Pre-Sowing Treatments Improve Germinability of South Texas Native Plant Seeds
Abstract
:1. Introduction
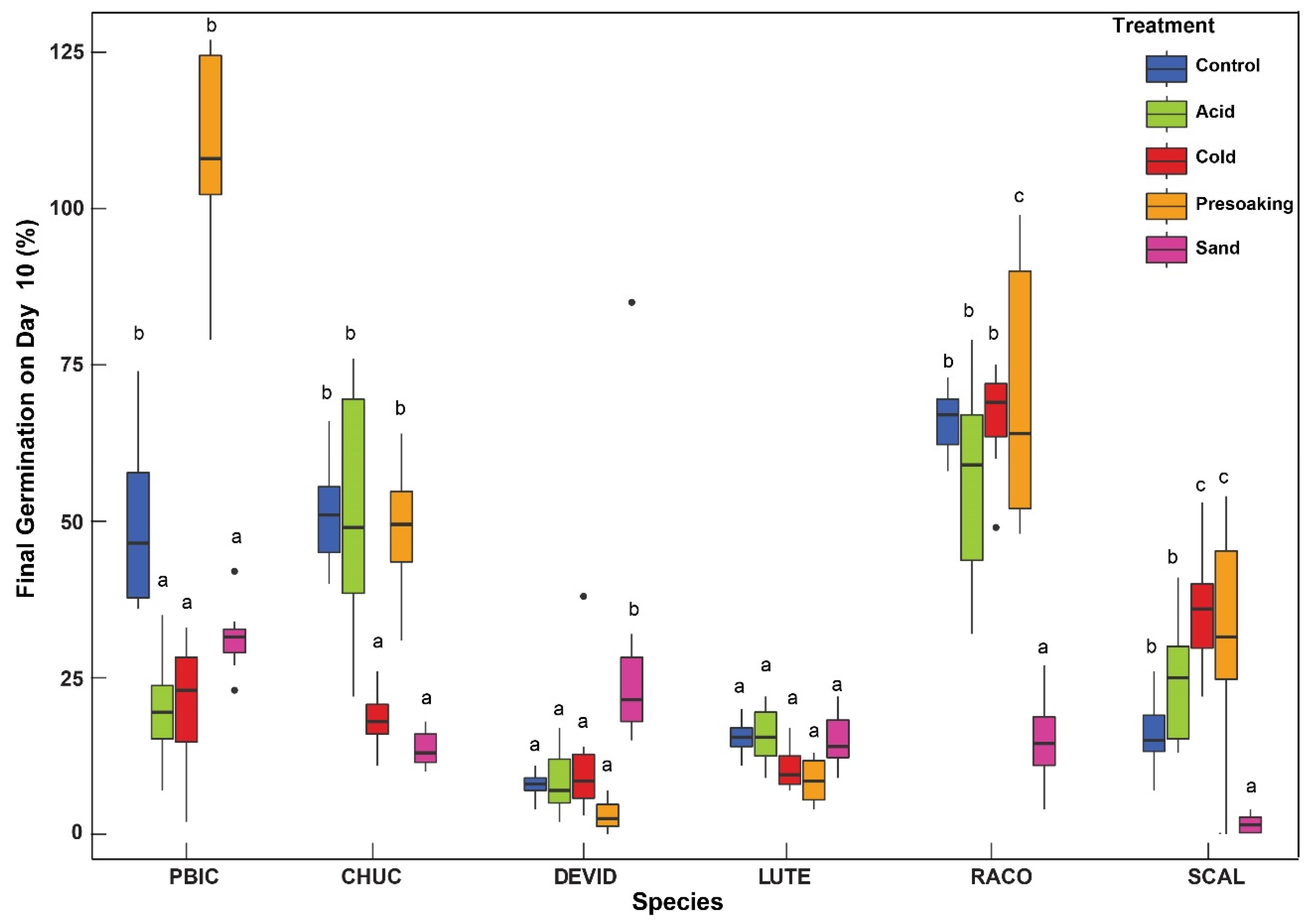
2. Results
Time to Germination
3. Discussion
4. Materials and Methods
4.1. Study Species
4.2. Seed Viability
4.3. Germination Trials
- (a)
- Sandpaper Scarification (SS)
- (b)
- Acid Scarification (AS)
- (c)
- Cold Stratification (CS)
- (d)
- Seed Presoaking (SP)
- (e)
- Control
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorner, J. An Introduction to Using Native Plants in Restoration Projects. Center for Urban Horticulture, University of Washington, Seattle. Available online: http://www.nps.gov/plants/restore/pubs/intronatplant (accessed on 5 October 2021).
- Bratcher, C.B.; Dole, J.M.; Cole, J.C. Stratification Improves Seed Germination of Five Native Wildflower Species. HortScience 1993, 28, 899–901. [Google Scholar] [CrossRef] [Green Version]
- Meyer, S.E. Strategies for seed propagation of native forbs. In National Proceedings: Forest and Conservation Nursery Associations-2005; Riley, L.E., Dumroese, R.K., Landis, T.D., Eds.; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006; Volume 43, pp. 3–9. [Google Scholar]
- Helmers, M.J.; Zhou, X.B.; Asbjornsen, H.; Kolka, R.; Tomer, M.D.; Cruse, R.M. Sediment removal by prairie filter strips in row-cropped ephemeral watersheds. J. Environ. Qual. 2012, 41, 1531. [Google Scholar] [CrossRef] [Green Version]
- Geertsema, W.; Rossing, A.H.W.; Landis, D.A.; Bianchi, F.J.J.A.; van Rijn, P.C.J.; Schaminée, J.H.J.; Tscharntke, T.; van der Werf, W. Actionable knowledge for ecological intensification of agriculture. Front. Ecol. Environ. 2016, 14, 209–216. [Google Scholar] [CrossRef]
- Schellhorn, N.A.; Glatz, R.V.; Wood, G.M. The risk of exotic and native plants as hosts for four pest thrips (Thysanoptera: Thripinae). Bull. Entomol. Res. 2010, 100, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Fielder, A.K.; Landis, D.A. Attractiveness of Michigan native plants to arthropod natural enemies and herbivores. Environ. Entomol. 2007, 36, 751–765. [Google Scholar]
- Martinez, L.; Soti, P.; Kaur, J.; Racelis, A.; Kariyat, R.R. Impact of Cover Crops on Insect Community Dynamics in Organic Farming. Agriculture 2020, 10, 209. [Google Scholar] [CrossRef]
- Elias, S.; Garay, A.; Schweitzer, L.; Hanning, S. Seed Quality Testing of Native Species. Nat. Plants J. 2006, 7, 15–19. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier, Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Ladouceur, E.; Jiménez-Alfaro, B.; Marin, M.; De Vitis, M.; Abbandonato, H.; Iannetta, P.P.; Bonomi, C.; Pritchard, H.W. Native Seed Supply and the Restoration Species Pool. Conserv. Lett. 2017, 11, e12381. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Toorop, P.; Powell, A.; Laverack, G. Tetrazolium staining predicts germination of commercial seed lots of European native species differing in seed quality. Seed Sci. Technol. 2017, 45, 151–166. [Google Scholar] [CrossRef]
- Humphries, T.; Chauhan, B.S.; Florentine, S.K. Environmental factors effecting the germination and seedling emergence of two populations of an aggressive agricultural weed; Nassella trichotoma. PLoS ONE 2018, 13, e0199491. [Google Scholar] [CrossRef]
- Barrera, D.; Luera, J.; Lavallee, K.; Soti, P. Influence of microbial priming and seeding depth on germination and growth of native wildflowers. Ecol. Process. 2021, 10, 19. [Google Scholar] [CrossRef]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, Postgermination Adapatation, and Species Ecological Ranges. Annu. Rev. Ecol. Evol. Systemat. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Silveira, F.A.; Fidelis, A.; Poschlod, P.; Commander, L. Seed germination traits can contribute better to plant community ecology. J. Veg. Sci. 2016, 27, 637–645. [Google Scholar] [CrossRef]
- Adkins, S.W.; Bellairs, S.M.; Loch, D.S. Seed dormancy mechanisms in warm season grass species. Euphytica 2002, 126, 13–20. [Google Scholar] [CrossRef]
- Olff, H.; Ritchie, M.E. Effects of herbivores on grassland plant diversity. Trends Ecol. Evol. 1998, 13, 261–265. [Google Scholar] [CrossRef] [Green Version]
- Cosyns, E.; Delporte, A.; Lens, L.; Hoffmann, M. Germination success of temperature grassland species after passage through ungulate and rabbit guts. J. Ecol. 2005, 93, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Ramos, M.E.; Robles, A.B.; Castro, J. Efficiency of endozoochorous seed dispersal in six dry-fruited species (Cistaceae): From seed ingestion to early seedling establishment. Plant Ecol. 2006, 185, 97–106. [Google Scholar] [CrossRef]
- Rehman, S.; Park, I.H. Effect of scarification, GA, and chilling on the germination of Goldenrain-tree (Koelreuteria paniculata Laxm.) seeds. Sci. Horticult. 2000, 85, 319–324. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Germinating Seeds of Wildflowers, an Ecological Perspective. HortTechnology 2004, 14, 467–473. [Google Scholar] [CrossRef]
- Pedrini, S.; Gibson-Roy, P.; Trivedi, C.; Gálvez-Ramírez, C.; Hardwick, K.; Shaw, N.; Dixon, K. Collection and production of native seeds for ecological restoration. Restor. Ecol. 2020, 28, S228–S238. [Google Scholar] [CrossRef]
- De Vitis, M.; Hay, F.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: Maintaining seed viability and vigor for restoration use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Hu, D.; Baskin, J.M.; Baskin, C.C.; Yang, X.; Huang, Z. Ecological role of physical dormancy in seeds of Oxytropis racemosa in a semiarid sandland with unpredictable rainfall. J. Plant Ecol. 2017, 11, 542–552. [Google Scholar] [CrossRef]
- Ali, A.S.; Elozeiri, A.A. Metabolic Processes during Seed Germination. Adv. Seed Biol. 2017, 141–166. [Google Scholar] [CrossRef] [Green Version]
- Jurado, E.; Westoby, M. Germination biology of selected central Australian plants. Austral Ecol. 1992, 17, 341–348. [Google Scholar] [CrossRef]
- Duncan, C.; Schultz, N.; Lewandrowski, W.; Good, M.K.; Cook, S. Lower dormancy with rapid germination is an important strategy for seeds in an arid zone with unpredictable rainfall. PLoS ONE 2019, 14, e0218421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wainwright, C.E.; Wolkovich, E.M.; Cleland, E.E. Seasonal priority effects: Implications for invasion and restoration in a semi-arid system. J. Appl. Ecol. 2012, 49, 234–241. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P.; Osborne, B.A. Timing is everything: Does early and late germination favor invasions by herbaceous alien plants? J. Plant Ecol. 2018, 11, 4–16. [Google Scholar] [CrossRef]
- Rincón-Rosales, R.; Culebro-Espinosa, N.R.; Gutierrez-Miceli, F.A.; Dendooven, L. Scarification of seeds of Acacia angustissima (Mill.) Kuntze and its effect on germination. Seed Sci. Technol. 2003, 31, 301–307. [Google Scholar] [CrossRef]
- Salazar, A. Effects of mechanical and acid scarification on germination performance of Schizolobium parahyba (Fabaceae –Caesalpinioideae) seeds. J. Trop. Biol. Conserv. (JTBC) 2019, 213–227. [Google Scholar]
- Islam, M.; Kimura, E. Seed Scarification Methods and their Use in Forage Legumes. Res. J. Seed Sci. 2012, 5, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Heuzé, V.; Tran, G.; Hassoun, P.; Lebas, F. Slender Grama (Bouteloua repens). Feedipedia. 2017. Available online: https://www.feedipedia.org/content/slender-grama-bouteloua-repens-0 (accessed on 5 October 2021).
- Smith, F. Plant Profile–Slender grama (Bouteloua repens). Nativ. Plants J. 2009, 10, 295–299. [Google Scholar] [CrossRef]
- Native American Seed. Chloris cuculatta—Hooded Windmill Grass. 2020. Available online: https://www.seedsource.com/catalog/detail.asp?PRODUCT_ID=2054 (accessed on 21 October 2021).
- Lloyd-Reilley, J. Plant Guide for Pink Pappusgrass (Pappophorum Bicolor); USDA-NRCS, E. “Kika” de la Garza Plant Material Center: Kingsville, TX, USA, 2010.
- USDA-NRCS. Welder Germplasm– Shortspike Windmillgrass; E. “Kika” de la Garza, Plant Materials Center: Kingsville, TX, USA, 2007.
- USDA-NRCS. Purple Prairie Clover, Dalea Purpurea Vent. Available online: https://www.westernnativeseed.com/plant%20guides/dalpurpg.pdf (accessed on 5 October 2021).
- Lloyd-Reilley, J. Plant Guide for Prairie Acacia (Acacia Angustissima var. hirta); USDA-NRCS; USDA Natural Resources Conservation Service, E. “Kika” de la Garza Plant Materials Center: Kingsville, TX, USA, 2011.
- Lloyd-Reilley, J.; Maher, S.D. Plant Fact Sheet- Prostrate Bundleflower (Desmanthus virgatus (L.) Willd. var. depressus (Willd.)); Turner, B.L., Ed.; USDA-NRCS. E. “Kika” del la Garza Plant Material Center: Kingsville, TX, USA, 2008.
- Parsons, J.M.; Davis, T.D.; George, S.W.; Mackay, W.A. Barbara Bush’ Bluebonnet (Lupinus texensis Hook.). HortScience 1994, 29, 1202. [Google Scholar] [CrossRef] [Green Version]
- Winslow, S. Plant Fact Sheet Prairie Coneflower (Ratibida columnifera) (Nutt.) Woot. & Standl; USDA-NRCS. Bridger Plant Materials Center: Bridger, MT, USA, 2006.
- Smith, J. Release Brochure for Plateau (Simsia Calva); USDA-NRCS, Plant Material Center: Knox City, TX, USA, 2012.
- Lloyd-Reilley, J.; Maher, S.D. Plant Fact Sheet–Orange Zexmenia (Wedelia Texana); Gray, A., Turner, B.L., Eds.; USDA-NRCS. E. “Kika” del la Garza Plant Material Center: Kingsville, TX, USA, 2008.
- Verma, P.; Majee, M. Seed Germination and Viability Test in Tetrazolium (TZ) Assay. Bio-Protocol 2013, 3, e884. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, B.E.; Rivero, L.; Calhoun, C.S.; Grotewold, E.; Brkljacić, J. Standardized Method for High-throughput Sterilization of Arabidopsis Seeds. J. Vis. Exp. 2017, 17, e56587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, A.; Demesyeux, L.; Moon, P.; Fu, Y. Optimization of miracle fruit (Synsepalum dulcificum) seed germination and mutagenesis. Afr. J. Food Sci. Technol. 2018, 9. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Liu, R. H2016. Effect of germination on lignin biosynthesis, and antioxidant and antiproliferative activities in flaxseed (Linum usitatissimum L.). Food Chem. 2016, 205, 170–177. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Chavana, J.; Singh, S.; Vazquez, A.; Christoffersen, B.; Racelis, A.; Kariyat, R.R. Local adaptation to continuous mowing makes the noxious weed Solanum elaeagnifolium a superweed candidate by improving fitness and defense traits. Sci. Rep. 2021, 11, 6634. [Google Scholar] [CrossRef]
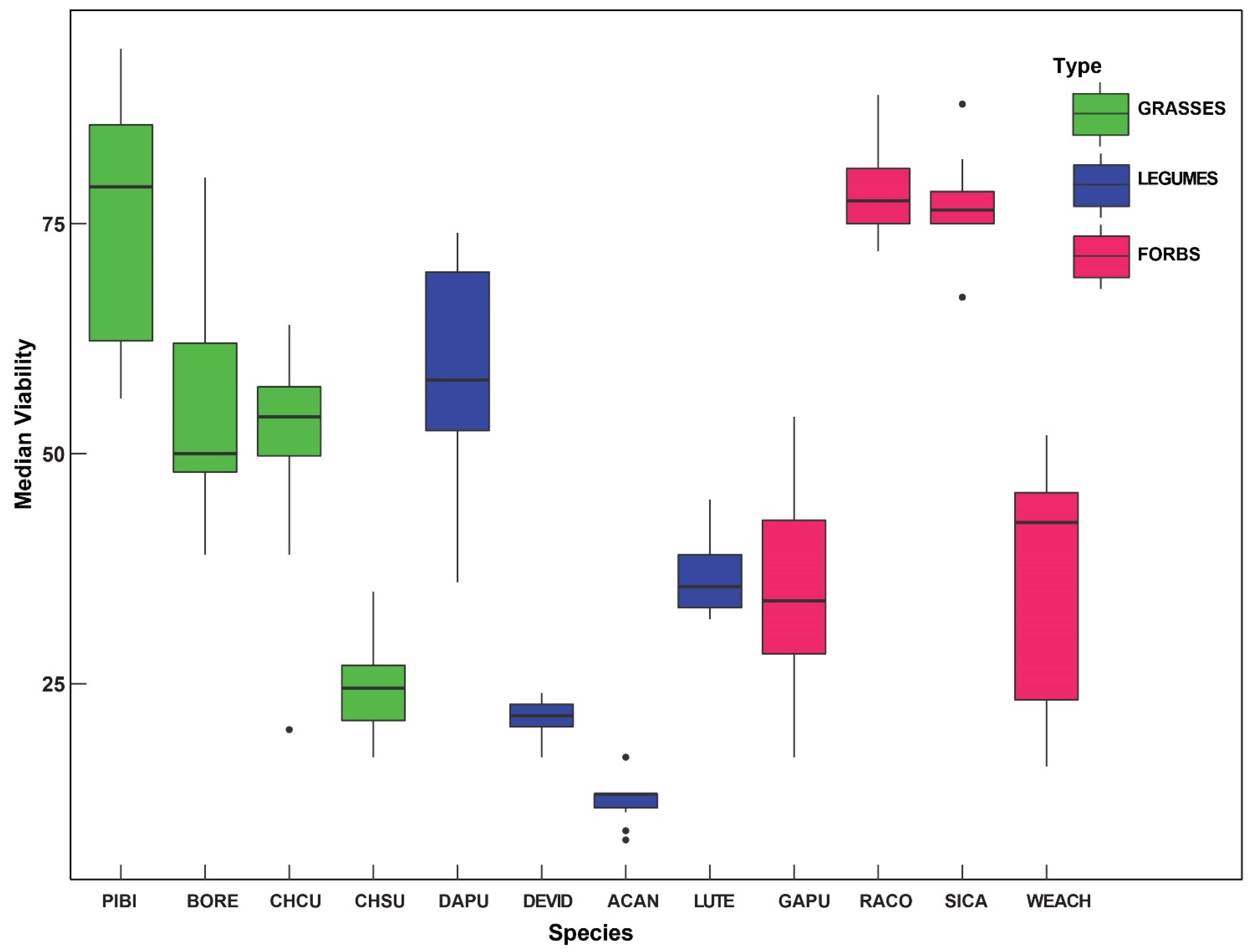

| Species | Mean/Median Germination (n = 100) Rate (SD) | Median Viability (%) |
|---|---|---|
| Ratibidia columnifera | 54.8/62 (24.2) | 78.7 |
| Chloris cuculllata | 36.7/40 (20.0) | 50.5 |
| Pappophorum bicolor | 46.4/32.5 (35.7) | 75.2 |
| Simsia calva | 22/21.5 (15.5) | 76.3 |
| Lupinus texensis | 13/13 (4.62) | 36.7 |
| Desmanthus virgatus var depressus | 11.8/8 (13.4) | 21.4 |
| Bouteloua repens | 7.38/6.5 (4.99) | 49.2 |
| Acaciella angustissima | 5.08/3 (4.91) | 12.3 |
| Chloris subdolistachya | 4.78/2 (6.52) | 24.8 |
| Dalea purpurea | 2.38/2 (2.07) | 59.0 |
| Gaillardia pulchella | 4.6/0 (9.99) | 35.5 |
| Wedelia acapulcensis var. hispida | 0.42/0 (1.2) | 36.6 |
| Plant Type | Common Name | Scientific Name | Characteristics | Reference |
|---|---|---|---|---|
| Grass | Slender Grama | Bouteloua repens | Drought tolerant Perennial grass Highly competitive with invasives | [34,35] |
| Hooded Windmill Grass | Chloris cuculllata | Perennial grass Livestock forage High potential for habitat restoration | [36] | |
| Pink Pappusgrass | Pappophorum bicolor | Perennial grass Livestock forage High potential for rangeland restoration | [37] | |
| Shortspike Windmill Grass | Chloris subdolistachya | Perennial grass Used in roadside and rangeland restoration Highly competitive with invasives | [38] | |
| Legume | Purple Prairie Clover | Dalea purpurea | Perennial herbaceous plant Nectar and pollen attract diverse insects Improves soil nutrient status | [39] |
| Prairie Acacia | Acaciella angustissima | Perennial herbaceous plant Wildlife and livestock forage High potential for habitat restoration, soil reclamation sites | [40] | |
| Prostrate Bundleflower | Desmanthus virgatus var. depressus | Perennial herb Forage for cattle and white-tailed deer Seeds are food for bobwhite quail, Rio Grande turkey | [41] | |
| Blue Bonnet | Lupinus texensis | Winter annual flowering plant Attractive to pollinators | [42] | |
| Forb | Indian Blanket | Gaillardia pulchella | Annual flowering plant Livestock forage Reseeds easily, easy to maintain Commonly used in roadside plantings | |
| Mexican Hat | Ratibidia columnifera | Perennial wildflower Young leaves used for livestock grazing The seeds feed birds and small mammals. Nectar and pollen attract diverse insects | [43] | |
| Bush Sunflower | Simsia calva | Simi-woody perennial forb Palatable to sheep, goat, deer, and bird. The border patch butterfly caterpillar feed on the leaves. Nectar plant for insects | [44] | |
| Orange Zexmenia | Wedelia acapulcensis var. hispida | Perennial flowering plant Drought tolerant Recommended for landscaping on roadsides and native gardens Browsed by deer, cattle, sheep, goats, bobwhite quail. Nectar plant for butterflies, bees, and other nectar-loving insects | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavallee, K.; Soti, P.G.; Rodrigo, H.; Kariyat, R.; Racelis, A. Pre-Sowing Treatments Improve Germinability of South Texas Native Plant Seeds. Plants 2021, 10, 2545. https://doi.org/10.3390/plants10112545
Lavallee K, Soti PG, Rodrigo H, Kariyat R, Racelis A. Pre-Sowing Treatments Improve Germinability of South Texas Native Plant Seeds. Plants. 2021; 10(11):2545. https://doi.org/10.3390/plants10112545
Chicago/Turabian StyleLavallee, Kaitlynn, Pushpa Gautam Soti, Hansapani Rodrigo, Rupesh Kariyat, and Alexis Racelis. 2021. "Pre-Sowing Treatments Improve Germinability of South Texas Native Plant Seeds" Plants 10, no. 11: 2545. https://doi.org/10.3390/plants10112545
APA StyleLavallee, K., Soti, P. G., Rodrigo, H., Kariyat, R., & Racelis, A. (2021). Pre-Sowing Treatments Improve Germinability of South Texas Native Plant Seeds. Plants, 10(11), 2545. https://doi.org/10.3390/plants10112545







