-Donoso, A.G.; et al. Pre-Anthesis Cytokinin Applications Increase Table Grape Berry Firmness by Modulating Cell Wall Polysaccharides. Plants 2021, 10, 2642. https://doi.org/10.3390/plants10122642
Rojas B, Suárez-Vega F, Saez-Aguayo S, Olmedo P, Zepeda B, Delgado-Rioseco J, Defilippi BG, Pedreschi R, Meneses C, Pérez-Donoso AG, et al. Pre-Anthesis Cytokinin Applications Increase Table Grape Berry Firmness by Modulating Cell Wall Polysaccharides. Plants. 2021; 10(12):2642. https://doi.org/10.3390/plants10122642
Chicago/Turabian StyleRojas, Bárbara, Felipe Suárez-Vega, Susana Saez-Aguayo, Patricio Olmedo, Baltasar Zepeda, Joaquín Delgado-Rioseco, Bruno G. Defilippi, Romina Pedreschi, Claudio Meneses, Alonso G. Pérez-Donoso, and et al. 2021. "Pre-Anthesis Cytokinin Applications Increase Table Grape Berry Firmness by Modulating Cell Wall Polysaccharides" Plants 10, no. 12: 2642. https://doi.org/10.3390/plants10122642
APA StyleRojas, B., Suárez-Vega, F., Saez-Aguayo, S., Olmedo, P., Zepeda, B., Delgado-Rioseco, J., Defilippi, B. G., Pedreschi, R., Meneses, C., Pérez-Donoso, A. G., & Campos-Vargas, R. (2021). Pre-Anthesis Cytokinin Applications Increase Table Grape Berry Firmness by Modulating Cell Wall Polysaccharides. Plants, 10(12), 2642. https://doi.org/10.3390/plants10122642







