Soybean Callus—A Potential Source of Tocopherols
Abstract
1. Introduction
2. Results
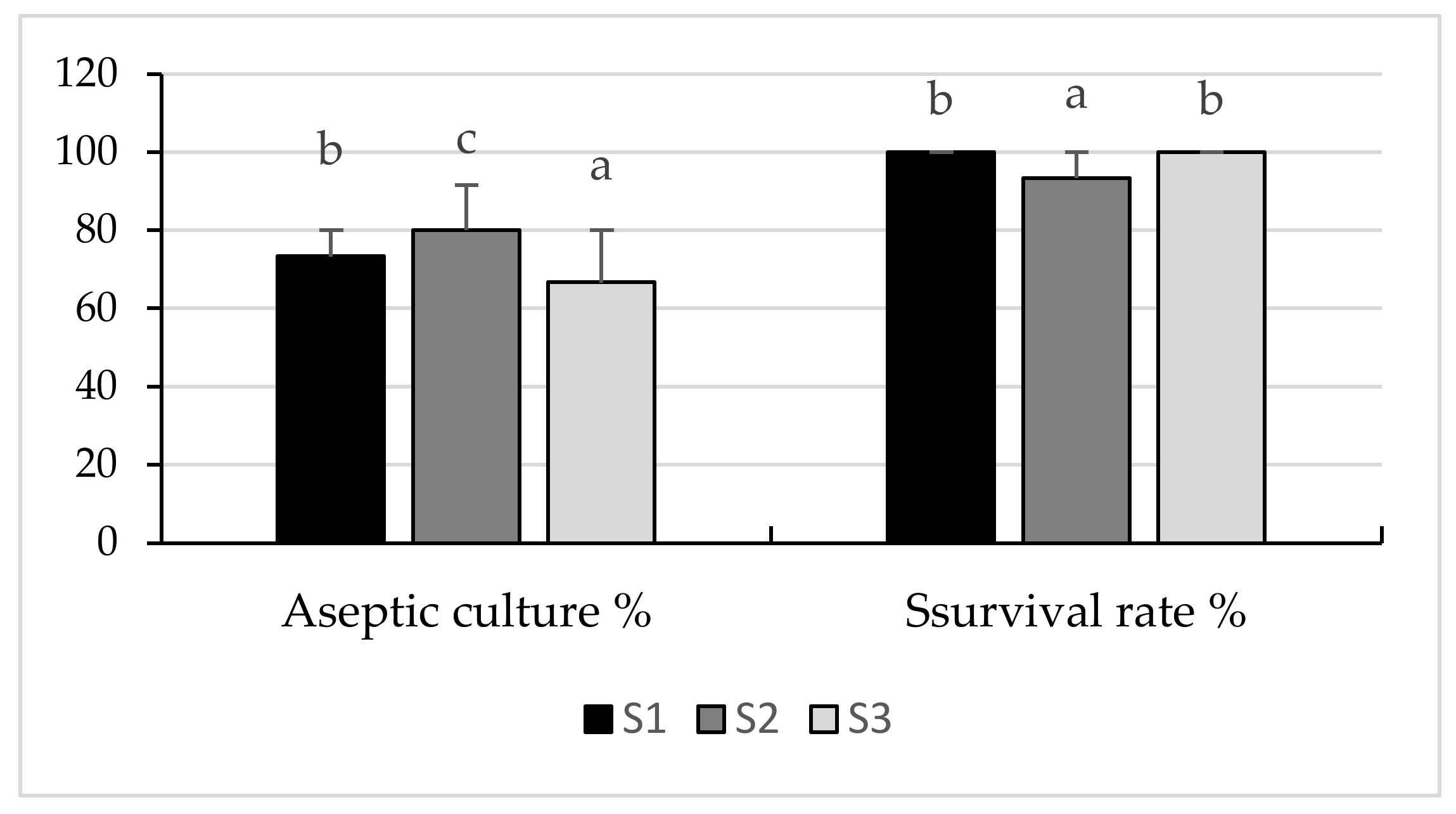
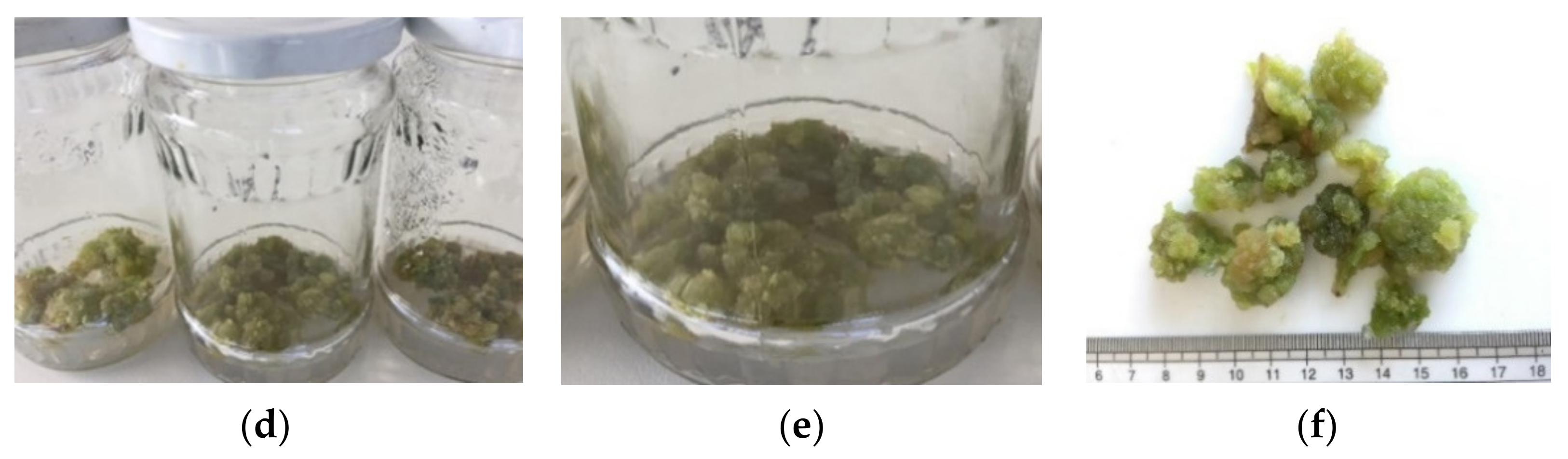
2.1. Callus Culture Initiation
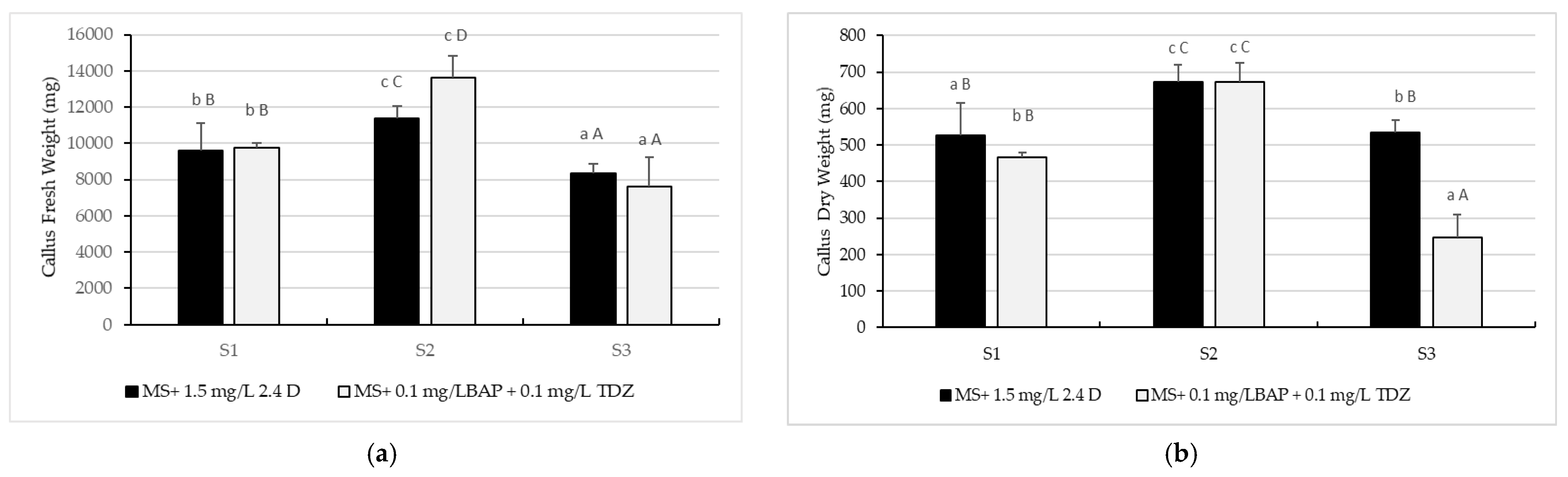
2.2. Callus Proliferation
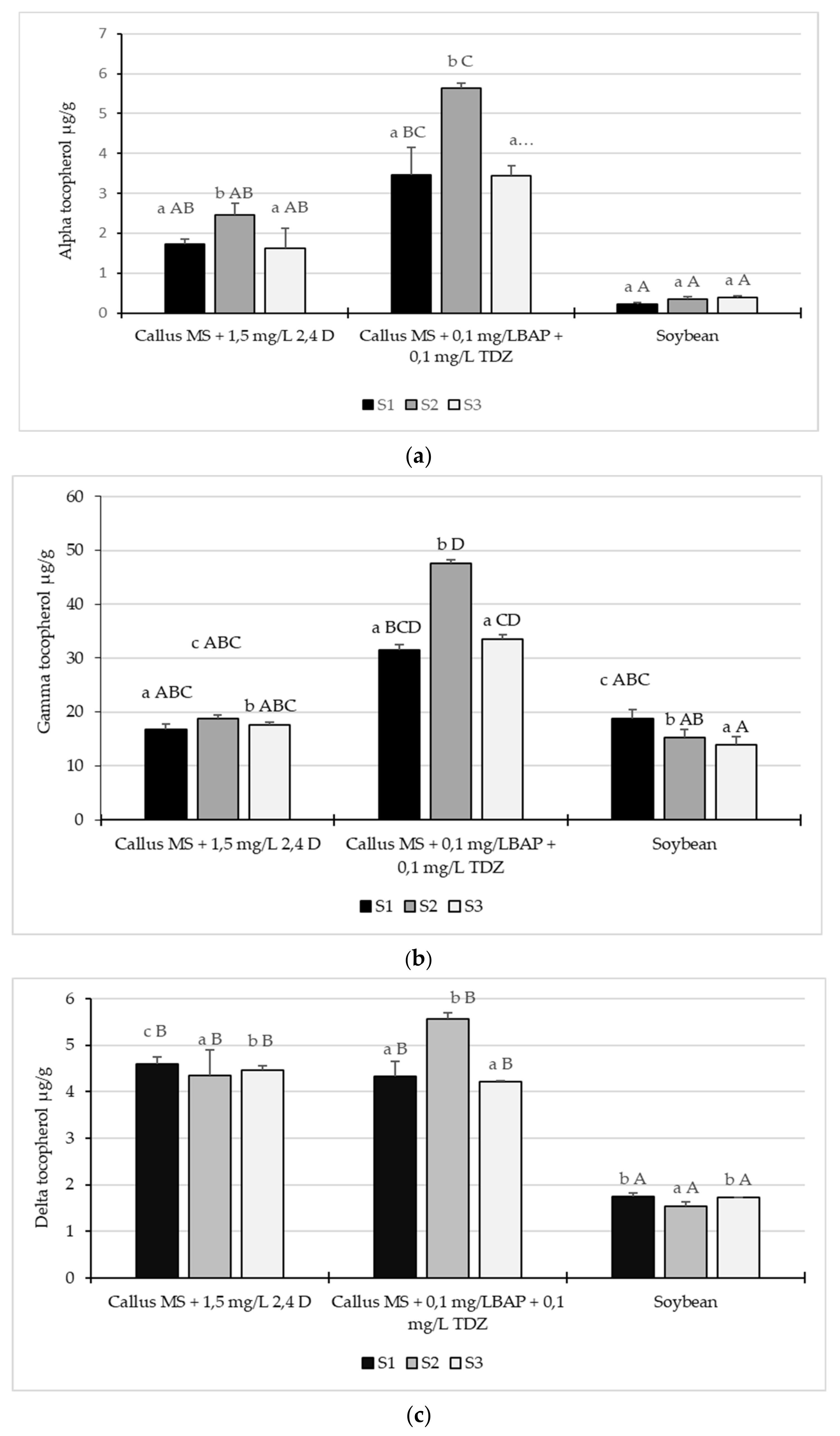
2.3. Tocopherol Content
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Callus Initiation and Subculture
4.3. Extraction and Analysis of Tocopherols
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Almonor, G.O.; Fenner, G.P.; Wilson, R.F. Temperature effects on tocopherols composition in soybeans with genetically improved oil quality. J. Am. Oil. Chem. Soc. 1998, 75, 591–596. [Google Scholar] [CrossRef]
- Van Eenennaam, A.L.; Lincoln, K.; Durrett, T.P.; Valentin, H.E.; Shewmaker, C.K.; Thorne, G.M.; Jiang, J.; Baszis, S.R.; Levering, C.K.; Aasen, E.D.; et al. Engineering Vitamin E Content: From Arabidopsis Mutant to Soy Oil. Plant Cell 2003, 15, 3007–3019. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000; p. 6. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225461/ (accessed on 23 January 2021).
- Brigelius-Flohé, R.; Kelly, F.J.; Salonen, J.T.; Neuzil, J.; Zingg, J.M.; Azzi, A. The European perspective on vitamin E: Current knowledge and future research. Am. J. Clin. Nutr. 2002, 76, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Traber, M.G. A history of vitamin E. Ann. Nutr. Metab. 2012, 61, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Sokol, R.J.; Ringel, S.P.; Neville, H.E.; Thellman, C.A.; Kayden, H.J. Lack of tocopherol in peripheral nerves of vitamin E-deficient patients with peripheral neuropathy. N. Engl. J. Med. 1987, 317, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.K.; Lauridsen, C. a-Tocopherol Stereoisomers. Vitam. Horm. 2007, 76, 281–308. [Google Scholar] [CrossRef]
- Ranard, K.M.; Erdman, J.W., Jr. Effects of dietary RRR α-tocopherol vs all-racemic α-tocopherol on health outcomes. Nutr. Rev. 2018, 76, 141–153. [Google Scholar] [CrossRef]
- Almagro, L.; Sabater-Jara, A.B.; Belchí-Navarro, S.; Pedreno, M.A. Recent trends in the biotechnological production of tocopherols using in vitro cultures. Phytochem. Rev. 2021, 20, 1193–1207. [Google Scholar] [CrossRef]
- Nazir, S.; Jan, H.; Tungmunnithum, D.; Drouet, S.; Zia, M.; Hano, C.; Abbasi, B.H. Callus Culture of Ocimum basilicum L. cv ‘Thai Basil’ Is an Effective Biological System for the Production of Antioxidants Compared to Leaves. Preprints 2020, 2020090285. [Google Scholar]
- Park, J.-S.; Seong, Z.-K.; Kim, M.-S.; Ha, J.-H.; Moon, K.-B.; Lee, H.-J.; Lee, H.-K.; Jeon, J.-H.; Park, S.U.; Kim, H.-S. Production of flavonoids in callus cultures of Sophora flavescens Aiton. Plants 2020, 9, 688. [Google Scholar] [CrossRef]
- Wahyuni, D.K.; Rahayu, S.; Zaidan, A.H.; Ekasari, W.; Prasongsuk, S.; Purnobasuki, H. Growth, secondary metabolite production, and in vitro antiplasmodial activity of Sonchus arvensis L. callus under dolomite [CaMg (CO3) 2] treatment. PLoS ONE 2021, 16, e0254804. [Google Scholar] [CrossRef] [PubMed]
- Parihar, S. Protein profiling of regenerative and non regenerative callus cultures of Glossonema varians: A rare, endemic and edible plant of Indian Thar Desert. Vegetos 2020, 33, 385–389. [Google Scholar] [CrossRef]
- Kamarul Zaman, M.A.; Azzeme, A.M.; Ramle, I.K.; Normanshah, N.; Ramli, S.N.; Shaharuddin, N.A.; Ahmad, S.; Abdullah, S.N.A. Induction, multiplication, and evaluation of antioxidant activity of Polyalthia bullata callus, a woody medicinal plant. Plants 2020, 9, 1772. [Google Scholar] [CrossRef] [PubMed]
- Ashokhan, S.; Othman, R.; Abd Rahim, M.H.; Karsani, S.A.; Yaacob, J.S. Effect of plant growth regulators on coloured callus formation and accumulation of azadirachtin, an essential biopesticide in Azadirachta indica. Plants 2020, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Dan, Y.; Reichert, N.A. Organogenic regeneration of soybean from hypocotyls explants. In Vitro Cell Dev. Biol. Plant 1998, 34, 14–21. [Google Scholar] [CrossRef]
- Sairam, R.V.; Franklin, G.; Hassel, R.; Smith, B.; Meeker, K.; Kashikar, N.; Parani, M.; Al.Abed, D.; Ismail, S.; Berry, K.; et al. A study on the effect of genotypes, plant growth regulators and sugars in promoting plant regeneration via organogenesis from soybean cotyledonary nodal callus. Plant Cell Tissue Organ. Cult. 2003, 75, 79–85. [Google Scholar] [CrossRef]
- Shan, Z.; Raemakers, K.; Tzitzikas, E.N.; Ma, Z.; Visser, R.F.G. Development of a highly efficient, repetitive system of organogenesis in soybean (Glycine max (L.) Merr.). Plant Cell Rep. 2005, 24, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Ranjithakumari, B.D. Callus induction and plant regeneration of Indian soybean (Glycine max (L.) Merr. cv. CO3) via half seed explant culture. J. Agri. Technol. 2007, 3, 287–297. [Google Scholar]
- Yang, C.; Zhao, T.; Yu, D.; Gai, J. Somatic embryogenesis and plant regeneration in Chinese soybean [Glycine max (L.) Merr.]—Ompacts of mannitol, abscisic acid and explants age. In Vitro Cell Dev. Bio. Plant 2009, 45, 180–188. [Google Scholar] [CrossRef]
- Joyner, E.Y.; Boykin, L.S.; Lodhi, M.A. Callus Induction and Organogenesis in Soybean [Glycine max (L.) Merr.] cv. Pyramid from Mature Cotyledons and Embryos. Open Plant Sci. J. 2010, 4, 18–21. [Google Scholar] [CrossRef][Green Version]
- Loganathan, M.; Maruthasalam, S.; Shiu, Y.L.; Lien, W.C.; Hsu, W.H.; Lei, P.F.; Yu, C.W.; Lin, C.H. Regeneration of soybean (Glycine max L. Merrill) through direct somatic embryogenesis from the immature embryonic shoot tip. In Vitro Cell Dev. Bio. Plant 2010, 46, 265–273. [Google Scholar] [CrossRef][Green Version]
- Gueven, A.; Knorr, D. Isoflavonoid production by soy plant callus suspension culture. J. Food Eng. 2011, 103, 237–243. [Google Scholar] [CrossRef]
- Sansanelli, S.; Zanichelli, D.; Filippini, A.; Ferri, M.; Tassoni, A. Production of free and glycosylated isoflavones in in vitro soybean (Glycine max L.) hypocotyl cell suspensions and comparison with industrial seed extracts. Plant Cell Tissue Organ Cult. 2014, 119, 301–311. [Google Scholar] [CrossRef]
- Devi, M.A.; Kumar, G.; Giridhar, P. Effect of biotic and abiotic elicitors on isoflavone biosynthesis during seed development and in suspension cultures of soybean (Glycine max L.). 3 Biotech 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ajjawi, I.; Shintani, D. Engineered plants with elevated vitamin E: A nutraceutical success story. Trends Biotechnol. 2004, 22, 104–107. [Google Scholar] [CrossRef]
- Kadam, S.S.; Nerkar, Y.S.; Salunkhe, D.K. Subtoxic effects of certain chemicals on food crops. Crit. Rev. Food. Sci. Nutr. 1981, 14, 49–109. [Google Scholar] [CrossRef] [PubMed]
- Barwale, U.B.; Kerns, H.R.; Widholm, J.M. Plant regeneration from callus cultures of several soybean genotypes via embryogenesis and organogenesis. Planta 1986, 167, 473–481. [Google Scholar] [CrossRef]
- Islam, N.; Islam, T.; Hossain, M.M.; Bhattacharjee, B.; Islam, S.M.S. Embryogenic callus induction and efficient plant regeneration in three varieties of soybean (Glycine max). Plant Tiss. Cult. Biotech. 2017, 27, 41–50. [Google Scholar] [CrossRef]
- Phat, P.; Rehman, S.U.; Jung, H.I.; Ju, H.J. Optimization of soybean (Glycine max L.) regeneration for Korean cultivars. Pak. J. Bot. 2015, 47, 2379–2385. [Google Scholar]
- Kosturkova, G.P.; Dimitrova, M.S.; Todorova, R.V.; Tasheva, K.N.; Petrov, P.I.; Tidke, S.; Gokare, R.A. Soybean long-term callus cultures–potential for biotransformation and nutraceutical production. J. BioSci. Biotech. 2017, 6, 179–185. [Google Scholar]
- Mangena, P. Benzyl adenine in plant tissue culture-succinct analysis of the overall influence in soybean [Glycine max (L.) Merrill.] seed and shoot culture establishment. J. Biotech. Res. 2020, 11, 23–34. [Google Scholar]
- Kumari, B.D.R.; Settu, A.; Sujatha, G. Somatic embryogenesis and plant regeneration in soybean. Indian J. Biotechnol. 2006, 5, 243–245. [Google Scholar]
- Shahin, H. Callus formation and production of secondary metabolites by seedling explants of Chenopodium quinoa. Egypt. J. Bot. 2019, 59, 451–460. [Google Scholar] [CrossRef]
- Hegazi, G.A.; Ibrahim, W.M.; Hendawy, M.H.; Salem, H.M.; Ghareb, H.E. Improving α-tocopherol accumulation in Argania spinosa suspension cultures by precursor and nanoparticles feeding. Plant Arch. 2020, 20, 2431–2437. [Google Scholar]
- Caretto, S.; Speth, E.B.; Fachechi, C.; Gala, R.; Zacheo, G.; Giovinazzo, G. Enhancement of vitamin E production in sunflower cell cultures. Plant Cell Rep. 2004, 23, 174–179. [Google Scholar] [CrossRef]
- Caretto, S.; Nisi, R.; Paradiso, A.; De Gara, L. Tocopherol production in plant cell cultures. Mol. Nutr. Food Res. 2010, 54, 726–730. [Google Scholar] [CrossRef]
- Chavan, S.P.; Lokhande, V.H.; Nitnaware, K.M.; Nikam, T.D. Influence of growth regulators and elicitors on cell growth and α-tocopherol and pigment productions in cell cultures of Carthamus tinctorius L. Appl. Microbiol. Biotechnol. 2011, 89, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Miras-Moreno, B.; Almagro, L.; Pedreño, M.A.; Sabater-Jara, A.B. Enhanced accumulation of phytosterols and phenolic compounds in cyclodextrin-elicited cell suspension culture of Daucus carota. Plant Sci. 2016, 250, 154–164. [Google Scholar] [CrossRef]
- Shintani, D.; DellaPenna, D. Elevating the vitamin E content of plants through metabolic engineering. Science 1998, 282, 2098–2100. [Google Scholar] [CrossRef] [PubMed]
- Britz, S.J.; Kremer, D.F.; Kenworthy, W.J. Tocopherols in Soybean Seeds: Genetic Variation and Environmental Effects in Field-Grown Crops. J. Am. Oil Chem. Soc. 2008, 85, 931–936. [Google Scholar] [CrossRef]
- Giurizatto, M.I.K.; Ferrarese-Filho, O.; Ferrarese, M.L.L.; Robaina, A.D.; Gonçalves, M.C.; Cardoso, C.A.L. α-Tocopherol levels in natural and artificial aging of soybean seeds. Acta Sci. Agron. 2012, 34, 339–343. [Google Scholar] [CrossRef]
- Britz, S.; Kremer, D. Warm Temperatures or Drought during Seed Maturation Increase Free r-Tocopherol in Seeds of Soybean (Glycine max [L.] Merr.). J. Agric. Food Chem. 2002, 50, 6058–6063. [Google Scholar] [CrossRef] [PubMed]
- Seguin, P.; Tremblay, G.; Pageau, D.; Liu, W. Soybean Tocopherol Concentrations Are Affected by Crop Management. J. Agric. Food Chem. 2010, 58, 5495–5501. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, A.; Yamada, T.; Fujimoto, K.; Endo, Y.; Kitamura, K. Identification of soybean varieties with high α-tocopherol content. Breed. Sci. 2005, 55, 123–125. [Google Scholar] [CrossRef]
- Mureșan, L.; Clapa, D.; Borsai, O.; Rusu, T.; Wang, T.T.; Park, J.B. Potential Impacts of Soil Tillage System on Isoflavone Concentration of Soybean as Functional Food Ingredients. Land 2020, 9, 386. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Pradhan, S.; Singh, S.K.; Srivastav, M.; Prakash, J.; Lal, S.K.; Padaria, J.C.; Goswami, A.K.; Maurya, N.K. Poly ethylene glycol mediated in vitro screening and physico-biochemical changes induced in mango callus due to moisture stress. Plant Cell Tissue Organ. Cult. 2021, 145, 155–172. [Google Scholar] [CrossRef]
- Daffalla, H.M.; Elsheikh, A.M.; Ali, H.A.; Khalafalla, M.M. Callus maintenance and cell line selection of Grewia Tenax. J. Herbs Spices Med. Plants 2019, 25, 218–235. [Google Scholar] [CrossRef]
- Kim, J.A.; Chung, I.M. Changes in isoflavones concentration of soybean (Glycine max) seeds at different growth stages. J. Sci. Food Agric. 2007, 87, 496–503. [Google Scholar] [CrossRef]
- Wang, S.; Kanamaru, K.; Li, W.; Abe, J.; Yamada, T.; Kitamura, K. Simultaneous accumulation of high contents of α-tocopherol and lutein is possible in seeds of soybean (Glycine max (L.) Merr.). Breed Sci. 2007, 57, 297–304. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mureșan, L.; Clapa, D.; Rusu, T.; Wang, T.T.Y.; Park, J.B. Soybean Callus—A Potential Source of Tocopherols. Plants 2021, 10, 2571. https://doi.org/10.3390/plants10122571
Mureșan L, Clapa D, Rusu T, Wang TTY, Park JB. Soybean Callus—A Potential Source of Tocopherols. Plants. 2021; 10(12):2571. https://doi.org/10.3390/plants10122571
Chicago/Turabian StyleMureșan, Liliana, Doina Clapa, Teodor Rusu, Thomas T. Y. Wang, and Jae B. Park. 2021. "Soybean Callus—A Potential Source of Tocopherols" Plants 10, no. 12: 2571. https://doi.org/10.3390/plants10122571
APA StyleMureșan, L., Clapa, D., Rusu, T., Wang, T. T. Y., & Park, J. B. (2021). Soybean Callus—A Potential Source of Tocopherols. Plants, 10(12), 2571. https://doi.org/10.3390/plants10122571







