Impacts of Nicotiana glauca Graham Invasion on the Vegetation Composition and Soil: A Case Study of Taif, Western Saudi Arabia
Abstract
1. Introduction
2. Results
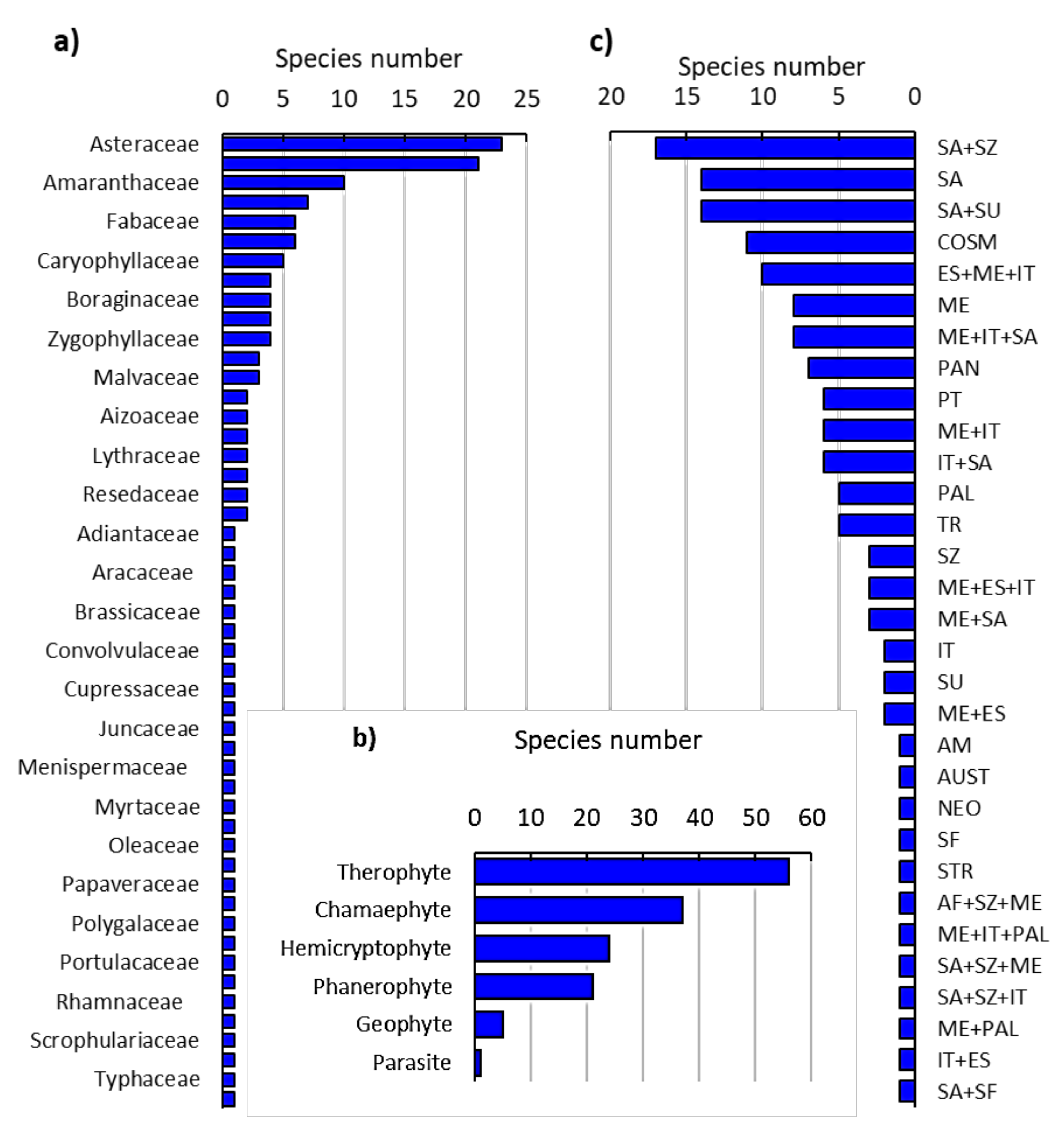
2.1. Floristic Analysis of the Invaded Locations by N. glauca
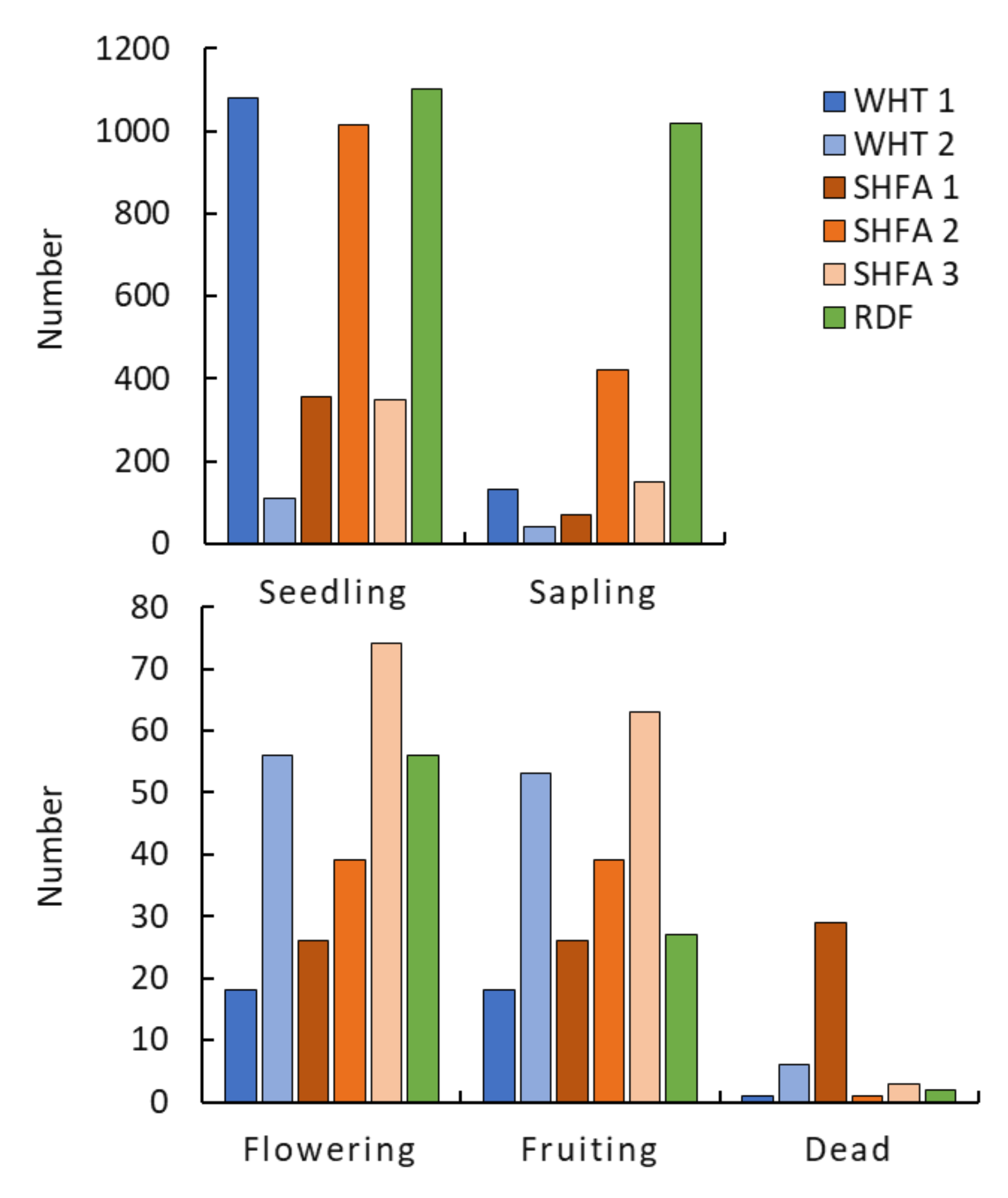
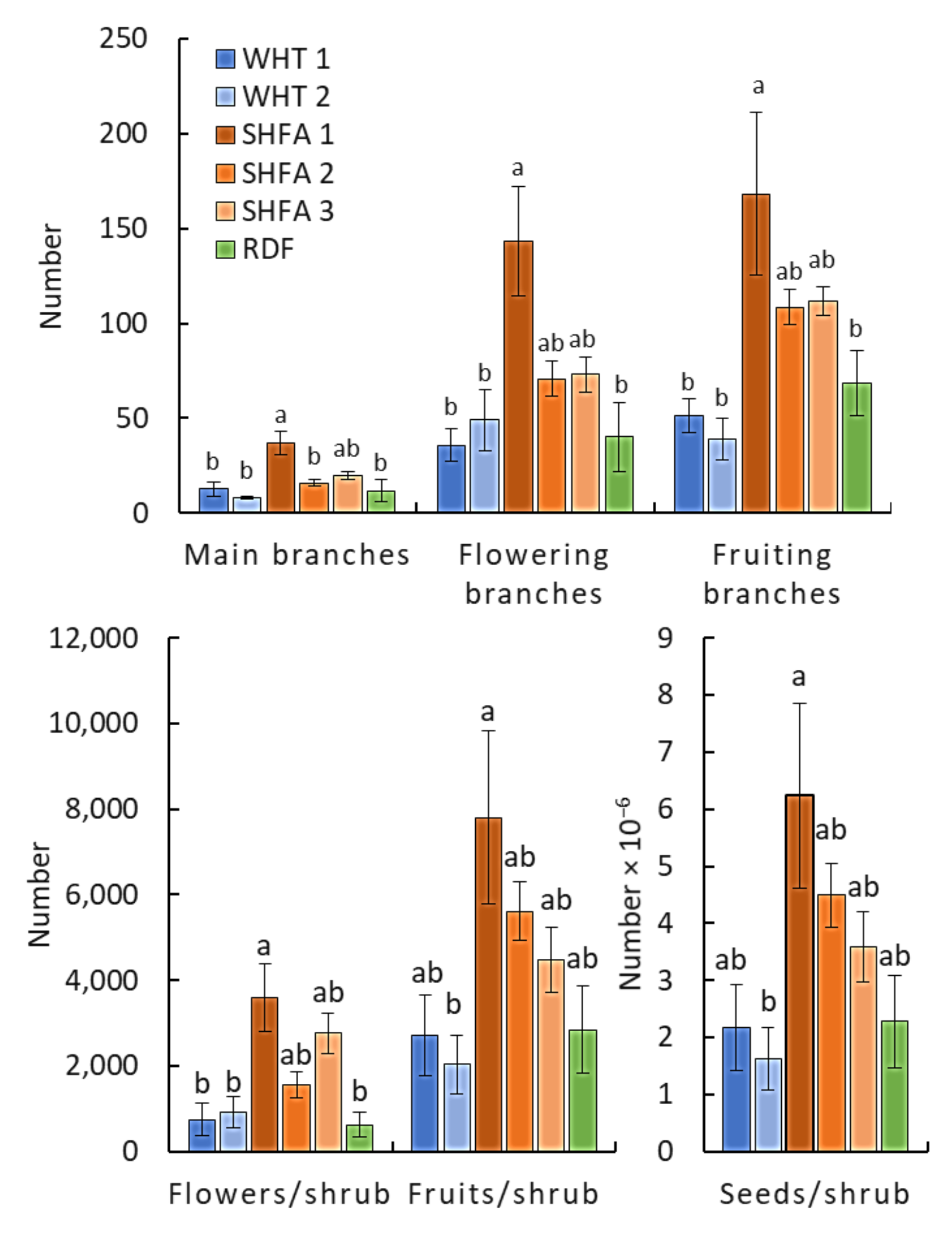
2.2. Nicotiana glauca Shrub Measurements
2.3. Vegetation Analysis of the Invaded Locations by N. glauca
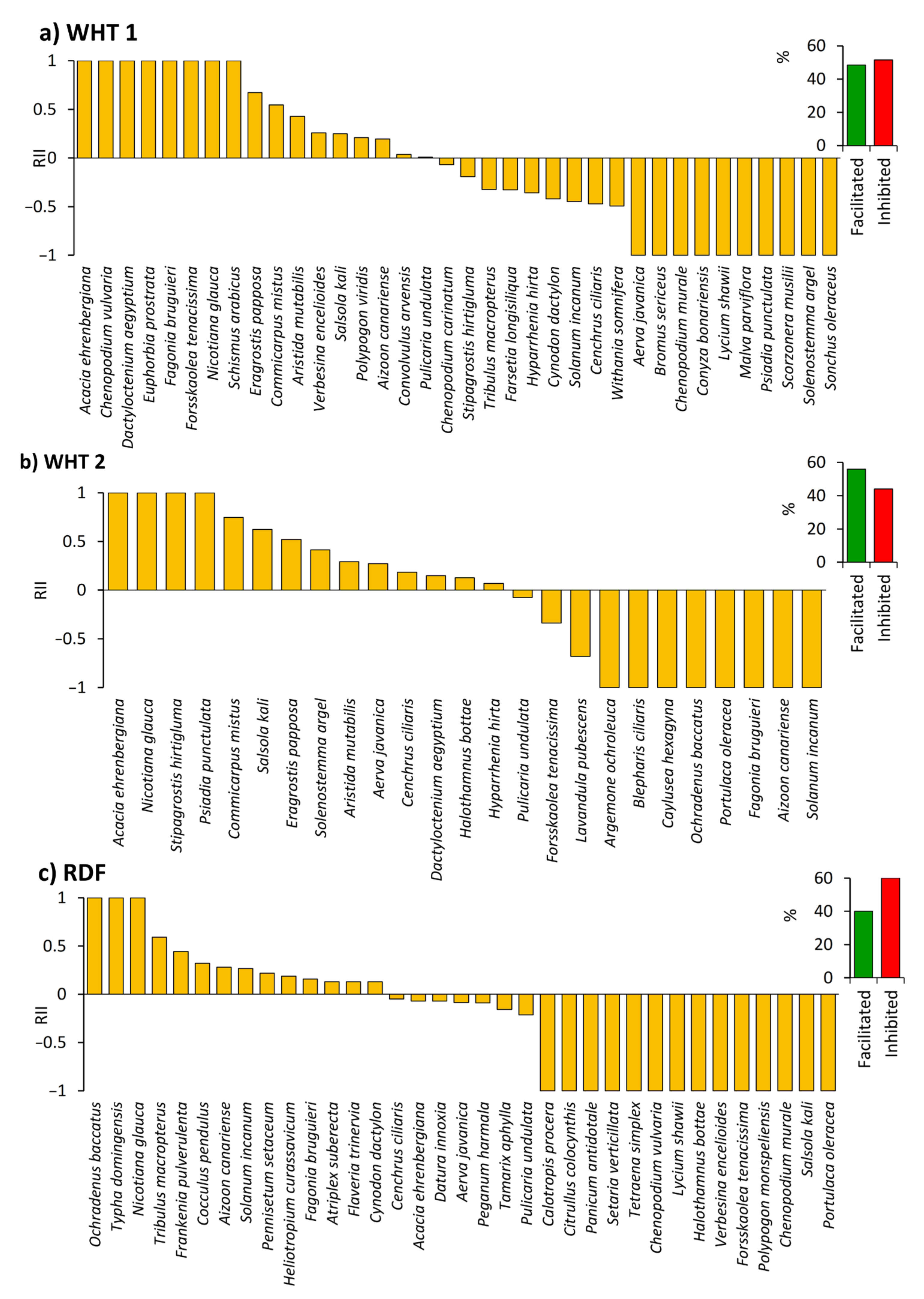
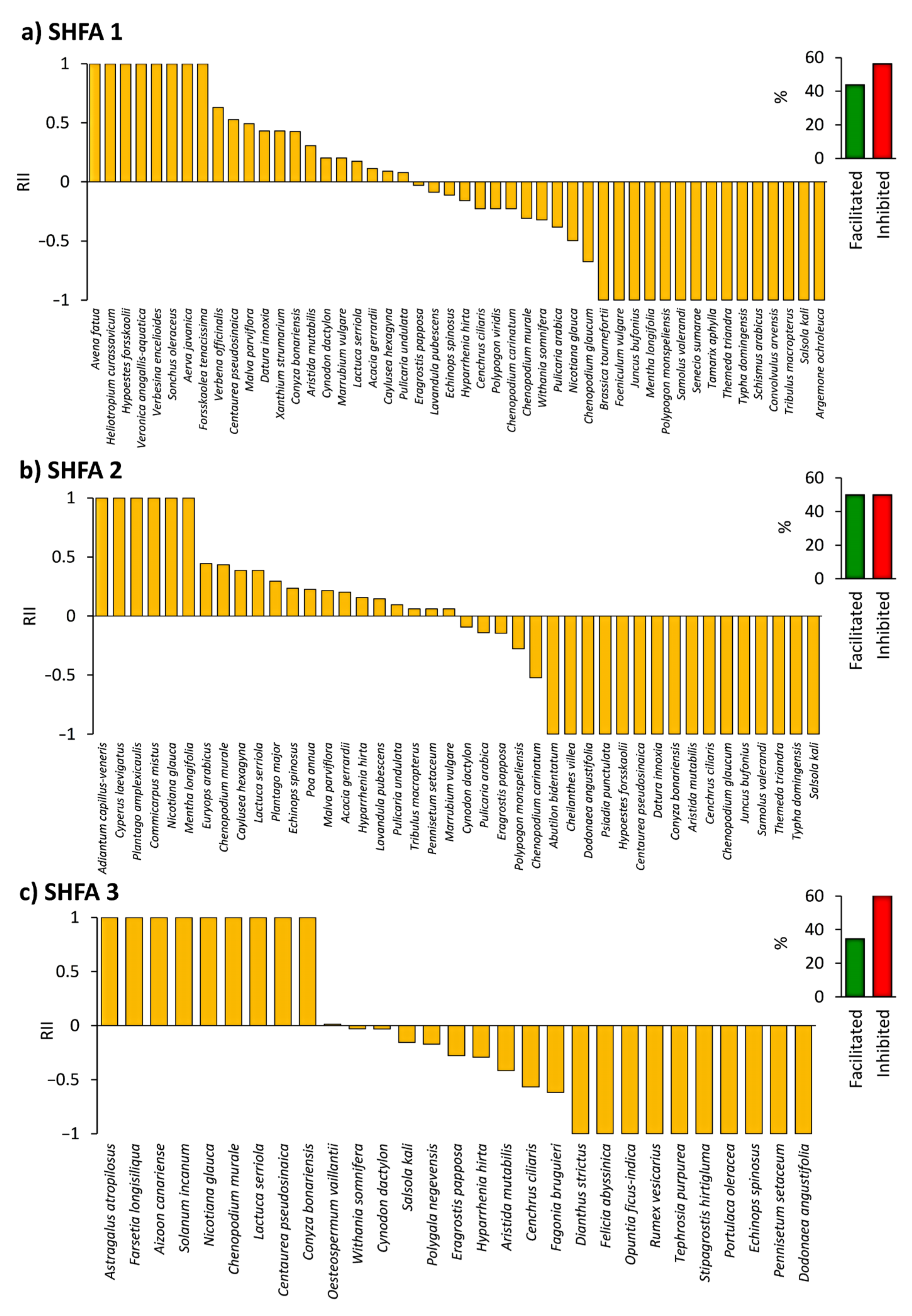
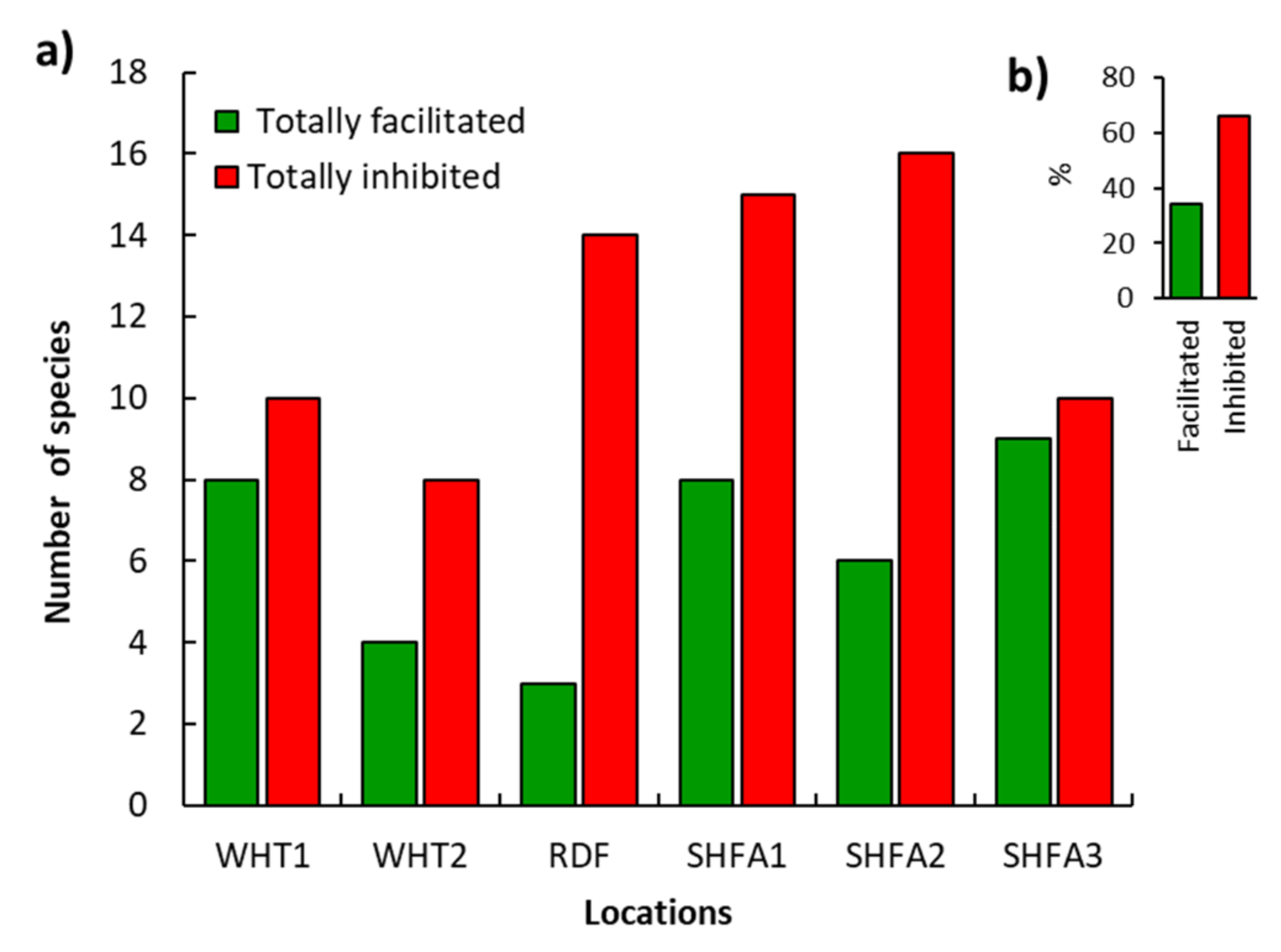
2.4. Influence of N. glauca on the Native Understory Plant Species
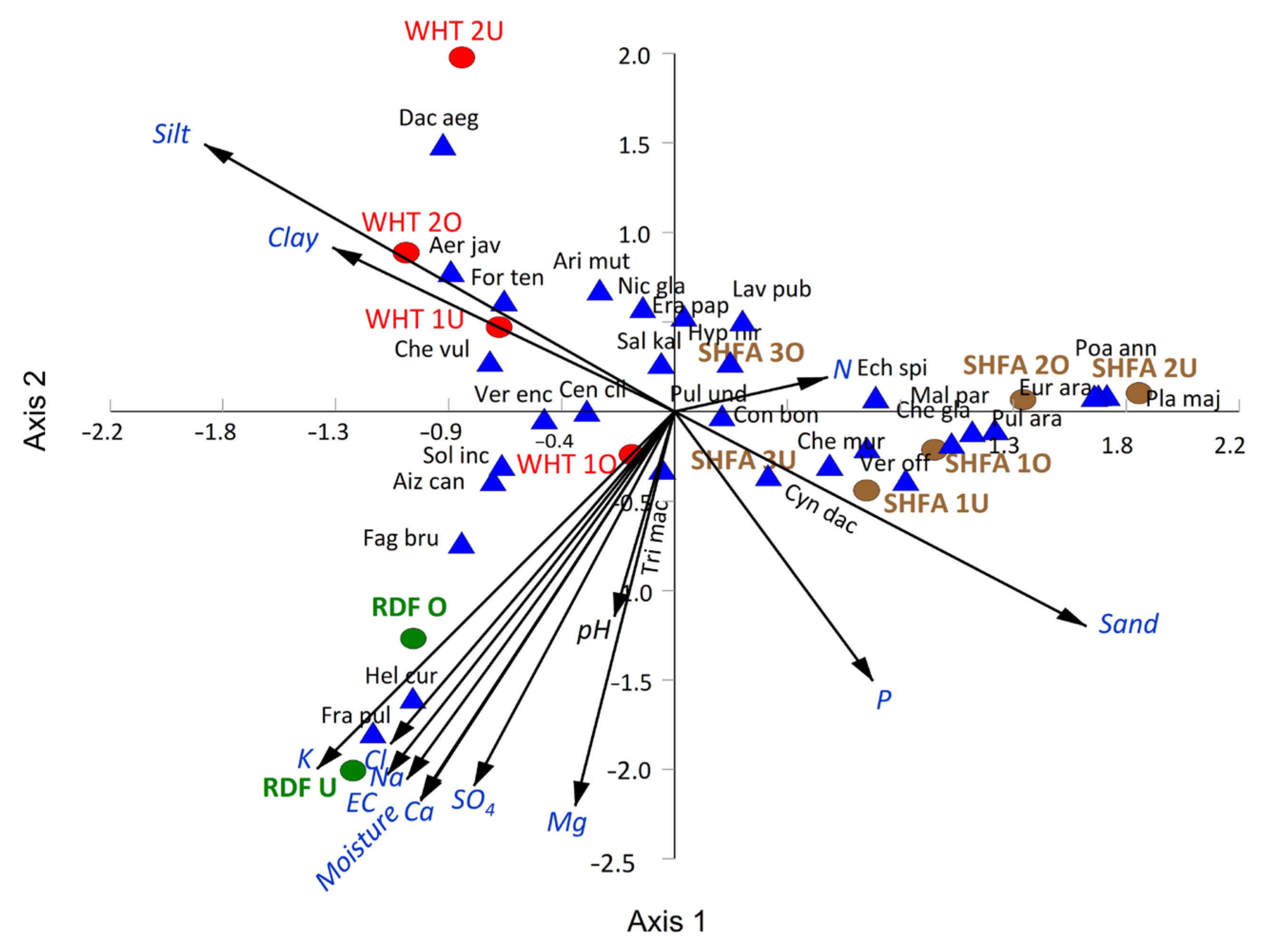
2.5. Vegetation–Soil Relationship
3. Discussion
4. Materials and Methods
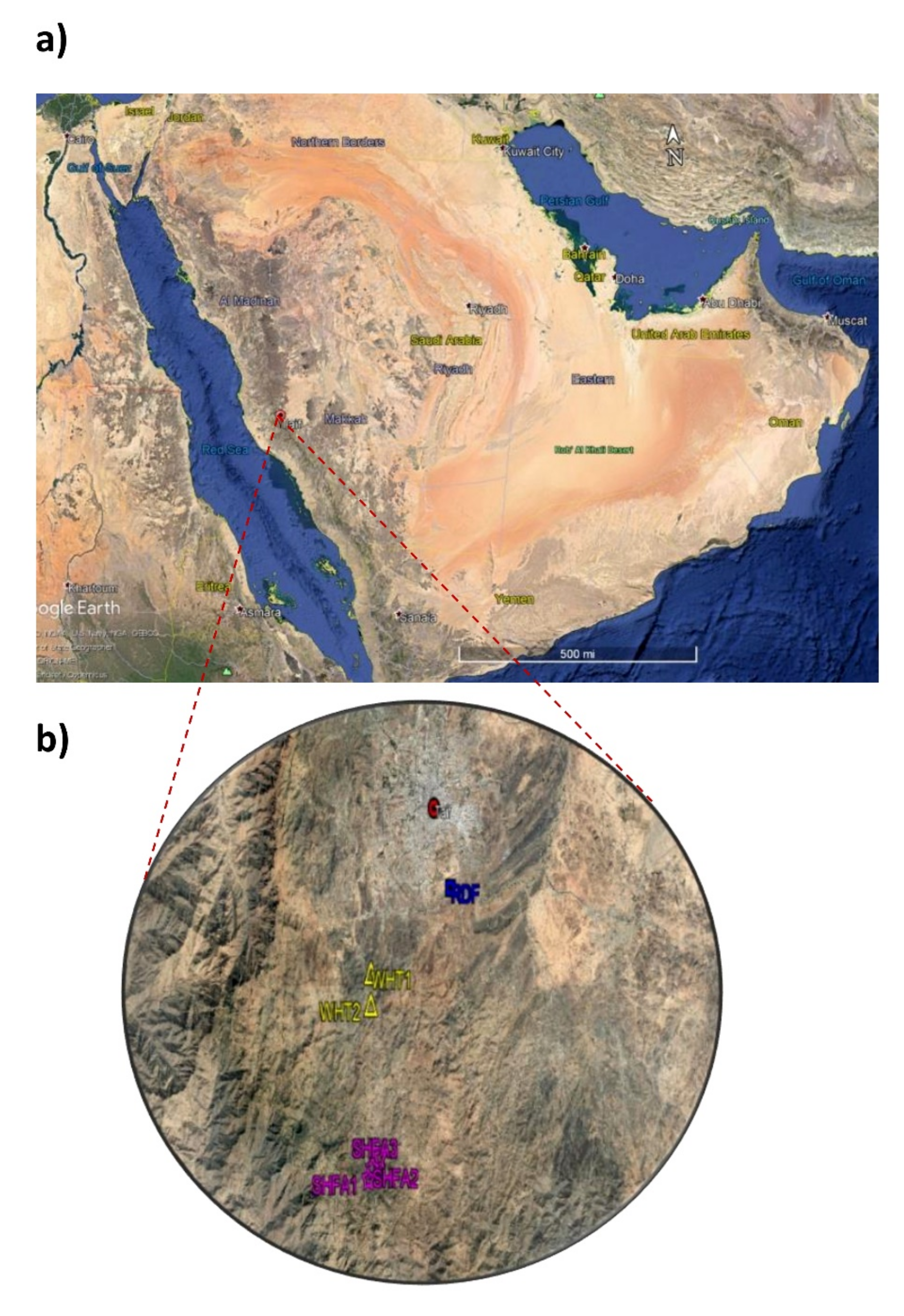
4.1. Study Area
4.2. Studied Locations and Nicotiana glauca Shrub Measurements
4.3. Vegetation Analysis of the Invaded Locations
4.4. Soil Analysis
4.5. Data Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Wilgen, B.; Le Maitre, D.C.; Cowling, R. Ecosystem services, efficiency, sustainability and equity: South Africa’s Working for Water programme. Trends Ecol. Evol. 1998, 13, 378. [Google Scholar] [CrossRef]
- Keller, R.P.; Geist, J.; Jeschke, J.M.; Kühn, I. Invasive species in Europe: Ecology, status, and policy. Environ. Sci. Eur. 2011, 23, 23. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Linders, T.E.W.; Schaffner, U.; Eschen, R.; Abebe, A.; Choge, S.K.; Nigatu, L.; Mbaabu, P.R.; Shiferaw, H.; Allan, E. Direct and indirect effects of invasive species: Biodiversity loss is a major mechanism by which an invasive tree affects ecosystem functioning. J. Ecol. 2019, 107, 2660–2672. [Google Scholar] [CrossRef]
- Duenas, M.-A.; Ruffhead, H.J.; Wakefield, N.H.; Roberts, P.D.; Hemming, D.J.; Diaz-Soltero, H.J.B. The role played by invasive species in interactions with endangered and threatened species in the United States: A systematic review. Biodivers. Conserv. 2018, 27, 3171–3183. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. BioScience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Gaertner, M.; Den Breeyen, A.; Cang, H.; Richardson, D.M. Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: A meta-analysis. Prog. Phys. Geogr. 2009, 33, 319–338. [Google Scholar] [CrossRef]
- Liao, C.; Peng, R.; Luo, Y.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.; Pyšek, P.; Jarošík, V.; Pergl, J.; Schaffner, U.; Vila, M. Bias and error in current knowledge of plant invasions impacts. Trends Ecol. Evol. 2013, 28, 212–218. [Google Scholar] [CrossRef]
- Abd El Gawad, A.; El-Amier, Y. Allelopathy and potential impact of invasive Acacia saligna (Labill.) Wendl. on plant diversity in the Nile Delta coast of Egypt. Int. J. Environ. Res. 2015, 9, 923–932. [Google Scholar]
- Bonanomi, G.; Incerti, G.; Abd El-Gawad, A.M.; Sarker, T.C.; Stinca, A.; Motti, R.; Cesarano, G.; Teobaldelli, M.; Saulino, L.; Cona, F. Windstorm disturbance triggers multiple species invasion in an urban Mediterranean forest. iFor. Biogeosci. For. 2018, 11, 64–71. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Chen, D.; Yan, R.; Yu, F.-H.; van Kleunen, M. Invasive alien clonal plants are competitively superior over co-occurring native clonal plants. Perspect. Plant Ecol. Evol. Syst. 2019, 40, 125484. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, J. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecol. Indic. 2020, 111, 106020. [Google Scholar]
- Weigelt, A.; Weisser, W.W.; Buchmann, N.; Scherer-Lorenzen, M. Biodiversity for multifunctional grasslands: Equal productivity in high-diversity low-input and low-diversity high-input systems. Biogeosciences 2009, 6, 1695–1706. [Google Scholar] [CrossRef]
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Ann. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Funk, J.L.; Standish, R.J.; Stock, W.D.; Valladares, F. Plant functional traits of dominant native and invasive species in mediterranean-climate ecosystems. Ecol. Lett. 2016, 97, 75–83. [Google Scholar] [CrossRef]
- Blossey, B.; Notzold, R. Evolution of increased competitive ability in invasive nonindigenous plants: A hypothesis. J. Ecol. 1995, 83, 887–889. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Cassey, P.; Blackburn, T. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 2005, 20, 223–228. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Petruzzella, A.; Tauany, A.; van Leeuwen, C.H.; de Assis Esteves, F.; Figueiredo-Barros, M.P.; Bakker, E.S. Species identity and diversity effects on invasion resistance of tropical freshwater plant communities. Sci. Rep. 2020, 10, 5626. [Google Scholar] [CrossRef]
- Williamson, M. Invasions. Ecography 1999, 22, 5–12. [Google Scholar] [CrossRef]
- Issaly, E.A.; Sérsic, A.N.; Pauw, A.; Cocucci, A.A.; Traveset, A.; Benitez-Vieyra, S.M.; Paiaro, V. Reproductive ecology of the bird-pollinated Nicotiana glauca across native and introduced ranges with contrasting pollination environments. Biol. Invasions 2020, 22, 485–498. [Google Scholar] [CrossRef]
- Bogdanović, S.; Mitić, B.; Ruščić, M.; Dolina, K. Nicotiana glauca Graham (Solanaceae), a new invasive plant in Croatia. Acta Bot. Croat. 2006, 65, 203–209. [Google Scholar]
- Schueller, S.K. Self-pollination in island and mainland populations of the introduced hummingbird-pollinated plant, Nicotiana glauca (Solanaceae). Am. J. Bot. 2004, 91, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Florentine, S.; Westbrooke, M.; Gosney, K.; Ambrose, G.; O’Keefe, M. The arid land invasive weed Nicotiana glauca R. Graham (Solanaceae): Population and soil seed bank dynamics, seed germination patterns and seedling response to flood and drought. J. Arid Environ. 2006, 66, 218–230. [Google Scholar] [CrossRef][Green Version]
- Nattero, J.; Cocucci, A.A. Geographical variation in floral traits of the tree tobacco in relation to its hummingbird pollinator fauna. Biol. J. Linn. Soc. 2007, 90, 657–667. [Google Scholar] [CrossRef]
- Scharenberg, F.; Stegemann, T.; Çiçek, S.S.; Zidorn, C. Sequestration of pyridine alkaloids anabasine and nicotine from Nicotiana (Solanaceae) by Orobanche ramosa (Orobanchaceae). Biochem. Syst. Ecol. 2019, 86, 103908. [Google Scholar] [CrossRef]
- Thomas, J.; El-Sheikh, M.A.; Alfarhan, A.H.; Alatar, A.A.; Sivadasan, M.; Basahi, M.; Al-Obaid, S.; Rajakrishnan, R. Impact of alien invasive species on habitats and species richness in Saudi Arabia. J. Arid Environ. 2016, 127, 53–65. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.M.; Hutchison, M.A.; Ewers, R.M.; Gemmell, N.J. Are invasive species the drivers of ecological change? Trends Eco. Evol. 2005, 20, 470–474. [Google Scholar] [CrossRef]
- Weidlich, E.W.; Flórido, F.G.; Sorrini, T.B.; Brancalion, P.H. Controlling invasive plant species in ecological restoration: A global review. J. Appl. Ecol. 2020, 57, 1806–1817. [Google Scholar] [CrossRef]
- Abdel Khalik, K.; Al-Gohary, I.; Al-Sodany, Y. Floristic composition and vegetation: Environmental relationships of Wadi Fatimah, Mecca, Saudi Arabia. Arid Land Res. Manag. 2017, 31, 316–334. [Google Scholar] [CrossRef]
- Collenette, S. Wildflowers of Saudi Arabia; National Commission for Wildlife Conservation and Development (NCWCD): Riyadh, Saudi Arabia, 1999. [Google Scholar]
- Alharthi, A.S.; Abd-ElGawad, A.M.; Assaeed, A.M. Influence of the invasive shrub Nicotiana glauca Graham on the plant seed bank in various locations in Taif region, western of Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 360–370. [Google Scholar] [CrossRef]
- Khalik, K.A.; El-Sheikh, M.; El-Aidarous, A. Floristic diversity and vegetation analysis of wadi Al-Noman, Mecca, Saudi Arabia. Turk. J. Bot. 2013, 37, 894–907. [Google Scholar] [CrossRef]
- Osman, A.K.; Al-Ghamdi, F.; Bawadekji, A. Floristic diversity and vegetation analysis of Wadi Arar: A typical desert Wadi of the Northern Border region of Saudi Arabia. Saudi J. Biol. Sci. 2014, 21, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Tarhouni, M.; Ben Hmida, W.; Neffati, M. Long-term changes in plant life forms as a consequence of grazing exclusion under arid climatic conditions. Land Degrad. Dev. 2017, 28, 1199–1211. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M. Ecology and allelopathic control of Brassica tournefortii in reclaimed areas of the Nile Delta, Egypt. Turk. J. Bot. 2014, 38, 347–357. [Google Scholar] [CrossRef]
- Alsherif, E.A.; Fadl, M.A. Floristic study of the Al-shafa highlands in Taif, Western Saudi Arabia. Flora 2016, 225, 20–29. [Google Scholar] [CrossRef]
- Wan, J.-Z.; Zhang, Z.-X.; Wang, C.-J. Effects of ecoregional vulnerability on habitat suitability of invasive alien plants: An assessment using 13 species on a global scale. Environ. Earth Sci. 2019, 78, 180. [Google Scholar] [CrossRef]
- Liao, H.; Luo, W.; Peng, S.; Callaway, R.M. Plant diversity, soil biota and resistance to exotic invasion. Divers. Distrib. 2015, 21, 826–835. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Chan, Z. Comparative proteomic and physiological analyses reveal the protective effect of exogenous polyamines in the bermudagrass (Cynodon dactylon) response to salt and drought stresses. J. Proteome Res. 2013, 12, 4951–4964. [Google Scholar] [CrossRef] [PubMed]
- Juraimi, A.S.; Drennan, S.D.; Anuar, N. Competitive effect of Cynodon dactylon (L.) Pers. on four crop species, soybean [Glycine max (L.) Merr.], maize (Zea mays), spring wheat (Triticum aestivum) and faba bean [Vicia faba (L.)]. Asian J. Plant Sci. 2005, 4, 90–94. [Google Scholar] [CrossRef][Green Version]
- Liang, Z.; Li, X.; Zhang, H.; Li, J.; Bian, X.; Xu, J. Allelopathic effects of Bermuda grass (Cynodon dactylon L.) root exudates on seed germination and seedling growth of tall fescue (Festuca arundinacea Schreb). Allelopathy J. 2018, 44, 25–34. [Google Scholar] [CrossRef]
- Ward, J.S.; Williams, S.C.; Linske, M.A. Influence of invasive shrubs and deer browsing on regeneration in temperate deciduous forests. Can. J. For. Res. 2018, 48, 58–67. [Google Scholar] [CrossRef]
- Medina-Villar, S.; Alonso, Á.; Castro-Díez, P.; Pérez-Corona, M.E. Allelopathic potentials of exotic invasive and native trees over coexisting understory species: The soil as modulator. Plant Ecol. 2017, 218, 579–594. [Google Scholar] [CrossRef]
- Woodworth, G.R.; Ward, J.N.; Carr, D.E. Exotic tree and shrub invasions alter leaf-litter microflora and arthropod communities. Oecologia 2020, 193, 177–187. [Google Scholar] [CrossRef]
- Alshahrani, S.T. Effect of aqueous extract of the invasive species tobacco (Nicotiana glauca L.) on seedlings growth of Juniper (Juniperus procera L.). Emir. J. Food Agric. 2008, 20, 10–17. [Google Scholar] [CrossRef]
- El-Kenany, E.T.; El-Darier, S.M.; Abdellatif, A.A.; Shaklol, S.M. Allelopathic potential of invasive species: Nicotiana glauca Graham on some ecological and physiological aspects of Medicago sativa L. and Triticum aestivum L. Rend. Lincei 2017, 28, 159–167. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Evergetis, E.; Papachristos, D.; Vangelatou, O.; Antonatos, S.; Milonas, P.; Haroutounian, S.A.; Machera, K. An essay on ecosystem availability of Nicotiana glauca graham alkaloids: The honeybees case study. BMC Ecol. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Lucero, J.E.; Noble, T.; Haas, S.; Westphal, M.; Butterfield, H.S.; Lortie, C.J. The dark side of facilitation: Native shrubs facilitate exotic annuals more strongly than native annuals. NeoBiota 2019, 44, 75–93. [Google Scholar] [CrossRef]
- Green, P.T.; O’Dowd, D.J.; Abbott, K.L.; Jeffery, M.; Retallick, K.; Mac Nally, R. Invasional meltdown: Invader-invader mutualism facilitates a secondary invasion. Ecology 2011, 92, 1758–1768. [Google Scholar] [CrossRef]
- Rodriguez, L.F. Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biol. Invasions 2006, 8, 927–939. [Google Scholar] [CrossRef]
- Wundrow, E.J.; Carrillo, J.; Gabler, C.A.; Horn, K.C.; Siemann, E. Facilitation and competition among invasive plants: A field experiment with alligatorweed and water hyacinth. PLoS ONE 2012, 7, e48444. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Brunel, C.; van Kleunen, M. Soil-microorganism-mediated invasional meltdown in plants. Nat. Ecol. Evol. 2020, 4, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Shumway, S.W. Facilitative effects of a sand dune shrub on species growing beneath the shrub canopy. Oecologia 2000, 124, 138–148. [Google Scholar] [CrossRef]
- Assaeed, A.M.; Al-Rowaily, S.L.; El-Bana, M.I.; Hegazy, A.K.; Dar, B.A.; Abd-ElGawad, A.M. Functional traits plasticity of the invasive herb Argemone ochroleuca Sweet in different arid habitats. Plants 2020, 9, 1268. [Google Scholar] [CrossRef]
- Milanović, M.; Knapp, S.; Pyšek, P.; Kühn, I. Linking traits of invasive plants with ecosystem services and disservices. Ecosyst. Serv. 2020, 42, 101072. [Google Scholar] [CrossRef]
- Incerti, G.; Cartenì, F.; Cesarano, G.; Sarker, T.C.; El-Gawad, A.; Ahmed, M.; D’Ascoli, R.; Bonanomi, G.; Giannino, F. Faster N release, but not C loss, from leaf litter of invasives compared to native species in Mediterranean ecosystems. Front. Plant Sci. 2018, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Weidenhamer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef]
- Rout, M.E.; Callaway, R.M. Interactions between exotic invasive plants and soil microbes in the rhizosphere suggest that ‘everything is not everywhere’. Ann. Bot. 2012, 110, 213–222. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Wren, I.F.; Herman, D.J.; Firestone, M.K. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol. Lett. 2005, 8, 976–985. [Google Scholar] [CrossRef] [PubMed]
- McCormick, L.; McMillan, M.; Lodge, G. Coolatai grass (Hyparrhenia hirta) control. Aust. Weeds Res. Newsl. 1992, 41, 36–38. [Google Scholar]
- Chaudhary, S.A. Grasses of Saudi Arabia; Ministry of Agriculture and Water: Riyadh, Saudi Arabia, 1989.
- Vincent, P. Saudi Arabia: An Environmental Overview; Taylor & Francis: Leiden, The Netherlands, 2008. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Bonham, C.D. Measurements for Terrestrial Vegetation; John Wiley & Sons: New York, NY, USA, 2013. [Google Scholar]
- Chaudhary, S.A. Flora of the Kingdom of Saudi Arabia; Ministry of Agriculture and Water: Riyadh, Saudi Arabia, 1999; Volume 1.
- Raunkiaer, C. Plant Life Forms; Clarendon Press: Oxford, UK, 1937. [Google Scholar]
- Zohary, M. Geobotanical Foundations of the Middle East; Gustav Fischer Verlag: Stuttgart, Germany, 1973. [Google Scholar]
- Fadl, M.A.; Farrag, H.F.; Al-Sherif, E. Floristic composition and vegetation analysis of wild legumes in Taif district, Saudi Arabia. Int. Res. J. Agric. Sci. Soil Sci. 2015, 5, 74–80. [Google Scholar]
- Farrag, H.F. Floristic composition and vegetation-soil relationships in Wadi Al-Argy of Taif region, Saudi Arabia. Int. Res. J. Plant Sci. 2012, 3, 147–157. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Rowell, D. Soil Science: Method and Applications; Pearson Education Limited: Essex, UK, 1994. [Google Scholar]
- Rhoades, J. Soluble salts. In Methods of Soil Analysis: Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Constable and Co Ltd.: London, UK, 1962. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Interscience Publishers, Inc.: New York, NY, USA, 1947. [Google Scholar]
- Nelson, D.; Sommers, L. Chemical and microbiological properties. In Methods for Soil Analysis; Page, A.L., Ed.; ASA Monograph: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Bremner, J.; Mulvaney, C. Total nitrogen. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Armas, C.; Ordiales, R.; Pugnaire, F.I. Measuring plant interactions: A new comparative index. Ecology 2004, 85, 2682–2686. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Schaffers, A.P. Co-correspondence analysis: A new ordination method to relate two community compositions. Ecology 2004, 85, 834–846. [Google Scholar] [CrossRef]

| Location | Total Species no. | Richness (Simpson Index) | Evenness (Shannon-Evenness) | Most Dominant | Second Most Dominant | Important Species | |
|---|---|---|---|---|---|---|---|
| WHT 1 | U | 36 | 0.96 ab | 0.94 b | Chenopodium vulvaria (8.93 *) | Aizoon canariense (8.33) | Verbesina encelioides (7.54) Salsola kali (7.38) Aristida mutabilis (6.80) Tribulus macropterus (6.19) |
| O | 28 | 0.96 ab | 0.95 b | Tribulus macropterus (12.11) | Cynodon dactylon (7.00) | Cenchrus ciliaris (6.61) Aizoon canariense (5.60) Hyparrhenia hirta (4.67) Solanum incanum (4.76) | |
| WHT 2 | U | 17 | 0.93 de | 0.92 c | Aerva javanica (16.80) | Nicotiana glauca (10.35) | Eragrostis papposa (9.90) Dactyloctenium aegyptium (9.00) Aristida mutabilis (8.70) |
| O | 21 | 0.92 ef | 0.89 e | Aizoon canariense (18.78) | Forsskaolea tenacissima (10.85) | Aerva javanica (9.62) Lavandula pubescens (9.39) Dactyloctenium aegyptium (6.68) | |
| SHFA 1 | U | 33 | 0.91 fg | 0.86 g | Cynodon dactylon (26.74) | Conyza bonariensis (7.43) | Malva parviflora (4.96) Verbena officinalis (4.96) |
| O | 40 | 0.95 bc | 0.89 ef | Cynodon dactylon (17.67) | Chenopodium glaucum (7.31) | Chenopodium murale (5.34) Pulicaria arabica (5.27) Nicotiana glauca (4.22) | |
| SHFA 2 | U | 26 | 0.96 a | 0.95 b | Chenopodium murale (9.18) | Poa annua (7.65) | Cynodon dactylon (7.04) Plantago major (7.10) Euryops arabicus (6.28) Pulicaria undulata (6.24) |
| O | 36 | 0.97 a | 0.97 a | Cynodon dactylon (8.49) | Pulicaria undulata (5.16) | Poa annua (4.83) Pulicaria arabica (4.34) | |
| SHFA 3 | U | 19 | 0.90 g | 0.87 f | Aizoon canariense (19.36) | Cynodon dactylon (18.72) | Chenopodium murale (10.65) Salsola kali (6.99) Nicotiana glauca (6.29) |
| O | 20 | 0.92 ef | 0.90 d | Cynodon dactylon (19.99) | Eragrostis papposa (10.50) | Salsola kali (9.58) Echinops spinosus (7.76) Hyparrhenia hirta (7.30) | |
| RDF | U | 21 | 0.94 cd | 0.92 c | Aizoon canariense (15.58) | Heliotropium curassavicum (10.38) | Cynodon dactylon (9.71) Fagonia bruguieri (8.26) Frankenia pulverulenta (7.08) |
| O | 32 | 0.96 ab | 0.94 b | Aizoon canariense (8.73) | Cynodon dactylon (7.49) | Heliotropium curassavicum (7.10) Fagonia bruguieri (6.00) | |
| F value 0.05 | 22.26 *** | 65.54 *** | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assaeed, A.M.; Alharthi, A.S.; Abd-ElGawad, A.M. Impacts of Nicotiana glauca Graham Invasion on the Vegetation Composition and Soil: A Case Study of Taif, Western Saudi Arabia. Plants 2021, 10, 2587. https://doi.org/10.3390/plants10122587
Assaeed AM, Alharthi AS, Abd-ElGawad AM. Impacts of Nicotiana glauca Graham Invasion on the Vegetation Composition and Soil: A Case Study of Taif, Western Saudi Arabia. Plants. 2021; 10(12):2587. https://doi.org/10.3390/plants10122587
Chicago/Turabian StyleAssaeed, Abdulaziz M., Abdullah S. Alharthi, and Ahmed M. Abd-ElGawad. 2021. "Impacts of Nicotiana glauca Graham Invasion on the Vegetation Composition and Soil: A Case Study of Taif, Western Saudi Arabia" Plants 10, no. 12: 2587. https://doi.org/10.3390/plants10122587
APA StyleAssaeed, A. M., Alharthi, A. S., & Abd-ElGawad, A. M. (2021). Impacts of Nicotiana glauca Graham Invasion on the Vegetation Composition and Soil: A Case Study of Taif, Western Saudi Arabia. Plants, 10(12), 2587. https://doi.org/10.3390/plants10122587








