Hypolignification: A Decisive Factor in the Development of Hyperhydricity
Abstract
1. Introduction
2. Results
2.1. The Effect of p-Coumaric Acid on Apoplastic Water and Air Volumes in Arabidopsis thaliana Col-0 Seedlings
2.2. The Effect of p-Coumaric Acid on Lignin Production and Root Growth Linked to the Development of HH
2.3. The Effect of Inhibiting Lignin Biosynthesis on HH
2.4. PAL Activity in Normal and Hyperhydric Arabidopsis thaliana Col-0 Seedlings
2.5. Leaf Anatomy
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials
5.2. Experimental Setup
5.3. Extraction of Cell Walls and Total Lignin Determination
5.4. Evaluation of Apoplastic Water and Air Volumes in Leaves
5.5. Root Growth Determination
5.6. Enzyme Extraction and C4H Activity Assay
5.7. Quantitative Real-Time PCR (qPCR)
5.8. Determination of Phenylalanine Ammonia-Lyase (PAL) Activity
5.9. Microscopy
5.10. Statistical Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziv, M. Quality of micropropagated plants—Vitrification. In Vitro Cell. Dev. Biol. Plant 1991, 27, 64–69. [Google Scholar] [CrossRef]
- Franck, T.; Crèvecoeur, M.; Wuest, J.; Greppin, H.; Gaspar, T. Cytological Comparison of Leaves and Stems of Prunus avium L. Shoots Cultured on a Solid Medium with Agar or Gelrite. Biotech. Histochem. 1998, 73, 32–43. [Google Scholar] [CrossRef]
- Olmos, E.; Hellín, E. Ultrastructural differences of hyperhydric and normal leaves from regenerated carnation plants. Sci. Hortic. 1998, 75, 91–101. [Google Scholar] [CrossRef]
- Picoli, E.A.; Otoni, W.; Figueira, M.L.; Carolino, S.M.; Almeida, R.S.; Silva, E.A.; Carvalho, C.R.; Fontes, E. Hyperhydricity in in vitro eggplant regenerated plants: Structural characteristics and involvement of BiP (Binding Protein). Plant Sci. 2001, 160, 857–868. [Google Scholar] [CrossRef]
- Dewir, Y.; Chakrabarty, D.; Ali, M.; Hahn, E.; Paek, K.Y. Lipid peroxidation and antioxidant enzyme activities of Euphorbia millii hyperhydric shoots. Environ. Exp. Bot. 2006, 58, 93–99. [Google Scholar] [CrossRef]
- Kevers, C.; Coumans, M.; Coumans-Gilles, M.-F.; Caspar, T. Physiological and biochemical events leading to vitrification of plants cultured in vitro. Physiol. Plant 1984, 61, 69–74. [Google Scholar] [CrossRef]
- Kevers, C.; Prat, R.; Gaspar, T. Vitrification of carnation in vitro: Changes in cell wall mechanical properties, cellulose and lignin content. Plant Growth Regul. 1987, 5, 59–66. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Franck, T.; Bisbis, B.; Billard, J.P.; Huault, C.; Le Dily, F.; Petit-Paly, G.; Rideau, M.; Penel, C.; et al. Paradoxical results in the analysis of hyperhydric tissues considered as being under stress: Questions for a debate. Bulg. J. Plant Physiol. 1995, 21, 80–97. [Google Scholar]
- Komatsu, S.; Kobayashi, Y.; Nishizawa, K.; Nanjo, Y.; Furukawa, K. Comparative proteomics analysis of differentially expressed proteins in soybean cell wall during flooding stress. Amino Acids 2010, 39, 1435–1449. [Google Scholar] [CrossRef]
- Barceló, A.R. Peroxidase and not lacasse is the enzyme responsible for cell wall linification in the secondary thickening of xylem vessels in Lupinus. Protoplasma 1995, 186, 41–44. [Google Scholar] [CrossRef]
- Sarkanen, K.V.; Ludwig, C.H. Lignins, Occurrence, Formation, Structure and Reactions; Wiley-Interscience: New York, NY, USA, 1971. [Google Scholar]
- Donaldson, L.A. Lignification and lignin topochemistry—An ultrastructural view. Phytochemistry 2001, 57, 859–876. [Google Scholar] [CrossRef]
- Vanholme, R.; Storme, V.; Vanholme, B.; Sundin, L.; Christensen, J.H.; Goeminne, G.; Halpin, C.; Rohde, A.; Morreel, K.; Boerjan, W. A Systems Biology View of Responses to Lignin Biosynthesis Perturbations in Arabidopsis. Plant Cell 2012, 24, 3506–3529. [Google Scholar] [CrossRef] [PubMed]
- Schilmiller, A.L.; Stout, J.; Weng, J.-K.; Humphreys, J.; Ruegger, M.O.; Chapple, C. Mutations in the cinnamate 4-hydroxylase gene impact metabolism, growth and development in Arabidopsis. Plant J. 2009, 60, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Dolan, W.L.; Anderson, N.A.; Chapple, C. Indole Glucosinolate Biosynthesis Limits Phenylpropanoid Accumulation in Arabidopsis thaliana. Plant Cell 2015, 27, 1529–1546. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef]
- Bell-Lelong, D.A.; Cusumano, J.C.; Meyer, K.; Chapple, C. Cinnamate-4-Hydroxylase Expression in Arabidopsis (Regulation in Response to Development and the Environment). Plant Physiol. 1997, 113, 729–738. [Google Scholar] [CrossRef]
- Schmid, M.; Davison, T.S.; Henz, S.R.; Pape, U.J.; Demar, M.; Vingron, M.; Schölkopf, B.; Weigel, D.; Lohmann, J. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 2005, 37, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Landry, L.G.; Chapple, C.; Last, R. Arabidopsis Mutants Lacking Phenolic Sunscreens Exhibit Enhanced Ultraviolet-B Injury and Oxidative Damage. Plant Physiol. 1995, 109, 1159–1166. [Google Scholar] [CrossRef]
- Chapple, C.C.S.; Vogt, T.; Ellis, B.E.; Somerville, C.R. An Arabidopsis Mutant Defective in the General Phenylpropanoid Pathway. Plant Cell 1992, 4, 1413–1424. [Google Scholar] [CrossRef]
- Ruegger, M.; Chapple, C. Mutations that reduce sinapoylmalate accumulation in Arabidopsis thaliana define loci with diverse roles in phenylpropanoid metabolism. Genetics 2001, 159, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, J.M.; Chapple, C. Rewriting the lignin road-map. Curr. Opin. Plant Biol. 2002, 5, 224–229. [Google Scholar] [CrossRef]
- Nair, R.B.; Bastress, K.L.; Ruegger, M.O.; Denault, J.W.; Chapple, C. The Arabidopsis thaliana REDUCED EPIDERMAL FLUORESCENCE1 Gene Encodes an Aldehyde Dehydrogenase Involved in Ferulic Acid and Sinapic Acid Biosynthesis. Plant Cell 2004, 16, 544–554. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The Phenylpropanoid Pathway in Arabidopsis. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2011; pp. 1–19. [Google Scholar]
- Taylor, N.G.; Scheible, W.-R.; Cutler, S.; Somerville, C.R.; Turner, S.R. The irregular xylem3 Locus of Arabidopsis Encodes a Cellulose Synthase Required for Secondary Cell Wall Synthesis. Plant Cell 1999, 11, 769–780. [Google Scholar] [CrossRef]
- Jones, L.; Ennos, A.R.; Turner, S.R. Cloning and characterization of irregular xylem4 (irx4): A severely lignin-deficient mutant of Arabidopsis. Plant J. 2001, 26, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.; Ord, B. Influence of phenolic acids on morphological changes in roots of Pisum sativum. J. Sci. Food Agric. 1990, 52, 289–299. [Google Scholar] [CrossRef]
- Politycka, B. Ethylene-dependent activity of phenylalanine ammonia-lyase and lignin formation in cucumber roots exposed to phenolic allelochemicals. Acta Soc. Bot. Pol. 1999, 68, 123–127. [Google Scholar] [CrossRef]
- Baleroni, C.R.S.; Ferrarese, M.L.L.; Braccini, A.L.; Scapim, C.A.; Ferrarese-Filho, O. Effects of ferulic and caffeic acids on canola (Brassica napus L. cv. Hyola 401) seed germination. Seed Sci. Technol. 2000, 28, 201–207. [Google Scholar]
- Salvador, V.H.; Lima, R.; dos Santos, W.; Soares, A.R.; Böhm, P.A.F.; Marchiosi, R.; Ferrarese, M.D.L.L.; Ferrarese-Filho, O. Cinnamic Acid Increases Lignin Production and Inhibits Soybean Root Growth. PLoS ONE 2013, 8, e69105. [Google Scholar] [CrossRef]
- dos Santos, W.; Ferrarese, M.D.L.L.; Finger, A.; Teixeira, A.C.N.; Ferrarese-Filho, O. Lignification and Related Enzymes in Glycine max Root Growth-Inhibition by Ferulic Acid. J. Chem. Ecol. 2004, 30, 1203–1212. [Google Scholar] [CrossRef]
- Zanardo, D.I.L.; Lima, R.B.; Ferrarese, M.D.L.L.; Bubna, G.A.; Ferrarese-Filho, O. Soybean root growth inhibition and lignification induced by p-coumaric acid. Environ. Exp. Bot. 2009, 66, 25–30. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis: A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Fahrendorf, T.; Ni, W.; Shorrosh, B.S.; Dixon, R.A. Stress responses in alfalfa (Medicago sativa L.) XIX. Transcriptional activation of oxidative pentose phosphate pathway genes at the onset of the isoflavonoid phytoalexin response. Plant Mol. Biol. 1995, 28, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Teutsch, H.G.; Hasenfratz, M.P.; Lesot, A.; Stoltz, C.; Garnier, J.M.; Jeltsch, J.M.; Durst, F.; Werck-Reichhart, D. Isolation and sequence of a cDNA encoding the Jerusalem artichoke cinnamate 4-hydroxylase, a major plant cytochrome P450 involved in the general phenylpropanoid pathway. Proc. Natl. Acad. Sci. USA 1993, 90, 4102–4106. [Google Scholar] [CrossRef] [PubMed]
- Anterola, A.M.; Lewis, N.G. Trends in lignin modification: A comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity. Phytochemistry 2002, 61, 221–294. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Pazos-Malvido, E. Phytotoxic Effects of 21 Plant Secondary Metabolites on Arabidopsis thaliana Germination and Root Growth. J. Chem. Ecol. 2007, 33, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Peña, M.J.; Revilla, G.; Zarra, I. Changes in Dehydrodiferulic Acids and Peroxidase Activity against Ferulic Acid Associated with Cell Walls during Growth of Pinus pinaster Hypocotyl. Plant Physiol. 1996, 111, 941–946. [Google Scholar] [CrossRef]
- Janovicek, K.J.; Vyn, T.J.; Voroney, R.P.; Allen, O.B. Early corn seedling growth response to phenolic acids. Can. J. Plant Sci. 1997, 77, 391–393. [Google Scholar] [CrossRef][Green Version]
- Ng, P.L.L.; Ferrarese, M.L.L.; Huber, D.; Ravagnani, A.; Ferrarese-Filho, O. Canola (Brassica napus L.) seed germination influenced by cinnamic and benzoic acids and derivatives: Effects on peroxidase. Seed Sci. Technol. 2003, 31, 39–46. [Google Scholar] [CrossRef]
- Lipetz, J.; Garro, A.J. Ionic Effects on Lignification and Peroxidase in Tissue Cultures. J. Cell Biol. 1965, 25, 109–116. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Y.; Zhang, Y.; Huang, S.; Liu, Z.; Fei, D.; Feng, H. A missense mutation of plastid RPS4 is associated with chlorophyll deficiency in Chinese cabbage (Brassica campestris ssp. pekinensis). BMC Plant Biol. 2018, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Chen, H.; Zhang, Y.; Zhang, Z.; Zheng, N.; Yin, B.; Yan, H.; Zhu, L.; Zhao, X.; Yuan, M.; et al. Knockout of the AtCESA2 Gene Affects Microtubule Orientation and Causes Abnormal Cell Expansion in Arabidopsis. Plant Physiol. 2007, 143, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, Z.; Lin, Y.; Liu, W.; Guo, H.; Zhang, W.; Zhang, C. Comparative study on antioxidative system in normal and vitrified shoots of Populus suaveolens in tissue culture. For. Stud. 2004, 6, 1–8. [Google Scholar] [CrossRef]
- Saher, S.; Piqueras, A.; Hellin, E.; Olmos, E. Hyperhydricity in micropropagated carnation shoots: The role of oxidative stress. Physiol. Plant 2004, 120, 152–161. [Google Scholar] [CrossRef]
- Kevers, C.; Gaspar, T. Vitrification on Carnation in vitro: Changes in Ethylene Production. Plant Cell Tissue Organ Cult. 1985, 4, 215–223. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Estrada, B.; Bernal, M.A.; Díaz, J.; Pomar, F.; Merino, F. Fruit development in Capsicum annuum: Changes in capsaicin, lignin, free phenolics, and peroxidase patterns. J. Agric. Food Chem. 2000, 48, 6234–6239. [Google Scholar] [CrossRef]
- Fukushima, R.S.; Kerley, M.S. Use of Lignin Extracted from Different Plant Sources as Standards in the Spectrophotometric Acetyl Bromide Lignin Method. J. Agric. Food Chem. 2011, 59, 3505–3509. [Google Scholar] [CrossRef]
- Terry, M.E.; Bonner, B.A. An Examination of Centrifugation as a Method of Extracting an Extracellular Solution from Peas, and Its Use for the Study of Indoleacetic Acid-induced Growth. Plant Physiol. 1980, 66, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dries, N.; Giannì, S.; Czerednik, A.; Krens, F.A.; De Klerk, G.-J.M. Flooding of the apoplast is a key factor in the development of hyperhydricity. J. Exp. Bot. 2013, 64, 5221–5230. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Wang, D.; Jin, C.; Sun, W.; Liu, X.; Zhang, S.; Gao, F.; Khanizadeh, S. Cinnamate-4-Hydroxylase Gene Is Involved in the Step of Lignin Biosynthesis in Chinese White Pear. J. Am. Soc. Hortic. Sci. 2015, 140, 573–579. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sundin, L.; Vanholme, R.; Geerinck, J.; Goeminne, G.; Höfer, R.; Kim, H.; Ralph, J.; Boerjan, W. Mutation of the Inducible ARABIDOPSIS THALIANA CYTOCHROME P450 REDUCTASE2 Alters Lignin Composition and Improves Saccharification. Plant Physiol. 2014, 166, 1956–1971. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B. Tissue and method specificities of phenylalanine ammonia-lyase assay. J. Plant Physiol. 2012, 169, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
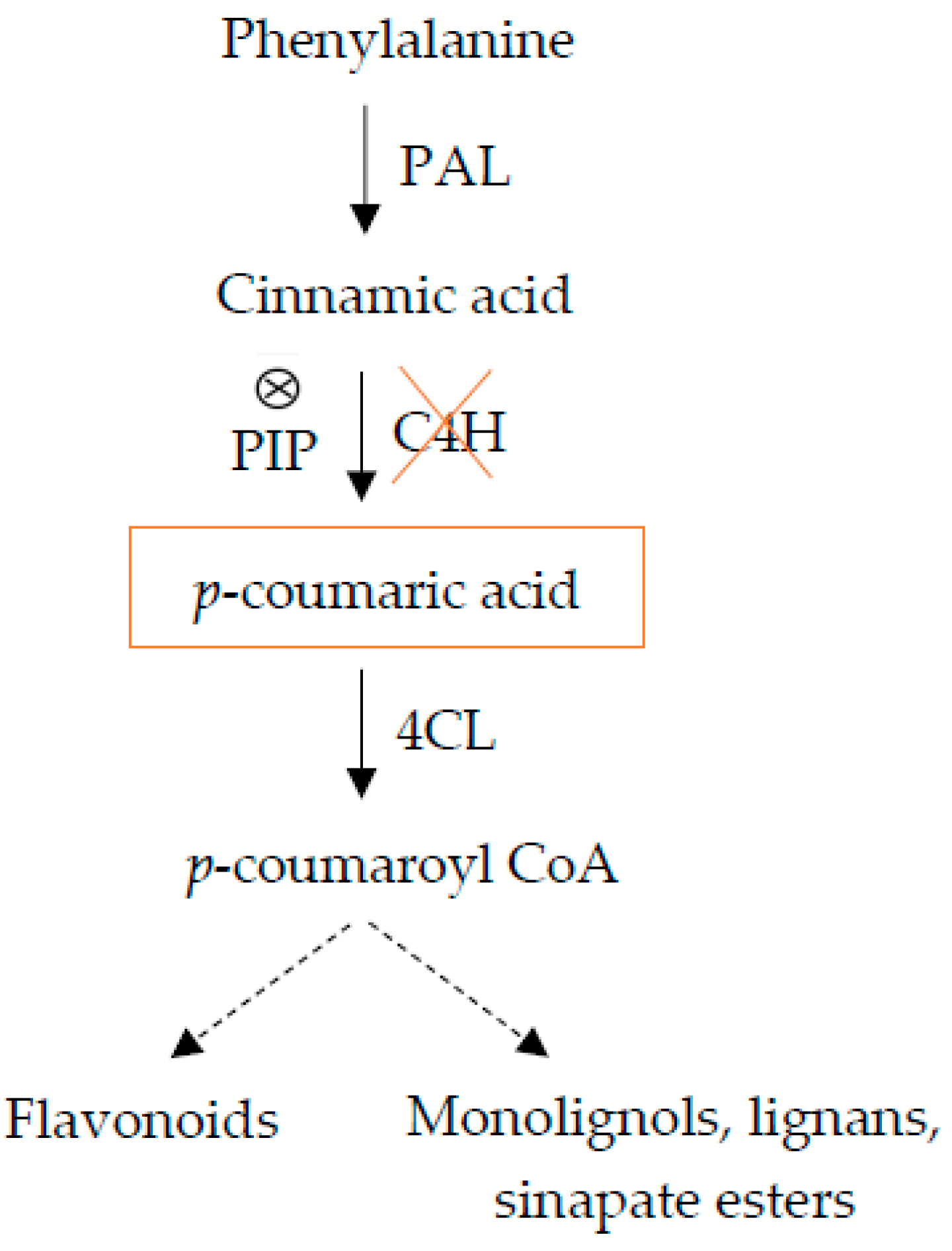









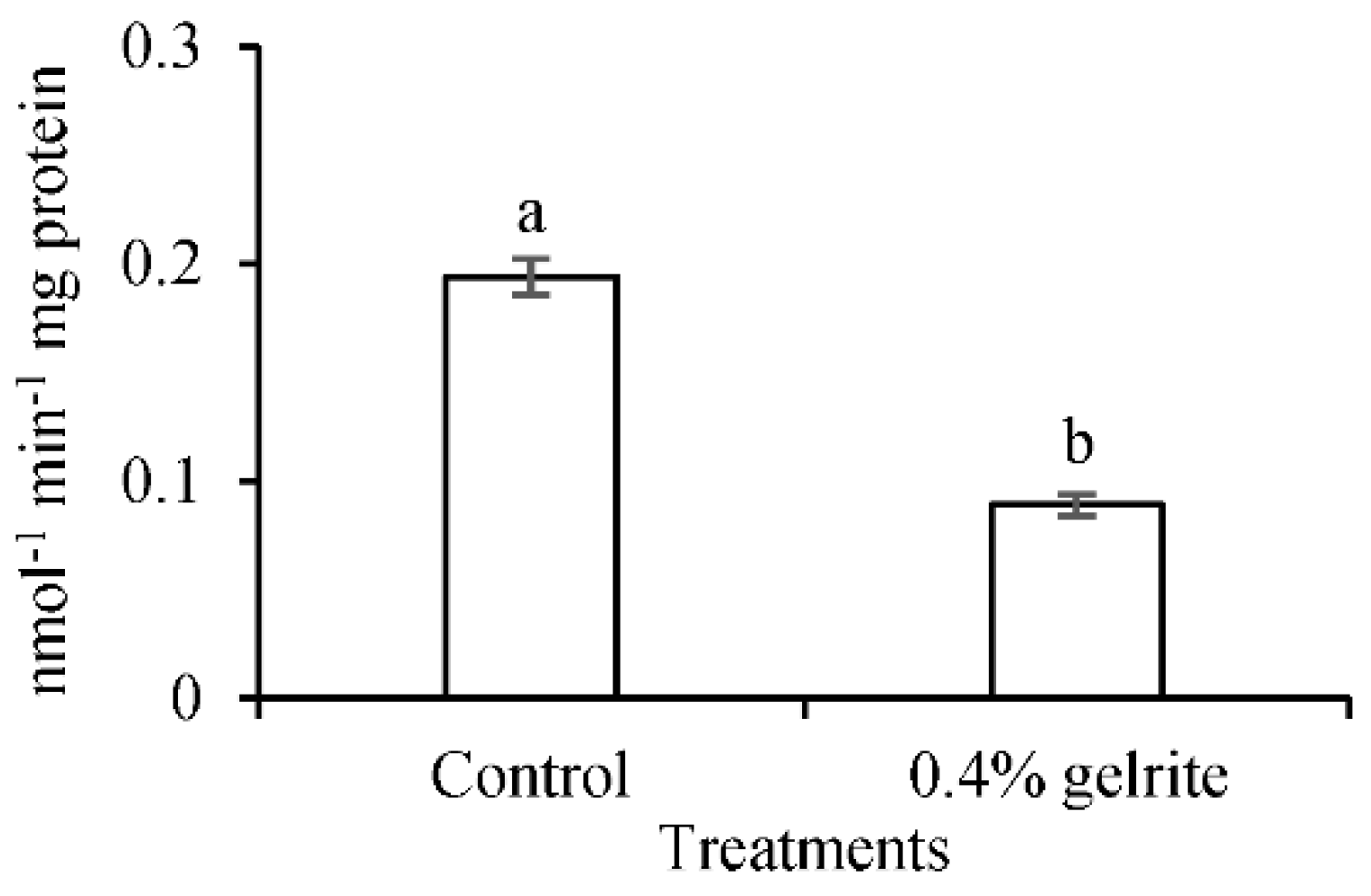

| Line | Lignin (A280 mg g−1 Cell Walls) |
|---|---|
| Ler 0.7% Micro-agar (control) | 0.0182 ± 0.0002 a |
| Ler 0.4% Gelrite | 0.0084 ± 0.0006 d |
| Ler 0.4% Gelrite + 100 µM p-coumaric acid | 0.0123 ± 0.0004 b |
| ref3-1 Micro-agar | 0.0076 ± 0.0006 d,e |
| ref3-1 0.4% Gelrite | 0.0067 ± 0.0005 e |
| ref3-1 0.4% Gelrite + 100 µM p-coumaric acid | 0.0102 ± 0.0003 c |
| Col-0 0.7% Micro-agar (control) | 0.0225 ± 0.0004 a |
| Col-0 0.4% Gelrite | 0.0119 ± 0.0002 c |
| Col-0 0.4% Gelrite + 100 µM p-coumaric acid | 0.0146 ± 0.0007 b |
| ref3-3 Micro-agar | 0.0098 ± 0.0003 d |
| ref3-3 0.4% Gelrite | 0.0088 ± 0.0003 d |
| ref 3-3 0.4% Gelrite + 100 µM p-coumaric acid | 0.0138 ± 0.0004 b |
| Line | Lignin (A280 mg g−1 Cell Walls) |
|---|---|
| Ler 0.7% Micro-agar (control) | 0.0196 ± 0.0004 a |
| Ler Micro-agar + 100 µM PIP | 0.0090 ± 0.0007 b |
| ref3-1 Micro-agar | 0.0080 ± 0.0003 b |
| Col-0 0.7% Micro-agar (control) | 0.0216 ± 0.0005 a |
| Col-0 Micro-agar + 100 µM PIP | 0.0099 ± 0.0008 b |
| ref3-3 Micro-agar | 0.0087 ± 0.0004 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemat, N.; Visser, R.G.F.; Krens, F.A. Hypolignification: A Decisive Factor in the Development of Hyperhydricity. Plants 2021, 10, 2625. https://doi.org/10.3390/plants10122625
Kemat N, Visser RGF, Krens FA. Hypolignification: A Decisive Factor in the Development of Hyperhydricity. Plants. 2021; 10(12):2625. https://doi.org/10.3390/plants10122625
Chicago/Turabian StyleKemat, Nurashikin, Richard G. F. Visser, and Frans A. Krens. 2021. "Hypolignification: A Decisive Factor in the Development of Hyperhydricity" Plants 10, no. 12: 2625. https://doi.org/10.3390/plants10122625
APA StyleKemat, N., Visser, R. G. F., & Krens, F. A. (2021). Hypolignification: A Decisive Factor in the Development of Hyperhydricity. Plants, 10(12), 2625. https://doi.org/10.3390/plants10122625







