Developmental Stages-Specific Response of Anise Plants to Laser-Induced Growth, Nutrients Accumulation, and Essential Oil Metabolism
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Experimental Conditions
2.2. Determination of Photosynthetic Rate
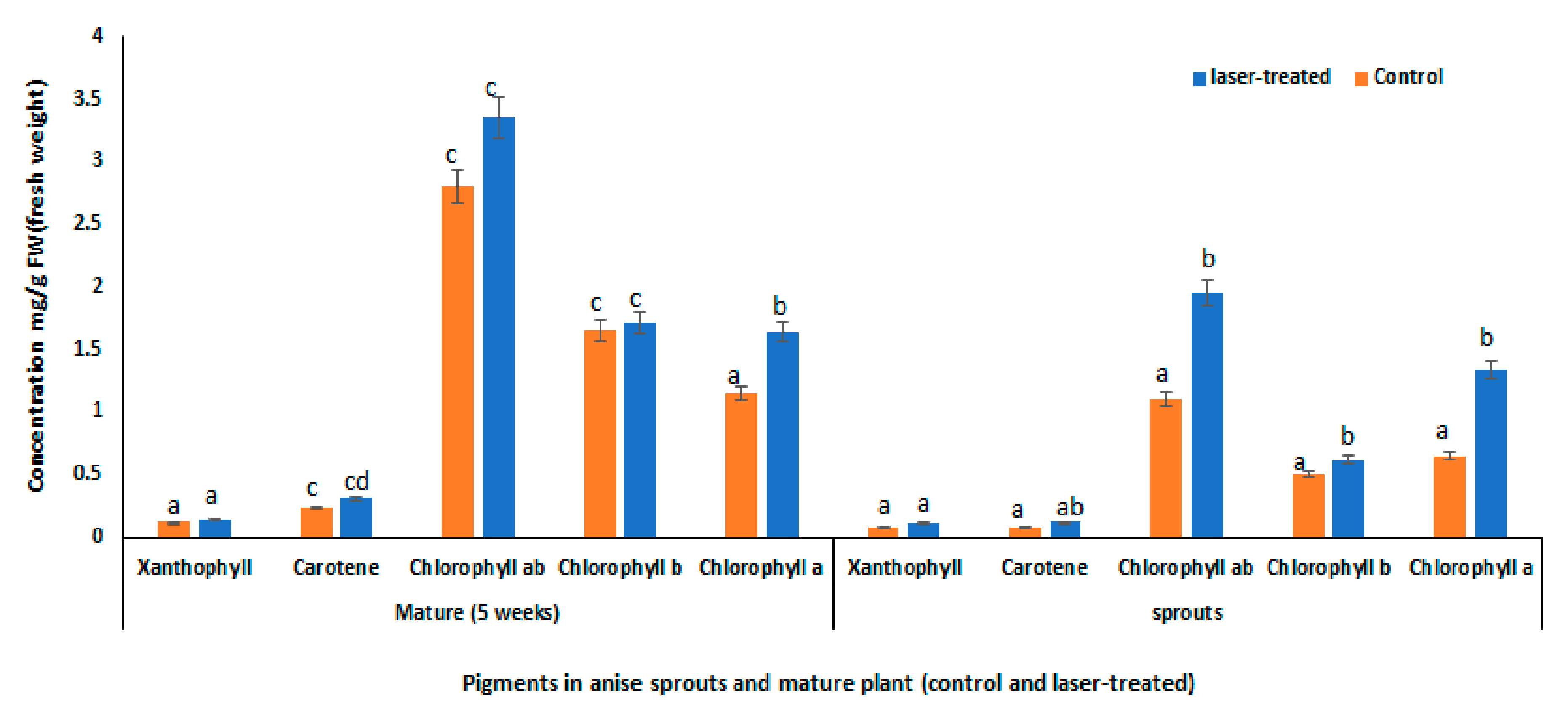
2.3. Pigment Analysis
2.4. Elemental Analysis
2.5. Vitamins Analysis
2.6. Essential oil Analysis
2.6.1. GC-MS Analysis
2.6.2. Determination of DAHPS
2.6.3. Determination of PAL
2.7. Determination of Phenolic Profile
2.7.1. Determination of Total Phenolic Content
2.7.2. HPLC Analysis
2.8. Biological Activities
2.8.1. Antioxidant Activities
2.8.2. Hypocholesterolaemic Activity
Inhibition of Micellar Solubility of Cholesterol
Pancreatic Lipase Inhibition Assay
2.9. Statistical Analyses
3. Results and Discussion
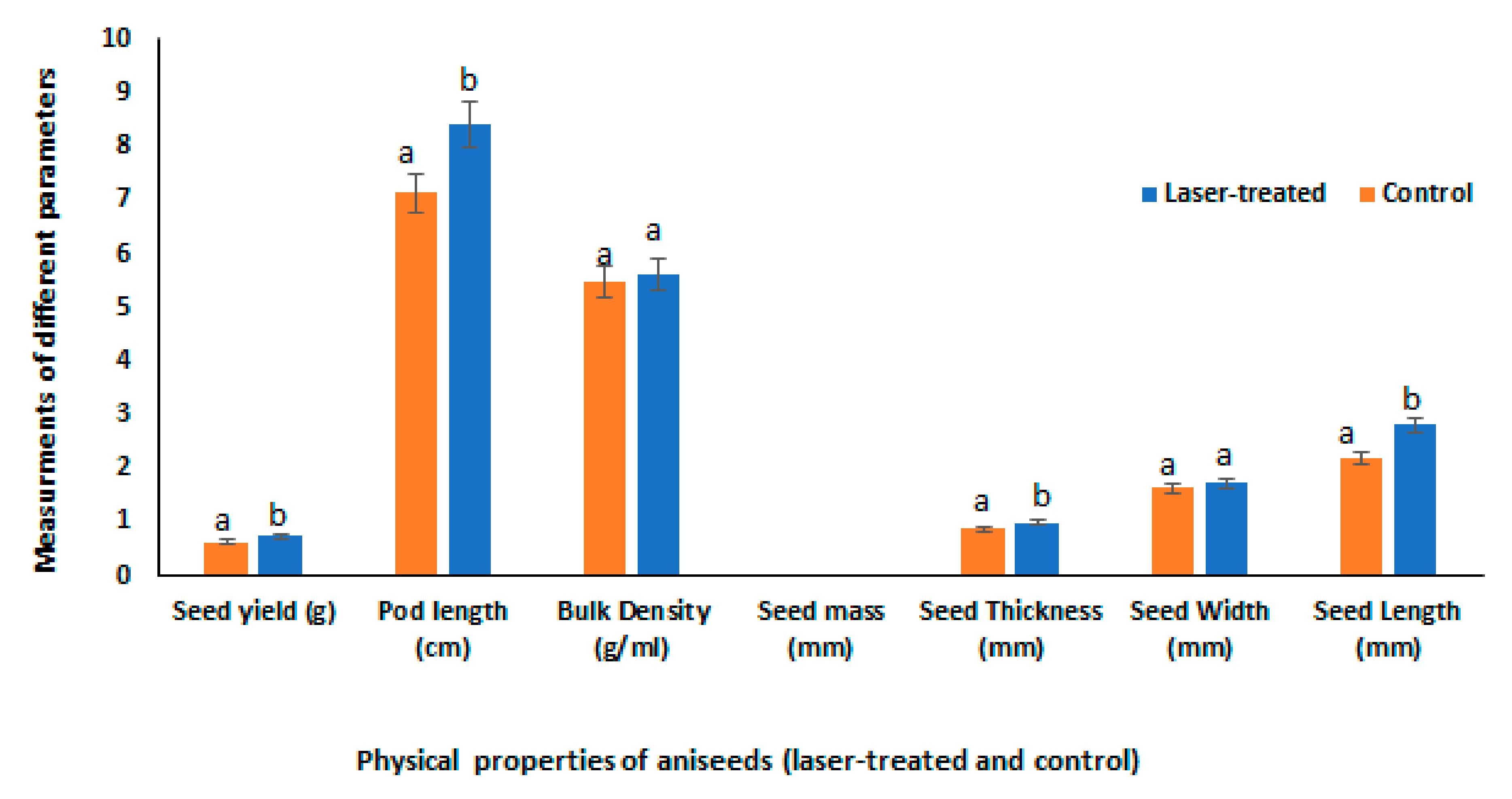
3.1. Physical Properties and Biomass Accumulation of Anise Fruits, Sprouts, and Mature Plants as Affected by Laser Light Treatment
3.2. Sprouting and Laser Light Induced a More Pronounced Effect on Nutritive Values of Anise
3.3. Laser Light Increased the Antioxidant Potential of Anise Sprouts and Mature Plants by Enhancing Their Phenolic Content
3.4. Laser Light Improved the Anti-Lipidemic Activity Particularly in Anise Sprouts
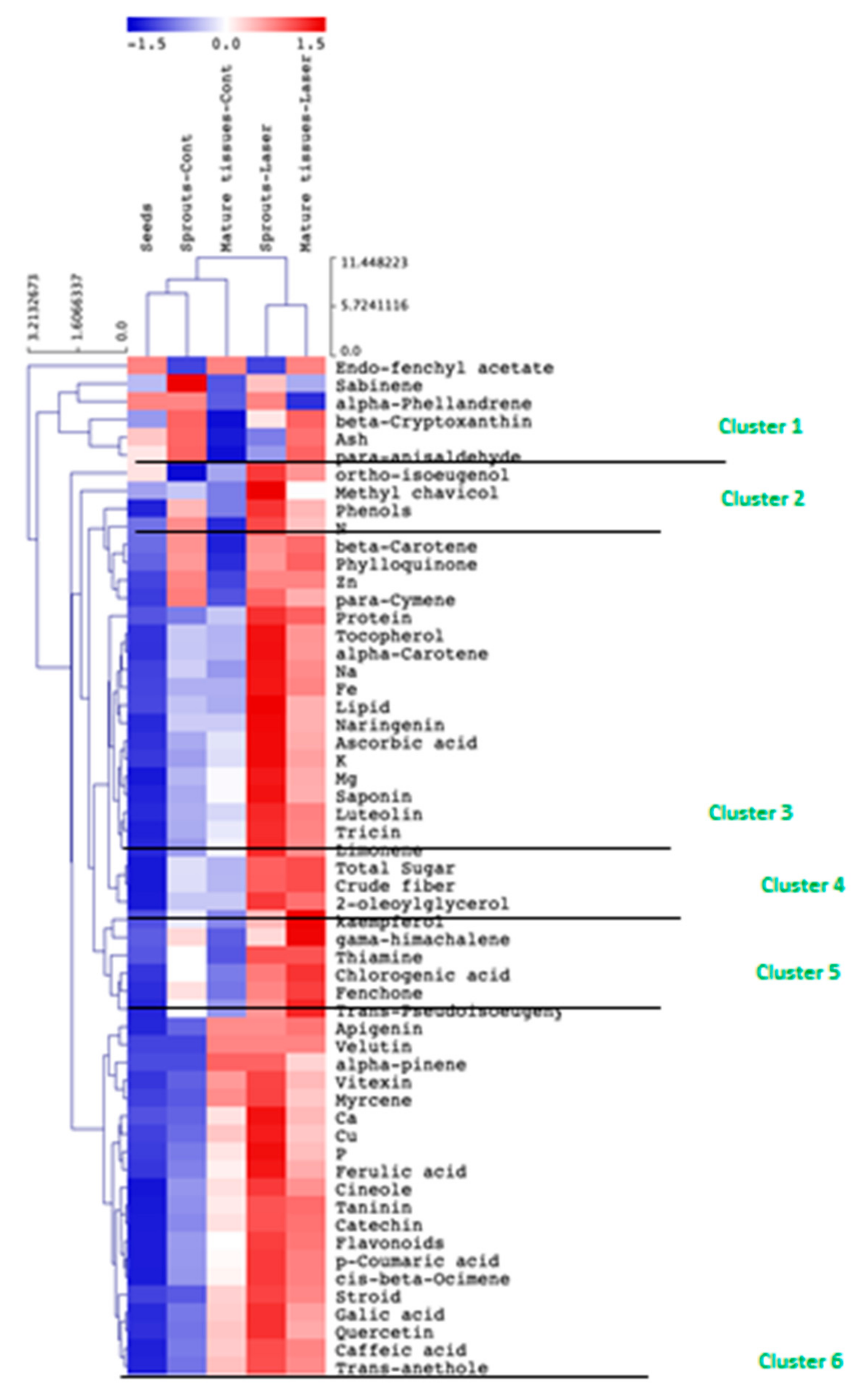
3.5. Tissue-Specific Response to Laser Light Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manchali, S.; Murthy, K.N.C.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Marton, M.; Mandoki, Z.S.; Csapo-Kiss, Z.S.; Csapo, J. The role of sprouts in human nutrition. A review. Acta Univ. Sapientiae 2010, 3, 81–117. [Google Scholar]
- Almuhayawi, M.S.; Al Jaouni, S.K.; Almuhayawi, S.M.; Selim, S.; Abdel-Mawgoud, M. Elevated CO2 improves the nutritive value, antibacterial, anti-inflammatory, antioxidant and hypocholestecolemic activities of lemongrass sprouts. Food Chem. 2021, 357, 129730. [Google Scholar] [CrossRef] [PubMed]
- Almuhayawi, M.S.; Hassan, A.H.A.; Abdel-Mawgoud, M.; Khamis, G.; Selim, S.; Al Jaouni, S.K.; AbdElgawad, H. Laser light as a promising approach to improve the nutritional value, antioxidant capacity and anti-inflammatory activity of flavonoid-rich buckwheat sprouts. Food Chem. 2020, 345, 128788. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.M.; Abdel-Mawgoud, M.; Hassan, A.R.; Habeeb, T.H.; Yehia, R.S.; AbdElgawad, H. Global metabolic changes induced by arbuscular mycorrhizal fungi in oregano plants grown under ambient and elevated levels of atmospheric CO2. Plant Physiol. Biochem. 2020, 152, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Yue, M.; Wang, X.-L. Influence of He–Ne laser irradiation on seeds thermodynamic parameters and seedlings growth of Isatis indogotica. Plant Sci. 2005, 168, 601–606. [Google Scholar] [CrossRef]
- Perveen, R.; Ali, Q.; Ashraf, M.; Al-Qurainy, F.; Jamil, Y.; Raza Ahmad, M. Effects of different doses of low power continuous wave He–Ne laser radiation on some seed thermodynamic and germination parameters, and potential enzymes involved in seed germination of sunflower (Helianthus annuus L.). Photochem. Photobiol. 2010, 86, 1050–1055. [Google Scholar] [CrossRef]
- Podleśny, J.; Stochmal, A.; Podleśna, A.; Misiak, L.E. Effect of laser light treatment on some biochemical and physiological processes in seeds and seedlings of white lupine and faba bean. Plant Growth Regul. 2012, 67, 227–233. [Google Scholar] [CrossRef]
- Osman, Y.A.H.; El-Tobgy, K.M.K.; El-Sherbini, E.S.A. Effect of laser radiation treatments on growth, yield and chemical constituents of fennel and coriander plants. J. Appl. Sci. Res. 2009, 5, 244–252. [Google Scholar]
- Asghar, T.; Jamil, Y.; Iqbal, M.; Abbas, M. Laser light and magnetic field stimulation effect on biochemical, enzymes activities and chlorophyll contents in soybean seeds and seedlings during early growth stages. J. Photochem. Photobiol. B Biol. 2016, 165, 283–290. [Google Scholar] [CrossRef]
- Okla, M.K.; El-Tayeb, M.A.; Qahtan, A.A.; Abdel-Maksoud, M.A.; Elbadawi, Y.B.; Alaskary, M.K.; Balkhyour, M.A.; Hassan, A.H.A.; AbdElgawad, H. Laser Light Treatment of Seeds for Improving the Biomass Photosynthesis, Chemical Composition and Biological Activities of Lemongrass Sprouts. Agronomy 2021, 11, 478. [Google Scholar] [CrossRef]
- Tepe, A.S.; Tepe, B. Traditional use, biological activity potential and toxicity of Pimpinella species. Ind. Crops Prod. 2015, 69, 153–166. [Google Scholar] [CrossRef]
- Ullah, H.; Honermeier, B. Fruit yield, essential oil concentration and composition of three anise cultivars (Pimpinella anisum L.) in relation to sowing date, sowing rate and locations. Ind. Crops Prod. 2013, 42, 489–499. [Google Scholar] [CrossRef]
- Rebey, I.B.; Wannes, W.A.; Kaab, S.B.; Bourgou, S.; Tounsi, M.S.; Ksouri, R.; Fauconnier, M.L. Bioactive compounds and antioxidant activity of Pimpinella anisum L. accessions at different ripening stages. Sci. Hortic. 2019, 246, 453–461. [Google Scholar] [CrossRef]
- Rajeshwari, U.; Shobha, I.; Andallu, B. Comparison of aniseeds and coriander seeds for antidiabetic, hypolipidemic and antioxidant activities. Spat. DD 2011, 1, 9–16. [Google Scholar] [CrossRef]
- Rajeshwari, C.U.; Abirami, M.; Andallu, B. In vitro and in vivo antioxidant potential of aniseeds (Pimpinella anisum). Asian J. Exp. Biol. Sci. 2011, 2, 80–89. [Google Scholar]
- Gülçın, İ.; Oktay, M.; Kıreçcı, E.; Küfrevıoǧlu, Ö.İ. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Ozek, T.; Kirimer, N.; Baser, K.H.C.; Bedir, E.; Khan, I.A.; Wedge, D.E. Gas chromatographic–mass spectrometric analysis of essential oils from Pimpinella species gathered from Central and Northern Turkey. J. Chromatogr. A 2006, 1117, 194–205. [Google Scholar] [CrossRef]
- El Tobgy, K.M.K.; Osman, Y.A.H.; El Sherbini, E.A. Effect of laser radiation on growth, yield and chemical constituents of anise and cumin plants. J. Appl. Sci. Res. 2009, 5, 522–528. [Google Scholar]
- Habeeb, T.H.; Abdel-Mawgoud, M.; Yehia, R.S.; Khalil, A.M.A.; Saleh, A.M.; AbdElgawad, H. Interactive Impact of Arbuscular Mycorrhizal Fungi and Elevated CO2 on Growth and Functional Food Value of Thymus vulgare. J. Fungi 2020, 6, 168. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, S.; Wang, Y.; Liu, J.; Ma, H.; Wang, Y.; Tian, Y.; Hou, W. Effects of light irradiation on essential oil biosynthesis in the medicinal plant Asarum heterotropoides Fr. Schmidt var. mandshuricum (Maxim) Kitag. PLoS ONE 2020, 15, e0237952. [Google Scholar] [CrossRef] [PubMed]
- Naudts, K.; Van den Berge, J.; Farfan Vignolo, E.R.; Rose, P.; Abdelgawad, H.; Ceulemans, R.; Janssens, I.A.; Asard, H.; Nijs, I. Future climate alleviates stress impact on grassland productivity through altered antioxidant capacity. Environ. Exp. Bot. 2013, 99, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Casasole, G.; Raap, T.; Costantini, D.; AbdElgawad, H.; Asard, H.; Pinxten, R.; Eens, M. Neither artificial light at night, anthropogenic noise nor distance from roads are associated with oxidative status of nestlings in an urban population of songbirds. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 210, 14–21. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, H.; Zong, Y.; Li, F.Y.; Han, Y.; Hao, X. Photosynthesis and metabolite responses of Isatis indigotica Fortune to elevated [CO2]. Crop. J. 2017, 5, 345–353. [Google Scholar] [CrossRef]
- Aladjadjiyan, A. The use of physical methods for plant growing stimulation in Bulgaria. J. Cent. Eur. Agric. 2007, 8, 369–380. [Google Scholar]
- Wu, J.; Gao, X.; Zhang, S. Effect of laser pretreatment on germination and membrane lipid peroxidation of Chinese pine seeds under drought stress. Front. Biol. China 2007, 2, 314–317. [Google Scholar] [CrossRef]
- Tuan, P.A.; Thwe, A.A.; Kim, Y.B.; Kim, J.K.; Kim, S.-J.; Lee, S.; Chung, S.-O.; Park, S.U. Effects of white, blue, and red light-emitting diodes on carotenoid biosynthetic gene expression levels and carotenoid accumulation in sprouts of tartary buckwheat (Fagopyrum tataricum Gaertn.). J. Agric. Food Chem. 2013, 61, 12356–12361. [Google Scholar] [CrossRef]
- Perveen, R.; Jamil, Y.; Ashraf, M.; Ali, Q.; Iqbal, M.; Ahmad, M.R. He-Ne Laser-Induced Improvement in Biochemical, Physiological, Growth and Yield Characteristics in Sunflower (Helianthus annuus L.). Photochem. Photobiol. 2011, 87, 1453–1463. [Google Scholar] [CrossRef]
- Nam, T.-G.; Lim, Y.J.; Eom, S.H. Flavonoid accumulation in common buckwheat (Fagopyrum esculentum) sprout tissues in response to light. Hortic. Environ. Biotechnol. 2018, 59, 19–27. [Google Scholar] [CrossRef]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO2 induces a global metabolic change in basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant. Physiol. 2018, 224, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Sacala, E.; Demczuk, A.; Grzys, E.; Prosba-Bialczyk, U.; Szajsner, H. Impact of presowing laser irradiation of seeds on sugar beet properties. Int. Agrophysics 2012, 26, 295–300. [Google Scholar] [CrossRef]
- Rauf, M.; Yoon, H.; Lee, S.; Lee, M.-C.; Oh, S.; Choi, Y.-M. Evaluation of sprout growth traits and flavonoid content in common and tartary buckwheat germplasms. Plant. Breed. Biotechnol. 2019, 7, 375–385. [Google Scholar] [CrossRef]
- Dinani, H.J.; Eslami, P.; Mortazaeinezhad, F.; Ghahrizjani, R.T. Effects of laser radiation on the growth indicators of Kelussia odoratissima Mozaff. Medical plant. In Proceedings of the 2019 Photonics North (PN), Quebec City, QC, Canada, 21–23 May 2019; pp. 1–8. [Google Scholar]
- Shafique, H.; Jamil, Y.; ul Haq, Z.; Mujahid, T.; Khan, A.U.; Iqbal, M.; Abbas, M. Low power continuous wave-laser seed irradiation effect on Moringa oleifera germination, seedling growth and biochemical attributes. J. Photochem. Photobiol. B Biol. 2017, 170, 314–323. [Google Scholar]
- Bettaieb Rebey, I.; Bourgou, S.; Aidi Wannes, W.; Hamrouni Selami, I.; Saidani Tounsi, M.; Marzouk, B.; Fauconnier, M.-L.; Ksouri, R. Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant. Biosyst. Int. J. Deal. All Asp. Plant. Biol. 2018, 152, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.K.; Mattar, Z.A.; Abdel-Khalek, H.H.; Azzam, Y.M. Evaluation of antibacterial efficacy of anise wastes against some multidrug resistant bacterial isolates. J. Radiat. Res. Appl. Sci. 2017, 10, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Mosavat, S.H.; Jaberi, A.R.; Sobhani, Z.; Mosaffa-Jahromi, M.; Iraji, A.; Moayedfard, A. Efficacy of Anise (Pimpinella anisum L.) oil for migraine headache: A pilot randomized placebo-controlled clinical trial. J. Ethnopharmacol. 2019, 236, 155–160. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant. Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant. Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Vialart, G.; Hehn, A.; Olry, A.; Ito, K.; Krieger, C.; Larbat, R.; Paris, C.; Shimizu, B.; Sugimoto, Y.; Mizutani, M. A 2-oxoglutarate-dependent dioxygenase from Ruta graveolens L. exhibits p-coumaroyl CoA 2′-hydroxylase activity (C2′ H): A missing step in the synthesis of umbelliferone in plants. Plant. J. 2012, 70, 460–470. [Google Scholar] [CrossRef]
- Balkhyour, M.A.; Hassan, A.H.A.; Halawani, R.F.; Summan, A.S.; AbdElgawad, H. Effect of Elevated CO2 on Seed Yield, Essential Oil Metabolism, Nutritive Value, and Biological Activity of Pimpinella anisum L. Accessions at Different Seed Maturity Stages. Biology 2021, 10, 979. [Google Scholar] [CrossRef]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Christova-Bagdassarian, V.L.; Bagdassarian, K.S.; Atanassova, M.S. Phenolic compounds and antioxidant capacity in Bulgarian plans (dry seeds). Int. J. Adv. Res. 2013, 1, 186–197. [Google Scholar]
- Sakr, A.A.E.; Taha, K.M.; Abozid, M.M. Comparative Study Between Anise Seeds and Mint Leaves (Chemical Composition, Phenolic Compounds and Flavonoids). Menoufia J. Agric. Biotechnol. 2019, 4, 53–60. [Google Scholar] [CrossRef]
- Tsurunaga, Y.; Takahashi, T.; Katsube, T.; Kudo, A.; Kuramitsu, O.; Ishiwata, M.; Matsumoto, S. Effects of UV-B irradiation on the levels of anthocyanin, rutin and radical scavenging activity of buckwheat sprouts. Food Chem. 2013, 141, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Park, K.-J.; Kim, B.-K.; Jeong, J.-W.; Kim, H.-J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K. Antioxidant properties of quercetin. In Oxygen Transport to Tissue XXXII; LaManna, J., Puchowicz, M., Xu, K., Harrison, D., Bruley, D., Eds.; Springer: Boston, MA, USA, 2011; Volume 701, pp. 283–289. [Google Scholar]
- Oboh, G.; Ademosun, A.O.; Akinleye, M.; Omojokun, O.S.; Boligon, A.A.; Athayde, M.L. Starch composition, glycemic indices, phenolic constituents, and antioxidative and antidiabetic properties of some common tropical fruits. J. Ethn. Foods 2015, 2, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Zahoor, M.; Bari, W.U.; Zeb, A.; Khan, I. Toxicological, anticholinesterase, antilipidemic, antidiabetic and antioxidant potentials of Grewia optiva Drummond ex Burret extracts. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 1–16. [Google Scholar] [CrossRef]
| Metabolite | Fruits | Sprouts Control Laser-Treated | Mature Control Laser-Treated | ||
|---|---|---|---|---|---|
| Total primary metabolite (mg/g FW) | |||||
| Lipid | 84.7 ± 13.7a | 109.1 ± 9.7a | 173.2 ± 5.9b | 104.4 ± 18.3b | 138.2 ± 9.9c |
| Protein | 163 ± 19.9a | 171.4 ± 10.7a | 239.5 ± 4.4b | 185.9 ± 26a | 230.3 ± 14.4b |
| Total Sugar | 192.4 ± 26.8a | 247.9 ± 8.5a | 304 ± 31.6b | 235.9 ± 34.1b | 309.3 ± 19.3c |
| Ash | 2.4 ± 0.16a | 2.6 ± 0.2a | 2 ± 0.4a | 1.8 ± 0.93a | 2.58 ± 0.16a |
| Crude fiber | 2.02 ± 0.28a | 2.6 ± 0.1a | 3.2 ± 0.5b | 2.48 ± 0.36ab | 3.25 ± 0.2b |
| Total secondary metabolite (mg/g FW) | |||||
| Phenols | 6.67 ± 0.9a | 10.1 ± 0.9a | 11.6 ± 0.1b | 7.66 ± 1.2a | 10.1 ± 0.76a |
| Flavonoids | 0.26 ± 0.04a | 0.33 ± 0a | 0.5 ± 0b | 0.39 ± 0.05a | 0.47 ± 0.03a |
| Taninin | 41 ± 6a | 47.33 ± 0.4a | 63.3 ± 6.2b | 55 ± 7a | 62 ± 4.5a |
| Saponin | 4.62 ± 0.82a | 7 ± 0.4a | 12.9 ± 1.2b | 8.51 ± 1.23b | 10.15 ± 0.7c |
| Stroid | 8.8 ± 1a | 9.1 ± 0.6a | 13.9 ± 0.7b | 12 ± 2b | 13 ± 1bc |
| Vitamins (mg/g FW) | |||||
| Tocopherol (Vit E) | 0.49 ± 0.07a | 0.63 ± 0.1a | 0.91 ± 0.1b | 0.61 ± 0.09a | 0.79 ± 0.05ab |
| α-Carotene (Vit A) | 0.28 ± 0.04a | 0.36 ± 0.1a | 0.52 ± 0.1b | 0.35 ± 0.05a | 0.45 ± 0.03a |
| β-Carotene (Vit A) | 0.09 ± 0.01a | 0.16 ± 0a | 0.16 ± 0a | 0.07 ± 0.02a | 0.17 ± 0.01b |
| β-Cryptoxanthin (Vit A) | 0.05 ± 0.01a | 0.07 ± 0a | 0.06 ± 0a | 0.04 ± 0.01a | 0.07 ± 0b |
| Thiamine (Vit B) | 0.05 ± 0.01a | 0.06 ± 0a | 0.07 ± 0a | 0.05 ± 0.01a | 0.07 ± 0a |
| Phylloquinone (Vit K) | 0.09 ± 0.01a | 0.14 ± 0a | 0.14 ± 0a | 0.08 ± 0.01a | 0.15 ± 0.01b |
| Ascorbic Acid (Vit C) | 1.2 ± 0.2a | 1.4 ± 0.1a | 1.96 ± 0.1ab | 1.5 ± 0.2a | 1.7 ± 0.1ab |
| Minerals (mg/g DW) | |||||
| K | 10.2 ± 1.4a | 11.7 ± 1a | 16.89 ± 1b | 12.7 ± 1.7a | 14.7 ± 0.9b |
| P | 3 ± 0.5a | 3.46 ± 0.4a | 6.28 ± 0.5b | 4.7 ± 0.6ab | 5 ± 0.3ab |
| Ca | 2.2 ± 0.3a | 2.24 ± 0.3a | 3.19 ± 0.5b | 2.7 ± 0.3a | 2.8 ± 0.2a |
| Mg | 0.8 ± 0.17a | 1.39 ± 0.5a | 2.55 ± 0.4ab | 1.65 ± 0.23b | 2.01 ± 0.13c |
| Na | 0.32 ± 0.04a | 0.37 ± 0.1a | 0.47 ± 0.1b | 0.35 ± 0.05a | 0.43 ± 0.03a |
| Fe | 0.14 ± 0.02a | 0.15 ± 0.02a | 0.18 ± 0.02a | 0.15 ± 0.02a | 0.17 ± 0.01a |
| Zn | 0.02 ± 0a | 0.04 ± 0.01a | 0.04 ± 0.01a | 0.02 ± 0a | 0.04 ± 0b |
| Cu | 0.07 ± 0.01a | 0.08 ± 0.02a | 0.17 ± 0.01b | 0.13 ± 0.02a | 0.13 ± 0.01a |
| N | 24.5 ± 2.9a | 32.51 ± 3a | 34.58 ± 3a | 22.4 ± 3.6a | 30.9 ± 2.2b |
| Essential oils (mg/g FW) | |||||
| α-pinene | 0.02 ± 0a | 0.02 ± 0.01a | 0.05 ± 0.02b | 0.05 ± 0.01b | 0.04 ± 0b |
| Sabinene | 0.26 ± 0.03a | 0.46 ± 0.22a | 0.34 ± 0.07a | 0.2 ± 0.04a | 0.25 ± 0.03a |
| Myrcene | 0.13 ± 0.03a | 0.15 ± 0.03a | 0.45 ± 0.09b | 0.39 ± 0.05b | 0.34 ± 0.02b |
| Fenchone | 3.3 ± 0.43a | 4.38 ± 0.67a | 4.79 ± 0.75a | 3.61 ± 0.55a | 5.09 ± 0.32b |
| p-cymene | 0.47 ± 0.06a | 0.61 ± 0.18a | 0.62 ± 0.1a | 0.48 ± 0.07a | 0.59 ± 0.04ab |
| o-isoeugenol | 3.9 ± 0.36ab | 2.97 ± 0.6a | 4.47 ± 0.7b | 3.47 ± 0.48a | 4.17 ± 0.26ab |
| 1,8-cineole | 1.24 ± 0.18a | 1.41 ± 0.2a | 1.84 ± 0.4a | 1.61 ± 0.21ab | 1.72 ± 0.11ab |
| Cis-β-ocimene | 0.26 ± 0.04a | 0.33 ± 0.04a | 0.51 ± 0.11b | 0.4 ± 0.05a | 0.47 ± 0.03a |
| Aα-phellandrene | 0 ± 0a | 0 ± 0a | 0 ± 0a | 00.14 ± 0a | 0.012 ± 0a |
| Methyl chavicol | 0.18 ± 0.03a | 0.2 ± 0.04a | 0.39 ± 0.08 b | 0.16 ± 0.04a | 0.23 ± 0.02ab |
| Endo-fenchyl acetate | 0 ± 0a | 0.001 ± 0a | 0.002 ± 0b | 0 ± 0a | 0 ± 0a |
| p-anisaldehyde | 0.07 ± 0.01ab | 0.08 ± 0.04a | 0.06 ± 0.01a | 0.05 ± 0.01a | 0.08 ± 0a |
| Limonene | 0.12 ± 0.02a | 0.18 ± 0.03a | 0.35 ± 0.05b | 0.23 ± 0.03ab | 0.3 ± 0.02c |
| Stearic acid | 0.03 ± 0a | 0.03 ± 0.01a | 0.03 ± 0.01a | 0.03 ± 0a | 0.03 ± 0a |
| 2-oleoylglycerol | 0.19 ± 0.03a | 0.29 ± 0a | 0.44 ± 0.05b | 0.29 ± 0.04a | 0.41 ± 0.03a |
| γ-himachalene | 0.1 ± 0.01a | 0.11 ± 0.03a | 0.11 ± 0.01a | 0.1 ± 0.01a | 0.12 ± 0.01a |
| Trans-Pseudoisoeugenyl | 0.13 ± 0.02a | 0.15 ± 0.06a | 0.16 ± 0.01a | 0.14 ± 0.02a | 0.17 ± 0.01b |
| Trans-anethole | 44 ± 7a | 49.99 ± 8.36a | 75.17 ± 2.4b | 66.4 ± 8b | 70.5 ± 4bc |
| Amino acids | |||||
| Phenylalanine | 2.0 ± 0.1a | 3.2 ± 0.12b | 5.8 ± 0.8d | 2.8 ± 0.07b | 4.2 ± 0.4c |
| L-phenylalanine aminolyase | 25.9 ± 1.8a | 33.1 ± 2.1a | 57.1 ± 5.4c | 28.3 ± 3.1a | 49.2 ± 5.41b |
| DAHPS | 0.1 ± 0.02a | 0.44 ± 0b | 0.95 ± 0.01c | 0.41 ± 0b | 0.8 ± 0.05c |
| Other related compounds | |||||
| Cinnamic acid | 2.8 ± 0.1b | 1.6 ± 0a | 2.7 ± 0.1b | 3.1 ± 0.1b | 4.07 ± 0.2c |
| Shikimic acid | 33.9 ± 1.1a | 58.3 ± 6.8b | 74 ± 4.0c | 40.5 ± 6.2a | 60.8 ± 7.4b |
| Sprouts | Mature | ||||
|---|---|---|---|---|---|
| Compound | Fruits | Control | Laser-Treated | Control | Laser-Treated |
| Phenolic acids (μg/g DW) | |||||
| Caffeic acid | 3.86 ± 0.59a | 4.41 ± 0.2a | 6.44 ± 0.1b | 5.64 ± 0.73ab | 6.08 ± 0.38b |
| Ferulic acid | 0.03 ± 0.01a | 0.04 ± 0a | 0.09 ± 0c | 0.06 ± 0.01b | 0.07 ± 0b |
| Catechin | 1.17 ± 0.17a | 1.34 ± 0.3a | 1.82 ± 0a | 1.59 ± 0.21a | 1.77 ± 0.11a |
| Galic acid | 3.76 ± 0.71a | 4.84 ± 0.3a | 9.72 ± 0.2b | 7.54 ± 0.98ab | 8.16 ± 0.51b |
| p-Coumaric acid | 1.02 ± 0.16a | 1.31 ± 0.1a | 2.04 ± 0a | 1.58 ± 0.22a | 1.88 ± 0.8a |
| Flavonoids (μg/g DW) | |||||
| kaempferol | 0.44 ± 0.06a | 0.82 ± 0a | 1.05 ± 0.2a | 0.57 ± 0.1a | 1.5 ± 0.07a |
| Chlorogenic acid | 0.11 ± 0.01a | 0.14 ± 0a | 0.16 ± 0a | 0.12 ± 0.02a | 0.17 ± 0.01a |
| Quercetin | 1.54 ± 0.3a | 1.98 ± 0.1a | 4.28 ± 0.1b | 3.32 ± 0.4ab | 3.51 ± 0.2ab |
| Luteolin | 0.04 ± 0.01a | 0.07 ± 0a | 0.14 ± 0b | 0.08 ± 0.01a | 0.12 ± 0.01ab |
| Apigenin | 0.16 ± 0.03a | 0.21 ± 0a | 0.44 ± 0b | 0.44 ± 0.04b | 0.46 ± 0.02b |
| Naringenin | 0.78 ± 0.14a | 1.44 ± 0.1b | 2.67 ± 0.1c | 1.44 ± 0.23b | 2.0 ± 0.14ab |
| Velutin | 0.01 ± 0a | 0.01 ± 0a | 0.02 ± 0b | 0.02 ± 0a | 0.02 ± 0a |
| Tricin | 0.77 ± 0.13a | 1.17 ± 0.1a | 2.06 ± 0ab | 1.36 ± 0.2a | 1.81 ± 0.11a |
| vitexin | 0.51 ± 0.1a | 0.58 ± 0a | 1.21 ± 0b | 1.06 ± 0.13a | 1 ± 0.06a |
| Activity | Fruits | Sprouts Control Laser-Treated | Mature Control Laser-Treated | ||
|---|---|---|---|---|---|
| Antioxidant | |||||
| DPPH (%) | 36.5 ± 0.37a | 50.2 ± 2.956a | 65.61 ± 2.6b | 37.41 ± 0.4a | 54.27 ± 0.2b |
| FRAP | 6.4 ± 2.3a | 15.3 ± 0.6a | 27.6 ± 1.9b | 14.18 ± 3.1b | 24.05 ± 1.5c |
| Total antioxidant capacity (TAC) (nmol/g FW) | 9.39 ± 1.3a | 10.72 ± 0.061a | 13.23 ± 0.2b | 11.5 ± 1.5a | 13.4 ± 0.84b |
| Anti-lipid peroxidation | 2.5 ± 0.37a | 3.22 ± 0.275a | 4.39 ± 0.07b | 3.41 ± 0.4b | 4.27 ± 0.2c |
| (ORAC) | 571 ± 80a | 865.7 ± 34.3a | 1064.6 ± 85.5b | 821 ± 65b | 1082 ± 68c |
| % inhibation of LDL oxidation (TBARS) | 13 ± 2a | 14.3 ± 0.9a | 28.6 ± 3.7b | 24 ± 2ab | 25 ± 3b |
| % inhibation of LDL oxidation (conjugated dienes) | 15 ± 3a | 15.4 ± 0.3a | 28.5 ± 3.4b | 17 ± 3a | 25 ± 2b |
| Anti-lipidemic | |||||
| Anti-Amylase IC50(mg/mL) | 3.1 ± 0.2a | 2.7 ± 0.1a | 1.5 ± 0.1b | 2.96 ± 0.22a | 2.35 ± 0.1b |
| Anti-Lipase IC50(mg/mL) | 1.32 ± 0.1a | 1.7 ± 0.3a | 0.97 ± 0b | 1.75 ± 0.14a | 1.0 ± 0.1b |
| Anti-chlostrol | 39 ± 5.77a | 50.2 ± 1.9a | 68.7 ± 3.7b | 43 ± 7.5a | 67 ± 4.17b |
| Anti-hemolytic activity | |||||
| % inhibation of hemolysis | 11 ± 1.78a | 14 ± 1.1a | 22.6 ± 1.8b | 18 ± 2.38a | 21 ± 1.28a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okla, M.K.; Abdel-Mawgoud, M.; Alamri, S.A.; Abbas, Z.K.; Al-Qahtani, W.H.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Hassan, A.H.A.; Selim, S.; Alruhaili, M.H.; et al. Developmental Stages-Specific Response of Anise Plants to Laser-Induced Growth, Nutrients Accumulation, and Essential Oil Metabolism. Plants 2021, 10, 2591. https://doi.org/10.3390/plants10122591
Okla MK, Abdel-Mawgoud M, Alamri SA, Abbas ZK, Al-Qahtani WH, Al-Qahtani SM, Al-Harbi NA, Hassan AHA, Selim S, Alruhaili MH, et al. Developmental Stages-Specific Response of Anise Plants to Laser-Induced Growth, Nutrients Accumulation, and Essential Oil Metabolism. Plants. 2021; 10(12):2591. https://doi.org/10.3390/plants10122591
Chicago/Turabian StyleOkla, Mohammad K., Mohamed Abdel-Mawgoud, Saud A. Alamri, Zahid Khorshid Abbas, Wahidah H. Al-Qahtani, Salem Mesfir Al-Qahtani, Nadi Awad Al-Harbi, Abdelrahim H. A. Hassan, Samy Selim, Mohammed H. Alruhaili, and et al. 2021. "Developmental Stages-Specific Response of Anise Plants to Laser-Induced Growth, Nutrients Accumulation, and Essential Oil Metabolism" Plants 10, no. 12: 2591. https://doi.org/10.3390/plants10122591
APA StyleOkla, M. K., Abdel-Mawgoud, M., Alamri, S. A., Abbas, Z. K., Al-Qahtani, W. H., Al-Qahtani, S. M., Al-Harbi, N. A., Hassan, A. H. A., Selim, S., Alruhaili, M. H., & AbdElgawad, H. (2021). Developmental Stages-Specific Response of Anise Plants to Laser-Induced Growth, Nutrients Accumulation, and Essential Oil Metabolism. Plants, 10(12), 2591. https://doi.org/10.3390/plants10122591









