Changes in Berry Tissues in Monastrell Grapevines Grafted on Different Rootstocks and Their Relationship with Berry and Wine Phenolic Content
Abstract
1. Introduction
- (1)
- To analyze the changes in the accumulation of secondary metabolites (polyphenols) in the different berry tissues (skins and seeds) due to the rootstock, irrigation method, and their interaction.
- (2)
- To study the anatomical/morphological changes in skins and seeds due to the rootstock and irrigation method.
- (3)
- To study the concentration/dilution effects of changes in the berry size/weight and the skin/pulp/berry ratio, and their influence on the final must and wine polyphenolics concentrations.
- (4)
- To look for significant relationships between berry tissue morphological, anatomical, and metabolomic changes and the final must and wine composition.
2. Materials and Methods
2.1. Field Conditions, Plant Materials, and Irrigation Treatments
2.2. Physicochemical Determinations in Grapes
2.3. Determination of Sugars in Grapes
2.4. Determination of the Total Phenolic Compounds and Total Anthocyanins in the Skin and Seeds
2.5. Histological Analysis of the Grapes
2.6. Tasting and Sensory Analysis of Grapes
2.7. Microvinifications and Quality Attributes of Wine
2.8. Statistical Analysis
3. Results
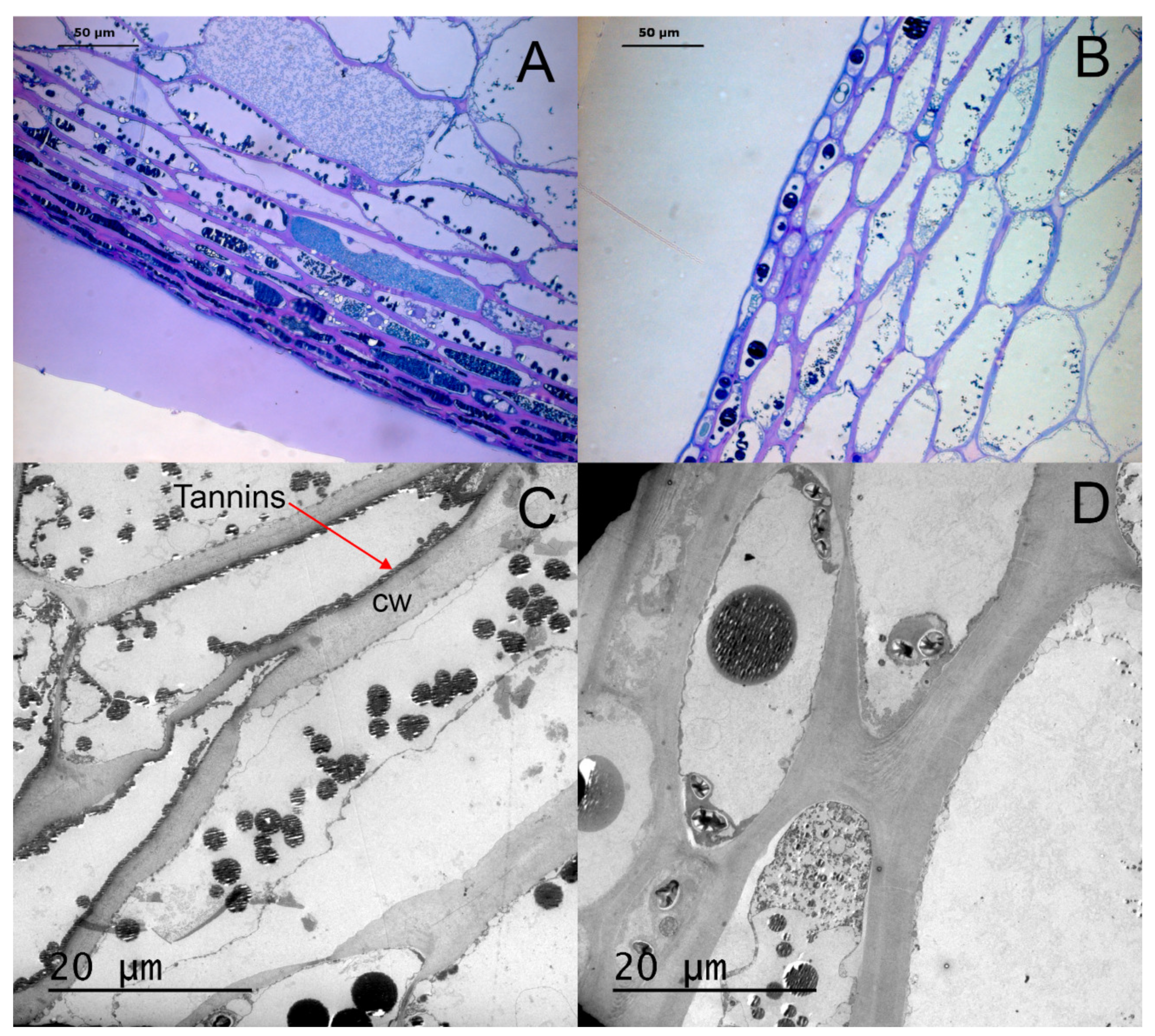
3.1. Histological Analysis of Berry Skin
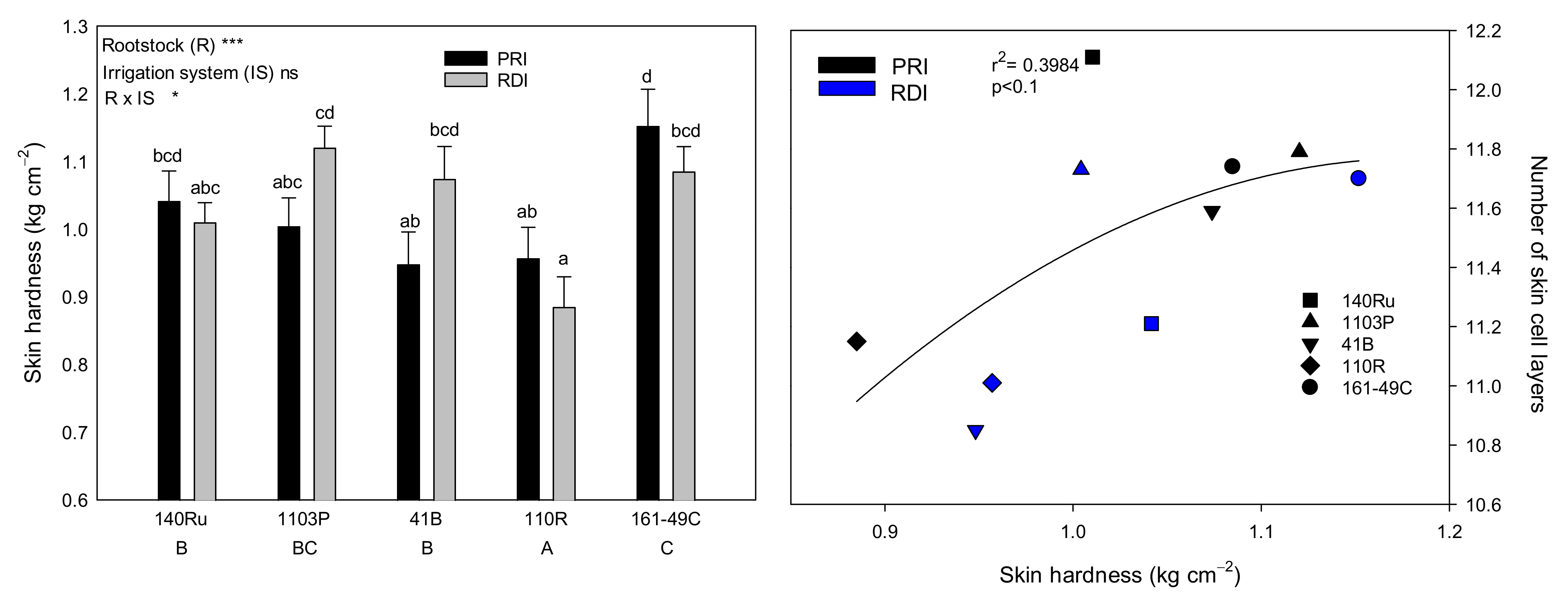
3.2. Grape Morphological Characteristics and Texture Analysis
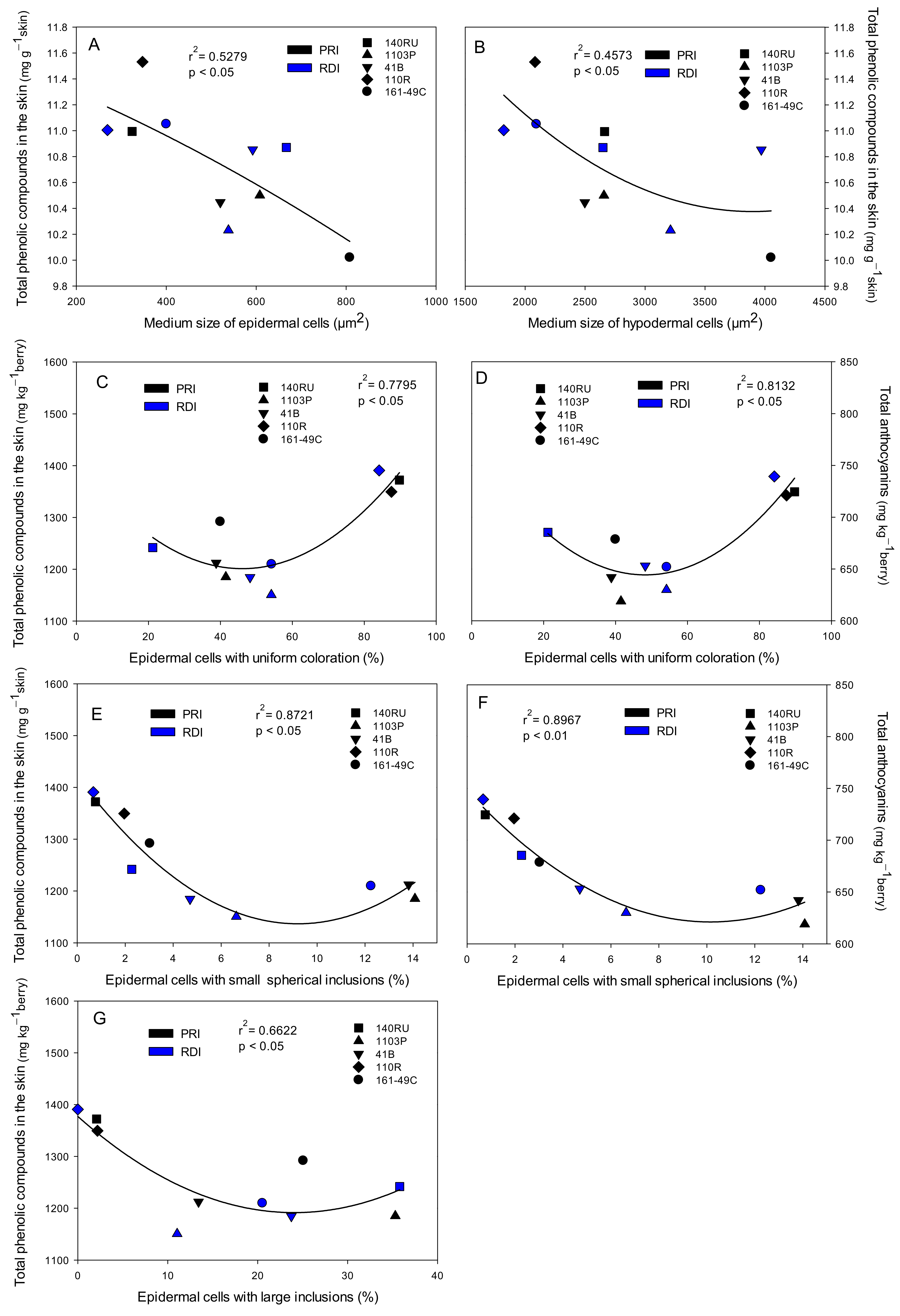
3.3. Total Phenolic Compounds and Total Anthocyanins in Seeds, Skin, and Whole Berries
3.4. Carbohydrate Concentration in Grapes and Grape Sensorial Analysis
3.5. Must Color
3.6. Skin Characteristics and Grape, Must, and Wine Quality Relationships
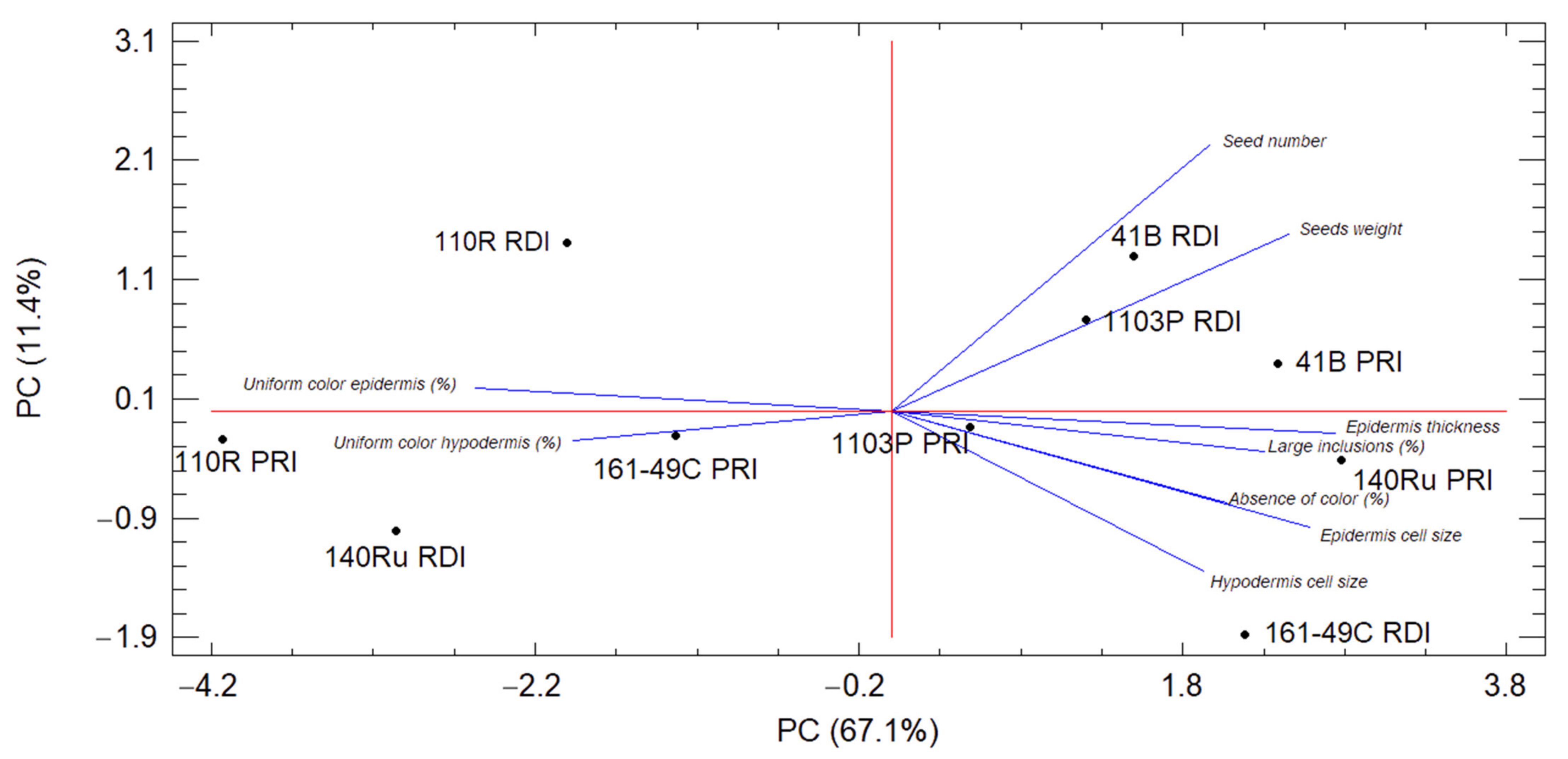
3.7. Principal Component Analysis of the Berry Characteristics
4. Discussion
4.1. Rootstock Effect
4.1.1. Cuticle and Cell Wall Thickness
4.1.2. Skin Thickness and Skin Cell Size
4.1.3. Typology of Cells in the Skin
4.2. Effects of the Irrigation Method and Its Interaction with the Rootstock
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Focus OIV. Distribution of the World’s Grapevine Varieties; International Organisation of Vine and Wine: Paris, France, 2017; p. 54. Available online: https://www.oiv.int (accessed on 17 August 2021).
- Romero, P.; Fernández-Fernández, J.I.; Martinez-Cutillas, A. Physiological thresholds for efficient regulated deficit-irrigation management in wine grapes grown under semiarid conditions. Am. J. Enol. Vitic. 2010, 61, 300–312. [Google Scholar]
- Romero, P.; Gil-Muñoz, R.; del Amor, F.; Valdés, E.; Fernández-Fernández, J.I.; Martínez-Cutillas, A. Regulated deficit irrigation based upon optimum water status improves phenolic composition in Monastrell grapes and wines. Agric. Water Manag. 2013, 121, 85–101. [Google Scholar] [CrossRef]
- Guiot, J.; Cramer, W. Climate change: The 2015 Paris agreement thresholds and Mediterranean basin ecosystems. Science 2016, 354, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Fraga, H.; Malheiro, A.C.; Mountinho-Pereira, J.; Santos, J.A. An overview of climate change impacts on European viticulture. Food Energy Secur. 2013, 1, 94–110. [Google Scholar] [CrossRef]
- Iglesias, A.; Garrote, L. Adaptation strategies for agricultural water management under climate change in Europe. Agric. Water Manag. 2015, 155, 113–124. [Google Scholar] [CrossRef]
- Resco, P.; Iglesias, A.; Bardají, I.; Sotés, V. Exploring adaptation choices for grapevine regions in Spain. Reg. Environ. Chang. 2016, 16, 979–993. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; García de Cortazar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.A.; Lacombe, T. From Pinotto Xinomavro in the world’s future wine-growing regions. Nat. Clim. Chang. 2018, 8, 29–37. [Google Scholar] [CrossRef]
- Alsina, M.M.; Smart, D.R.; Bauerle, T.; de Herralde, F.; Biel, C.; Stockert, C.; Negron, C.; Save, R. Seasonal changes of whole root system conductance by a drought-tolerant grape root system. J. Exp. Bot. 2011, 62, 99–109. [Google Scholar] [CrossRef]
- Jones, H.G. How do rootstocks control shoot water relations? New Phytol. 2012, 194, 301–303. [Google Scholar] [CrossRef]
- Bianchi, D.; Caramanico, L.; Grossi, D.; Brancadoro, L.; Lorenzis, G.D. How do novel M-Rootstock (Vitis spp.) genotypes cope with drought? Plants 2020, 9, 1385. [Google Scholar] [CrossRef]
- Berdeja, M.; Hilbert, G.; Dai, Z.; Lafontaine, M.; Stoll, M.; Schultz, H.; Delrot, S. Rootstock and water stress affect berry quality. Aust. J. Grape Wine Res. 2014, 20, 409–421. [Google Scholar] [CrossRef]
- Habran, A.; Commisso, M.; Helwi, P.; Hilbert, G.; Negri, S.; Ollat, N.; Gomes, E.; van Leeuwen, C.; Guzzo, F.; Delrot, S. Rootstock/scion/nitrogen interactions affect secondary metabolism in the grape berry. Front. Plant Sci. 2016, 7, 1134. [Google Scholar] [CrossRef]
- Stevens, R.M.; Pech, J.M.; Taylor, J.; Clingeleffer, P.; Walker, R.R.; Nicholas, P.R. Effects of irrigation and rootstock on Vitis vinifera (L.) cv. Shiraz berry composition and shrivel, and wine composition and wine score. Aust. J. Grape Wine Res. 2016, 22, 124–136. [Google Scholar] [CrossRef]
- Romero, P.; Botía, P.; Navarro, J.M. Selecting rootstocks to improve vine performance and vineyard sustainability in deficit irrigated Monastrell grapevines under semiarid conditions. Agric. Water Manage. 2018, 209, 73–93. [Google Scholar] [CrossRef]
- Romero, P.; Botía, P.; del Amor, F.; Gil-Muñoz, R.; Flores, P.; Navarro, J.M. Interactive effects of the rootstock and the deficit irrigation technique on wine composition, nutraceutical potential, aromatic profile, and sensory attributes under semiarid and water limiting conditions. Agric. Water Manag. 2019, 225, 105733. [Google Scholar] [CrossRef]
- Grimplet, J.; Wheatley, M.D.; Jouira, H.B.; Deluc, L.G.; Cramer, G.R.; Cushman, J.C. Proteomic and selected metabolite analysis of grape berry tissues under well watered and water-deficit stress conditions. Proteomics 2009, 9, 2503–2528. [Google Scholar] [CrossRef]
- Deluc, L.G.; Qulici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficits alters differentially metabolic pathways affecting important flavour and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. A first approach towards the relationship between grape skin cell-wall composition and anthocyanin extractability. Anal. Chim. Acta 2006, 563, 26–32. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortin, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Changes in skin cell wall composition during the maturation of four premium wine grape varieties. J. Sci. Food Agric. 2008, 88, 420–428. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gil-Muñoz, R. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef]
- Battista, F.; Tomas, D.; Porro, D.; Caicci, F.; Giacosa, S.; Rolle, L. Winegrape berry skin thickness determination comparison between histological observation and texture analysis determination. Ital. J. Food Sci. 2015, 27, 136–141. [Google Scholar]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M. Cell wall compounds of red grapes skins and their grape marcs from three different winemaking techniques. Food Chem. 2015, 187, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M. The composition of cell walls from grape marcs is affected by grape origin and enological technique. Food Chem. 2015, 167, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cascales, I.; Ortega-Regules, A.; Lopez-Roca, J.; Fernandez-Fernandez, J.; Gomez-Plaza, E. Differences in anthocyanins extractability from grapes to wines according to variety. Am. J. Enol. Vitic. 2005, 56, 212–219. [Google Scholar]
- Cheynier, V.; Fulcrand, H.; Brossaud, F.; Asselin, C.; Moutounet, M. Phenolic composition as related to red wine flavour. In Chemistry of Wine Flavour; Waterhouse, A.L., Ebeler, S.E., Eds.; American Chemistry Society: Washington, DC, USA, 1998; pp. 124–141. [Google Scholar]
- Ayuso, T.; Urbano, M.L.; Valdés, E.; Uriarte, D.; Moreno, D.; Giraldo, E.; Alarcón, M.V. Influencia del riego en las células tánicas del hollejo de Vitis vinifera L. cv Tempranillo. In VII Congreso Ibérico de Agroingeniería y Ciencias Hortícolas; Ayuga, F., Masaguer, A., Mariscal, I., Villarroel, M., Ruiz-Altisent, M., Riquelme, F., Correa, E.V., Eds.; Fundación General de la Universidad Politécnica de Madrid: Madrid, Spain, 2013; pp. 139–144. [Google Scholar]
- Romero, P.; Fernández-Fernández, J.I.; Botía, P. Interannual climatic variability effects on yield, berry and wine quality indices in long-term deficit irrigated grapevines, determined by multivariate analysis. Int. J. Wine Res. 2016, 8, 3–17. [Google Scholar] [CrossRef][Green Version]
- Peppi, M.C.; Fidelibus, M.W.; Dokoozlian, N. Abscisic acid application timing and concentration affect firmness, pigmentation and color of ‘Flame Seedless’ grapes. HortScience 2006, 41, 1440–1445. [Google Scholar] [CrossRef]
- Navarro, J.M.; Morte, A. Mycorrhizal effectiveness in Citrus macrophylla at low phosphorus fertilization. J. Plant Physiol. 2018, 232, 201–210. [Google Scholar] [CrossRef]
- Rustioni, L.; Magradze, D.; Popescu, C.F.; Cola, G.; Abashidze, E.; Aroutiounian, R.; Brazao, J.; Coletti, S.; Cornea, V.; Dejeu, L.; et al. First results of the European grapevine collections collaborative network: Validation of a standard eno-carpological phenotyping method. Vitis 2014, 53, 219–226. [Google Scholar]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M. and Gómez-Plaza, E. Differences in morphology and composition of skin and pulp cell walls from grapes (Vitis vinifera L.): Technological implications. Eur. Food Res. Technol. 2008, 227, 223–231. [Google Scholar] [CrossRef]
- Hardie, W.; O’Brien, T.; Jaudzems, V. Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust. J. Grape Wine Res. 1996, 2, 97–142. [Google Scholar] [CrossRef]
- Chevalier, M.; Perrochon, E.; Clement, A.; Dubot, A.L.; Tellier, M.; Cadot, Y.; Barbeau, G. Utilisation de l’analyse d’image por l’étude des composes phénoliques de la pellicule de baie de raisin (Vitis vinifera L., cv. Cabernet franc). J. Int. Sci. Vigne Vin 2003, 37, 61–65. [Google Scholar]
- Cadot, Y.; Miñana Castelló, M.T.; Chevalier, M. Flavan-3-ol compositional changes in grape berries (Vitis vinifera L. cv Cabernet Franc) before veraison, using two complementary analytical approaches, HPLC reversed phase and histochemistry. Anal. Chim. Acta 2006, 563, 65–75. [Google Scholar] [CrossRef]
- Cadot, Y.; Chevalier, M.; Barbeau, G. Evolution of the localisation and composition of phenolics in grape skin between veraison and maturity in relation to water availability and some climatic conditions. J. Sci. Food Agric. 2011, 91, 1963–1976. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.D.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; DiGaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grape vine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdied, D. Handbook of Enology Vol 2: The Chemistry of Wine and Stabilization and Treatments; John Wiley and Sons, Ltd.: Chichester, UK, 2006; p. 405. [Google Scholar]
- Koundouras, S.; Hatzidimitriou, E.; Karamolegkou, M.; Dimopoulou, E.; Kallithraka, S.; Tsialtas, J.T.; Zioziou, E.; Nikolaou, N.; Kotseridis, Y. Irrigation and rootstock effects on the phenolic concentration and aroma potential of Vitis vinifera L. cv. Cabernet Sauvignon grapes. J. Agric. Food Chem. 2009, 57, 7805–7813. [Google Scholar] [CrossRef]
- Amrani Joutei, K.; Glories, Y. Tannins and anthocyanins of grape berries: Localization and extraction technique. Rev. Française D’oenologie. 1995, 153, 28–31. [Google Scholar]
- Bautista-Ortín, A.B.; Lopez-Roca, J.M.; Fernandez-Fernandez, E.; Gómez-Plaza, J.I. Pigments in Food. More than Colors; Dufosse, L., Ed.; Universite de Bretagne Occidentale: Quimper, France, 2004; p. 300. [Google Scholar]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, L.; Fan, X.; Zheng, T.; Dong, T.; Liu, C.; Fang, J. Anatomical characteristics associated with different degrees of berry cracking in grapes. Sci. Hortic. 2020, 261, 108992. [Google Scholar] [CrossRef]
- Río Segade, S.; Giacosa, S.; Gerbi, V.; Rolle, L. Berry skin thickness as main texture parameter to predict anthocyanin extractability in winegrapes. LWT-Food Sci. Technol. 2011, 44, 392–398. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Relationship between skin cell wall composition and anthocyanin extractability of Vitis vinifera L. cv. Tempranillo at different grape ripeness degree. Food Chem. 2014, 146, 41–47. [Google Scholar] [CrossRef] [PubMed]
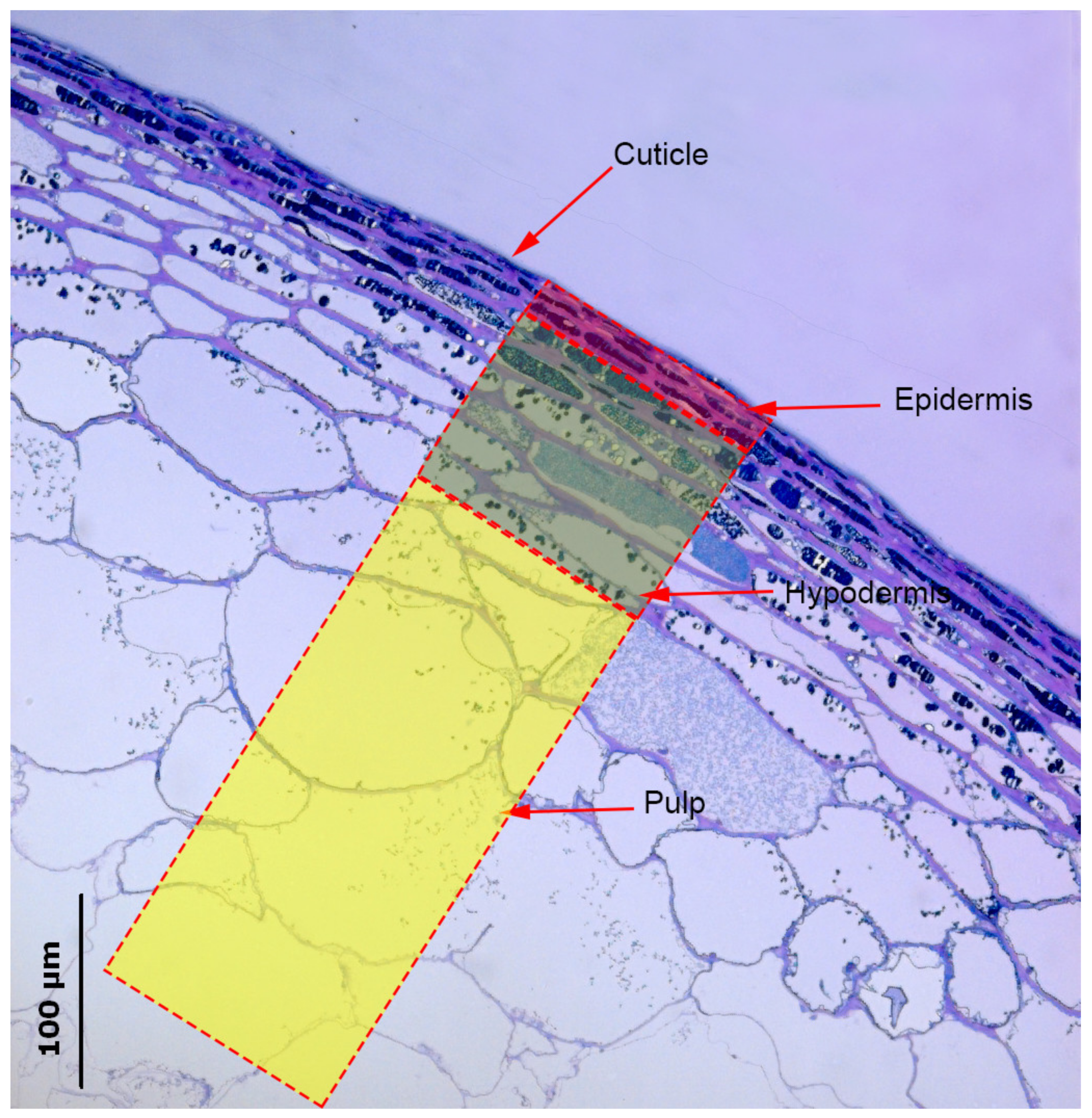



| Rootstock (R) | Cuticle Thickness (µm) | Cell Wall Thickness (µm) | Thickness (µm) | Cell Size (µm2) | Epidermis Cell Size/Cell Wall Thickness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole Skin | Epidermis | Hypodermis | Whole Skin | Epidermis | Hypodermis | Whole Skin | Epidermis | Hypodermis | Hypodermis/Epidermis | Pulp | Pulp/Skin | ||||
| 140Ru | 2.50 b | 2.80 | 2.08 | 3.11 ab | 156 | 17.7 b | 138 | 2008 | 496 ab | 2656 ab | 6.2 | 17,064 | 9.9 | 237 b | |
| 1103P | 2.31 a | 2.87 | 2.16 | 3.18 ab | 163 | 17.9 b | 145 | 2226 | 573 b | 2935 ab | 5.3 | 24,479 | 11.7 | 271 b | |
| 41B | 2.18 a | 2.69 | 2.33 | 2.85 a | 162 | 19.6 b | 142 | 2431 | 556 b | 3234 b | 5.8 | 25,193 | 10.9 | 256 b | |
| 110R | 2.13 a | 2.78 | 2.14 | 3.06 a | 134 | 13.0 a | 121 | 1459 | 308 a | 1952 a | 6.9 | 22,462 | 16.5 | 150 a | |
| 161-49C | 2.27 a | 3.07 | 2.24 | 3.44 b | 195 | 18.4 b | 177 | 2283 | 604 b | 3073 b | 5.1 | 17,606 | 11.3 | 245 b | |
| Irrigation strategy (IS) | |||||||||||||||
| PRI | 2.39 | 2.73 | 2.12 | 2.99 | 165 | 17.4 | 147 | 2053 | 493 | 2750 | 5.8 | 20,199 | 11.6 | 240 | |
| RDI | 2.17 | 2.96 | 2.26 | 3.27 | 159 | 17.2 | 142 | 2110 | 521 | 2790 | 5.9 | 22,524 | 12.6 | 223 | |
| R× IS | |||||||||||||||
| 140Ru | PRI | 2.50 cd | 2.67 | 2.05 | 2.93 | 159 | 21.0 c | 138 | 2054 ab | 667 bc | 2649 ab | 4.3 | 12,590 | 7.1 | 335 c |
| RDI | 2.50 cd | 2.93 | 2.10 | 3.29 | 152 | 14.5 ab | 138 | 1961 ab | 324 ab | 2663 ab | 8.1 | 21,538 | 12.7 | 140 a | |
| 1103P | PRI | 2.39 bcd | 2.70 | 2.24 | 2.90 | 187 | 17.0 bc | 170 | 2410 ab | 538 abc | 3212 ab | 6.0 | 21,836 | 9.9 | 254 abc |
| RDI | 2.23 abc | 3.05 | 2.08 | 3.46 | 139 | 18.8 c | 121 | 2042 ab | 608 abc | 2657 ab | 4.5 | 27,123 | 13.5 | 288 bc | |
| 41B | PRI | 2.35 bcd | 2.78 | 2.15 | 3.06 | 189 | 20.4 c | 168 | 2957 b | 592 abc | 3970 b | 6.8 | 25,912 | 9.0 | 288 bc |
| RDI | 2.01 a | 2.60 | 2.51 | 2.64 | 135 | 18.7 bc | 116 | 1905 ab | 520 abc | 2498 ab | 4.8 | 24,475 | 12.9 | 225 abc | |
| 110R | PRI | 2.12 a | 2.59 | 1.93 | 2.88 | 124 | 11.6 a | 112 | 1356 a | 269 a | 1822 a | 6.8 | 21,343 | 16.0 | 149 a |
| RDI | 2.14 ab | 2.98 | 2.34 | 3.25 | 145 | 14.5 ab | 131 | 1562 a | 347 ab | 2083 a | 7.1 | 23,581 | 17.0 | 150 a | |
| 161-49C | PRI | 2.60 d | 2.88 | 2.23 | 3.19 | 167 | 17.1 bc | 150 | 1488 a | 400 ab | 2094 a | 5.3 | 19,312 | 15.8 | 177 ab |
| RDI | 1.95 a | 3.25 | 2.24 | 3.70 | 224 | 19.8 c | 204 | 3078 b | 808 c | 4051 b | 4.9 | 15,900 | 6.8 | 314 c | |
| ANOVA | |||||||||||||||
| R | *** | ns | ns | * | ns | ** | ns | ns | *⊥ | *⊥ | ns | ns | ns | * | |
| IS | *** | * | ns | * | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| R × IS | ** | ns | ns | ns | ns | * | ns | * | * | * | ns | ns | ns | ** | |
| Rootstock (R) | Epidermis (1–3 Layers) | Hypodermis (4–10 Layers) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Absence of Coloration (%) | Uniform Coloration (%) | Fine Granulation (%) | Small Spherical Inclusions (%) | Large Inclusions (%) | Absence of Coloration (%) | Uniform Coloration (%) | Fine Granulation (%) | Small Spherical Inclusions (%) | Large Inclusions (%) | ||
| 140Ru | 23.2 | 55.5 a | 0.9 | 1.5 | 18.9 b | 39.8 | 3.7 a | 7.0 | 41.0 | 8.5 | |
| 1103P | 16.8 | 47.8 a | 1.8 | 10.4 | 23.26 b | 34.4 | 4.0 a | 13.0 | 42.6 | 6.1 | |
| 41B | 26.6 | 43.5 a | 2.0 | 9.3 | 18.6 b | 34.9 | 2.1 a | 18.7 | 40.5 | 3.8 | |
| 110R | 11.8 | 85.8 b | 0.0 | 1.3 | 1.1 a | 37.0 | 13.3 b | 6.0 | 37.0 | 6.7 | |
| 161-49C | 21.9 | 47.1 a | 0.6 | 7.6 | 22.8 b | 32.9 | 7.9 ab | 8.5 | 44.2 | 6.5 | |
| Irrigation strategy (IS) | |||||||||||
| PRI | 23.0 | 52.4 | 1.1 | 5.3 | 18.2 | 37.8 | 7.8 | 10.6 | 39.5 | 4.3 | |
| RDI | 17.1 | 59.5 | 1.0 | 6.7 | 15.6 | 33.8 | 4.6 | 10.6 | 42.6 | 8.4 | |
| R× IS | |||||||||||
| 140Ru | PRI | 38.9 c | 21.2 a | 1.8 | 2.3 | 35.8 c | 36.1 | 2.4 a | 12.4 ab | 45.5 | 3.5 |
| RDI | 7.4 a | 89.8 c | 0.0 | 0.8 | 2.1 ab | 43.6 | 5.0 a | 1.5 a | 36.5 | 13.4 | |
| 1103P | PRI | 24.5 abc | 54.2 abc | 3.6 | 6.6 | 11.0 ab | 37.2 | 0.8 a | 15.4 bc | 43.2 | 3.4 |
| RDI | 9.1 ab | 41.5 a | 0.0 | 14.1 | 35.3 c | 31.6 | 7.2 a | 10.6 ab | 42.0 | 8.7 | |
| 41B | PRI | 23.3 abc | 48.3 ab | 0.0 | 4.7 | 23.7 abc | 50.4 | 1.0 a | 7.3 ab | 39.7 | 1.6 |
| RDI | 30.0 abc | 38.8 a | 4.0 | 13.8 | 13.4 abc | 19.4 | 3.2 a | 30.1 c | 41.2 | 6.0 | |
| 110R | PRI | 15.2 ab | 84.1 bc | 0.0 | 0.7 | 0.0 a | 35.8 | 22.5 b | 7.9 ab | 29.2 | 4.7 |
| RDI | 8.3 ab | 87.5 c | 0.0 | 2.0 | 2.2 ab | 38.2 | 4.1 a | 4.2 ab | 44.8 | 8.7 | |
| 161-49C | PRI | 13.0 ab | 54.2 abc | 0.0 | 12.3 | 20.5 abc | 29.6 | 12.3 a | 10.3 ab | 39.8 | 8.0 |
| RDI | 30.7 bc | 40.0 a | 1.2 | 3.0 | 25.1 bc | 36.2 | 3.5 a | 6.8 ab | 48.6 | 5.0 | |
| ANOVA | |||||||||||
| R | ns | ** | ns | ns | * | ns | * | ns | ns | ns | |
| IS | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| R × IS | ** | ** | ns | ns | ** | ns | * | * | ns | ns | |
| Rootstock (R) | H2O (%) | mg Pulp Berry−1 | mg Skin Berry−1 | mg Seed Berry−1 | Seed Number | mg Seed Seed−1 | Skin/Pulp Ratio | Skin/Seeds Ratio | Skin (%) | Pulp (%) | Seed (%) | Must (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 140Ru | 71.0 | 1370 | 185 | 80.6 a | 2.04 a | 40.1 c | 0.149 b | 2.27 c | 11.9 bc | 83.0 | 5.2 | 63.3 b | |
| 1103P | 70.7 | 1321 | 176 | 83.7 ab | 2.26 bc | 37.4 ab | 0.137 a | 2.16 ab | 11.2 a | 83.4 | 5.4 | 60.9 a | |
| 41B | 71.1 | 1343 | 177 | 87.5 b | 2.37 c | 37.0 a | 0.139 a | 2.14 a | 11.3 ab | 83.7 | 5.6 | 60.2 a | |
| 110R | 70.1 | 1294 | 181 | 78.9 a | 2.12 ab | 37.3 ab | 0.149 b | 2.26 c | 12.1 c | 82.6 | 5.3 | 64.1 b | |
| 161-49C | 70.7 | 1298 | 181 | 80.4 a | 2.09 a | 38.8 bc | 0.143 ab | 2.23 bc | 11.9 abc | 82.9 | 5.3 | 60.9 a | |
| Irrigation strategy (IS) | |||||||||||||
| PRI | 70.7 | 1338 | 178 | 81.9 | 2.17 | 38.0 | 0.139 | 2.19 | 11.4 | 83.3 | 5.3 | 62.0 | |
| RDI | 70.7 | 1313 | 182 | 82.6 | 2.18 | 38.2 | 0.148 | 2.24 | 12.0 | 82.9 | 5.4 | 61.8 | |
| Year | |||||||||||||
| 2012 | - | 1319 b | 184 b | 82.0 b | 2.11 ab | 39.2 bc | 0.140 | 2.29 | 11.6 c | 83.2 b | 5.2 b | 68.4 b | |
| 2013 | - | 1636 c | 202 c | 93.5 c | 2.40 c | 39.4 c | 0.124 | 2.21 | 10.5 b | 84.6 c | 4.9 ab | 58.9 a | |
| 2014 | - | 1335 b | 146 a | 71.9 a | 2.02 a | 36.0 a | 0.110 | 2.06 | 9.5 a | 85.9 d | 4.7 a | - | |
| 2016 | 70.7 | 1012 a | 188 b | 81.4 b | 2.18 b | 37.9 b | 0.195 | 2.33 | 15.1 d | 78.9 a | 6.5 c | 58.4 a | |
| R× IS | |||||||||||||
| 140Ru | PRI | 70.6 | 1421 | 186 | 84.8 | 2.22 cde | 38.6 b | 0.139 a | 2.18 | 11.3 a | 83.5 | 5.2 | 62.3 |
| RDI | 71.4 | 1318 | 183 | 76.3 | 1.86 a | 41.5 c | 0.159 c | 2.36 | 12.4 bc | 82.5 | 5.1 | 64.3 | |
| 1103P | PRI | 70.8 | 1309 | 173 | 82.3 | 2.21 cde | 37.4 ab | 0.137 a | 2.16 | 11.1 a | 83.6 | 5.3 | 61.0 |
| RDI | 70.5 | 1333 | 180 | 85.2 | 2.31 de | 37.3 ab | 0.137 a | 2.16 | 11.3 a | 83.3 | 5.4 | 60.9 | |
| 41B | PRI | 71.5 | 1355 | 173 | 87.2 | 2.37 e | 37.1 ab | 0.134 a | 2.07 | 10.9 a | 83.6 | 5.5 | 60.4 |
| RDI | 70.8 | 1332 | 181 | 87.8 | 2.37 e | 37.0 ab | 0.144 ab | 2.21 | 11.7 ab | 83.9 | 5.6 | 60.0 | |
| 110R | PRI | 70.8 | 1276 | 183 | 76.4 | 1.99 ab | 38.6 ab | 0.157 bc | 2.35 | 12.5 bc | 82.2 | 5.3 | 64.7 |
| RDI | 69.3 | 1312 | 180 | 81.3 | 2.26 cde | 36.1 a | 0.141 abc | 2.16 | 11.7 ab | 83.0 | 5.3 | 63.4 | |
| 161-49C | PRI | 69.9 | 1328 | 173 | 78.7 | 2.07 bc | 38.3 ab | 0.128 a | 2.17 | 11.0 a | 83.9 | 5.1 | 61.5 |
| RDI | 71.4 | 1269 | 188 | 82.2 | 2.10 bcd | 39.3 b | 0.159 c | 2.29 | 12.7 c | 81.8 | 5.5 | 60.3 | |
| ANOVA | |||||||||||||
| R | ns | ns | ns | * | *** | *** | * | ** | * | ns | ns | *** | |
| IS | ns | ns | ns | ns | ns | ns | ** | ns | ** | ns | ns | ns | |
| Year | - | *** | *** | *** | *** | *** | *** | ** | *** | *** | *** | *** | |
| R × IS | ns | ns | ns | ns | *** | * | ** | ns | ** | ns | ns | ns | |
| Total Anthocyanins | ||||
|---|---|---|---|---|
| Rootstock (R) | mg g−1 Skin | mg Berry−1 | mg g−1 Berry | mg g−1 Must |
| 140Ru | 6.00 b | 1.08 bc | 0.71 bc | 1.21 bc |
| 1103P | 5.32 a | 0.93 a | 0.60 a | 1.01 a |
| 41B | 5.84 b | 0.99 ab | 0.65 ab | 1.10 ab |
| 110R | 6.09 b | 1.09 c | 0.73 c | 1.24 c |
| 161-49C | 5.67 ab | 1.00 ab | 0.67 b | 1.13 abc |
| Irrigation strategy (IS) | ||||
| PRI | 5.83 | 1.01 | 0.66 | 1.13 |
| RDI | 5.74 | 1.03 | 0.68 | 1.14 |
| Year | ||||
| 2012 | 7.19 c | 1.30 c | 0.83 c | 1.21 b |
| 2013 | 4.25 a | 0.86 a | 0.44 | 0.74 a |
| 2014 | 5.96 b | 0.86 a | 0.56 b | - |
| 2016 | 5.74 b | 1.05 b | 0.85 c | 1.45 c |
| ANOVA | ||||
| Rootstock (R) | * | ** | *** | ** |
| Irrigation strategy (IS) | ns | ns | ns | ns |
| Year | *** | *** | *** | *** |
| Interaction (R × IS) | ns | ns | ns | ns |
| Total Phenolic Compounds Content | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Seeds | Berry | Contribution | |||||||||
| Rootstock (R) | mg g−1 Skin | mg Berry−1 | mg g−1 Berry | mg g−1 Must | mg g−1 Seed | mg Berry−1 | mg g−1 Berry | mg g−1 Must | mg Berry−1 | mg g−1 Must | % Skin | % Seed |
| 140Ru | 10.9 ab | 1.98 b | 1.31 bc | 2.28 bc | 4.8 | 0.38 | 0.26 | 0.45 | 2.37 bc | 2718 | 83.5 | 16.5 |
| 1103P | 10.4 a | 1.80 a | 1.17 a | 2.02 a | 5.6 | 0.45 | 0.31 | 0.59 | 2.26 ab | 2589 | 80.0 | 20.0 |
| 41B | 10.7 a | 1.82 a | 1.20 a | 2.08 ab | 4.6 | 0.39 | 0.27 | 0.51 | 2.19 a | 2476 | 83.0 | 17.0 |
| 110R | 11.3 b | 2.00 b | 1.37 c | 2.32 c | 5.7 | 0.43 | 0.31 | 0.55 | 2.45 c | 2827 | 82.8 | 17.2 |
| 161-49C | 10.5 a | 1.86 a | 1.25 ab | 2.16 abc | 6.0 | 0.46 | 0.32 | 0.58 | 2.31 ab | 2713 | 80.4 | 19.6 |
| Irrigation strategy (IS) | ||||||||||||
| PRI | 10.8 | 1.88 | 1.24 | 2.14 | 5.6 | 0.44 | 0.30 | 0.53 | 2.31 | 2621 | 81.3 | 18.7 |
| RDI | 10.7 | 1.91 | 1.28 | 2.21 | 5.2 | 0.41 | 0.29 | 0.54 | 2.32 | 2708 | 82.6 | 17.4 |
| Year | ||||||||||||
| 2012 | 13.0 d | 2.36 | 1.50 c | 2.20 b | 3.9 ab | 0.31 | 0.20 a | 0.29 a | 2.67 b | 2490 b | 88.3 c | 11.7 a |
| 2013 | 7.5 a | 1.50 | 0.78 a | 1.32 a | 3.7 a | 0.35 | 0.18 a | 0.32 a | 1.85 a | 1615 a | 81.0 b | 19.0 b |
| 2014 | 10.7 b | 1.55 | 1.01 b | - | 4.6 b | 0.32 | 0.22 a | - | 1.88 a | - | 82.6 b | 17.4 b |
| 2016 | 11.8 c | 2.17 | 1.76 d | 3.00 c | 9.2 c | 0.72 | 0.57 b | 0.989 b | 2.86 c | 3890 c | 75.9 a | 24.1 c |
| ANOVA | ||||||||||||
| Rootstock (R) | * | *** | *** | * | ns | ns | ns | ns | ** | ns | ns | ns |
| Irrigation strategy (IS) | Ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Year | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Interaction (R × IS) | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Epidermis | Hypodermis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Absence of Coloration (%) | Uniform Coloration (%) | Fine Granulation (%) | Small Spherical Inclusions (%) | Large Inclusions (%) | Absence of Coloration (%) | Uniform Coloration (%) | Fine Granulation (%) | Small Spherical Inclusions (%) | Large Inclusions (%) | |
| Skin total anthocyanins (mg g−1 skin) | −0.27 | 0.37 | −0.60 | −0.37 | −0.20 | 0.30 | 0.25 | −0.39 | −0.32 | 0.20 |
| Skin total anthocyanins (mg berry−1) | −0.16 | 0.37 | −0.53 | −0.58 | −0.22 | 0.21 | 0.32 | −0.45 | −0.22 | 0.27 |
| Skin total anthocyanins (mg g−1 berry) | −0.26 | 0.58 | −0.56 | −0.69 * | −0.46 | 0.25 | 0.46 | −0.53 | −0.38 | 0.35 |
| Skin total phenolic compounds (mg g−1 skin) | −0.53 | 0.59 | −0.59 | −0.32 | −0.41 | 0.25 | 0.32 | −0.43 | −0.37 | 0.35 |
| Skin total phenolic compounds (mg berry−1) | −0.37 | 0.72 * | −0.46 | −0.70 * | −0.63 * | 0.20 | 0.49 | −0.54 | −0.41 | 0.46 |
| Skin total phenolic compounds (mg g−1 berry) | −0.14 | 0.55 | −0.36 | −0.78 ** | −0.50 | 0.24 | 0.38 | −0.50 | −0.30 | 0.32 |
| Berry total phenolic compounds (mg g−1 berry) | −0.49 | 0.76 * | −0.53 | −0.57 | −0.64 * | 0.10 | 0.69 * | −0.55 | −0.49 | 0.46 |
| Berry total phenolic compounds (mg berry−1) | 0.19 | 0.13 | −0.05 | 0.23 | 0.44 | 0.85 | 0.09 | 0.11 | 0.43 | 0.23 |
| Rootstock (R) | Glucose | Fructose | Sucrose | G+F+S | G/F | Starch | |
|---|---|---|---|---|---|---|---|
| 140Ru | 90 abc | 95 ab | 9.6 b | 192 ab | 0.94 | 5.08 a | |
| 1103P | 87 ab | 90 a | 8.3 a | 186 a | 0.95 | 4.79 a | |
| 41B | 86 a | 90 a | 8.3 a | 184 a | 0.95 | 5.20 ab | |
| 110R | 95 bc | 96 ab | 9.2 ab | 198 ab | 0.96 | 4.94 a | |
| 161-49C | 99 c | 102 b | 8.0 a | 209 b | 0.95 | 5.61 b | |
| Irrigation strategy (IS) | |||||||
| PRI | 93 | 95 | 8.9 | 195 | 0.95 | 5.21 | |
| RDI | 90 | 94 | 8.5 | 193 | 0.95 | 5.04 | |
| Year | |||||||
| 2014 | 77 b | 84 a | 3.5 a | 165 b | 0.92 | 5.60 b | |
| 2015 | 69 a | 83 a | 4.1 a | 152 a | 0.82 | 5.26 b | |
| 2016 | 128 c | 116 b | 18.5 b | 265 c | 1.10 | 4.52 a | |
| R × IS | |||||||
| 140Ru | PRI | 94 abc | 97 a | 9.9 | 202 abc | 0.94 | 5.39 bc |
| RDI | 85 ab | 93 a | 9.3 | 183 ab | 0.94 | 4.78 ab | |
| 1103P | PRI | 80 a | 85 a | 7.8 | 173 a | 0.94 | 5.07 abc |
| RDI | 93 abc | 96 a | 8.9 | 199 ab | 0.95 | 4.50 a | |
| 41B | PRI | 85 ab | 89 a | 8.7 | 182 ab | 0.95 | 4.76 ab |
| RDI | 87 ab | 91 a | 7.8 | 186 ab | 0.95 | 5.64 cd | |
| 110R | PRI | 95 bc | 94 a | 9.3 | 193 ab | 0.95 | 4.67 ab |
| RDI | 96 bc | 98 ab | 9.1 | 203 bc | 0.96 | 5.22 abc | |
| 161-49C | PRI | 108 c | 111 b | 8.6 | 227 c | 0.95 | 6.16 d |
| RDI | 90 ab | 94 a | 7.5 | 191 ab | 0.94 | 5.06 abc | |
| ANOVA | |||||||
| R | * | * | * | * | ns | * | |
| IS | ns | ns | ns | ns | ns | ns | |
| Year | *** | *** | *** | *** | *** | *** | |
| R × IS | * | * | ns | ** | ns | *** | |
| Rootstock (R) | OD620 | OD520 | OD420 | IC | Tone | L* | a* | b* | C* | h* | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 140Ru | 0.53 a | 1.98 a | 1.98 a | 4.49 a | 1.04 d | 22.8 | 15.2 a | 3.2 ab | 15.6 a | 11.0 a | |
| 1103P | 0.61 b | 2.18 bc | 2.22 b | 5.02 b | 1.03 d | 23.0 | 16.2 ab | 3.6 abc | 16.7 ab | 11.7 ab | |
| 41B | 0.52 a | 2.16 b | 2.00 a | 4.68 a | 0.94 b | 23.3 | 19.2 c | 4.8 c | 20.1 c | 13.5 b | |
| 110R | 0.62 b | 2.32 cd | 2.27 b | 5.20 b | 0.99 c | 22.5 | 15.0 a | 3.0 a | 15.3 a | 10.9 a | |
| 161-49C | 0.55 a | 2.44 d | 2.12 a | 5.16 b | 0.88 a | 22.9 | 18.4 bc | 4.4 bc | 18.9 bc | 12.8 ab | |
| Irrigation strategy (IS) | |||||||||||
| PRI | 0.53 | 2.20 | 2.03 | 4.77 | 0.95 | 23.1 | 18.2 | 4.3 | 18.7 | 12.8 | |
| RDI | 0.60 | 2.25 | 2.20 | 5.05 | 1.00 | 22.8 | 15.4 | 3.3 | 15.9 | 11.1 | |
| Year | |||||||||||
| 2012 | 0.53 c | 1.97 b | 1.98 b | 4.47 b | 1.00 c | - | - | - | - | - | |
| 2013 | 0.33 -a | 1.57 a | 1.51 a | 3.41 a | 0.99 c | - | - | - | - | - | |
| 2014 | 0.82 e | 2.42 d | 2.83 d | 6.07 c | 1.18 d | - | - | - | - | - | |
| 2015 | 0.42 b | 2.18 c | 1.86 b | 4.46 b | 0.90 b | - | - | - | - | - | |
| 2016 | 0.72 d | 2.94 e | 2.38 c | 6.04 c | 0.81 a | 22.9 | 16.8 | 3.8 | 17.3 | 12.0 | |
| Interaction (R × IS) | |||||||||||
| 140Ru | PRI | 0.49 a | 2.00 ab | 1.90 | 4.39 a | 1.00 bc | 23.0 | 18.0 | 4.3 | 18.6 | 12.8 |
| RDI | 0.57 ab | 1.96 a | 2.06 | 4.59 ab | 1.08 d | 22.6 | 12.4 | 2.1 | 12.6 | 9.2 | |
| 1103P | PRI | 0.55 ab | 2.08 abc | 2.05 | 4.68 abc | 0.99 b | 23.5 | 19.0 | 4.6 | 19.6 | 13.3 |
| RDI | 0.67 c | 2.28 bc | 2.40 | 5.35 d | 1.07 cd | 22.6 | 13.5 | 2.7 | 13.8 | 10.0 | |
| 41B | PRI | 0.48 a | 2.05 ab | 1.90 | 4.43 ab | 0.94 b | 23.5 | 19.7 | 5.0 | 20.4 | 13.7 |
| RDI | 0.55 ab | 2.27 bc | 2.10 | 4.93 bcd | 0.94 b | 23.1 | 18.6 | 4.5 | 19.8 | 13.3 | |
| 110R | PRI | 0.61 bc | 2.26 abc | 2.23 | 5.10 cd | 1.00 bc | 22.6 | 16.1 | 3.4 | 16.4 | 11.5 |
| RDI | 0.63 bc | 2.37 cd | 2.31 | 5.31 d | 0.98 b | 22.4 | 14.0 | 2.7 | 14.2 | 10.3 | |
| 161-49C | PRI | 0.54 ab | 2.62 d | 2.09 | 5.24 d | 0.83 a | 22.6 | 18.3 | 4.1 | 18.7 | 12.5 |
| RDI | 0.57 ab | 2.37 abcc | 2.15 | 5.08 abc | 0.93 b | 23.2 | 18.5 | 4.7 | 19.1 | 13.0 | |
| ANOVA | |||||||||||
| R | *** | *** | *** | *** | *** | ns | * | * | * | * | |
| IS | *** | ns | *** | * | *** | ns | ** | * | ** | * | |
| Year | *** | *** | *** | *** | *** | - | - | - | - | - | |
| R × IS | *** | ** | ns | * | ** | ns | ns | ns | ns | ns | |
| Grape Quality Parameters | Wine Quality Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenols | EA | TA | SM index | QIphenolic | CI | TPI | Anthocyanins | Tannins | QIwine | |
| Skin cell size | −0.10 | −0.29 | −0.33 | 0.30 | −0.48 | −0.60 | −0.09 | −0.51 | 0.38 | 0.05 |
| Epidermis cell size | 0.02 | −0.21 | −0.32 | 0.49 | −0.24 | −0.67 * | 0.04 | −0.46 | 0.41 | 0.10 |
| Hypodermis cell size | −0.08 | −0.25 | −0.29 | 0.26 | −0.46 | −0.58 | −0.11 | −0.53 | 0.37 | 0.02 |
| Pulp cell size | 0.16 | −0.13 | −0.02 | 0.21 | 0.07 | 0.03 | −0.01 | 0.16 | 0.15 | 0.07 |
| Hypodermis cell size/epidermis cell size | −0.23 | −0.02 | 0.12 | −0.26 | −0.31 | 0.37 | −0.26 | 0.08 | −0.32 | −0.23 |
| Pulp cell size/skin cell size | 0.33 | 0.43 | 0.49 | −0.22 | 0.53 | 0.55 | −0.09 | 0.41 | −0.24 | −0.21 |
| Skin thickness | 0.10 | 0.09 | 0.00 | 0.07 | −0.14 | −0.46 | −0.09 | −0.54 | 0.15 | −0.02 |
| Epidermis thickness | 0.03 | −0.17 | −0.18 | 0.38 | −0.16 | −0.86 ** | −0.06 | −0.54 | 0.15 | −0.02 |
| Hypodermis thickness | 0.10 | 0.12 | 0.02 | 0.04 | −0.13 | −0.40 | −0.09 | −0.48 | 0.27 | −0.17 |
| Skin cell wall thickness | 0.08 | 0.20 | 0.13 | −0.06 | 0.02 | −0.29 | −0.34 | −0.68 * | 0.43 | −0.67 * |
| Epidermis cell wall thickness | 0.49 | 0.09 | 0.08 | 0.63 | 0.36 | −0.52 | −0.24 | −0.11 | −0.15 | −0.08 |
| Hypodermis cell wall thickness | −0.01 | 0.21 | 0.14 | −0.21 | −0.04 | −0.17 | −0.29 | −0.66 * | 0.46 | −0.65 * |
| Cuticle thickness | −0.41 | −0.01 | 0.10 | 0.76 ** | 0.15 | −0.02 | 0.22 | −0.19 | −0.24 | −0.27 |
| Epidermal cells without coloration | 0.02 | −0.21 | −0.30 | 0.49 | −0.27 | −0.43 | 0.10 | −0.02 | −0.06 | 0.46 |
| Epidermal cells with uniform coloration | −0.07 | 0.16 | 0.23 | −0.46 | −0.01 | 0.61 | −0.18 | 0.25 | −0.23 | −0.25 |
| Epidermal cells with fine granules | −0.02 | −0.46 | −0.55 | 0.65 * | 0.08 | −0.23 | 0.45 | 0.29 | 0.08 | 0.66 * |
| Epidermal cells with small spherical inclusions | 0.33 | 0.05 | 0.03 | 0.36 | 0.67 * | −0.25 | 0.22 | −0.02 | 0.37 | 0.10 |
| Epidermal cells with large inclusions | −0.02 | −0.07 | −0.11 | 0.18 | −0.03 | −0.58 | 0.10 | −0.45 | 0.30 | −0.05 |
| Hypodermal cells without coloration | −0.34 | −0.09 | 0.02 | −0.49 | −0.66 * | −0.05 | −0.19 | −0.27 | −0.18 | −0.27 |
| Hypodermal cells with uniform coloration | 0.18 | 0.46 | 0.41 | −0.37 | 0.34 | 0.86 ** | 0.06 | 0.50 | 0.06 | 0.12 |
| Hypodermal cells with fine granules | 0.23 | −0.23 | −0.28 | 0.69 * | 0.32 | −0.21 | 0.29 | 0.33 | 0.06 | 0.60 |
| Hypodermal cells with small spherical inclusions | 0.16 | −0.05 | −0.13 | 0.45 | −0.03 | −0.76 * | −0.06 | −0.48 | 0.05 | −0.24 |
| Hypodermal cells with large inclusions | −0.32 | −0.05 | 0.01 | −0.43 | 0.23 | 0.16 | −0.22 | −0.33 | 0.10 | −0.59 |
| Must | Wine | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OD620 | OD520 | OD420 | CI | Tone | Polyphenols Content (mg L−1) | Extractable Anthocyanins (mg L−1) | Total Anthocyanins (mg L−1) | AE Index | SM Index | CI | |
| Total anthocyanins (mg g−1 skin) | 0.33 * | 0.30 | 0.32 * | 0.33 * | 0.04 | 0.47 ** | 0.89 *** | 0.91 *** | −0.49 ** | −0.87 *** | 0.09 |
| Total anthocyanins (µg g−1 berry) | 0.36 * | 0.56 *** | 0.27 | 0.43 ** | −0.45 ** | 0.07 | 0.64 *** | 0.74 *** | −0.12 | −0.83 *** | 0.51 * |
| Total anthocyanins (µg g−1 must) | 0.79 *** | 0.80 *** | 0.75 *** | 0.81 *** | −0.50 ** | 0.15 | 0.56 *** | 0.63 *** | −0.22 | −0.74 *** | −0.43 |
| Skin TPC (mg g−1 skin) | 0.46 ** | 0.51 *** | 0.41 ** | 0.49 ** | −0.19 | 0.39 * | 0.87 *** | 0.89 *** | −0.46 ** | −0.92 *** | 0.75 *** |
| Skin TPC (µg g−1 berry) | 0.41 ** | 0.64 *** | 0.30 | 0.49 ** | −0.51 *** | −0.02 | 0.52 *** | 0.62 *** | −0.06 | −0.75 *** | 0.57 ** |
| Skin TPC (µg g−1 must) | 0.83 *** | 0.85 *** | 0.78 *** | 0.85 *** | −0.54 ** | 0.06 | 0.44 * | 0.50 ** | −0.16 | −0.64 *** | −0.46 |
| Seed TPC (mg g−1 seed) | 0.39 * | 0.72 *** | 0.28 | 0.51 *** | −0.56 *** | −0.23 | 0.03 | 0.14 | 0.17 | −0.28 | 0.83 *** |
| Seed TPC (µg g−1 berry) | 0.38 * | 0.73 *** | 0.26 | 0.51 *** | −0.63 *** | −0.30 | 0.01 | 0.13 | 0.22 | −0.29 | 0.79 *** |
| Seed TPC (µg g−1 must) | 0.68 *** | 0.84 *** | 0.61 *** | 0.76 *** | −0.69 *** | −0.26 | −0.02 | 0.07 | 0.15 | −0.20 | 0.38 |
| Total TPC (µg g−1 berry) | 0.43 ** | 0.72 *** | 0.31 | 0.53 *** | −0.58 *** | −0.11 | 0.40 * | 0.51 *** | 0.03 | −0.66 *** | 0.66 ** |
| Total TPC (µg g−1 must) | 0.22 | 0.54 *** | 0.10 | 0.32 * | −0.64 *** | −0.04 | 0.31 | 0.39 * | −0.07 | 0.53 ** | 0.77 *** |
| Wine | ||||
|---|---|---|---|---|
| Berry | TPI | Tannins | Anthocyanins | QIwine |
| Polyphenols content | −0.14 | −0.26 | 0.44 * | −0.22 |
| Extractable anthocyanins | 0.27 | −0.07 | 0.83 *** | 0.22 |
| Total anthocyanins | 0.36 * | 0.03 | 0.83 *** | 0.30 |
| AE Index | 0.17 | 0.40 * | −0.48 ** | 0.15 |
| SM Index | −0.38 * | 0.01 | −0.81 *** | −0.37 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, J.M.; Botía, P.; Romero, P. Changes in Berry Tissues in Monastrell Grapevines Grafted on Different Rootstocks and Their Relationship with Berry and Wine Phenolic Content. Plants 2021, 10, 2585. https://doi.org/10.3390/plants10122585
Navarro JM, Botía P, Romero P. Changes in Berry Tissues in Monastrell Grapevines Grafted on Different Rootstocks and Their Relationship with Berry and Wine Phenolic Content. Plants. 2021; 10(12):2585. https://doi.org/10.3390/plants10122585
Chicago/Turabian StyleNavarro, Josefa M., Pablo Botía, and Pascual Romero. 2021. "Changes in Berry Tissues in Monastrell Grapevines Grafted on Different Rootstocks and Their Relationship with Berry and Wine Phenolic Content" Plants 10, no. 12: 2585. https://doi.org/10.3390/plants10122585
APA StyleNavarro, J. M., Botía, P., & Romero, P. (2021). Changes in Berry Tissues in Monastrell Grapevines Grafted on Different Rootstocks and Their Relationship with Berry and Wine Phenolic Content. Plants, 10(12), 2585. https://doi.org/10.3390/plants10122585







