Geometric Models for Seed Shape Description and Quantification in the Cactaceae
Abstract
:1. Introduction
2. Results
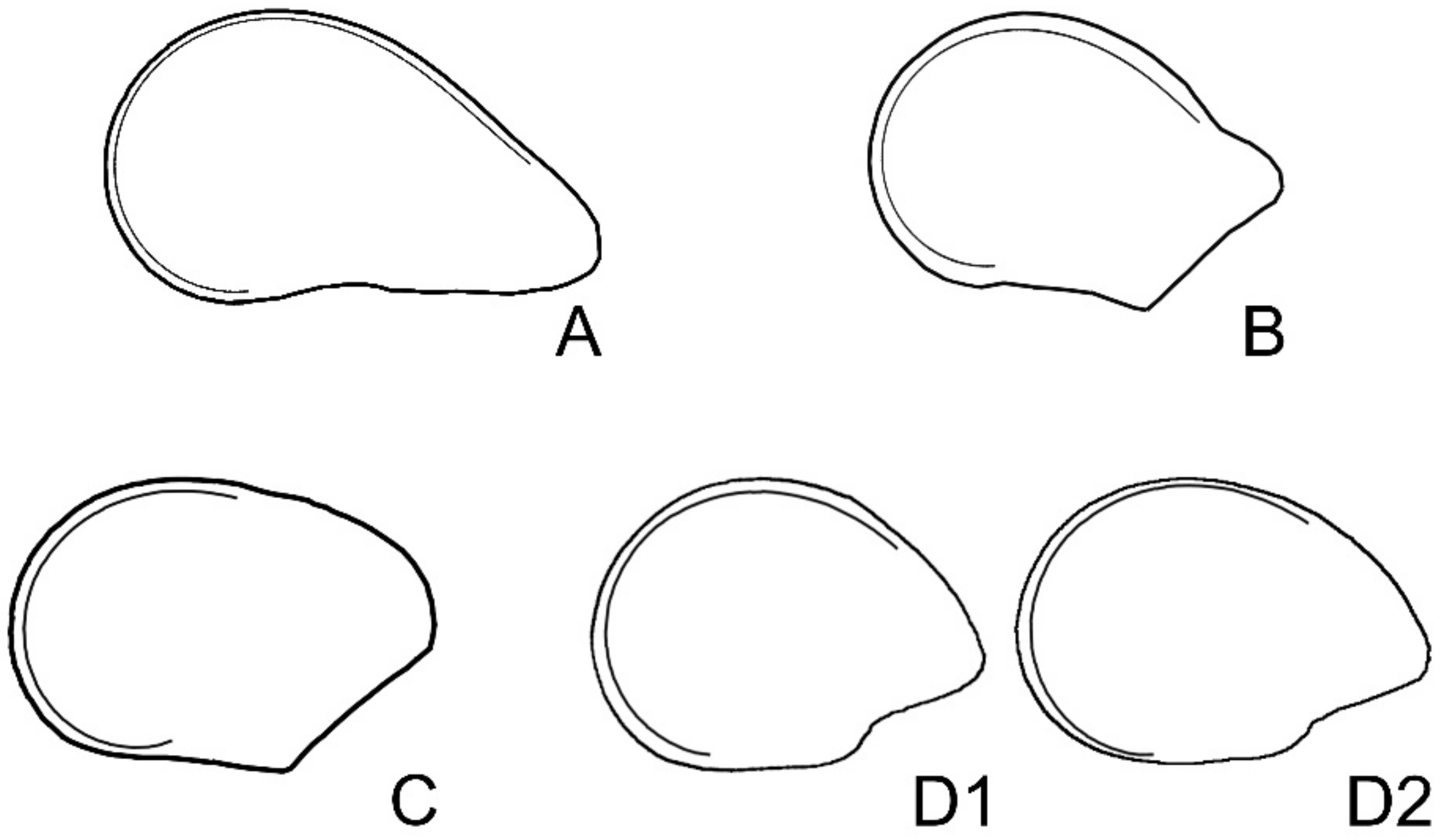
2.1. New Geometric Models Based on the Equation of an Ellipse
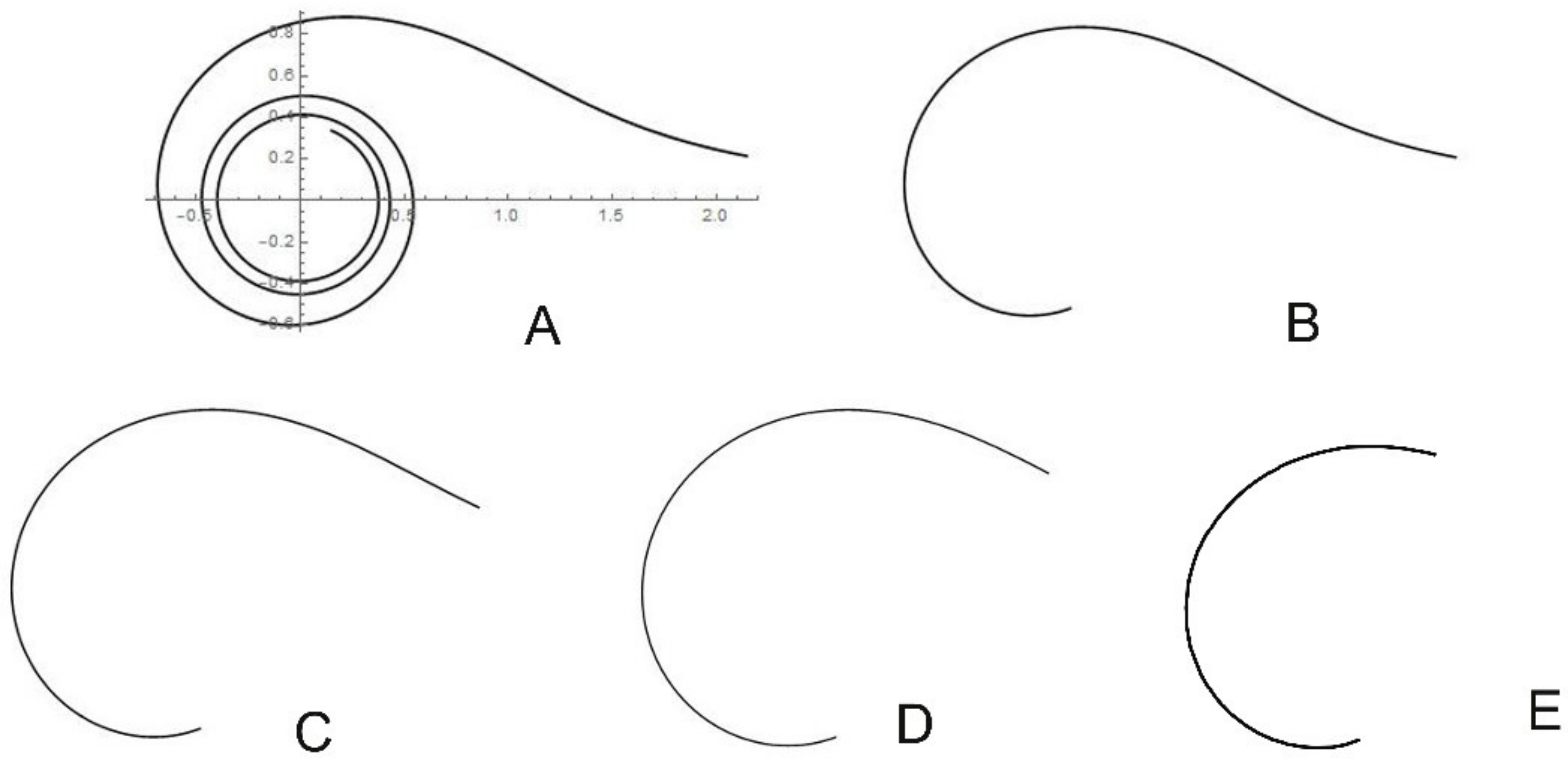
2.2. New Geometric Models Based on the Archimedean Spiral
2.3. Application of the Models to Seed Shape Quantification
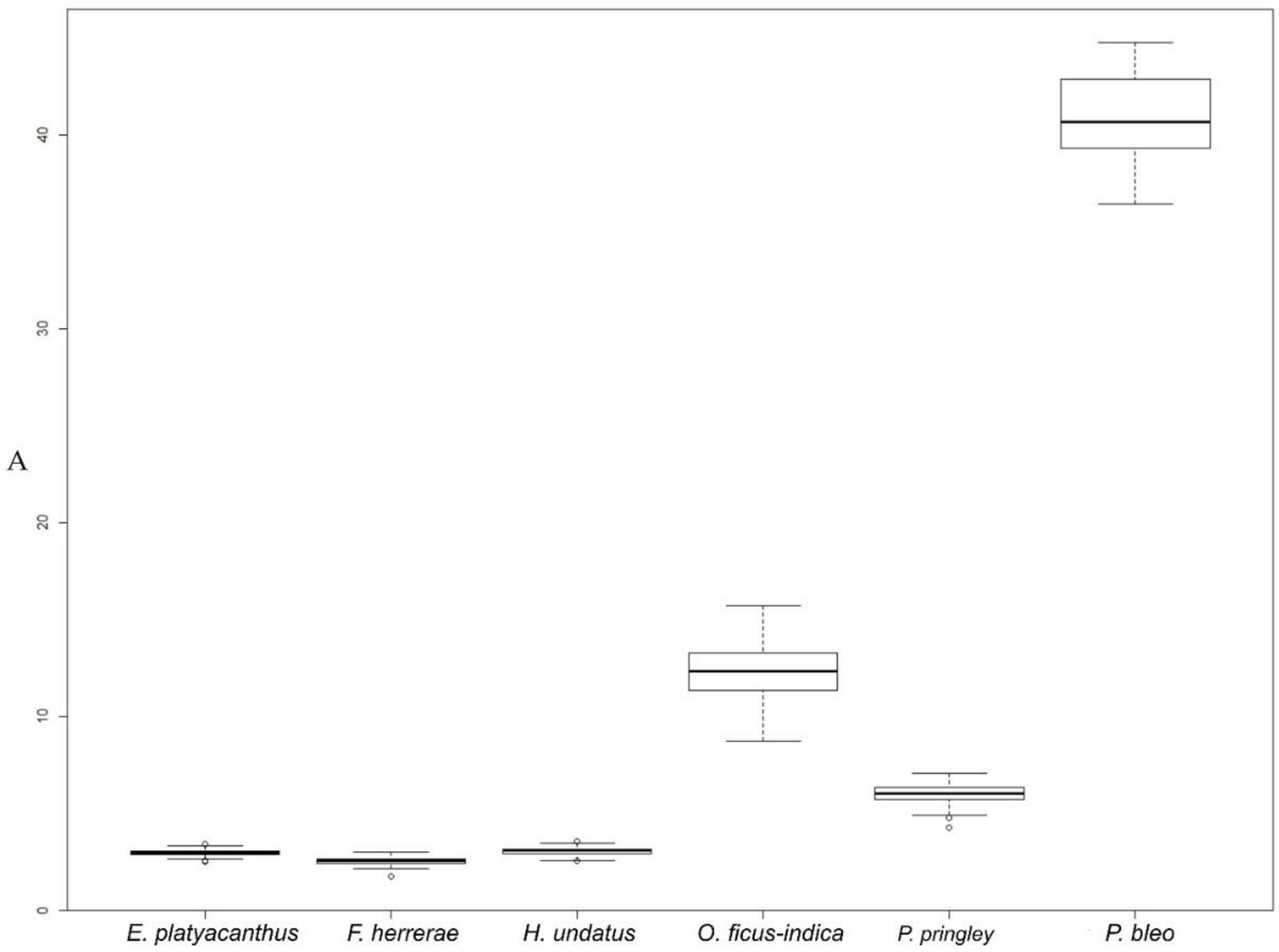
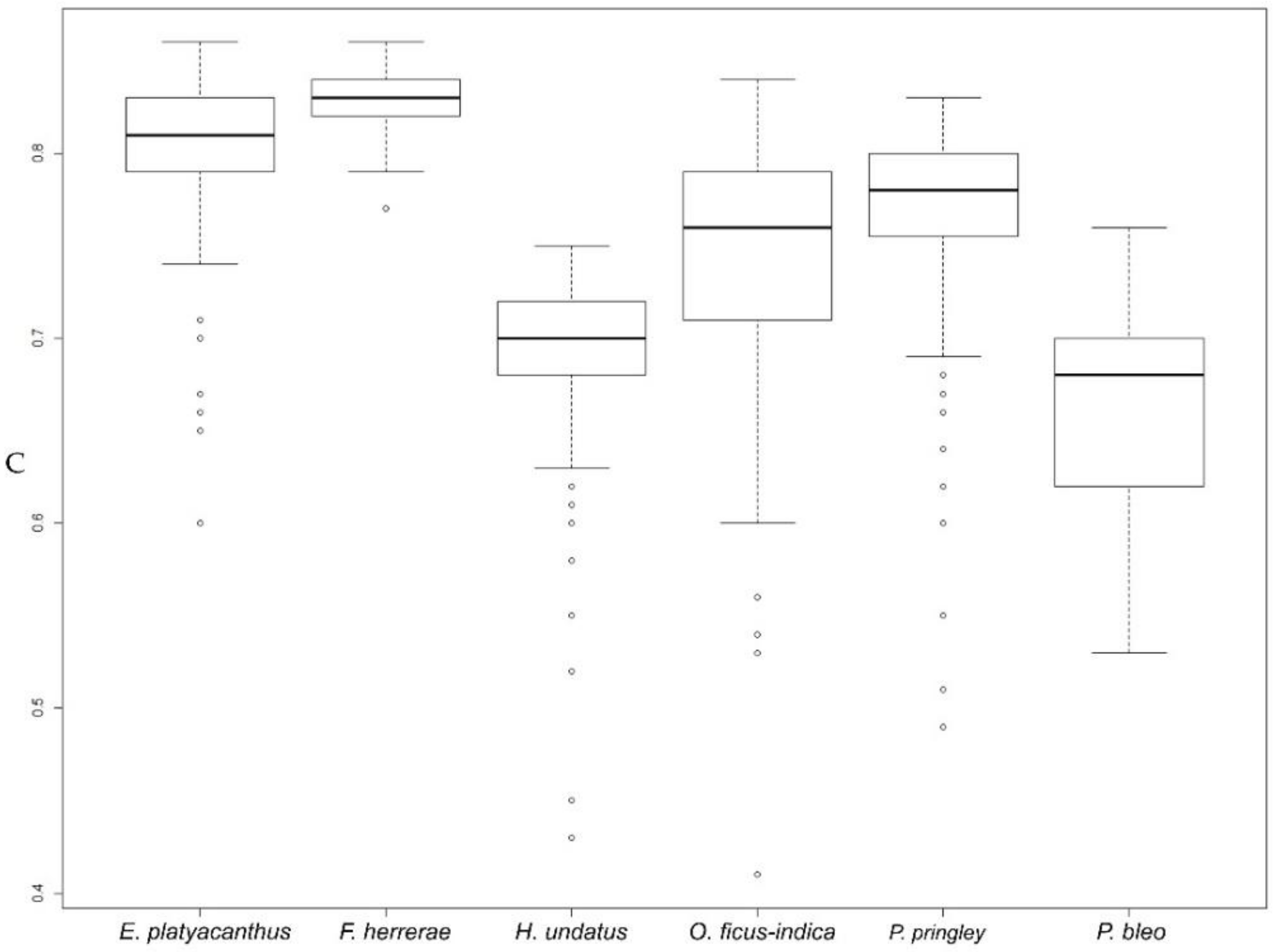
2.3.1. Overview of Seed Size and Shape in Some Cactus Species
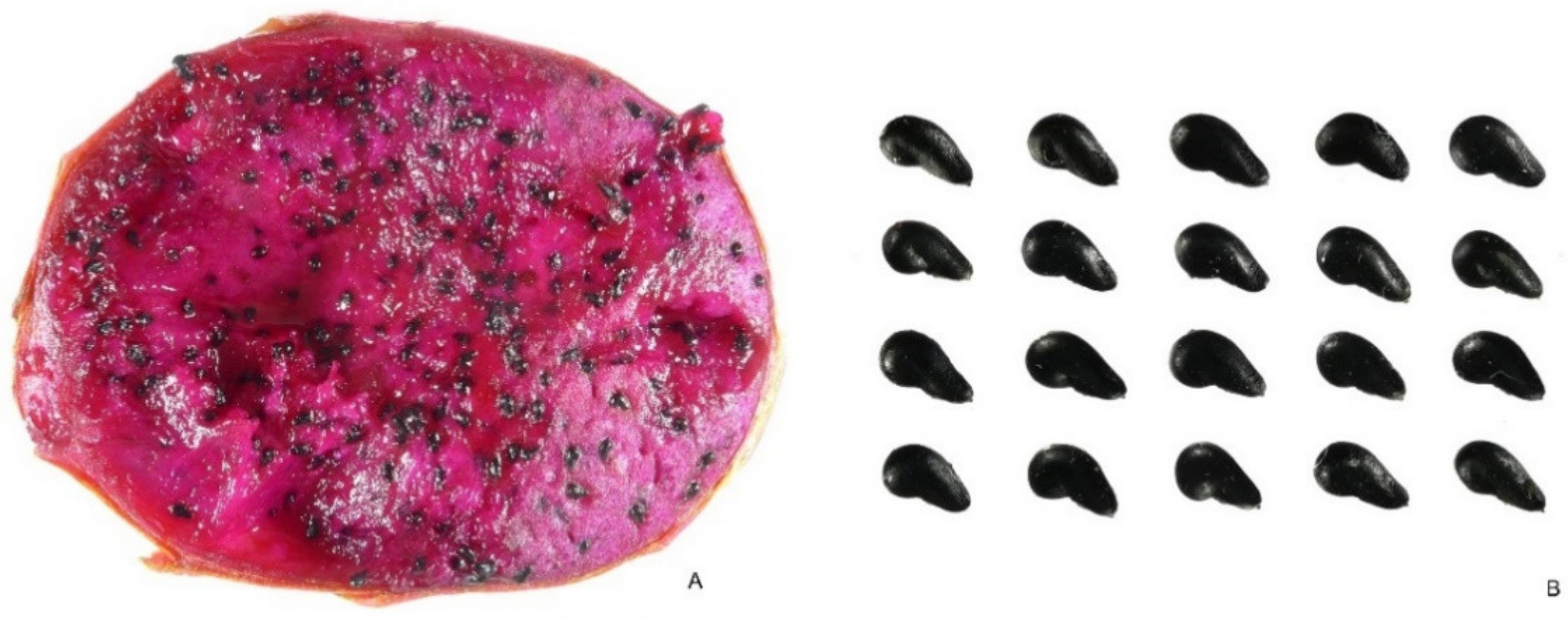
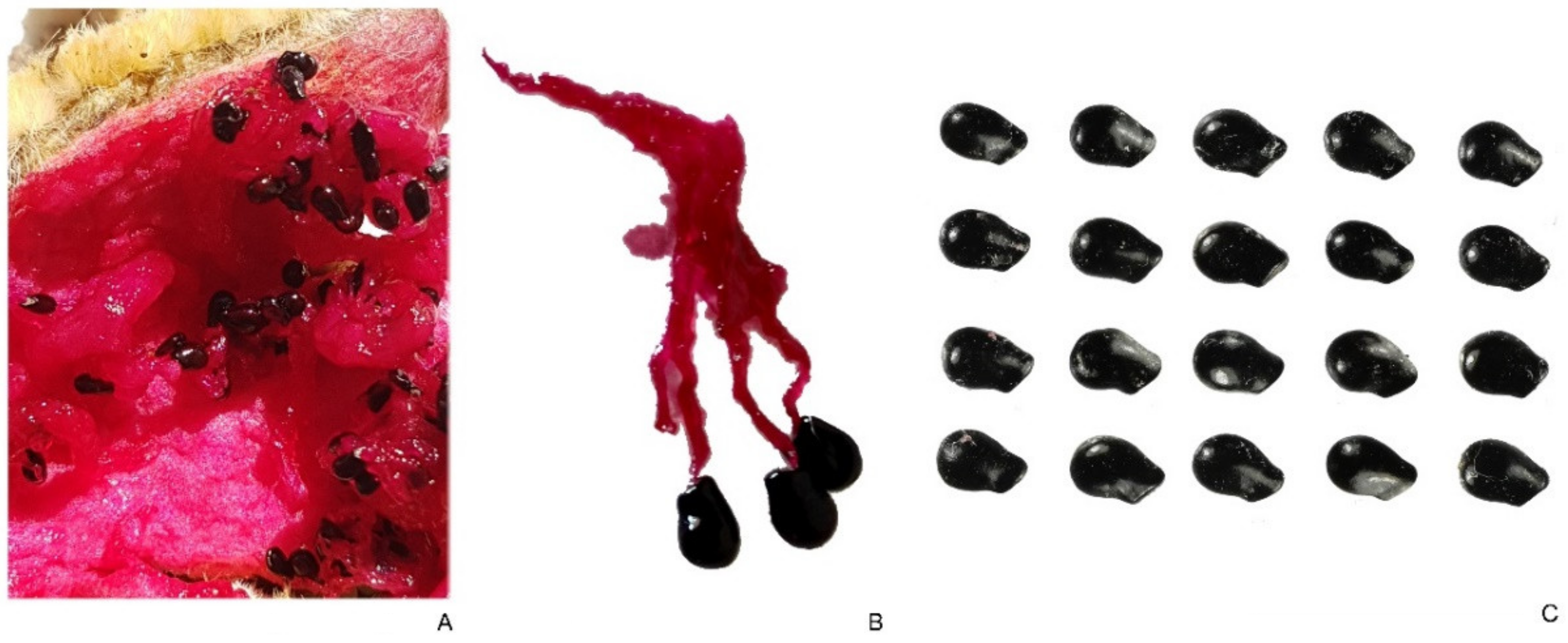
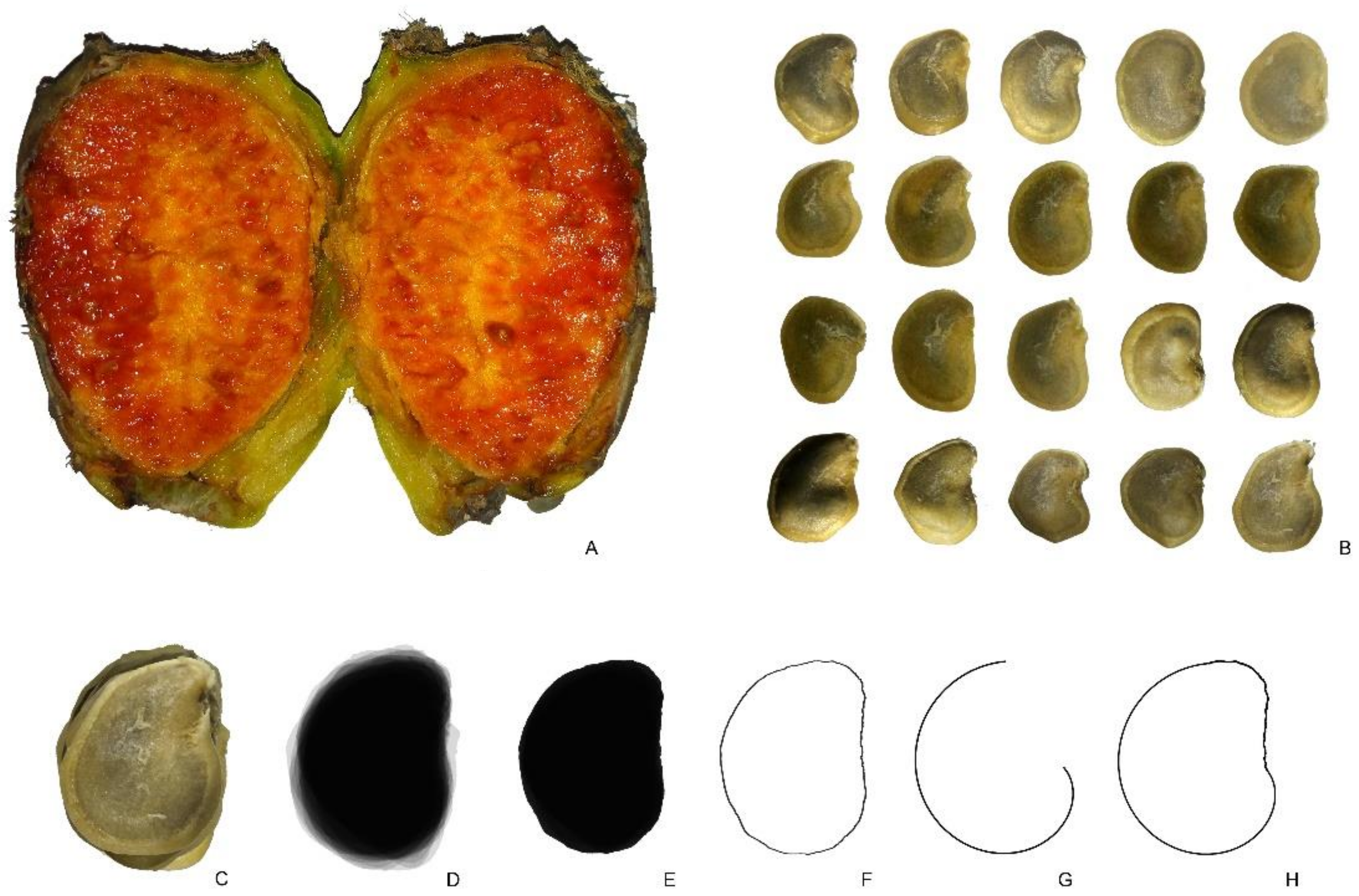
2.3.2. Hylocereus undatus
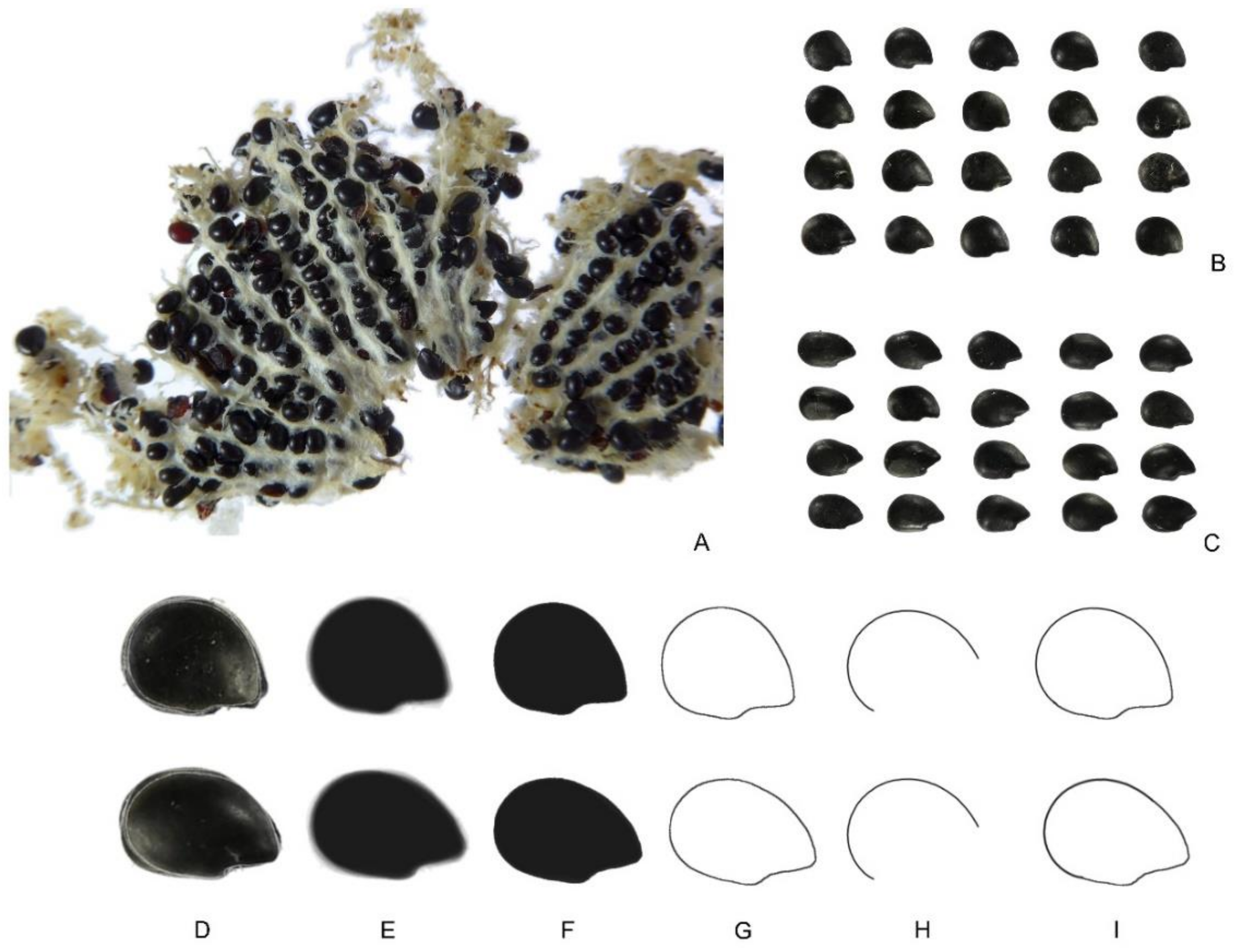
2.3.3. Pachycereus pringlei
2.3.4. Ferocactus herrerae
2.3.5. Echinocactus platyacanthus
2.3.6. Cardioid-Derived Models for Opuntia ficus-indica
2.3.7. Models for Species of Pereskia and Maihuenia
3. Discussion
3.1. The Relationship between Seed Shape and Taxonomy
3.2. Seed Geometry in the Cactaceae
4. Materials and Methods
4.1. Plant Material
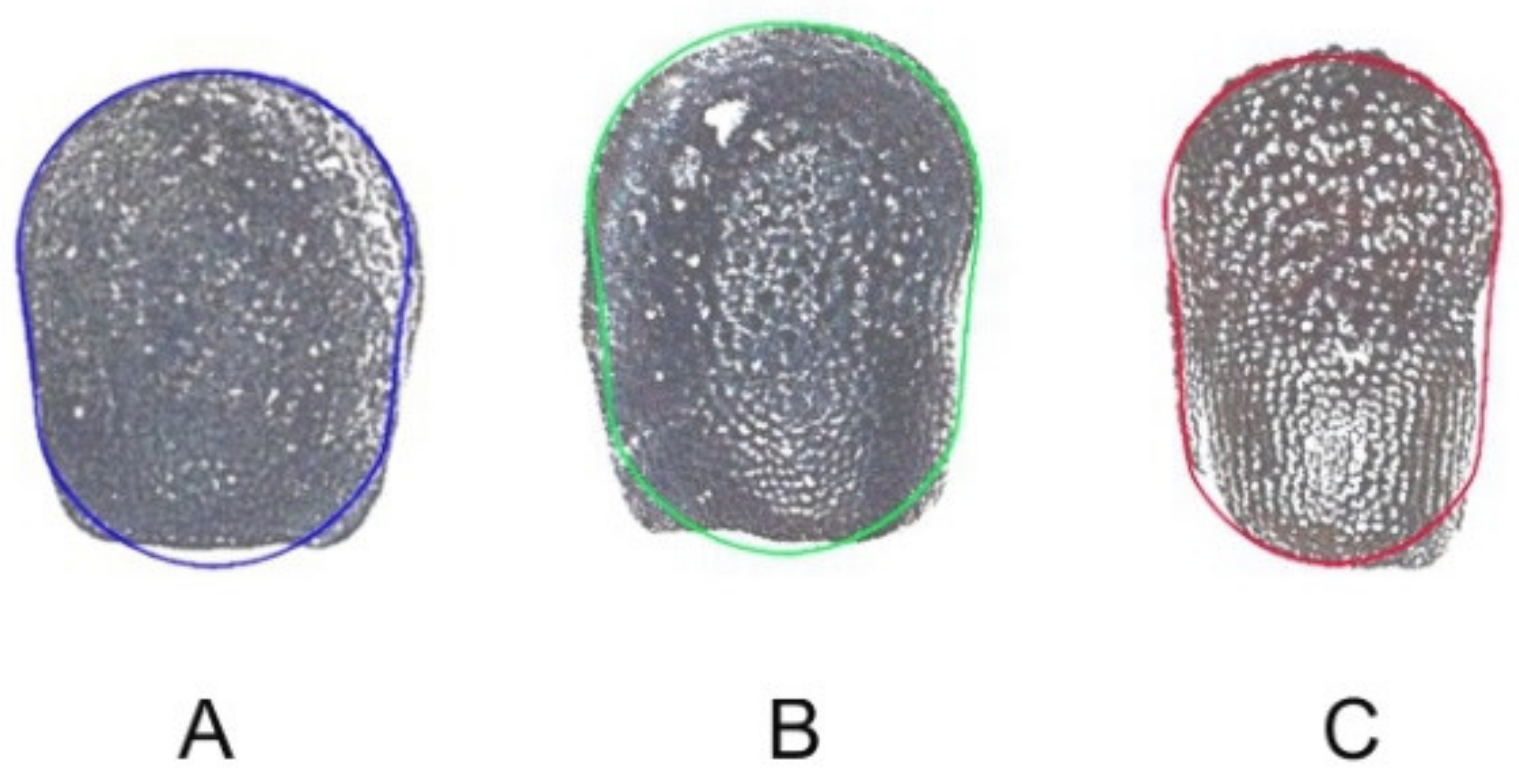
4.2. Photography and Image Analysis
4.3. Obtention of an Average Silhouette for Each Group of Seeds
4.4. Geometric Models
4.4.1. Geometric Models for the Symmetric Seeds of Rebutia and Echinopsis
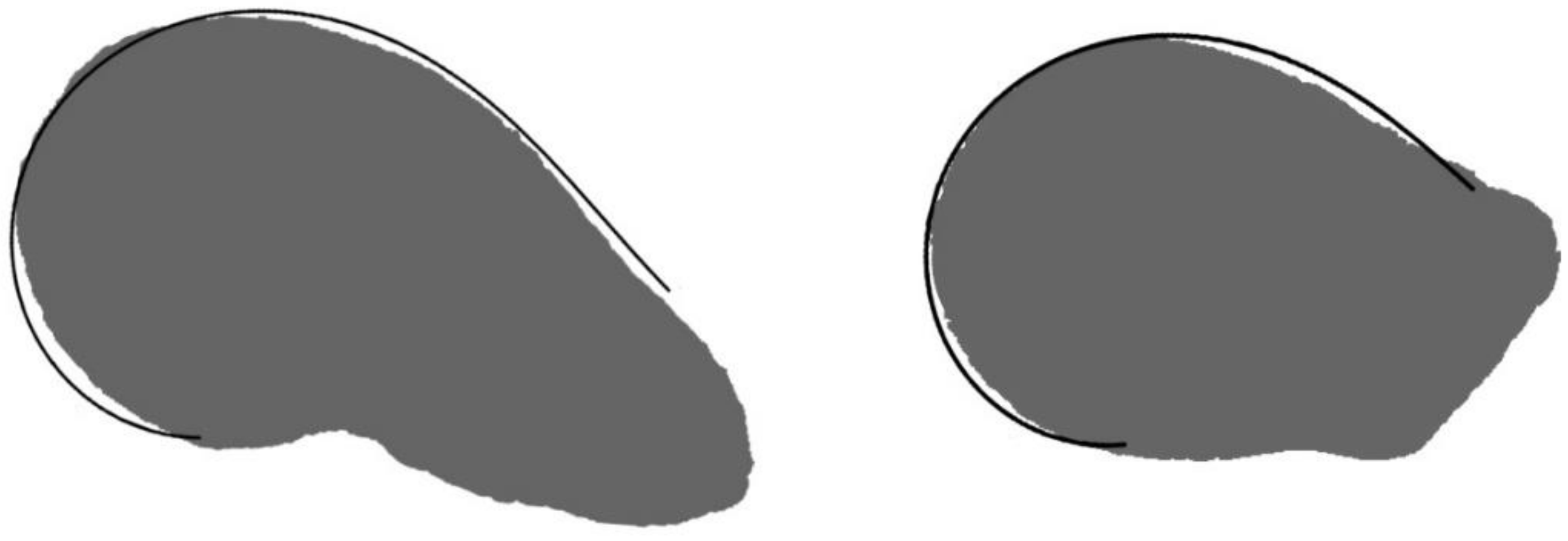
4.4.2. Geometric Models for the Asymmetric Seeds of Echinocactus, Ferocactus, Hylocereus, Opuntia ficus-indica, Pachycereus, and Pereskia
4.4.3. Drawing of the Models
4.5. Seed Shape Quantification and Testing of the Models: J Index
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Cronquist, A.; Thorne, R.F. Nomenclature and taxonomic history. In Caryophyllales: Evolution and Systematics; Behnke, H., Mabry, T.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 5–25. [Google Scholar]
- Nyffeler, R.; Eggli, U.; Ogburn, M.; Edwards, E. Variations on a theme: Repeated evolution of succulent life forms in the Portulacineae (Caryophyllales). Haseltonia 2008, 14, 26–36. [Google Scholar] [CrossRef]
- Nyffeler, R.; Eggli, U. Disintegrating Portulacaceae: A new familial classification of the suborder Portulacineae (Caryophyllales) based on molecular and morphological data. Taxon 2010, 59, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Nyffeler, R.; Eggli, U. A farewell to dated ideas and concepts: Molecular phylogenetics and a revised suprageneric classification of the family Cactaceae. Schumannia 2010, 6, 109–149. [Google Scholar]
- Rosas-Reinhold, I.; Piñeyro-Nelson, A.; Rosas, U.; Arias, S. Blurring the Boundaries between a Branch and a Flower: Potential Developmental Venues in Cactaceae. Plants 2021, 10, 1134. [Google Scholar] [CrossRef] [PubMed]
- Garcia de Almeida, O.J.; Sartori-Paoli, A.A.; de Souza, L.A. Morfo-anatomía de la flor de Epiphyllum phyllanthus (Cactaceae). Rev. Mex. Biodivers. 2010, 81, 65–80. [Google Scholar] [CrossRef]
- Copetti, D.; Búrquez, A.; Bustamante, E.; Charboneau, J.L.M.; Childs, K.L.; Eguiarte, L.E.; Lee, S.; Liu, T.L.; McMahon, M.M.; Whiteman, N.K.; et al. Extensive gene tree discordance and hemiplasy shaped the genomes of North American columnar cacti. Proc. Nat. Acad. Sci. USA 2017, 114, 12003–12008. [Google Scholar] [CrossRef] [Green Version]
- Bárcenas, R.T.; Hawkins, J. Nomenclatural Proliferation and the Superabundance of Homotypic Synonyms in Grusonia (Opuntioideae, Cactaceae). Haseltonia 2017, 23, 27–34. [Google Scholar] [CrossRef]
- Britton, N.L.; Rose, J.N. The Genus Cereus and its allies in North America. Contr. U.S. Natl. Herb. 1909, 10, 413–437. [Google Scholar]
- Arias, S.; Terrazas, T. Seed morphology and variation in the genus Pachycereus (Cactaceae). J. Plant Res. 2004, 117, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Berger, A. A systematic revision of the genus Cereus Mill. Rep. Annu. Missouri Bot. Gard. 1905, 16, 57–86. [Google Scholar] [CrossRef]
- Crozier, B.S. Subfamilies of Cactaceae Juss., including Blossfeldioideae subfam. nov. Phytologia 2004, 86, 52–64. [Google Scholar]
- Stuppy, W. Seed characters and the classification of the Opuntioideae. Succ. Plant Res. 2002, 6, 22–58. [Google Scholar]
- Taylor, N.P.; Clarke, J.Y. Seed-morphology and classification in Ferocactus subg. Ferocactus. Bradleya 1983, 1983, 13–16. [Google Scholar] [CrossRef]
- Arroyo-Cosultchi, G.; Terrazas, T.; Arias, S.; López-Mata, L. Morfología de las semillas en Neobuxbaumia (Cactaceae). Bot. Sci. 2007, 81, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Cosultchi, G.; Terrazas, T.; Arias, S.; Arreola-Nava, H.J. The systematic significance of seed morphology in Stenocereus (Cactaceae). Taxon 2006, 55, 983–992. [Google Scholar] [CrossRef]
- Lemus-Barrios, H.; Barrios, D.; García-Beltrán, J.A.; Arias, S.; Majure, L.C. Taxonomic implications of seed morphology in Melocactus (Cactaceae) from Cuba. Willdenowia 2021, 51, 91–113. [Google Scholar] [CrossRef]
- Barrios, D.; Sánchez, J.A.; Flores, J.; Jurado, E. Seed traits and germination in the Cactaceae family: A review across Americas. Bot. Sci. 2020, 98, 417–440. [Google Scholar] [CrossRef]
- Barthlott, W.; Hunt, D.R. Seed-Diversity in the Cactaceae: Subfamily Cactoideae; Series: Succulent Plant Research Volume: 5 D; Hunt: Sherborne, UK, 2000. [Google Scholar]
- Rojas-Arechiga, M.; Vazquez-Yanes, C. Cactus seed germination: A review. J. Arid Environ. 2000, 44, 85–104. [Google Scholar] [CrossRef] [Green Version]
- Cervantes, E.; Martín-Gómez, J.J.; Saadaoui, E. Updated methods for seed shape analysis. Scientifica 2016, 2016, 5691825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervantes, E.; Martín-Gómez, J.J. Seed Shape Description and Quantification by Comparison with Geometric Models. Horticulturae 2019, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- Cervantes, E.; Martin-Gómez, J.J.; Ardanuy, R.; de Diego, J.G.; Tocino, A. Modeling the Arabidopsis seed shape by a cardioid: Efficacy of the adjustment with a scale change with factor equal to the Golden Ratio and analysis of seed shape in ethylene mutants. J. Plant Physiol. 2010, 167, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, E.; Martin-Gómez, J.J.; Chan, P.K.; Gresshoff, P.M.; Tocino, A. Seed shape in model legumes: Approximation by a cardioid reveals differences in ethylene insensitive mutants of Lotus japonicus and Medicago truncatula. J. Plant Physiol. 2012, 169, 1359–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Gómez, J.J.; Tocino, Á.; Ardanuy, R.; de Diego, J.G.; Cervantes, E. Dynamic analysis of Arabidopsis seed shape reveals differences in cellulose mutants. Acta Physiol. Plant. 2014, 36, 1585–1592. [Google Scholar] [CrossRef]
- Saadaoui, E.; Martín-Gómez, J.J.; Cervantes, E. Seed morphology in Tunisian wild populations of Capparis spinosa L. Acta Biol. Cracov. Bot. 2013, 55, 99–106. [Google Scholar]
- Saadaoui, E.; Martín-Gómez, J.J.; Tlili, E.N.; Khaldi, A.; Cervantes, E. Effect of Climate in Seed Diversity of Wild Tunisian Rhus tripartita (Ucria) Grande. J. Adv. Biol. Biotechnol. 2017, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Martín-Gómez, J.J.; Rewicz, A.; Goriewa-Duba, K.; Wiwart, M.; Tocino, Á.; Cervantes, E. Morphological Description and Classification of Wheat Kernels Based on Geometric Models. Agronomy 2019, 9, 399. [Google Scholar] [CrossRef] [Green Version]
- Martín-Gómez, J.J.; Saadaoui, E.; Cervantes, E. Seed Shape of Castor Bean (Ricinus communis L.) Grown in Different Regions of Tunisia. J. Agric. Ecol. 2016, 8, 1–11. [Google Scholar]
- Saadaoui, E.; Martín-Gómez, J.J.; Bouazizi, R.; Romdhane, C.B.; Grira, M.; Abdelkabir, S.; Khouja, M.L.; Cervantes, E. Phenotypic variability and seed yield of Jatropha curcas L. Introduced to Tunisia. Acta Bot. Mex. 2015, 110, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Martín-Gómez, J.J.; Rewicz, A.; Cervantes, E. Seed Shape Diversity in families of the Order Ranunculales. Phytotaxa 2019, 425, 193–207. [Google Scholar] [CrossRef]
- Cervantes, E.; Martín-Gómez, J.J. Seed shape quantification in the order Cucurbitales. Modern Phytomorphol. 2018, 12, 1–13. [Google Scholar] [CrossRef]
- Gutiérrez del Pozo, D.; Martín-Gómez, J.J.; Tocino, Á.; Cervantes, E. Seed Geometry in the Arecaceae. Horticulturae 2020, 6, 64. [Google Scholar] [CrossRef]
- Cervantes, E.; Martín-Gómez, J.J.; Gutiérrez Del Pozo, D.; Tocino, A. Seed Geometry in the Vitaceae. Plants 2021, 10, 1695. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 2018. Available online: http://imagej.nih.gov/ij/ (accessed on 22 November 2021).
- Cervantes, E.; Martín-Gómez, J.J.; Espinosa-Roldán, F.E.; Muñoz-Organero, G.; Tocino, Á.; Cabello-Sáenz de Santamaría, F. Seed Morphology in Key Spanish Grapevine Cultivars. Agronomy 2021, 11, 734. [Google Scholar] [CrossRef]
- Guerrero, P.C.; Majure, L.C.; Cornejo-Romero, A.; Hernández-Hernández, T. Phylogenetic Relationships and Evolutionary Trends in the Cactus Family. J. Hered. 2019, 110, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Baes, P.; Sühring, S.; Sajama, I.; Sotola, E.; Alonso-Pedano, M.; Bravo, S.; Godínez-Alvarez, H. Diversity and conservation in the cactus family. In Desert Plants; Ramawat, K.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 157–173. [Google Scholar]
- Cervantes, E.; Martín-Gómez, J.J.; Gutiérrez del Pozo, D.; Silva Dias, L. An Angiosperm Species Dataset Reveals Relationships between Seed Size and Two-Dimensional Shape. Horticulturae 2019, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Baskin, C.C.; Baskin, J.M. Martin’s peripheral embryo—Unique but not a phylogenetic ‘orphan’ at the base of his family tree: A tribute to the insight of a pioneer seed biologist. Seed Sci. Res. 2019, 29, 155–156. [Google Scholar] [CrossRef]
- Martin, A.C. The comparative internal morphology of seeds. Am. Midl. Nat. 1946, 36, 513–660. [Google Scholar] [CrossRef]
- Dunham, D. Hyperbolic Spirals and Spirals Patterns. In Proceedings of the Meeting Alhambra, ISAMA-Bridges, University of Granada, Granada, Spain, 23–25 July 2003; pp. 521–529. [Google Scholar]
- Archibald, E.E.A. The development of the ovule and seed of Jointed Cactus (Opuntia aurantiaca, Lindley). S. Afr. J. Sci. 1939, 36, 211. [Google Scholar]
- Thompson, D. On Growth and Form; Cambridge University Press: Cambridge, UK, 1961. [Google Scholar]
- Azpeitia, E.; Tichtinsky, G.; Le Masson, M.; Serrano-Mislata, A.; Lucas, J.; Gregis, V.; Gimenez, C.; Prunet, N.; Farcot, E.; Kater, M.M.; et al. Cauliflower fractal forms arise from perturbations of floral gene networks. Science 2021, 373, 192–197. [Google Scholar] [CrossRef]
- Martín-Gómez, J.J.; Rewicz, A.; Rodriguez-Lorenzo, J.L.; Janousek, B.; Cervantes, E. Seed Morphology in Silene Based on Geometric Models. Plants 2020, 9, 1787. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Sokal, R.R.; Braumann, C.A. Significance Tests for Coefficients of Variation and Variability Profiles. Syst. Zool. 1980, 29, 50. [Google Scholar] [CrossRef]




| N | A (mm2) | P (mm) | L (mm) | W (mm) | AR | C | R | |
|---|---|---|---|---|---|---|---|---|
| Echinocactus platyacanthus | 153 | 2.97 a (0.15) 2.50/3.42 | 6.83 b (0.26) 6.29/8.05 | 2.23 a (0.09) 2.03/2.54 | 1.70 b (0.07) 1.50/1.87 | 1.32 b (0.08) 1.17/1.54 | 0.80 cd (0.04) 0.60/0.86 | 0.76 c (0.05) 0.65/0.86 |
| Ferocactus herrerae | 121 | 2.54 a (0.20) 1.72/3.02 | 6.19 a (0.25) 5.09/6.73 | 2.16 a (0.09) 1.73/2.36 | 1.49 a (0.06) 1.27/1.63 | 1.45 c (0.04) 1.36/1.63 | 0.83 d (0.01) 0.77/0.86 | 0.69 b (0.02) 0.61/0.73 |
| Hylocereus undatus | 121 | 3.04 a (0.18) 2.53/3.56 | 7.46 c (0.38) 6.75/9.55 | 2.69 b (0.12) 2.33/2.96 | 1.44 a (0.06) 1.30/1.59 | 1.87 d (0.11) 1.62/2.17 | 0.69 a (0.05) 0.43/0.75 | 0.54 a (0.03) 0.46/0.62 |
| Opuntia ficus-indica | 117 | 12.34 c (1.41) 8.71/15.70 | 14.49 e (1.11) 12.34/19.92 | 4.57 d (0.37) 3.71/5.63 | 3.43 d (0.25) 2.82/4.00 | 1.34 b (0.14) 1.07/1.74 | 0.74 b (0.07) 0.41/0.84 | 0.76 c (0.08) 0.57/0.94 |
| Pachycereus pringley | 151 | 5.95 b (0.49) 4.25/7.04 | 9.89 d (0.63) 8.37/12.82 | 3.37 c (0.15) 2.95/3.72 | 2.25 c (0.11) 1.83/2.46 | 1.50 c (0.07) 1.30/1.72 | 0.77 bc (0.06) 0.49/0.83 | 0.67 b (0.03) 0.58/0.77 |
| Pereskia bleo | 13 | 39.61 d (3.83) 31.38/44.78 | 26.78 f (2.23) 22.38/29.93 | 7.81 e (0.51) 6.92/8.60 | 6.45 e (0.36) 5.77/7.05 | 1.21 a (0.08) 1.04/1.34 | 0.70 a (0.09) 0.53/0.83 | 0.83 d (0.06) 0.74/0.96 |
| N | CV(A) | CV(P) | CV(L) | CV(W) | CV(AR) | CV(C) | CV(R) | |
|---|---|---|---|---|---|---|---|---|
| Echinocactus platyacanthus | 153 | 5.13 | 3.85 | 3.92 | 4.01 | 6.11 | 5.19 | 5.97 |
| Ferocactus herrerae | 121 | 7.87 | 4.05 | 4.12 | 4.20 | 2.49 | 1.66 | 2.47 |
| Hylocereus undatus | 121 | 5.84 | 5.14 | 4.43 | 3.87 | 5.80 | 7.41 | 6.06 |
| Opuntia ficus-indica | 117 | 11.39 | 7.63 | 8.16 | 7.24 | 10.67 | 9.87 | 10.49 |
| Pachycereus pringley | 151 | 8.22 | 6.42 | 4.42 | 4.97 | 4.44 | 7.30 | 4.48 |
| Pereskia bleo | 13 | 9.67 | 8.33 | 6.47 | 5.59 | 6.64 | 12.84 | 6.97 |
| N | Area | P | L | W | AR | C | R | J Index SM | J Index LM | |
|---|---|---|---|---|---|---|---|---|---|---|
| S | 20 | 2.94 a (0.15) 2.65/3.21 | 6.69 a (0.21) 6.34/7.07 | 2.15 a (0.06) 2.05/2.28 | 1.74 b (0.05) 1.62/1.80 | 1.24 a (0.04) 1.18/1.30 | 0.83 b (0.02) 0.80/0.86 | 0.81 b (0.02) 0.77/0.85 | 92.1 b (1.12) 88.9/93.6 | 87.5 a (1.36) 84.8/90.3 |
| L | 20 | 2.99 a (0.14) 2.74/3.19 | 6.86 b (0.19) 6.56/7.25 | 2.36 b (0.07) 2.24/2.50 | 1.61 a (0.05) 1.48/1.69 | 1.46 b (0.06) 1.37/1.60 | 0.80 a (0.02) 0.75/0.83 | 0.68 a (0.03) 0.63/0.73 | 87.7 a (1.90) 83.7/90.6 | 91.7 b (1.49) 88.9/93.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Gómez, J.J.; del Pozo, D.G.; Tocino, Á.; Cervantes, E. Geometric Models for Seed Shape Description and Quantification in the Cactaceae. Plants 2021, 10, 2546. https://doi.org/10.3390/plants10112546
Martín-Gómez JJ, del Pozo DG, Tocino Á, Cervantes E. Geometric Models for Seed Shape Description and Quantification in the Cactaceae. Plants. 2021; 10(11):2546. https://doi.org/10.3390/plants10112546
Chicago/Turabian StyleMartín-Gómez, José Javier, Diego Gutiérrez del Pozo, Ángel Tocino, and Emilio Cervantes. 2021. "Geometric Models for Seed Shape Description and Quantification in the Cactaceae" Plants 10, no. 11: 2546. https://doi.org/10.3390/plants10112546
APA StyleMartín-Gómez, J. J., del Pozo, D. G., Tocino, Á., & Cervantes, E. (2021). Geometric Models for Seed Shape Description and Quantification in the Cactaceae. Plants, 10(11), 2546. https://doi.org/10.3390/plants10112546







