Ecological Impacts of Megaprojects: Species Succession and Functional Composition
Abstract
:1. Introduction
- Are plant communities able to regenerate naturally and return to their initial re-establishment trajectory state in terms of species occurrence and abundance after pipeline construction and are there changes in the occurrence and abundance of species classified as invasive?
- Are there any functional differences between the established vegetation and the original one before the megaproject?
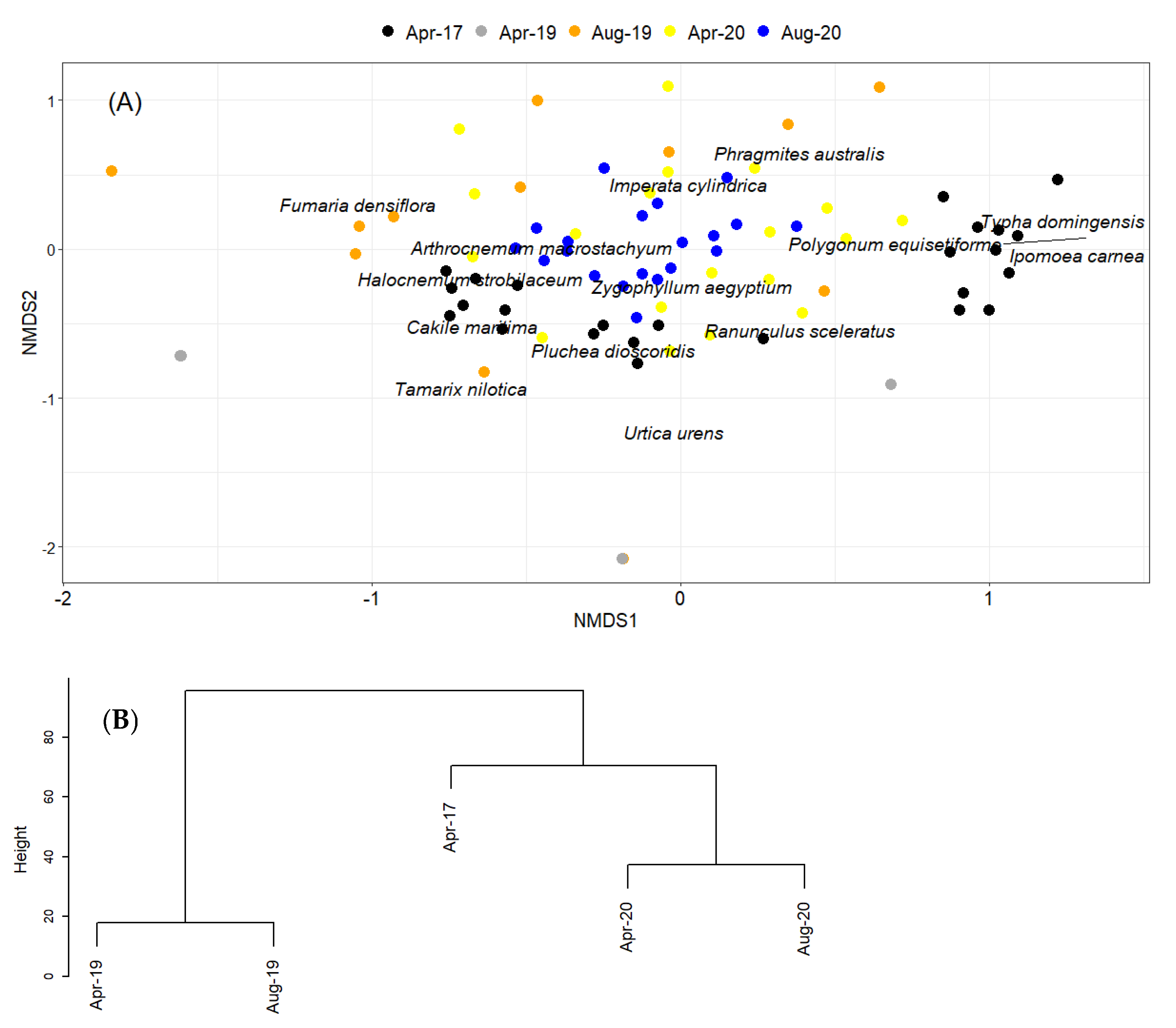
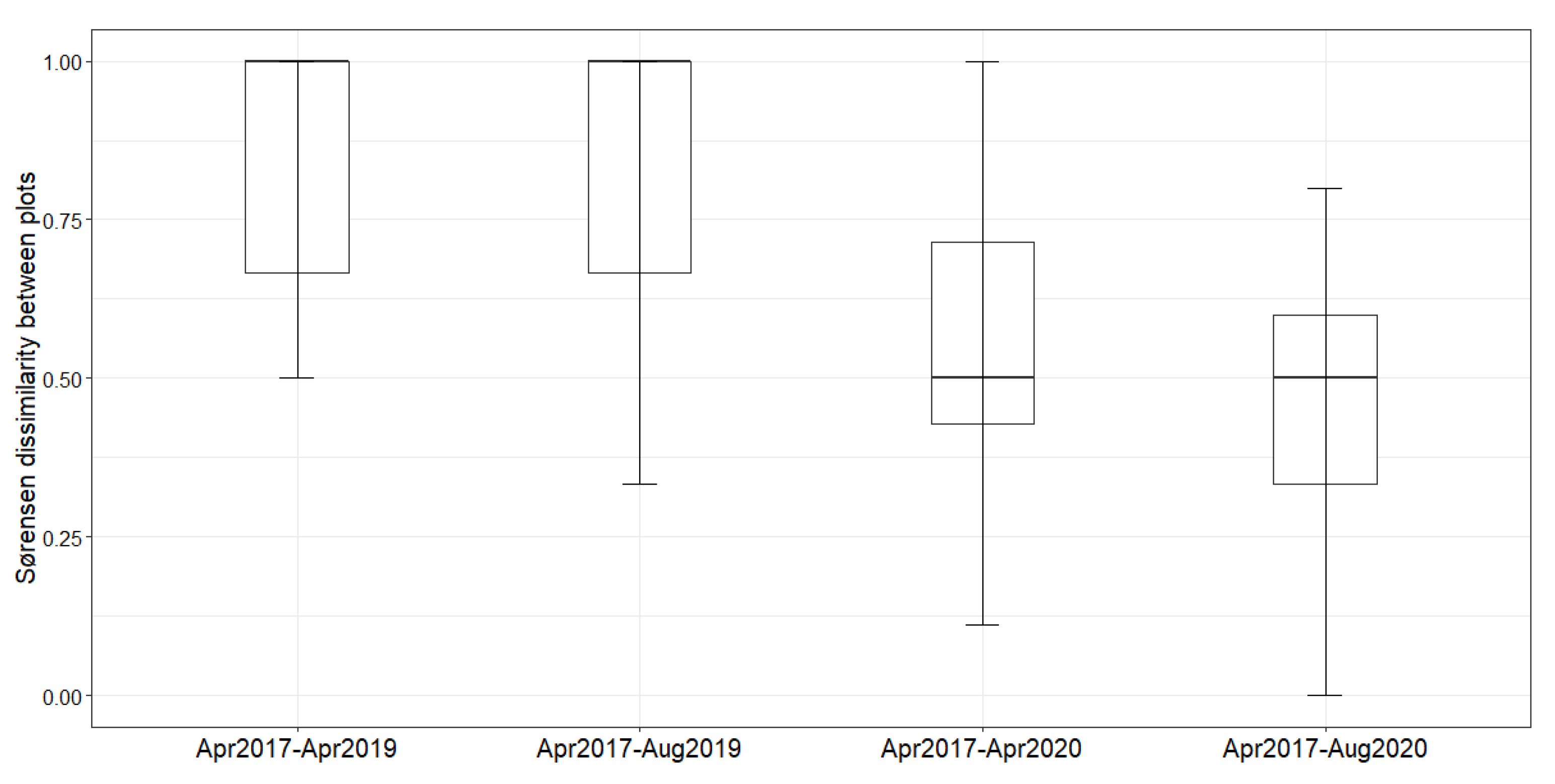
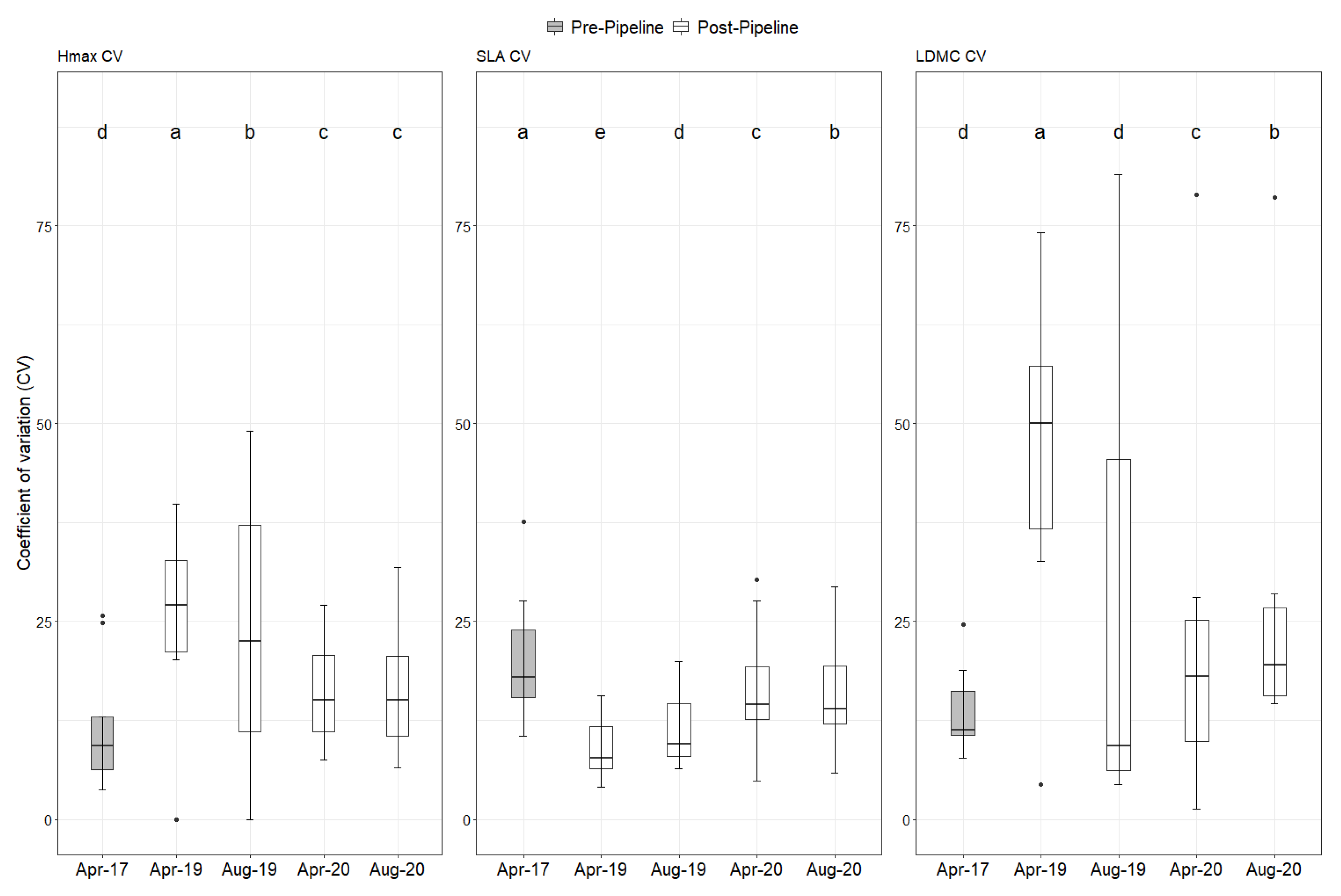
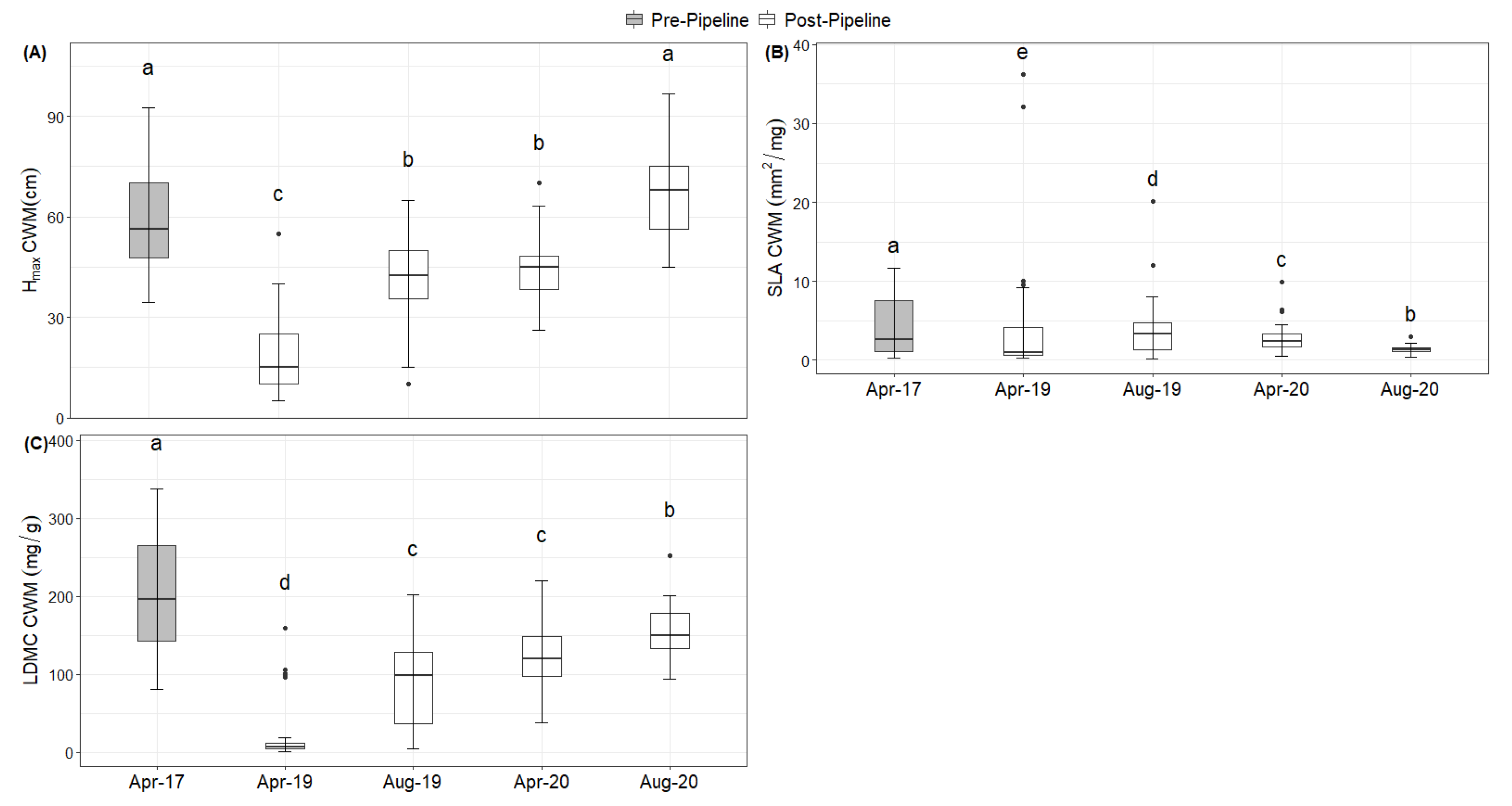
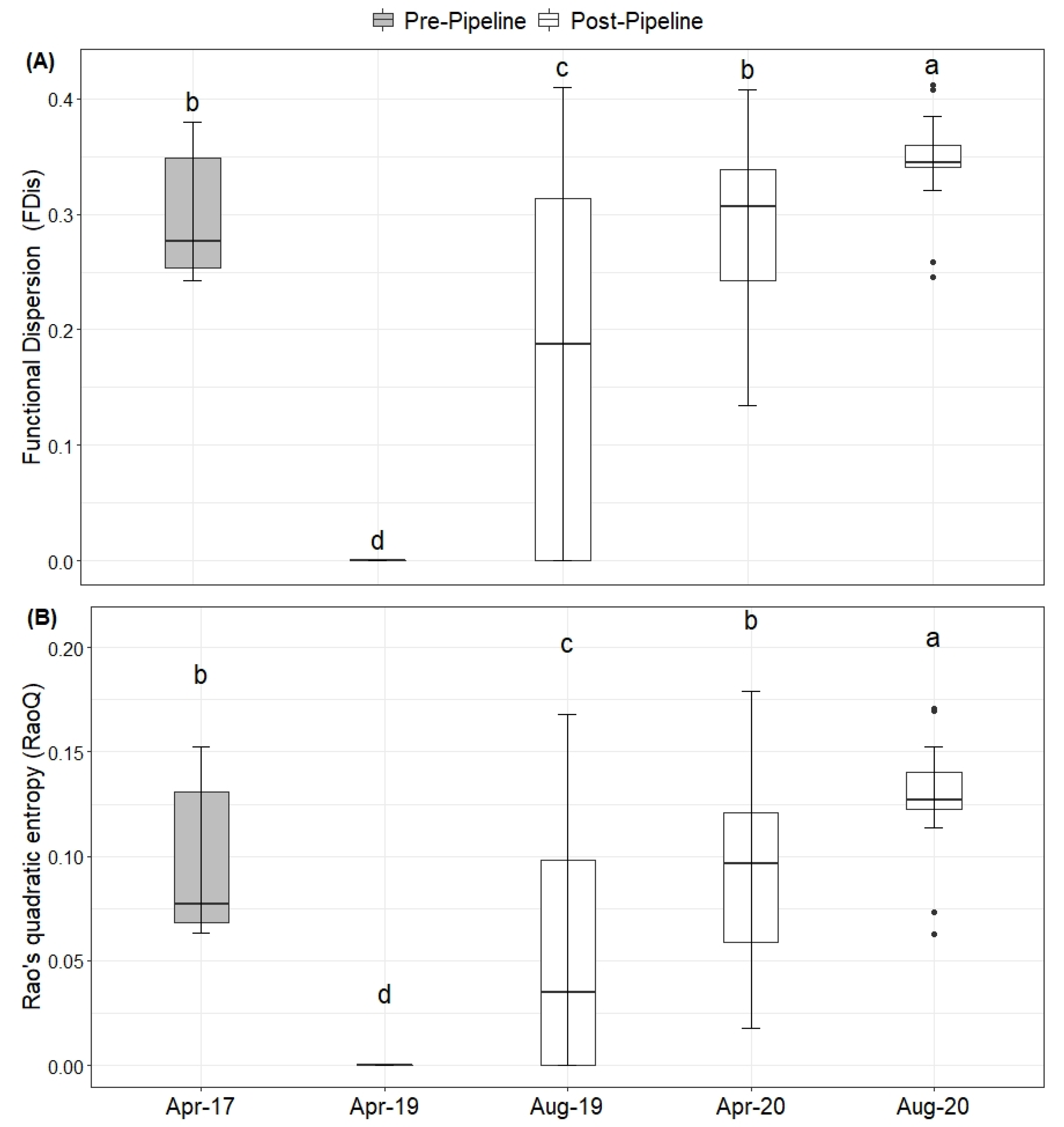
2. Results
Natural Regeneration after the Construction
3. Discussion
4. Materials and Methods
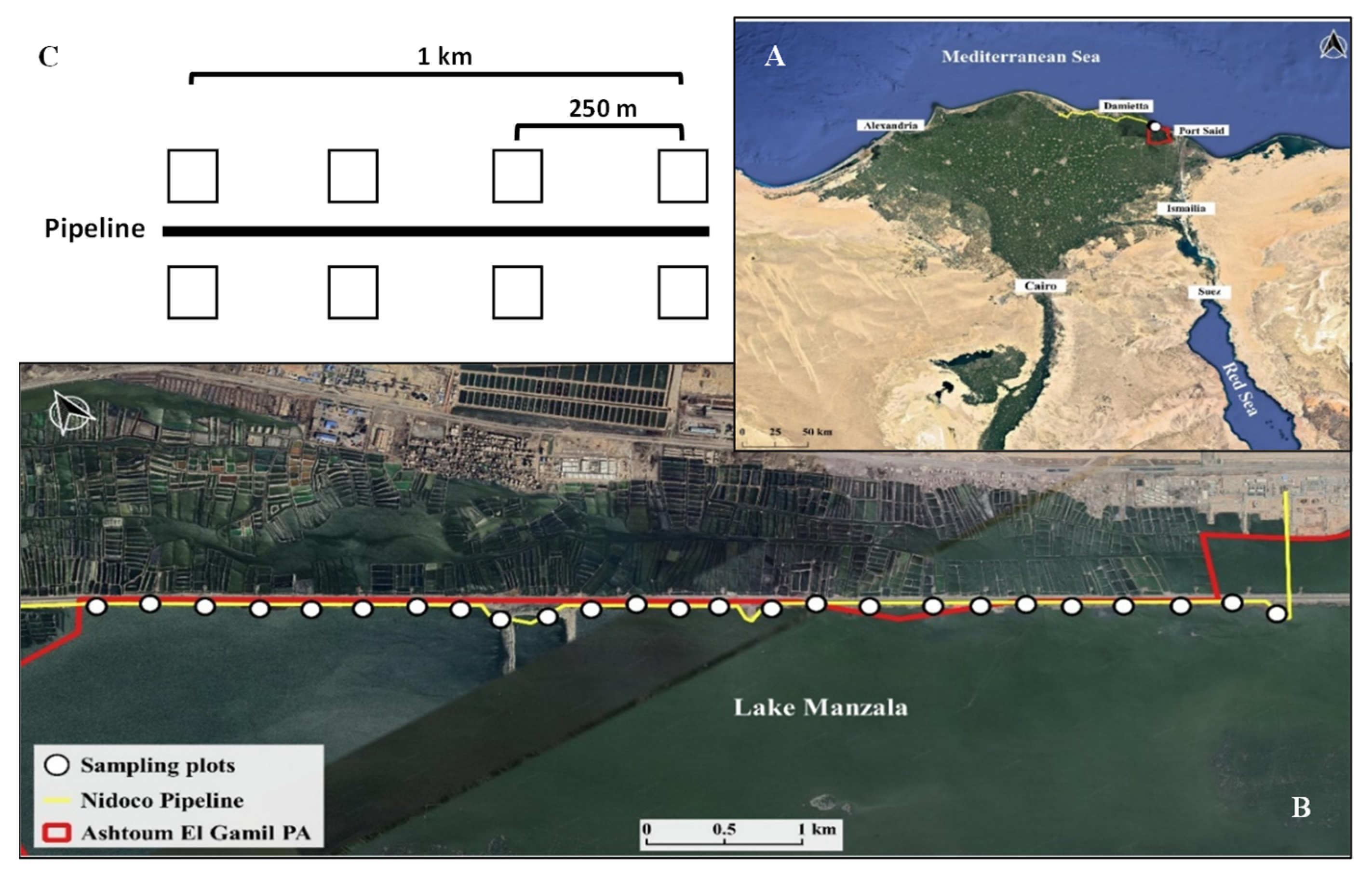
4.1. Site Description
4.2. Vegetation Surveys
4.3. Plant Functional Traits and Community Characteristics
4.4. Statistical Analyses
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fritch, R.A.; Sheridan, H.; Finn, J.; Kirwan, L.; Huallacháin, D.Ó. Methods of enhancing botanical diversity within field margins of intensively managed grassland: A 7-year field experiment. J. Appl. Ecol. 2011, 48, 551–560. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.; Borger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nat. Cell Biol. 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Kreft, H.; Weigelt, P.; Kartesz, J.; Nishino, M.; et al. Global Exchange and Accumulation of Non-Native Plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Berkes, F.; Davidson-Hunt, I.J. Biodiversity, Traditional Management Systems, and Cultural Landscapes: Examples from the Boreal Forest of Canada. Int. Soc. Sci. J. 2006, 58, 35–47. [Google Scholar] [CrossRef]
- Gao, H.; Ouyang, Z.; Chen, S.; Van Koppen, C.S.A. Role of culturally protected forests in biodiversity conservation in Southeast China. Biodivers. Conserv. 2013, 22, 531–544. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Naeem, S.; Duffy, J.E.; Zavaleta, E. The Functions of Biological Diversity in an Age of Extinction. Science 2012, 336, 1401–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, P.B.; Tilman, D.; Isbell, F.; Mueller, K.; Hobbie, S.E.; Flynn, D.F.; Eisenhauer, N. Impacts of Biodiversity Loss Escalate through Time as Redundancy Fades. Science 2012, 336, 589–592. [Google Scholar] [CrossRef] [Green Version]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of Changing Biodiversity. Nature 2000, 405, 234–242. [Google Scholar]
- Sala, O.E.; Chapin, F.S., 3rd; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Flyvbjerg, B. The Oxford Handbook of Megaproject Management; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Vilà, M.; Basnou, C.; Pyšek, P.; Josefsson, M.; Genovesi, P.; Gollasch, S.; Nentwig, W.; Olenin, S.; Roques, A.; Roy, D. How Well Do We Understand the Impacts of Alien Species on Ecosystem Services? A Pan-European, Cross-Taxa Assessment. Front. Ecol. Environ. 2010, 8, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Higgins, S.I.; Richardson, D. Invasive plants have broader physiological niches. Proc. Natl. Acad. Sci. USA 2014, 111, 10610–10614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappuccino, N.; Carpenter, D. Invasive exotic plants suffer less herbivory than non-invasive exotic plants. Biol. Lett. 2005, 1, 435–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carboni, M.; Münkemüller, T.; Gallien, L.; Lavergne, S.; Acosta, A.T.R.; Thuiller, W. Darwin’s naturalization hypothesis: Scale matters in coastal plant communities. Ecography 2013, 36, 560–568. [Google Scholar] [CrossRef]
- Perez- Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, L.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.; Craine, J.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- De Bello, F.; Lavorel, S.; Díaz, S.; Harrington, R.; Cornelissen, J.H.C.; Bardgett, R.D.; Berg, M.P.; Cipriotti, P.; Feld, C.K.; Hering, D.; et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Römermann, C.; Bernhardt-Römermann, M.; Kleyer, M.; Poschlod, P. Substitutes for grazing in semi-natural grasslands—Do mowing or mulching represent valuable alternatives to maintain vegetation structure? J. Veg. Sci. 2009, 20, 1086–1098. [Google Scholar] [CrossRef]
- Breitschwerdt, E.; Jandt, U.; Bruelheide, H. Do newcomers stick to the rules of the residents? Designing trait-based community assembly tests. J. Veg. Sci. 2014, 26, 219–232. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant Ecological Strategies: Some Leading Dimensions of Variation Between Species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef] [Green Version]
- Gaudet, C.L.; Keddy, P.A. A comparative approach to predicting competitive ability from plant traits. Nat. Cell Biol. 1988, 334, 242–243. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Garnier, E. Growth Analysis of Congeneric Annual and Perennial Grass Species. J. Ecol. 1992, 80, 665. [Google Scholar] [CrossRef]
- Poorter, H.; Remkes, C. Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 1990, 83, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Godoy, O.; Levine, J. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. USA 2015, 112, 797–802. [Google Scholar] [CrossRef] [Green Version]
- Diaz, S.; Cabido, M.; Casanoves, F. Plant functional traits and environmental filters at a regional scale. J. Veg. Sci. 1998, 9, 113–122. [Google Scholar] [CrossRef]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Venn, S.E.; Green, K.; Pickering, C.M.; Morgan, J.W. Using plant functional traits to explain community composition across a strong environmental filter in Australian alpine snowpatches. Plant Ecol. 2011, 212, 1491–1499. [Google Scholar] [CrossRef]
- Weiher, E.; Keddy, P.A. Assembly Rules, Null Models, and Trait Dispersion: New Questions from Old Patterns. Oikos 1995, 74, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Engst, K.; Baasch, A.; Erfmeier, A.; Jandt, U.; May, K.; Schmiede, R.; Bruelheide, H. Functional community ecology meets restoration ecology: Assessing the restoration success of alluvial floodplain meadows with functional traits. J. Appl. Ecol. 2016, 53, 751–764. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Joshi, C.; van Bodegom, P.; Bastow, Z.A.; Fulé, P.Z. A predictive model of community assembly that incorporates intraspecific trait variation. Ecol. Lett. 2012, 15, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E.J.P. The Ecology and Management of Field Margin Floras in England. Outlook Agric. 1988, 17, 178–182. [Google Scholar] [CrossRef]
- Moonen, A.C.; Marshall, E. The influence of sown margin strips, management and boundary structure on herbaceous field margin vegetation in two neighbouring farms in southern England. Agric. Ecosyst. Environ. 2001, 86, 187–202. [Google Scholar] [CrossRef]
- Ma, M.; Hietala, R.; Kuussaari, M.; Helenius, J. Impacts of edge density of field patches on plant species richness and community turnover among margin habitats in agricultural landscapes. Ecol. Indic. 2013, 31, 25–34. [Google Scholar] [CrossRef]
- Peres, G.; Cluzeau, D.; Menasseri-Aubry, S.; Soussana, J.-F.; Bessler, H.; Engels, C.; Habekost, M.; Gleixner, G.; Weigelt, A.; Weisser, W.; et al. Mechanisms linking plant community properties to soil aggregate stability in an experimental grassland plant diversity gradient. Plant Soil 2013, 373, 285–299. [Google Scholar] [CrossRef]
- Pohl, M.; Alig, D.; Körner, C.; Rixen, C. Higher plant diversity enhances soil stability in disturbed alpine ecosystems. Plant Soil 2009, 324, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.E.; Reineking, B.; Münkemüller, T. Effects of plant functional traits on soil stability: Intraspecific variability matters. Plant Soil 2016, 411, 359–375. [Google Scholar] [CrossRef]
- Aronson, M.; Galatowitsch, S. Long-term vegetation development of restored prairie pothole wetlands. Wetlands 2008, 28, 883–895. [Google Scholar] [CrossRef]
- Macdonald, G.E. Cogongrass (Imperata cylindrica)—Biology, Ecology, and Management. Crit. Rev. Plant Sci. 2004, 23, 367–380. [Google Scholar] [CrossRef]
- Shaltout, K.; Galal, T.M.; El-Komi, T.M. Phenology, biomass and nutrients of Imperata cylindrica and Desmostachya bipinnata along the water courses in Nile Delta, Egypt. Rend. Lincei 2016, 27, 215–228. [Google Scholar] [CrossRef]
- Mullié, W.C.; Meininger, P.L. Waterbird trapping and hunting in Lake Manzala, Egypt, with an outline of its economic significance. Biol. Conserv. 1983, 27, 23–43. [Google Scholar] [CrossRef]
- Bird Life International. Country Profile: Egypt. Available online: http://www.birdlife.org/datazone/country/egypt (accessed on 17 December 2020).
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967; pp. 1–205. [Google Scholar]
- Helm, J.; Dutoit, T.; Saatkamp, A.; Bucher, S.F.; Leiterer, M.; Römermann, C. Recovery of Mediterranean steppe vegetation after cultivation: Legacy effects on plant composition, soil properties and functional traits. Appl. Veg. Sci. 2019, 22, 71–84. [Google Scholar] [CrossRef]
- Wilson, J.B. Trait-Divergence Assembly Rules Have Been Demonstrated: Limiting Similarity Lives! A Reply to Grime. J. Veg. Sci. 2007, 18, 451–452. [Google Scholar] [CrossRef]
- Römermann, C.; Bucher, S.F.; Hahn, M.; Bernhardt-Römermann, M. Plant functional traits—Fixed facts or variable depending on the season? Folia Geobot. 2016, 51, 143–159. [Google Scholar] [CrossRef]
- Rosbakh, S.; Römermann, C.; Poschlod, P. Specific leaf area correlates with temperature: New evidence of trait variation at the population, species and community levels. Alp. Bot. 2015, 125, 79–86. [Google Scholar] [CrossRef]
- Albert, C.H.; Grassein, F.; Schurr, F.M.; Vieilledent, G.; Violle, C. When and how should intraspecific variability be considered in trait-based plant ecology? Perspect. Plant Ecol. Evol. Syst. 2011, 13, 217–225. [Google Scholar] [CrossRef]
- Jung, V.; Violle, C.; Mondy, C.; Hoffmann, L.; Muller, S.D. Intraspecific variability and trait-based community assembly. J. Ecol. 2010, 98, 1134–1140. [Google Scholar] [CrossRef]
- Ahmed, M.H.; El Leithy, B.M.; Thompson, J.R.; Flower, R.J.; Ramdani, M.; Ayache, F.; Hassan, S.M. Application of remote sensing to site characterisation and environmental change analysis of North African coastal lagoons. Hydrobiologia 2009, 622, 147–171. [Google Scholar] [CrossRef]
- Elnaggar, A.A.; El-Alfy, M.A. Physiochemical Properties of Water and Sediments in Manzala Lake, Egypt. J. Environ. Sci. 2016, 45, 157–174. [Google Scholar]
- El Kafrawy, S.B.; Ahmed, M.H. Monitoring and Protection of Egyptian Northern Lakes Using Remote Sensing Technology. In Environmental Remote Sensing in Egypt; Elbeih, S.F., Negm, A.M., Kostianoy, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 231–284. [Google Scholar]
- Zahran, M.A.; Willis, A.J. The Vegetation of Egypt; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; Volume 2. [Google Scholar]
- Baselga, A.; Orme, C.D.L. Betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.; Garden, D.; Dorrough, J.; Berman, S.; Quétier, F.; Thébault, A.; Bonis, A. Assessing functional diversity in the field—Methodology matters! Funct. Ecol. 2007, 22, 134–147. [Google Scholar] [CrossRef]
- Garnier, E.; Lavorel, S.; Ansquer, P.; Castro, H.; Cruz, P.; Dolezal, J.; Eriksson, O.; Fortunel, C.; Freitas, H.; Golodets, C.; et al. Assessing the Effects of Land-Use Change on Plant Traits, Communities and Ecosystem Functioning in Grasslands: A Standardized Methodology and Lessons from an Application to 11 European Sites. Ann. Bot. 2007, 99, 967–985. [Google Scholar] [CrossRef] [Green Version]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Rao, C. Diversity and dissimilarity coefficients: A unified approach. Theor. Popul. Biol. 1982, 21, 24–43. [Google Scholar] [CrossRef]
- Leps, J.; de Bello, F.; Lavorel, S.; Berman, S. Quantifying and Interpreting Functional Diversity of Natural Communities: Practical Considerations Matter. Preslia 2006, 78, 481–501. [Google Scholar]
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Crawley, M.J. The R Book; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- R: A Language and Environment for Statistical Computing; Version 4.1.1; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- Vegan: Community Ecology Package R Package, Version 2.5–7. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 September 2021).
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0–12. 2014. Available online: https://www.researchgate.net/publication/312463190_FD_Measuring_functional_diversity_from_multiple_traits_and_other_tools_for_functional_ecology (accessed on 15 September 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, H.E.; Bucher, S.F. Ecological Impacts of Megaprojects: Species Succession and Functional Composition. Plants 2021, 10, 2411. https://doi.org/10.3390/plants10112411
Ali HE, Bucher SF. Ecological Impacts of Megaprojects: Species Succession and Functional Composition. Plants. 2021; 10(11):2411. https://doi.org/10.3390/plants10112411
Chicago/Turabian StyleAli, Hamada E., and Solveig Franziska Bucher. 2021. "Ecological Impacts of Megaprojects: Species Succession and Functional Composition" Plants 10, no. 11: 2411. https://doi.org/10.3390/plants10112411
APA StyleAli, H. E., & Bucher, S. F. (2021). Ecological Impacts of Megaprojects: Species Succession and Functional Composition. Plants, 10(11), 2411. https://doi.org/10.3390/plants10112411






