Biochemical Changes and Antioxidant Variations in Date Palm (Phoenix dactylifera L.) Varieties during Flower Induction and Development
Abstract
:1. Introduction
2. Results
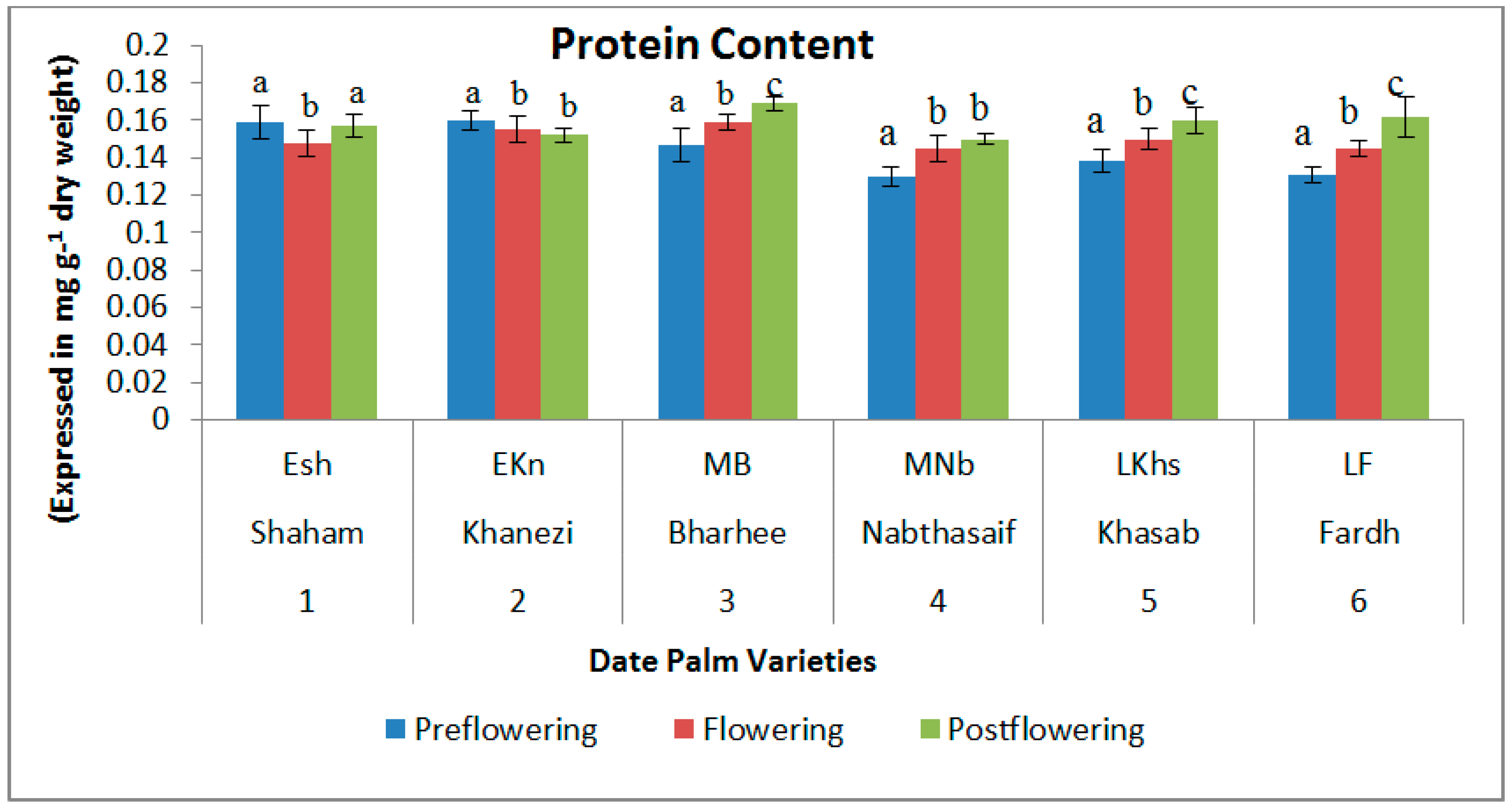
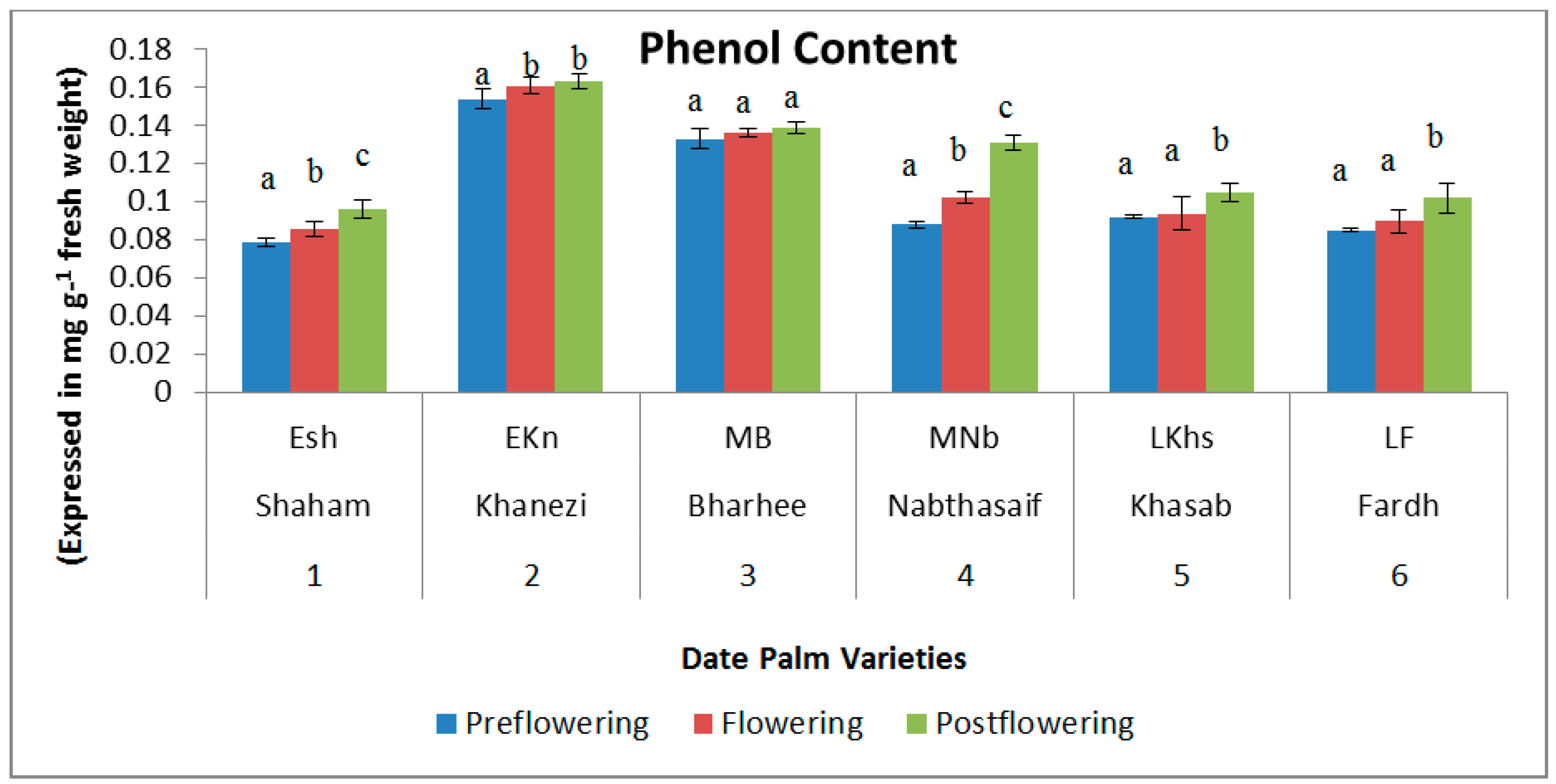
2.1. Biochemical Contents
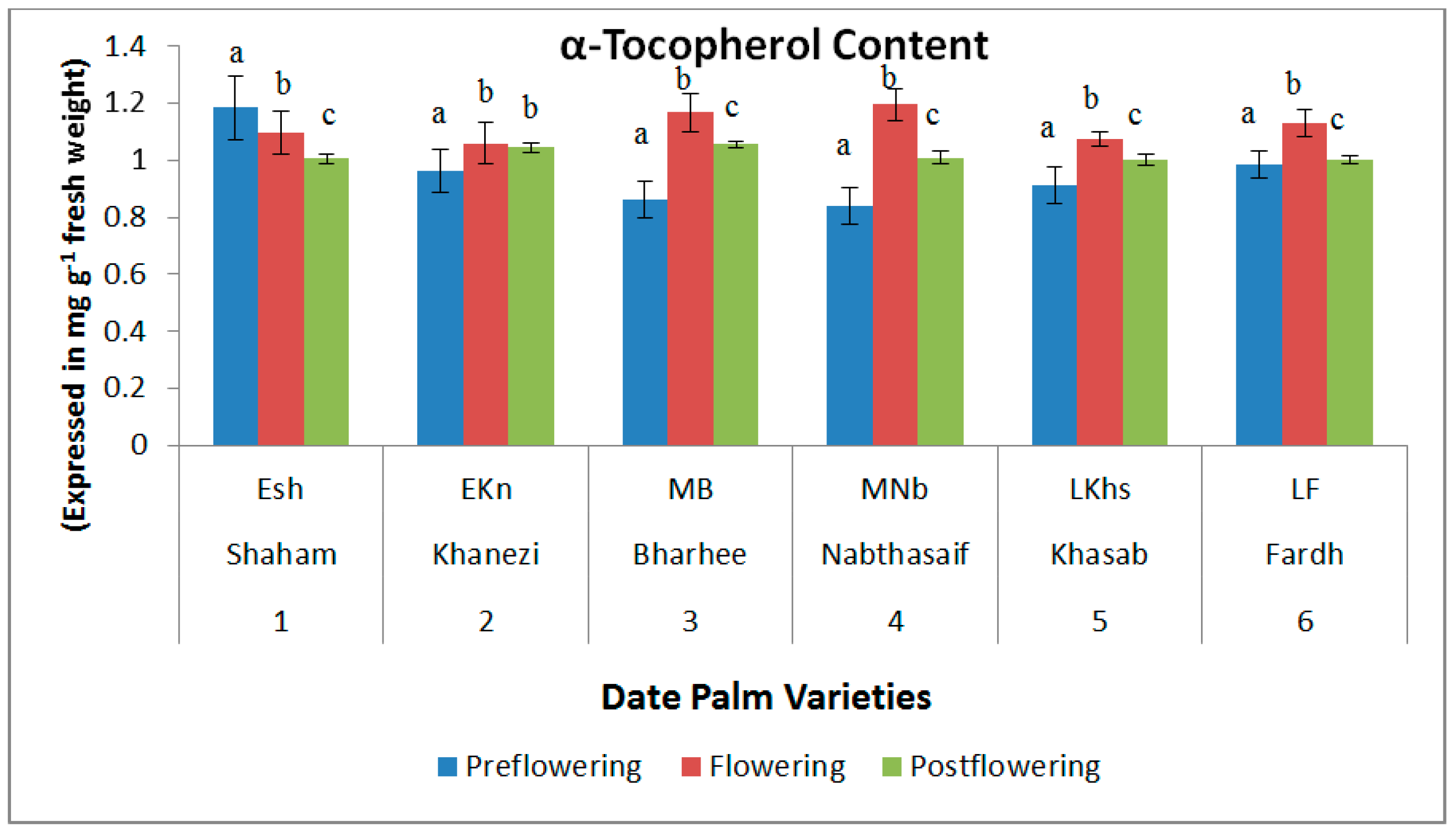
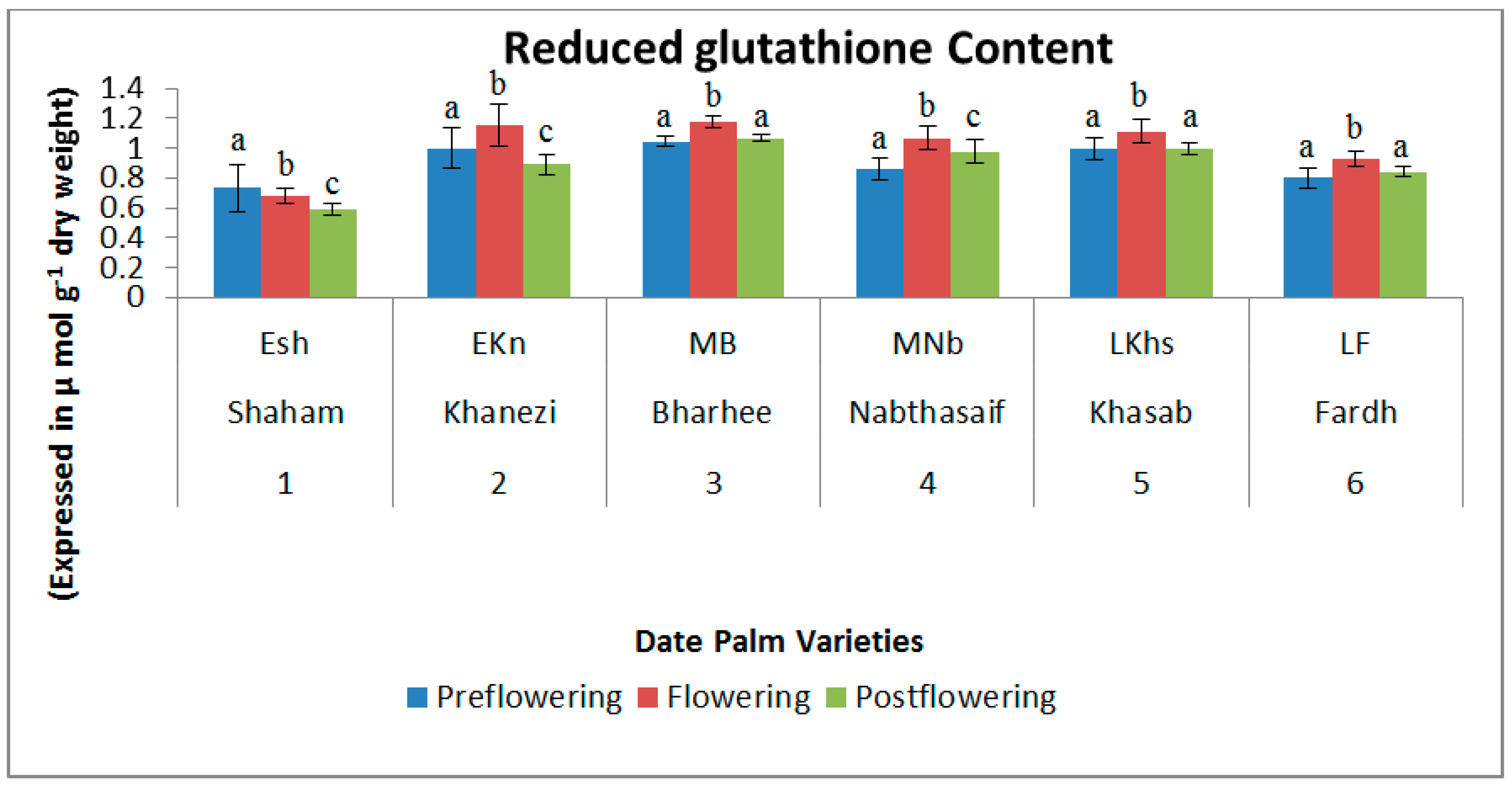
2.2. Non-Enzymatic Antioxidants
2.3. Antioxidant Enzymes
3. Discussion
4. Materials and Methods
- (i)
- Early season—Shaham (ESh) and Khanezi (EKn);
- (ii)
- Mid-late season—Barhee (MBr) and Nabthasaif (MNb);
- (iii)
- Late season—Khasab (LKh) and Fardh (LFr).
4.1. Biochemical Analysis
4.2. Non-Enzymatic Antioxidants
4.3. Antioxidant Enzymes
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaid, A.; De Wet, P.F. Chapter IV Climatic requirements of date palm. FAO Plant Prod. Prot. Pap. 1999, 156, 58–73. [Google Scholar]
- Krueger, R.R. Date Palm (Phoenix dactylifera L.) Biology and Utilization. In The Date Palm Genome; Springer: Cham, Switzerland, 2021; Volume 1, pp. 3–28. [Google Scholar]
- Al-Ameri, A.A.; Al-Qurainy, F.; Gaafar, A.R.Z.; Khan, S.; Nadeem, M. Male specific gene expression in dioecious Phoenix dactylifera (date palm) tree at flowering stage. Pak. J. Bot. 2016, 48, 131–135. [Google Scholar]
- Masmoudi-Allouche, F.; Meziou, B.; Kriaâ, W.; Gargouri-Bouzid, R.; Drira, N. In vitro flowering induction in date palm (Phoenix dactylifera L.). J. Plant Growth Regul. 2010, 29, 35–43. [Google Scholar] [CrossRef]
- Saleh, E.A.; Tawfik, M.S.; Abu-Tarboush, H.M. Phenolic contents and antioxidant activity of various date palm (Phoenix dactylifera L.) fruits from Saudi Arabia. Food Nutr. Sci. 2011, 2, 1134. [Google Scholar]
- Awad, M.A.; Al-Qurashi, A.D.; Mohamed, S.A. Mohamed. Antioxidant capacity, antioxidant compounds and antioxidant enzyme activities in five date cultivars during development and ripening. Sci. Hortic. 2011, 129, 688–693. [Google Scholar] [CrossRef]
- Othmani, A.; Collin, M.; Sellemi, A.; Jain, S.M.; Drira, N.; Aberlenc, F. First reported case of spontaneous hermaphrodism in female date palm (Phoenix dactylifera L.), cv ‘Alligue’. J. Hortic. Sci. Biotechnol. 2017, 92, 376–388. [Google Scholar]
- Cheruth, A.J.; Kurup, S.S.; Subramaniam, S. Variations in hormones and antioxidant status in relation to flowering in early, mid, and late varieties of date palm (Phoenix dactylifera) of United Arab Emirates. Sci. World J. 2015, 2015, 846104. [Google Scholar] [CrossRef] [Green Version]
- Purayil, F.T.; Robert, G.A.; Gothandam, K.M.; Kurup, S.S.; Subramaniam, S.; Jaleel, A. Genetic variability in selected date palm (Phoenix dactylifera L.) cultivars of United Arab Emirates using ISSR and DAMD markers. 3 Biotech 2018, 8, 109. [Google Scholar] [CrossRef]
- Yari, V.; Roein, Z.; Sabouri, A. Exogenous 5-azaCitidine accelerates flowering and external GA3 increases ornamental value in Iranian Anemone accessions. Sci. Rep. 2021, 11, 7478. [Google Scholar] [CrossRef]
- Salimonti, A.; Forgione, I.; Sirangelo, T.M.; Puccio, G.; Mauceri, A.; Mercati, F.; Sunseri, F.; Carbone, F. A complex gene network mediated by ethylene signal transduction tfs defines the flower induction and differentiation in Olea europaea L. Genes 2021, 12, 545. [Google Scholar] [CrossRef]
- Ebrahimzadeh, H.; Abrishamchi, P. Changes in IAA, phenolic compounds, peroxidase, IAA oxidase, and polyphenol oxidase in relation to flower formation in Crocus sativus. Russ. J. Plant Physiol. 2001, 48, 190–195. [Google Scholar] [CrossRef]
- Nakayama, T.; Yonekura-Sakakibara, K.; Sato, T.; Kikuchi, S.; Fukui, Y.; Fukuchi-Mizutani, M.; Ueda, T.; Nakao, M.; Tanaka, Y.; Kusumi, T.; et al. Aureusidin synthase: A polyphenol oxidase homolog responsible for flower coloration. Science 2000, 290, 1163–1166. [Google Scholar] [CrossRef]
- Chin, D.C.; Hsieh, C.C.; Lin, H.Y.; Yeh, K.W. A low glutathione redox state couples with a decreased ascorbate redox ratio to accelerate flowering in Oncidium orchid. Plant Cell Physiol. 2016, 57, 423–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslmoshtaghi, E.; Shahsavar, A.R. Biochemical changes involved in self-incompatibility in two cultivars of olive (Olea europaea L.) during flower development. J. Hortic. Sci. Biotechnol. 2016, 91, 189–195. [Google Scholar] [CrossRef]
- Selvarajan, E.; Veena, R.; Kumar, N.M. Polyphenol oxidase, beyond enzyme browning. In Microbial Bioprospecting for Sustainable Development; Springer: Singapore, 2018; pp. 203–222. [Google Scholar]
- Lin, C. Photoreceptors and regulation of flowering time. Plant Physiol. 2000, 123, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Notaguchi, M.; Abe, M.; Kimura, T.; Daimon, Y.; Kobayashi, T.; Yamaguchi, A.; Araki, T. Long-distance, graft-transmissible action of Arabidopsis flowering locus T protein to promote flowering. Plant Cell Physiol. 2008, 49, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuPont, F.M.; Altenbach, S.B. Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J. Cereal Sci. 2003, 38, 133–146. [Google Scholar] [CrossRef]
- Amasino, R. Seasonal and developmental timing of flowering. Plant J. 2010, 61, 1001–1013. [Google Scholar] [CrossRef]
- Kalra, G.; Lal, M.A. Physiology of Flowering. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2018; pp. 797–819. [Google Scholar]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Ferrarese-Filho, O. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Keller, M.; Hrazdina, G. Interaction of nitrogen availability during bloom and light intensity during veraison. II. Effects on anthocyanin and phenolic development during grape ripening. Am. J. Enol. Vitic. 1998, 49, 341–349. [Google Scholar]
- Del Baño, M.J.; Lorente, J.; Castillo, J.; Benavente-García, O.; del Río, J.A.; Ortuño, A.; Quirin, A.K.-W.; Gerard, D. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. J. Agric. Food Chem. 2003, 51, 4247–4253. [Google Scholar] [CrossRef]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; González, E.M.; Larrainzar, E. Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front. Plant Sci. 2017, 8, 1042. [Google Scholar] [CrossRef] [Green Version]
- Xiang, N.; Hu, J.; Wen, T.; Brennan, M.A.; Brennan, C.S.; Guo, X. Effects of temperature stress on the accumulation of ascorbic acid and folates in sweet corn (Zea mays L.) seedlings. J. Sci. Food Agric. 2020, 100, 1694–1701. [Google Scholar] [CrossRef]
- Bilska, K.; Wojciechowska, N.; Alipour, S.; Kalemba, E.M. Ascorbic Acid—The Little-Known Antioxidant in Woody Plants. Antioxidants 2019, 8, 645. [Google Scholar] [CrossRef] [Green Version]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Nagar, P.K. Changes in abscisic acid and phenols during flower development in two diverse species of rose. Acta Physiol. Plant. 2003, 25, 411–416. [Google Scholar] [CrossRef]
- Upadhyaya, D.C.; Bagri, D.S.; Upadhyaya, C.P.; Kumar, A.; Thiruvengadam, M.; Jain, S.K. Genetic engineering of potato (Solanum tuberosum L.) for enhanced α-tocopherols and abiotic stress tolerance. Physiol. Plant 2021, 173, 116–128. [Google Scholar] [CrossRef]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione Metabolism in Plants under Stress: Beyond Reactive Oxygen Species Detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, G.; Dong, Y.; Qian, X.; Li, J.; Xu, X.; Huang, H.; Xu, L.; Li, L. Screening of Key Proteins Affecting Floral Initiation of Saffron Under Cold Stress Using iTRAQ-Based Proteomics. Front. Plant Sci. 2021, 12, 708. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balfagón, D.; Zandalinas, S.I.; Baliño, P.; Muriach, M.; Gómez-Cadenas, A. Involvement of ascorbate peroxidase and heat shock proteins on citrus tolerance to combined conditions of drought and high temperatures. Plant Physiol. Biochem. 2018, 127, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Zhanassova, K.; Kurmanbayeva, A.; Gadilgereyeva, B.; Yermukhambetova, R.; Iksat, N.; Amanbayeva, U.; Bekturova, A.; Tleukulova, Z.; Omarov, R.; Masalimov, Z. ROS status and antioxidant enzyme activities in response to combined temperature and drought stresses in barley. Acta Physiol. Plant 2021, 43, 1–12. [Google Scholar] [CrossRef]
- Berwal, M.; Ram, C. Superoxide dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. Abiotic Biot. Stress Plants 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yaman, S.O.; Ayhanci, A. Lipid Peroxidation. In Lipid Peroxidation; IntechOpen: London, UK, 2021. [Google Scholar]
- Cembrowska-Lech, D.; Koprowski, M.; Kępczyński, J. Germination induction of dormant Avena fatua caryopses by KAR1 and GA3 involving the control of reactive oxygen species (H2O2 and O2−) and enzymatic antioxidants (superoxide dismutase and catalase) both in the embryo and the aleurone layers. J. Plant Physiol. 2015, 176, 169–179. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Mannino, G.; Beekwilder, J.; Contartese, V.; Karlova, R.; Bertea, C.M. The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants. Sci. Rep. 2021, 11, 354. [Google Scholar] [CrossRef]
- Abassi, R.; Namsi, A.; Bennasri, M.; Abdalah, H.B.; Mâachia, S.B.; Ouerghi, Z.; Duran-Vila, N. Manganese deficiency is associated with histological changes in date palm fronds showing brittle leaf disease symptoms. J. Plant Pathol. 2014, 96, 29–34. [Google Scholar]
- Tuzet, A.; Rahantaniaina, M.S.; Noctor, G. Analyzing the function of catalase and the ascorbate–glutathione pathway in H2O2 processing: Insights from an experimentally constrained kinetic model. Antioxid. Redox Signal. 2019, 30, 1238–1268. [Google Scholar] [CrossRef]
- Amor, N.; Jimenez, A.; Boudabbous, M.; Sevilla, F.; Abdelly, C. Implication of peroxisomes and mitochondria in the halophyte Cakile maritima tolerance to salinity stress. Biol. Plant 2019, 63, 113–121. [Google Scholar] [CrossRef]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A large family of class III plant peroxidases. Plant Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, R.; Kunkowska, A.B.; Schippers, J.H. Role of reactive oxygen species during cell expansion in leaves. Plant Physiol. 2016, 172, 2098–2106. [Google Scholar] [CrossRef] [Green Version]
- Kasraoui, M.F.; Duquesnoy, I.; Winterton, P.; Lamaze, T. Soluble and cell wall bound peroxidase activities are markers of flower bud development stages in lemon (Citrus limon L.). J. Appl. Bot. Food Qual. 2014, 87, 1–8. [Google Scholar]
- Liu, F.; Andersen, M.N.; Jensen, C.R. Root signal controls pod growth in drought-stressed soybean during the critical, abortion-sensitive phase of pod development. Field Crop Res. 2004, 85, 159–166. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–253. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Omaye, S.T.; Turnbull, J.D.; Sauberlich, H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979, 62, 3–11. [Google Scholar] [PubMed]
- Baker, H.; Frank, O.; DeAngelis, B.; Feingold, S. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr. Rep. Int. 1980, 21, 531–536. [Google Scholar]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Asada, K.; Takahashi, M. Production and scavenging of active oxygen in photosynthesis. In Photoinhibition; Kyle, D.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 227–287. [Google Scholar]
- Hwang, S.Y.; Lin, H.W.; Chern, R.H.; Lo, H.F.; Li, L. Reduced susceptibility to waterlogging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regul. 1999, 27, 167–172. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Cherry, J.H. Nucleic acid, mitochondria, & enzyme changes in cotyledons of peanut seeds during germination. Plant Physiol. 1963, 38, 440–446. [Google Scholar] [PubMed] [Green Version]
- Chandlee, J.M.; Scandalios, J.G. Analysis of variants affecting the catalase developmental program in maize scutellum. Theor. Appl. Gen. 1984, 69, 71–77. [Google Scholar] [CrossRef]
- Kumar, K.B.; Khan, P.A. Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Indian J. Exp. Bot. 1982, 20, 412–416. [Google Scholar]


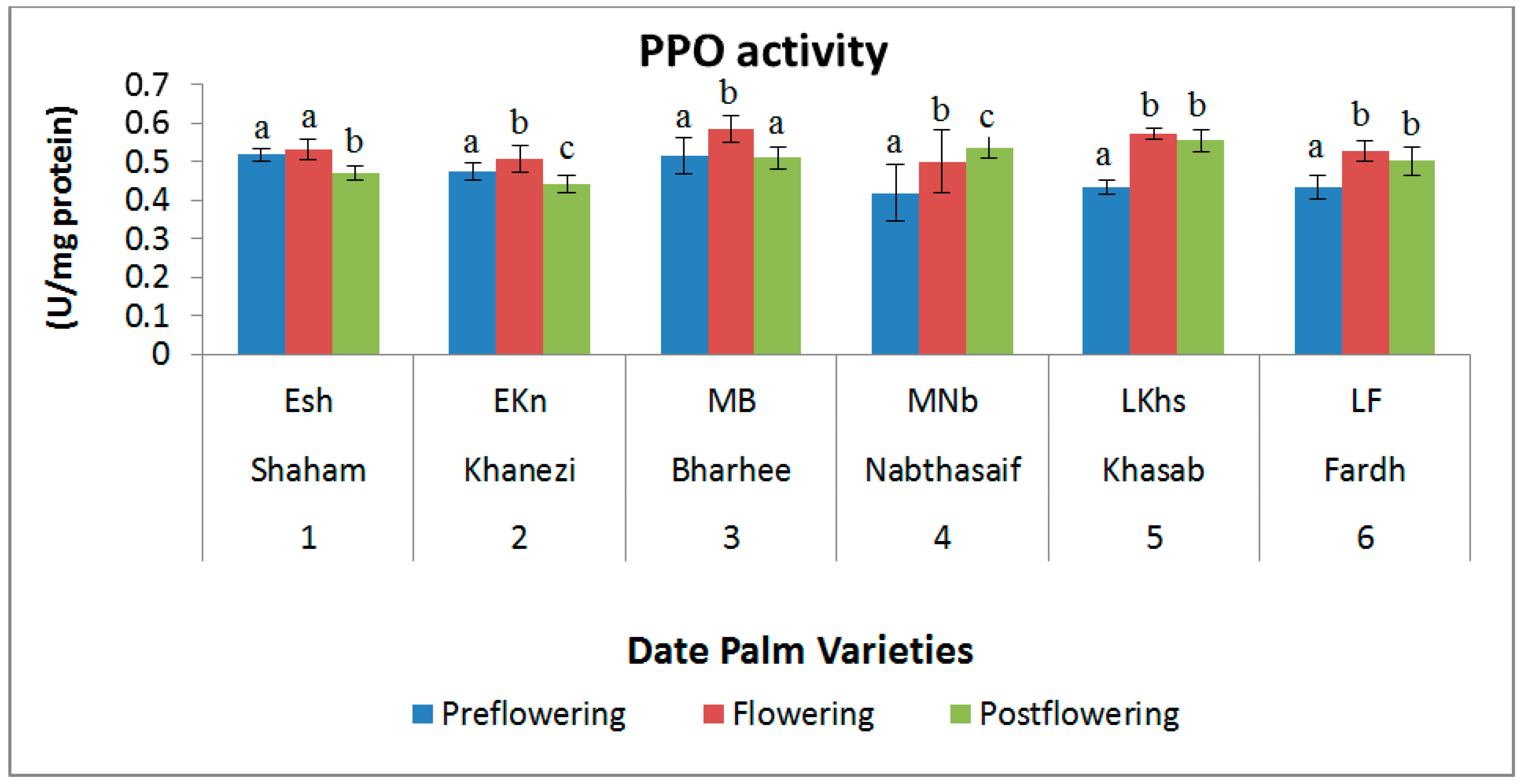
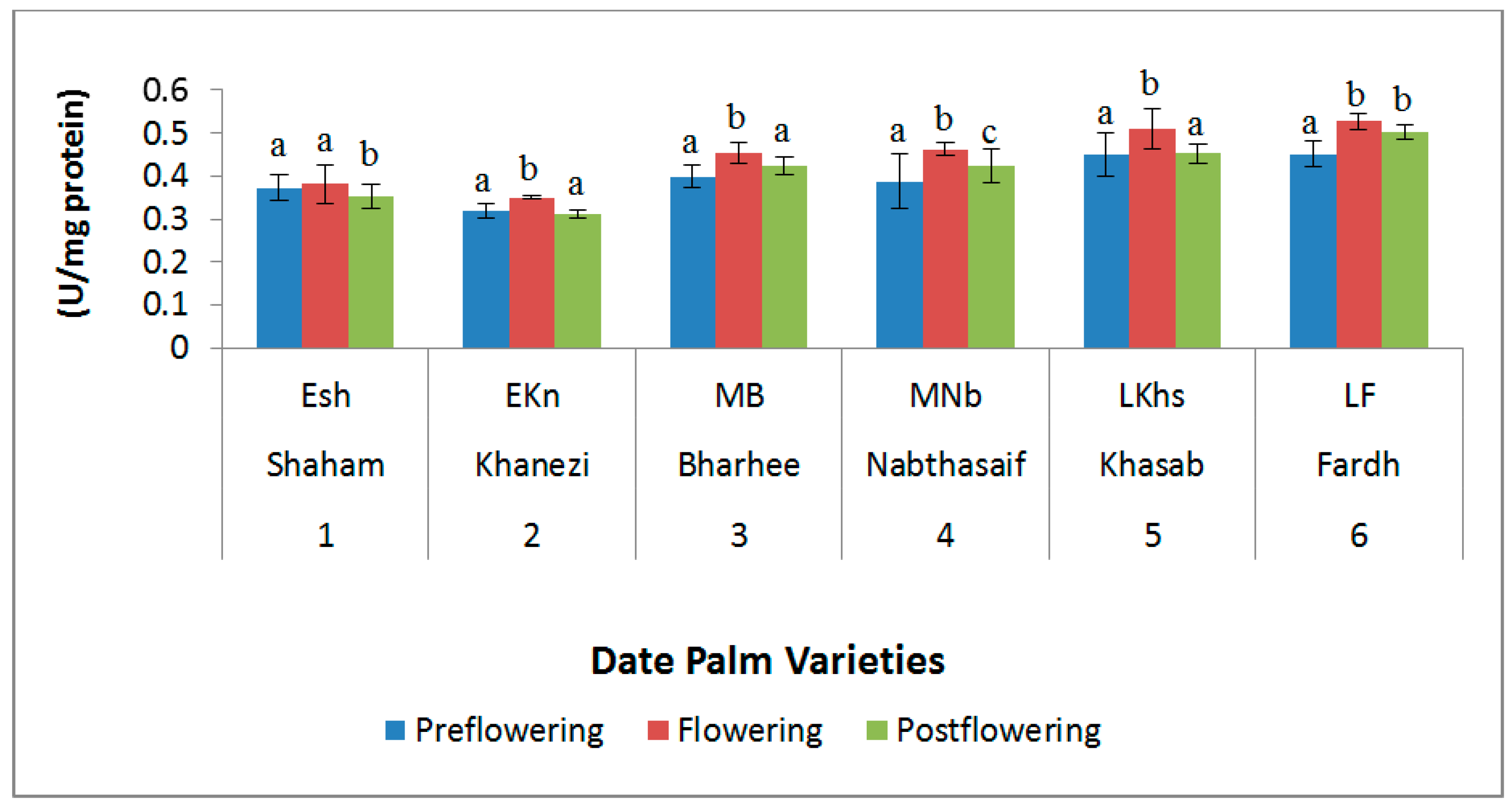
| S. No | Variety Name | Category | Flowering Stages | ||
|---|---|---|---|---|---|
| Preflowering | Flowering | Post Flowering | |||
| 1 | Shaham | Early Flowering | 1.146 ± 0.070 a | 0.768 ± 0.033 b | 0.794 ± 0.042 b |
| 2 | Khanezi | Early Flowering | 1.028 ± 0.097 a | 0.842 ± 0.026 b | 0.985 ± 0.038 b |
| 3 | Bharhee | Mid-late Flowering | 1.190 ± 0.031 a | 0.990 ± 0.040 b | 1.000 ± 0.023 b |
| 4 | Nabthasaif | Mid-late Flowering | 0.832 ± 0.039 a | 0.797 ± 0.028 a | 0.659 ± 0.063 c |
| 5 | Khasab | Late Flowering | 1.040 ± 0.074 a | 0.796 ± 0.024 b | 1.033 ± 0.050 a |
| 6 | Fardh | Late Flowering | 0.935 ± 0.040 a | 0.842 ± 0.064 b | 0.802 ± 0.022 b |
| S. No | Variety Name | Category | Flowering Stages | ||
|---|---|---|---|---|---|
| Preflowering | Flowering | Post Flowering | |||
| 1 | Shaham | Early Flowering | 0.429 ± 0.037 a | 0.371 ± 0.019 b | 0.300 ± 0.026 c |
| 2 | Khanezi | Early Flowering | 0.391 ± 0.024 a | 0.247 ± 0.017 b | 0.302 ± 0.018 c |
| 3 | Bharhee | Mid-late Flowering | 0.502 ± 0.006 a | 0.540 ± 0.026 b | 0.597 ± 0.015 c |
| 4 | Nabthasaif | Mid-late Flowering | 0.333 ± 0.027 a | 0.388 ± 0.032 b | 0.407 ± 0.026 c |
| 5 | Khasab | Late Flowering | 0.338 ± 0.027 a | 0.311 ± 0.020 a | 0.305 ± 0.044 a |
| 6 | Fardh | Late Flowering | 0.313 ± 0.022 a | 0.303 ± 0.044 b | 0.295 ± 0.020 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamsi, S.R.H.A.A.; Rabert, G.A.; Kurup, S.S.; Alyafei, M.A.M.; Jaleel, A. Biochemical Changes and Antioxidant Variations in Date Palm (Phoenix dactylifera L.) Varieties during Flower Induction and Development. Plants 2021, 10, 2550. https://doi.org/10.3390/plants10112550
Shamsi SRHAA, Rabert GA, Kurup SS, Alyafei MAM, Jaleel A. Biochemical Changes and Antioxidant Variations in Date Palm (Phoenix dactylifera L.) Varieties during Flower Induction and Development. Plants. 2021; 10(11):2550. https://doi.org/10.3390/plants10112550
Chicago/Turabian StyleShamsi, Saeed R. H. A. Al, Gabriel A. Rabert, Shyam S. Kurup, Mohammed Abdul Muhsen Alyafei, and Abdul Jaleel. 2021. "Biochemical Changes and Antioxidant Variations in Date Palm (Phoenix dactylifera L.) Varieties during Flower Induction and Development" Plants 10, no. 11: 2550. https://doi.org/10.3390/plants10112550
APA StyleShamsi, S. R. H. A. A., Rabert, G. A., Kurup, S. S., Alyafei, M. A. M., & Jaleel, A. (2021). Biochemical Changes and Antioxidant Variations in Date Palm (Phoenix dactylifera L.) Varieties during Flower Induction and Development. Plants, 10(11), 2550. https://doi.org/10.3390/plants10112550





