Abstract
Buzz pollination is a specialized pollination syndrome that requires vibrational energy to extract concealed pollen grains from poricidal anthers. Although a large body of work has examined the ecology of buzz pollination, whether acoustic properties of buzz pollinators affect pollen extraction is less understood, especially in weeds and invasive species. We examined the pollination biology of Silverleaf nightshade (Solanum elaeagnifolium), a worldwide invasive weed, in its native range in the Lower Rio Grande Valley (LRGV) in south Texas. Over two years, we documented the floral visitors on S. elaeagnifolium, their acoustic parameters (buzzing amplitude, frequency, and duration of buzzing) and estimated the effects of the latter two factors on pollen extraction. We found five major bee genera: Exomalopsis, Halictus, Megachile, Bombus, and Xylocopa, as the most common floral visitors on S. elaeagnifolium in the LRGV. Bee genera varied in their duration of total buzzing time, duration of each visit, and mass. While we did not find any significant differences in buzzing frequency among different genera, an artificial pollen collection experiment using an electric toothbrush showed that the amount of pollen extracted is significantly affected by the duration of buzzing. We conclude that regardless of buzzing frequency, buzzing duration is the most critical factor in pollen removal in this species.
1. Introduction
Buzz pollination, a specialized pollination syndrome, is found in ~6% of flowering plants, where pollen grains are concealed inside poricidal anthers [1,2,3,4]. It is suggested that the buzz pollinated plants are a typical example of convergent evolution, as similar flower morphologies appear to evolve among different unrelated families. For instance, Solanum-type flowers have evolved across Primilaceae, Gesneriaceae, and Ericaceae, in addition to Solanaceae with typical characteristics of buzz-pollinated species. These include the presence of poricidal anthers and the lack of nectar or other pollen rewards, which dispense pollen only to the authorized buzz pollinators [2,5,6,7]. Flowers from most of the Solanum spp. are exclusively buzz pollinated that control the rate of pollen removal as well as exclude pollen thieves such as hoverflies (Simosyrphus spp.) and stingless bees (Trigona spp.) [6,8,9,10,11]. Buzz pollination also provides us an excellent model system to understand the origin of complex floral modifications, the evolutionary ecology of pollen rewards, as well as biomechanics underlying plant-pollinator interactions [3,6,12,13,14]. Although a large body of previous work focused on understanding the role of plant and pollinator characteristics in the context of buzz pollination in various plant families, whether such interactions play a key role in the reproductive success of invasive species such as S. elaeagnifolium is less understood.
Even though buzz pollination is known for more than 100 years [15,16], only recently, the field has received a rejuvenated interest [12,13,17,18]. In the past decade, exceptional work has been done on understanding buzz pollination in different plant and bee species, their evolutionary and ecological consequences, as well as both plant and pollinator fitness [3,6,12,13,17,19,20,21,22,23,24]. For example, although bee vibrational acceleration (instantaneous changes in amplitude, ms−2) and frequency (number of cycles per second, Hz) are independent of bee mass and flower mass and vary between bee species, floral characteristics have been found to significantly affect their transmission to the flowers [3]. Moreover, the vibrations produced by the bumble bee (Bombus spp.), a major buzzing pollinator, with greater amplitude and longer duration, ejects more pollen in any given foraging effort [25]. Collectively, these studies demonstrate that bee and floral traits can affect acoustic properties of bees during buzz pollination [3].
Although previous studies documented the morphological and acoustic characteristics of buzzing bees and their host plants, we are still at the early stages of understanding such species-specific interactions at the community level [14,26,27,28,29]. This is more ecologically relevant in invasive weeds, where pollination success, and consequently seed set, is a driving force in invasion success. For instance, a comparison of S. elaeagnifolium populations within and beyond their ancestral range, Petanidou et al. (2018) found variations in resource allocation patterns that directly affect the pollinator visitation rate and fruit set [28]. Moreover, being self-incompatible and having nectar-less flowers, understanding the intricate details of the pollination biology of such weed species is important, as their reproduction ability, and hence propagule supply, plays a critical role in their uncontrollable spread and establishment [30]. To examine this, we documented the pollination biology of S. elaeagnifolium in the LRGV by identifying the major buzz pollinators, their buzzing acoustic parameters (buzzing frequency, amplitude, duration of buzzing), as well estimating the effects of frequency and duration of buzzing on pollen extraction. Following this, we then used electric toothbrushes [14] to simulate the natural buzzing frequency and duration of buzzing to validate the results from the native pollinators.
We hypothesized that buzz pollinators will vary in their buzzing properties (frequency, amplitude) during S. elaeagnifolium pollination and consequently aid in seed set. Apart from threatening the pollination success of other native plants [26,30], S. elaeagnifolium also serves as a reservoir host for ‘Candidatus Liberibacter solanacearum’ which causes Zebra Chip disease of the potato [31]. Understanding the role of S. elaeagnifolium pollinators and studying the effects of buzz pollination traits such as buzzing frequency on S. elaeagnifolium reproductive success in the native range will provide us better knowledge of how pollination traits affect the invasiveness of the species.
Specifically, we asked two major questions. 1) Who are the major buzz pollinators of S. elaeagnifolium in their native range, and 2) How do variations in bee buzzing traits affect pollen extraction? To answer these questions, we used a combination of field and lab studies over two years across the Lower Rio Grande Valley, Texas, USA, the native range of S. elaeagnifolium.
2. Results
2.1. Major Pollinators of S. elaeagnifolium in Its Native Range in LRGV, Texas
Bee specimens collected in field surveys were directly examined and visualized under a dissecting microscope. Upon identification, we found five different bee genera prevalent in the Lower Rio Grande Valley of Texas. These include (A) Exomalopsis, (B) Halictus, (C) Megachile, (D) Bombus, and (E) Xylocopa spp. We also identified a subset of the bees to the species level and found that they were predominantly Xylocopa mexicanorum, Halictus ligatus, Bombus pennsylvanicus, and Exomalopsis mellipes. The Bombus and Xylocopa are significantly bigger bees, while Exomalopsis, Halictus, and Megachile bees are comparatively smaller in size (Figure 1). The taxonomic confirmation of each collected bee to the species level would require additional resources and expertise and was not carried out for this study. The specimens are however stored for further analyses and DNA barcoding.
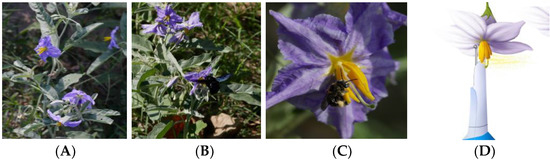
Figure 1.
Silverleaf nightshade (SLN; Solanum elaeagnifolium) flowers and pollinators in its native range in south Texas. (A) Inflorescence, (B) Xylocopa spp., and (C) Exomalopsis spp. buzz pollinating SLN, and (D) artificial pollination using electric toothbrush (modified from [14]).
2.2. Bee Visit Time
Bee visitation time (N = 40, Table 1) (the total time (seconds) spent by each bee on each flower) was estimated as the difference between when the bee landed on the flower and when the bee left the flower. Among all five genera, we found that Xylocopa, the largest bee genera surveyed, spent the lowest amount of time (Mean = 1.24 ± SE = 0.66; One-Way ANOVA; p < 0.0001) followed by the Bombus bee which spent significantly longer than Xylocopa (Mean = 4.05 ± SE = 0.81; p < 0.0001) but less than other small bees (Exomalopsis: Mean = 9.08 ± SE = 0.69; p = 0.0001, Megachile: Mean = 10.05 ± SE = 0.14; p = 0.0018, Halictus: Mean = 13.73 ± SE = 0.07; p = 0.0008 (Figure 2). The larger the bee mass/size, the higher the buzzing amplitude (i.e., the function of the physical condition of the bee), which gives an advantage to bee genera for efficient pollen extraction in less time [25]. However, there was no significant difference in bee visit time among Exomalopsis, Halictus, and Megachile (Figure 2), which also had comparable body mass as shown in Section 2.6.

Table 1.
Number of individuals (N) recorded for bee acoustic parameters.

Figure 2.
Box and whisker plot of the results of One-Way ANOVA and post-hoc Tukey’s HSD test of comparison of bee visit time (N = 40) among major five genera of buzz pollinating bees in LRGV, Texas. Different letters show significant differences among means (p < 0.05).
2.3. No. of Pulses Per Bee Visit and Buzz % over Visit Time
The analysis of bee vibrations using Audacity software showed that each bee visit included multiple pulses (bee buzzing) and time lags (bee not buzzing) within a single visit. After counting pulses/buzz among different genera, we found that Xylocopa (N = 22) produces significantly fewer pulses ~1 per buzz (Mean = 1 ± SE = 0; Kruskal–Wallis test; p < 0.001) as compared to other genera. However, we did not see any significant difference in the number of pulses/buzzes among Exomalopsis (N = 18), Halictus (N = 8), and Megachile (N = 16) genera (Figure 3A, Table 1). While estimating the number of pulses/buzzes in a single flower visit, we also measured the duration of each pulse, and the pooled pulse time was recorded as actual buzz time per flower visit. We calculated buzz % over visit time using the ratio of actual buzz time over total visit time. Interestingly, we found that Megachile had a significantly lower buzz % (Mean = 16.8 ± SE = 4.2) over the visit time as compared to Xylocopa (Mean = 27.45 ± SE = 4.20; One-way ANOVA; p < 0.0158), while there were no significant differences in buzz % over visit time among Exomaplosis, Halictus, and Xylocopa (Figure 3B).
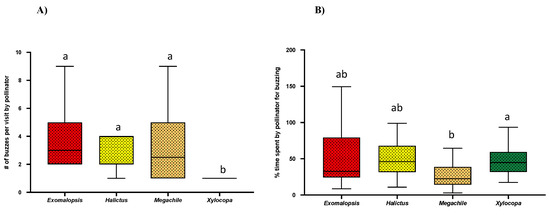
Figure 3.
Box and whisker plots of the results of Kruskal–Wallis, One-way ANOVA, and post-hoc Tukey’s HSD (p < 0.05), for comparison (A) No. of buzzes/visit and (B) Buzz % over visit time among different bee genera. Different letters show significant differences among means (p < 0.05).
2.4. Bee Buzzing Frequency and Buzzing Amplitude
Buzzing frequency (Hz) is the number of vibrations produced per second, while buzzing amplitude (dB) denotes how loud the vibrations are produced during buzzing. On vibrational analysis, we did not detect any significant differences in bee buzzing frequency (Exomalopsis: Mean = 126 ± SE = 29.68, Halictus: Mean = 144 ± SE = 50.73, Megachile: Mean = 119 ± SE = 29.66, and Xylocopa: Mean = 125 ± SE = 26.71; Kruskal–Wallis test; p = 0.4519) and buzzing amplitude (Exomalopsis: Mean = −50.6 ± SE = −11.9, Halictus: Mean = −50.1 ± SE = −17.7, Megachile: Mean = −53.8 ± SE = −13.44, and Xylocopa: Mean = −50.2 ± SE = −10.7; Kruskal–Wallis test; p = 0.3018) among all genera (Figure 4A,B). However, Bombus was not included in such analyses because we were unable to record their buzzing vibrations.

Figure 4.
Box and whisker plots of the results of non-parametric, Kruskal–Wallis test (p < 0.05) and post-hoc Dunn’s test for comparison of (A) Bee buzzing frequency and (B) Buzzing amplitude among different bee genera. Similar letters show non-significant differences (p < 0.05).
2.5. Acoustics of First and Last Buzz
Previous studies show that bees extract most of their pollen (~60%) in the first two pulses [2,25]. To find if there are any variations among buzzes, we compared buzzing acoustic parameters (frequency, amplitude, buzzing time) of the first and last pulse. In our pairwise comparisons, regardless of the bee genera, we found that bees produced the first pulse with a significantly lower frequency when compared to the last pulse frequency (Mean = 110 ± SE = 5.93; Mean = 136 ± SE = 9.24; t-test; p = 0.021) (Figure 5A). However, we found no significant differences in buzzing amplitude (Mean = −53.75 ± SE = −8.29; Mean = −53.04 ± SE = −8.18; t-test; p = 0.54) and buzzing time (Mean = 1.16 ± SE = 0.18; Mean = 1.16 ± SE = 0.18; t-test; p = 0.99) between the first and last buzz (Figure 5B,C).
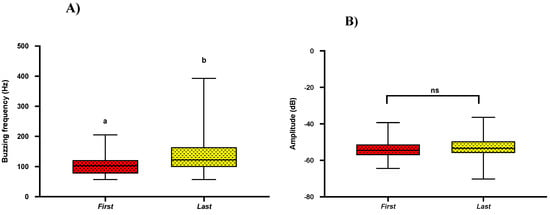
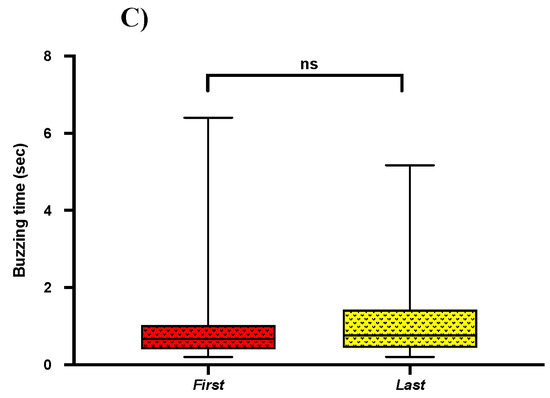
Figure 5.
Box and whisker plots of the results of t-test (p < 0.05) for comparison of (A) Buzzing frequency, (B) Buzzing amplitude, and (C) Buzzing time in between first and last buzz. Similar letters show the non-significant differences. Different letters show significant differences (p < 0.05).
2.6. Comparison of Bee Size among Different Genera
As bee size (bee mass and ITD: the distance between a bee’s wing bases or tegulae) is a key factor in determining bee buzzing acoustics (frequency, amplitude) [3,13,18,32,33,34], we compared the average bee mass (g) and ITD of all four genera (Figure 6A,B). We found that Xylocopa had a significant higher mass (Mean = 0.836 ± 0.18; Kruskal–Wallis test; p < 0.0001) as compared to other genera, while there was no significant difference among Exomalopsis (Mean = 0.066 ± 0.015), Halictus (Mean = 0.048 ± 0.017), and Megachile (Mean = 0.067 ± 0.017) (Figure 6A). Moreover, similar trends were observed for ITDs with having a significant higher ITD of Xylocopa (Mean = 365.9 ± 78; Kruskal–Wallis test; p < 0.0001), while there was insignificant difference among Exomalopsis (Mean = 146.33 ± 34.5), Halictus (Mean = 124.32 ± 43.95), and Megachile (Mean = 143.65 ± 35.91) (Figure 6B).
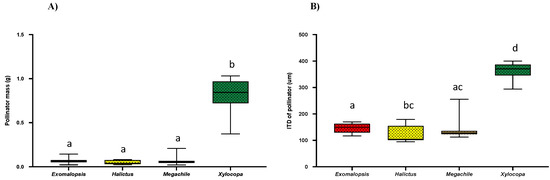
Figure 6.
Box and whisker plots of the results of non-parametric, Kruskal–Wallis test (p < 0.05) and post-hoc Dunn’s test for comparison of bee size; (A) Bee mass and (B) ITD. Different letters show significant differences among means (p < 0.05).
2.7. Artificial Pollen Extraction and Buzzing Time Intervals
Using electric toothbrushes in the lab, we examined if different frequency levels and buzzing time intervals affected the amount of pollen collected. We found that the amount of pollen collected was independent of buzzing frequency. On the other hand, buzzing time had a significant effect on the amount of pollen collected with longer buzzing time (10 s) resulting in more pollen extraction (Mean = 7.73 ± SE = 0.45; One-way ANOVA; p < 0.0001), while shorter buzzing time (~1.5 s) extracted a significantly lower amount of pollen (Mean = 2.61 ± SE = 0.22; One-way ANOVA; p < 0.0001; Figure 7A,B). Clearly, the length of buzzing time is a critical factor for efficient pollen collection.
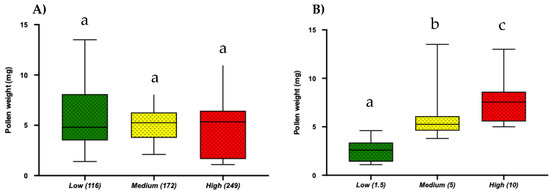
Figure 7.
Box and whisker plots of the results of One-way ANOVA, Tukey’s HSD (p < 0.05) for the effect of (A) Buzzing frequency and (B) Buzzing time (Low: 1.5 s, Medium: 5 s, High: 10 s) on artificial pollen extraction. Different letters show significant differences among means (p < 0.05).
3. Discussion
Our study provides valuable information on major floral visitors of S. elaeagnifolium as well as variation in their visit time and buzzing acoustics in their native range. As S. elaeagnifolium is a noxious and worldwide invasive weed threatening natural and agricultural ecosystems due to their high propagule supply [28], our study characterizing the time and acoustic parameters of buzzing bees will be of interest to understand the role and efficiency of pollinators in the invasion success of this species. In the field survey study, we found five major genera of buzz-pollinating bees: Exomalopsis, Halictus, Megachile, Bombus, and Xylocopa as the most prevalent in the LRGV of Texas. Exomalopsis, Xylocopa, and Bombus are members of the Apidae family, while Halictus and Megachile fall in Halictidae and Megachilidae, respectively. The Xylocopa and Bombus are comparatively bigger bees, while Exomalopsis, Halictus, and Megachile are smaller [13]. Previous studies demonstrated that these bees play a key role in pollinating other buzz-pollinated plants such as S. rostratum as well despite their size variations [11]. Interestingly, the absence of honeybees (Apis melifera) in S. elaeagnifolium visits shows how restricted pollen release is important, and only specialized pollinators can extract pollen, as pollen larceny is costly for plants [7,11,25]. The evolution of hereanthery (stamen dimorphism within angiosperms, lack of floral nectaries and poricidal anthers) within buzz pollination is possibly such a response to pollen thieves aimed at reducing pollen consumption by them [35].
Investigating how bee visit time varies among different genera, we found that small bees, i.e., Halictus, Exomalopsis, and Megachile, spent a significantly longer time than larger bees, i.e., Bombus and Xylocopa (Figure 2). This could be due to the competitive disadvantage of small bees due to their inability to generate vibrations at a higher magnitude when compared to larger bees. Consequently, they compensate it by spending a longer time on each flower to extract enough pollen in any given foraging effort [13,34,36]. The longer the time spent by a bee on each flower, anthers are stimulated with force longer; hence, more pollen is extracted [25]. Moreover, within each buzz, we found multiple pulses and time lags, a characteristic feature of a typical buzz (Figure 3A) [1,34]. Interestingly, among all bees, Megachile bees were found to have the lowest buzz % over visit time when compared to other genera although they visited each flower longer (Figure 3B), a possible effect of small bees trying to minimize their energy investment and extract more pollen in any given foraging effort.
In the recent past, more work has documented the effects of bee traits (size, species) on buzzing efficiency [3,6,12,17,18,37]. However, we lack any documentation of such characteristics in S. elaeagnifolium in its native range in south Texas. Over two years of experiments, we were able to compare the buzzing frequency and amplitude of four bee genera: Exomalopsis, Halictus, Megachile, and Xylocopa (Bombus was not included in such analyses because we were unable to record their buzzing vibrations), and surprisingly, we could not find any significant differences in buzzing frequency and buzzing amplitude among them (Figure 4A,B). Previous studies show that bees tend to extract maximum pollen (~60%) in the first and second pulses while the rest of the pulses only contribute to ~19% [2]. To study if such interactions prevail in S. elaeagnifolium pollination, we compared buzzing frequency, buzzing amplitude, and duration of buzzing time of the first and last pulses for all bees. We found a significant difference in buzzing frequency in the first and last pulses of visiting bees having a significantly lower frequency of the first pulse, as lower frequency (~124 Hz) is close to plant stamen frequency, which could result in better vibration transmission and, consequently, efficient pollen extraction (Figure 5A) [2]. It has been well documented that insect body size can influence flight and floral vibration frequencies and amplitude either negatively or positively [12,19,25,36]. Comparing bee size (bee mass and ITD) among different genera, the significantly heavier Xylocopa would have an advantage for efficient pollen collection with minimum foraging efforts [12,25].
To understand the effect of buzzing frequency and duration of buzzing time on the amount of pollen removed further [14], we ran an artificial pollen extraction experiment, which clearly demonstrated that pollen extraction is independent of buzzing frequency (Figure 7A), while the duration of buzzing time has a significant effect (Figure 7B). These findings also agree with [25], where they found that a longer duration of buzzes eject more pollen in S. rostratum, while frequency had no significant effect on the amount of pollen removed. As discussed before, small bees spend more time to extract pollen as compared to bigger bees, in which the latter can extract a similar amount of pollen in less time in any given foraging effort [13,17,25,38,39,40].
To conclude, using field-based insect-visitation surveys, acoustics assessment, and toothbrush-based artificial pollen extraction experiments, we conclude that the duration of buzzing time is the most critical factor in S. elaeagnifolium pollen extraction. We also show that although there are variations in bee size and their vibrational buzzing parameters, S. elaeagnifolium is visited by multiple bee genera in its native range and consequently results in a high seed set and propagule supply [30]. Future studies should be focused on fruit and/or seed-set to estimate the efficacy of pollination by the observed insect visitors experimentally, an area we are currently exploring. The comparison of S. elaeagnifolium-pollinator interactions in natural and managed agricultural systems would be useful for developing better management strategies.
4. Materials and Methods
4.1. Study System
S. elaeagnifolium is a noxious weed, native to the Southern United States and Northern Mexico while invasive in other continents [26,28,30,41]. It has blue-to-lilac hermaphrodite nectar-less flowers with poricidal anthers, which offers pollen as a reward to the buzz pollinators. S. elaeagnifolium propagate by seeds, dispersed by wind, water, birds, and grazing animals, and asexually through rhizomes [42,43,44] (Figure 1A). Previous studies show that S. elaeagnifolium is visited by various pollinators belonging to Apidae (Xylocopa spp, Bombus spp.), Halictidae (Nomina spp. and Pseudapis spp.), as well small bees of genus Megachile [28].
4.2. Field Survey and Bee Incidence
In this study, we surveyed silverleaf nightshade (S. elaeagnifolium) sub-populations across the Lower Rio Grande Valley, Texas, USA. In the Spring–Summer (April–August) of 2019 and 2020, we selected four field plots: (1) PPC Farms, City of Mission, TX, USA (26°16′84.25″N 98°31′45.47″W), (2) Edinburg, Texas, USA (26°20′18.1″N 98°11′17.5″W), (3) San Juan, Texas, USA (26°11′24.9″N 98°08′51.1″W), 4) Mission, Texas, USA (26°10′33.5″N 98°18′48.7″W), all within a 50-mile radius, as our study locations for pollinator assessment. S. elaeagnifolium populations were observed in small patches of various sizes measured in the area (m2) (PPC farm field: 100 m2, Edinburg: 60 m2, San Juan: 321 m2, Mission: 400 m2). Multiple field trips were made to observe major buzz pollinator incidence and record bee buzzing vibrations. Field visits were made early in the morning (7 a.m.–9 a.m.) to record maximum pollinator activity, and to confirm that we document first visitors once the flowers have opened [14]. During each visit, bee data were collected for their flower visit (number of visits), the time between landing and leaving a flower (visit bout), while simultaneously recording bee buzzes (explained in Section 4.3) followed by capturing a subset of bees for identification, bee mass and Intertegular distance (ITD) measurements carried out in the lab. Time spent between bee landing and bee leaving the flower was recorded as flower visit time. For each field visit, two researchers worked as a team to collect the data for 3 h/visit to minimize any recording errors.
4.3. Bee Vibrations Recording and Bee Capturing
When the bee buzzes, floral sonication is produced as a by-product of vibrations emitted from the bee exoskeleton transmitted to the anthers [1]. Previous studies established that floral sonication can be characterized by analyzing acoustic measures of duration and fundamental frequency [13]. To record and characterize floral sonication (produced in an audible sound), bees were observed in the early morning during their flower visits. Once the bee landed on the flower, a digital audio recorder Tascam DR-100 MK-III (TEAC America, Inc., Montebello, CA, USA) was held within 1–5 cm of the bee with the pointing microphone head towards the bee’s dorsal side until the bee left the flower, and these recordings were saved as wave files (.wav) (Sampling frequency = 10 recordings/h). After recording bee buzzing vibrations, bees were captured using an insect net and transferred to the 50 mL centrifuge tubes. Each tube had 50% ethanol dipped cotton balls, and each bee was later identified and labeled for mass and ITD measurements.
4.4. Estimating Vibration Frequency and Amplitude
Vibrational frequency and amplitude are characteristic perimeters of floral sonication vibrations. We used a freely accessible Audacity v. 2.1.3 (https://sourceforge.net/projects/audacity/, accessed on 23 September 2019) software to calculate bee vibrational frequency (Hertz or Hz), duration of vibration (s), and vibration amplitude (dB) [13]. Before analysis, recordings were listened to twice to identify extraneous noise, including wind, birds, and machinery. Then, this noise was filtered out (noise reduction = 12 dB, sensitivity = 6, frequency smoothing = 3) from a small portion of each recording, followed by a batch process filtration from the whole recording. Peak frequency/fundamental frequency (lowest frequency in the vibration with the largest amplitude) was calculated by using the “Plot Spectrum” function (FFT = 8192 Hz, Hamming window). Each vibration was analyzed by examining a spectrogram of the recording using the “Spectrogram” function (FFT = 8192 Hz, hamming window). Fundamental frequency and amplitude of a given vibration were determined with corresponding peak frequency with the largest amplitude in a spectrogram [20]. Duration of vibration was also calculated in Audacity by selecting the bee buzzing area in the spectrogram.
4.5. Bee Mass and Intertegular Distance (ITD)
Bee body mass and Intertegular distance (ITD) are reliable parameters of bee size characteristics [13]. All captured bees were stored in the refrigerator (4℃) for bee identification and body measurements (bee mass and ITD). Bee mass (g) was calculated by weighing each bee individually on an advanced digital balance (Accuris Series Dx, Model: W3101A-220, Benchmark Scientific, NJ, USA). For ITD measurements, each bee was observed under a compound microscope at 10X magnification. ITD (µm) was measured as the distance between the bee’s wing tegulae across the thoracic dorsum. After body mass and ITD measurements, each bee was placed back in corresponding tubes and stored at 4 °C.
4.6. Artificial Pollen Extraction
In addition to field surveys, a lab experiment was conducted to estimate the effect of different vibrational frequencies and duration of buzzing time on the amount of pollen extracted. To do so in the lab, we selected electric toothbrushes [14]; Figure 1D), which had vibrational frequency ranges (137 Hz–249 Hz), and the duration of toothbrushes use was determined based on data recorded from the field. We collected newly opened virgin flowers from the field early in the morning (to avoid any pollination by bees) and brought them to the lab. For pollen extraction, we used electric toothbrushes of different strokes, i.e., 14,000/min (Oral-B 3d White Action Power Toothbrush), 20,000/min (Colgate 360 powered toothbrush, Colgate Co. Pvt. Ltd.), and 30,000/min (Vivid Sonic Clean toothbrush) having three frequency levels (137 Hz, 173 Hz, and 249 Hz: [14]), respectively, for three buzzing times (1.5 s, 5 s, and 10 s) in a full factorial design. Collected pollen was weighed (in mg) and recorded for each treatment.
Statistical Analysis
All the collected data were checked for their distribution (normal or not), followed by parametric or non-parametric tests for analyses. Due to the non-normal nature of bee frequency, bee amplitude, bee mass, and ITD, data were analyzed using the Kruskal–Wallis test (p < 0.05). However, as bee visit time, bee buzz % of visit time followed a normal distribution, we used One-Way ANOVA followed by pairwise comparisons using Tukey’s HSD test (p < 0.05). On the other hand, the bee acoustic differences between the first and last buzz were analyzed using an unpaired t-test (p < 0.05). For artificial pollen extraction data, we used One-Way ANOVA followed by Tukey’s HSD (p < 0.05) to analyze the effect of frequency and length of buzzing time on the amount of pollen extracted. All data were analyzed in statistical software JMP (SAS Institute, Cary, NC, USA) and GraphPad PRISM (La Jolla, CA, USA).
Author Contributions
Conceptualization, M.T. and R.K.; methodology, M.T.; software, M.T.; validation, R.K., M.T.; formal analysis, R.K., M.T.; investigation, M.T.; resources, R.K.; data curation, M.T., R.K.; writing—original draft preparation, M.T.; writing—review and editing, M.T., R.K.; supervision, R.K.; funding acquisition, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by The University of Texas Rio Grande Valley College of Sciences Seed grant and Startup funds awarded to Rupesh Kariyat, and the Presidential Graduate Research Assistantship awarded to Mandeep Tayal.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data collected for this study are available from Dryad Digital Repository.
Acknowledgments
We thank Ernesto Herrera and Zachary Johnson for their assistance in insect identification, biomechanics data curation, and analysis. We would also like to thank Jesus Chavana and Sukhman Singh for their help in field data collection. We thank the three anonymous reviewers for their comments. The authors also thank the colleague who read the manuscript and provided suggestions on improving the writing.
Conflicts of Interest
The authors have no competing interests.
References
- Buchmann, S.L. Buzz pollination in angiosperms. In Handbook of Experimental Pollination Biology; Scientific and Academic Editions; Jones, C.E., Little, J.R., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1983; pp. 73–113. [Google Scholar]
- King, M.J.; Buchmann, S.L. Sonication Dispensing of Pollen from Solanum laciniatum Flowers. Funct. Ecol. 1996, 10, 449–456. [Google Scholar] [CrossRef]
- Arroyo-Correa, B.; Beattie, C.; Vallejo-Marín, M. Bee and floral traits affect the characteristics of the vibrations experienced by flowers during buzz-pollination. J. Exp. Biol. 2019, 222, jeb198176. [Google Scholar] [CrossRef] [Green Version]
- Cardinal, S.; Buchmann, S.L.; Russell, A.L. The evolution of floral sonication, a pollen foraging behavior used by bees (Anthophila). Evolution 2018, 72, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Harder, L.D.; Barclay, R.M.R. The Functional Significance of Poricidal Anthers and Buzz Pollination: Controlled Pollen Removal from Dodecatheon. Funct. Ecol. 1994, 8, 509–517. [Google Scholar] [CrossRef]
- De Luca, P.A.; Vallejo-Marín, M. What’s the “buzz” about? The ecology and evolutionary significance of buzz-pollination. Curr. Opin. Plant Biol. 2013, 16, 429–435. [Google Scholar] [CrossRef]
- Kariyat, R.R.; Bentley, T.G.; Nihranz, C.T.; Stephenson, A.G.; De Moraes, C.M.; Mescher, M.C. Inbreeding in Solanum carolinense alters floral attractants and rewards and adversely affects pollinator visitation. Am. J. Bot. 2021, 108, 74–82. [Google Scholar] [CrossRef]
- Vogel, S. Evolutionary shifts from reward to deception in pollen flowers. In The Pollination of Flowers by Insects; Richards, A.J., Ed.; Academic Press: London, UK, 1978; pp. 89–96. [Google Scholar]
- Mast, A.R.; Feller, D.M.S.; Kelso, S.; Conti, E. Buzz-pollinated Dodecatheon originated from within the heterostylous Primula subgenus Auriculastrum (Primulaceae): A seven-region cpDNA phylogeny and its implications for floral evolution. Am. J. Bot. 2004, 91, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C.H. Darwin’s legacy: The forms, function and sexual diversity of flowers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 351–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solís-Montero, L.; Vallejo-Marín, M. Does the morphological fit between flowers and pollinators affect pollen deposition? An experimental test in a buzz-pollinated species with anther dimorphism. Ecol. Evol. 2017, 7, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Marín, M. Buzz pollination: Studying bee vibrations on flowers. New Phytol. 2019, 224, 1068–1074. [Google Scholar] [CrossRef] [Green Version]
- De Luca, P.A.; Buchmann, S.; Galen, C.; Mason, A.C.; Vallejo-Marín, M. Does body size predict the buzz-pollination frequencies used by bees? Ecol. Evol. 2019, 9, 4875–4887. [Google Scholar] [CrossRef]
- Tayal, M.; Chavana, J.; Kariyat, R.R. Efficiency of using electric toothbrush as an alternative to a tuning fork for artificial buzz pollination is independent of instrument buzzing frequency. BMC Ecol. 2020, 20, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.A. The dehiscence of anthers by apical pores. In Missouri Botanical Garden Sixteenth Annual Report; Missouri Botanical Garden Press: St. Louis, MO, USA, 1905; pp. 167–257. [Google Scholar]
- Teppner, H. The first records of vibratory pollen-collection by bees. Phyton Horn 2018, 57, 135–141. [Google Scholar]
- Vallejo-Marín, M.; Vallejo, G.C. Comparison of defence buzzes in hoverflies and buzz-pollinating bees. J. Zool. 2021, 313, 237–249. [Google Scholar] [CrossRef]
- Pritchard, D.J.; Vallejo-Marín, M. Floral vibrations by buzz-pollinating bees achieve higher frequency, velocity and acceleration than flight and defence vibrations. J. Exp. Biol. 2020, 223, jeb220541. [Google Scholar] [CrossRef] [PubMed]
- Switzer, C.M.; Combes, S.A. Bumblebee sonication behavior changes with plant species and environmental conditions. Apidologie 2016, 48, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Morgan, T.; Whitehorn, P.; Lye, G.C.; Vallejo-Marín, M. Floral Sonication is an Innate Behaviour in Bumblebees that can be Fine-Tuned with Experience in Manipulating Flowers. J. Insect Behav. 2016, 29, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellinger, A.S.; Chartier, M.; Fernández-Fernández, D.; Penneys, D.S.; Alvear, M.; Almeda, F.; Michelangeli, F.A.; Staedler, Y.; Armbruster, W.S.; Schönenberger, J. Beyond buzz-pollination—Departures from an adaptive plateau lead to new pollination syndromes. New Phytol. 2019, 221, 1136–1149. [Google Scholar] [CrossRef] [Green Version]
- Dellinger, A.S.; Artuso, S.; Pamperl, S.; Michelangeli, F.A.; Penneys, D.S.; Fernández-Fernández, D.M.; Alvear, M.; Almeda, F.; Armbruster, W.S.; Staedler, Y.; et al. Author Correction: Modularity increases rate of floral evolution and adaptive success for functionally specialized pollination systems. Commun. Biol. 2019, 2. [Google Scholar] [CrossRef]
- Bochorny, T.; Bacci, L.F.; Dellinger, A.S.; Michelangeli, F.A.; Goldenberg, R.; Brito, V.L.G. Connective appendages in Huberia bradeana (Melastomataceae) affect pollen release during buzz pollination. Plant Biol. 2021, 23, 556–563. [Google Scholar] [CrossRef]
- Nevard, L.; Russell, A.L.; Foord, K.; Vallejo-Marín, M. Transmission of bee-like vibrations in buzz-pollinated plants with different stamen architectures. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- De Luca, P.A.; Bussière, L.F.; Souto-Vilaros, D.; Goulson, D.; Mason, A.C.; Vallejo-Marín, M. Variability in bumblebee pollination buzzes affects the quantity of pollen released from flowers. Oecologia 2013, 172, 805–816. [Google Scholar] [CrossRef]
- Boyd, J.W.; Murray, D.S.; Tyrl, R.J. Silverleaf nightshade, Solarium elaeagnifolium, origin, distribution, and relation to man. Econ. Bot. 1984, 38, 210–217. [Google Scholar] [CrossRef]
- Travlos, I.S. Responses of invasive silverleaf nightshade (Solanum elaeagnifolium) populations to varying soil water availability. Phytoparasitica 2013, 41, 41–48. [Google Scholar] [CrossRef]
- Petanidou, T.; Price, M.V.; Bronstein, J.L.; Kantsa, A.; Tscheulin, T.; Kariyat, R.; Krigas, N.; Mescher, M.C.; De Moraes, C.M.; Waser, N.M. Pollination and reproduction of an invasive plant inside and outside its ancestral range. Acta Oecologica 2018, 89, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Qasem, J.R.; Al Abdallat, A.M.; Hasan, S.M. Genetic diversity of Solanum elaeagnifolium, an invasive problematic weed in Jordan. Weed Res. 2019, 59, 222–234. [Google Scholar] [CrossRef]
- Chavana, J.; Singh, S.; Vazquez, A.; Christoffersen, B.; Racelis, A.; Kariyat, R.R. Local adaptation to continuous mowing makes the noxious weed Solanum elaeagnifolium a superweed candidate by improving fitness and defense traits. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Thinakaran, J.; Pierson, E.; Kunta, M.; Munyaneza, J.E.; Rush, C.M.; Henne, D.C. Silverleaf Nightshade (Solanum elaeagnifolium), a Reservoir Host for ‘Candidatus Liberibacter solanacearum’, the Putative Causal Agent of Zebra Chip Disease of Potato. Plant Dis. 2015, 99, 910–915. [Google Scholar] [CrossRef] [Green Version]
- Cane, J.H. Estimation of bee size using intertegular span (Apoidea). J. Kans. Entomol. Soc. 1987, 60, 145–147. [Google Scholar]
- King, M.J. Buzz foraging mechanism of bumble bees. J. Apic. Res. 1993, 32, 41–49. [Google Scholar] [CrossRef]
- King, M.J.; Buchmann, S.L. Floral Sonication by Bees: Mesosomal Vibration by Bombus and Xylocopa, but Not Apis (Hymenoptera: Apidae), Ejects Pollen from Poricidal Anthers. J. Kans. Entomol. Soc. 2003, 76, 295–305. [Google Scholar]
- Vallejo-Marin, M.; Manson, J.S.; Thomson, J.D.; Barrett, S.C. Division of labour within flowers: Heteranthery, a floral strategy to reconcile contrasting pollen fates. J. Evol. Biol. 2009, 22, 828–839. [Google Scholar] [CrossRef]
- Corbet, S.A.; Huang, S.-Q. Buzz pollination in eight bumblebee-pollinated Pedicularis species: Does it involve vibration-induced triboelectric charging of pollen grains? Ann. Bot. 2014, 114, 1665–1674. [Google Scholar] [CrossRef] [Green Version]
- Buehmann, S.L.; Cane, J.H. Bees assess pollen returns while sonicating Solanum flowers. Oecologia 1989, 81, 289–294. [Google Scholar] [CrossRef]
- Russell, A.L.; Buchmann, S.L.; Papaj, D.R. How a generalist bee achieves high efficiency of pollen collection on diverse floral resources. Behav. Ecol. 2017, 28, 991–1003. [Google Scholar] [CrossRef] [Green Version]
- Rosi-Denadai, C.A.; Araújo, P.C.S.; de Oliveira Campos, L.A.; Cosme, L., Jr.; Guedes, R.N.C. Buzz-pollination in Neotropical bees: Genus-dependent frequencies and lack of optimal frequency for pollen release. Insect Sci. 2020, 27, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekki, M. Biology, distribution and impacts of silverleaf nightshade (Solanum elaeagnifolium Cav.). EPPO Bull. 2007, 37, 114–118. [Google Scholar] [CrossRef]
- Bellue, M.K. Weed Seed Handbook; Bulletin of the California State Department of Agriculture Series VI; 1946; Volume 35, pp. 15–16. [Google Scholar]
- Cuthbertson, E.G.; Leys, A.R.; McMaster, G. Silverleaf nightshade—A potential threat to agriculture. Agric. Gaz. N. S. W. 1976, 87, 11–13. [Google Scholar]
- Molnar, V.M.; McKenzie, D.N. Progress report on silverleaf nightshade research. Pamphlet No. 61. In Vermin and Noxious Weeds Destruction Report; Board of Crown Lands and Survey: Victoria, Australia, 1976. [Google Scholar]
- Macior, L.W. Pollination adaptation in Pedicularis groenlandica. Am. J. Bot. 1968, 55, 927–932. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).