Interstitial Telomeric-like Repeats (ITR) in Seed Plants as Assessed by Molecular Cytogenetic Techniques: A Review
Abstract
1. Introduction
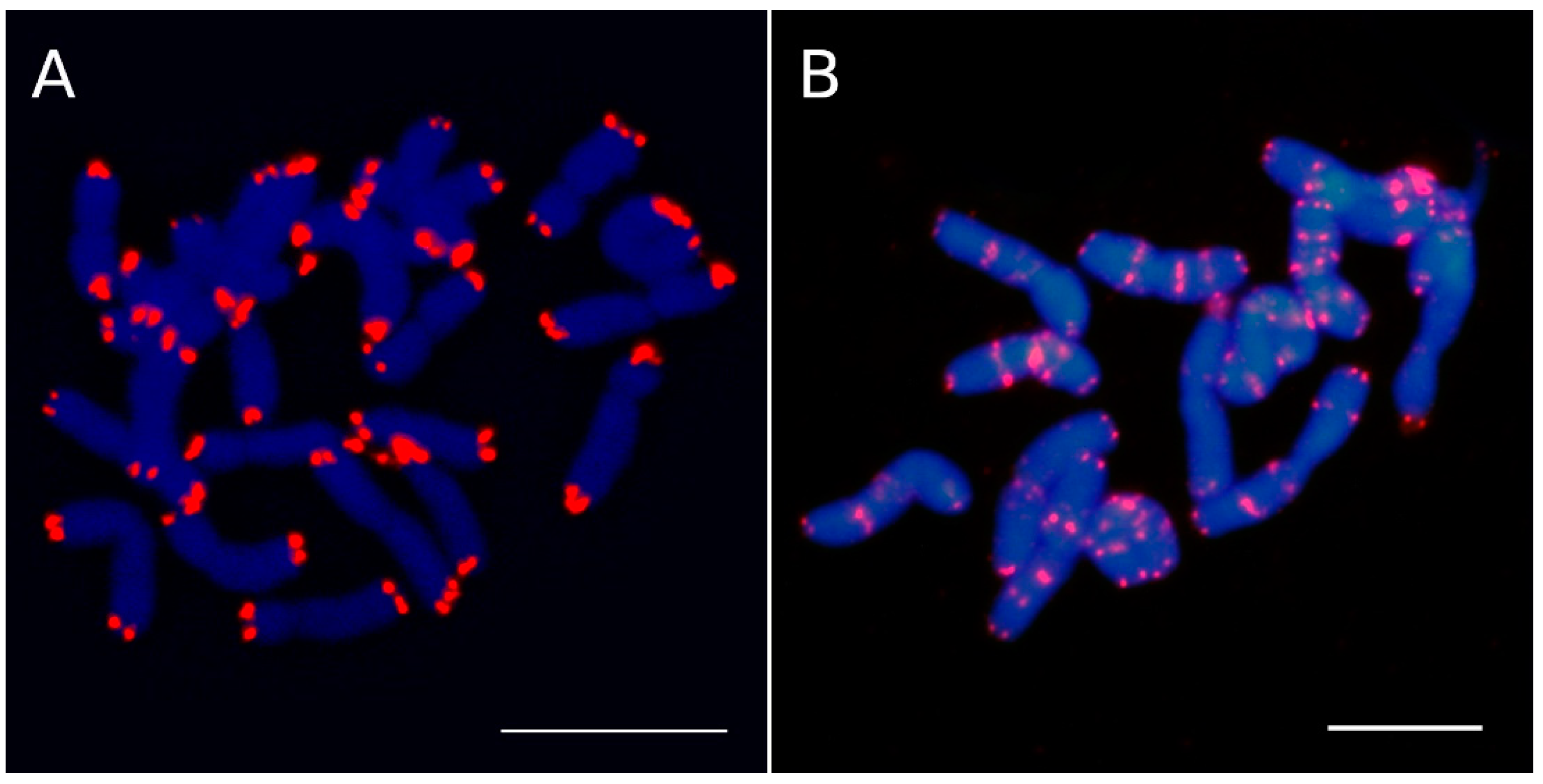
2. Molecular Cytogenetic Approaches Used in ITR Detection
3. ITR Sampling in Seed Plants
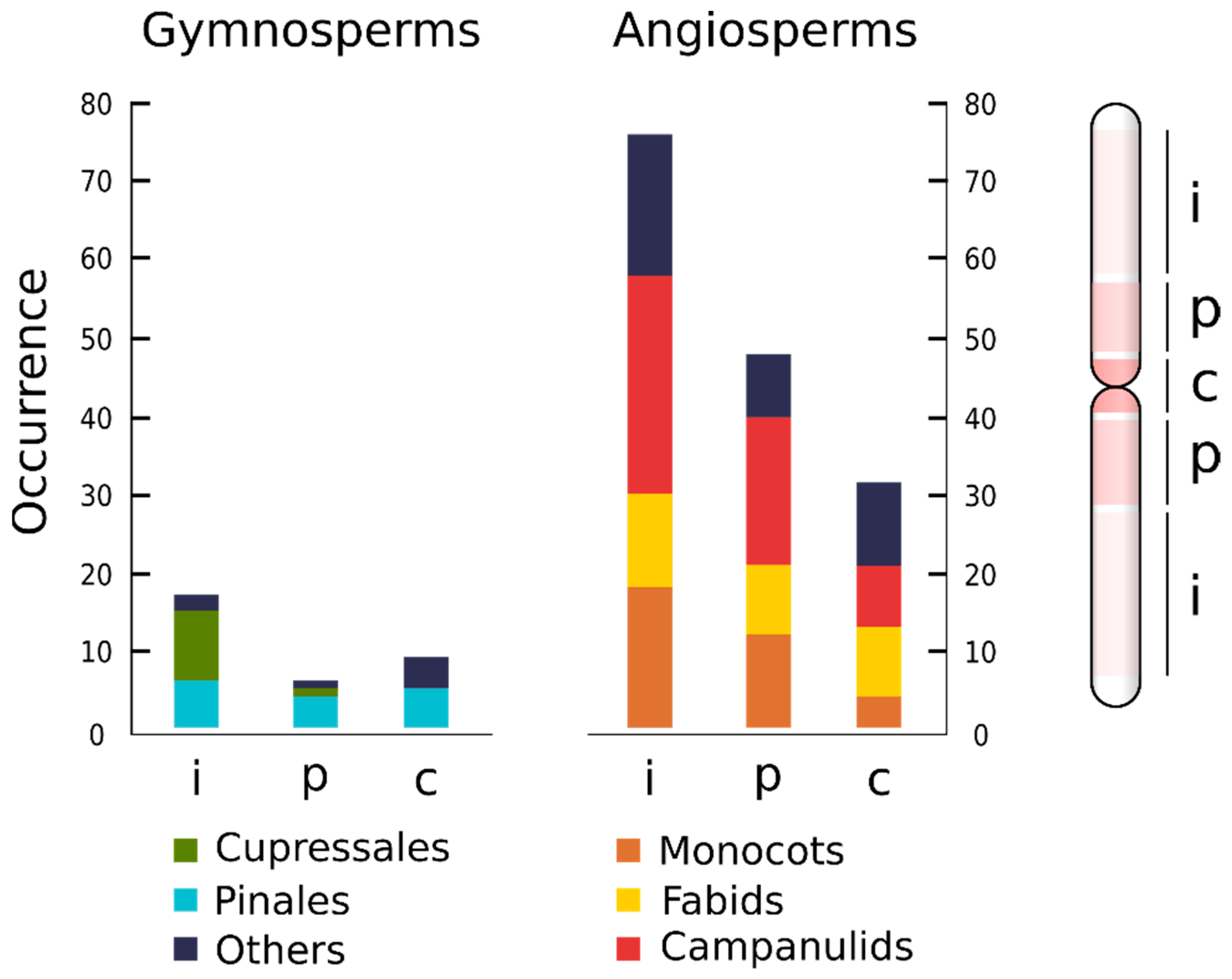
4. Taxonomic Distribution of ITRs Is Widespread among Major Lineages of Seed Plants
5. ITRs Preferentially Occur at Interstitial Chromosomal Arms
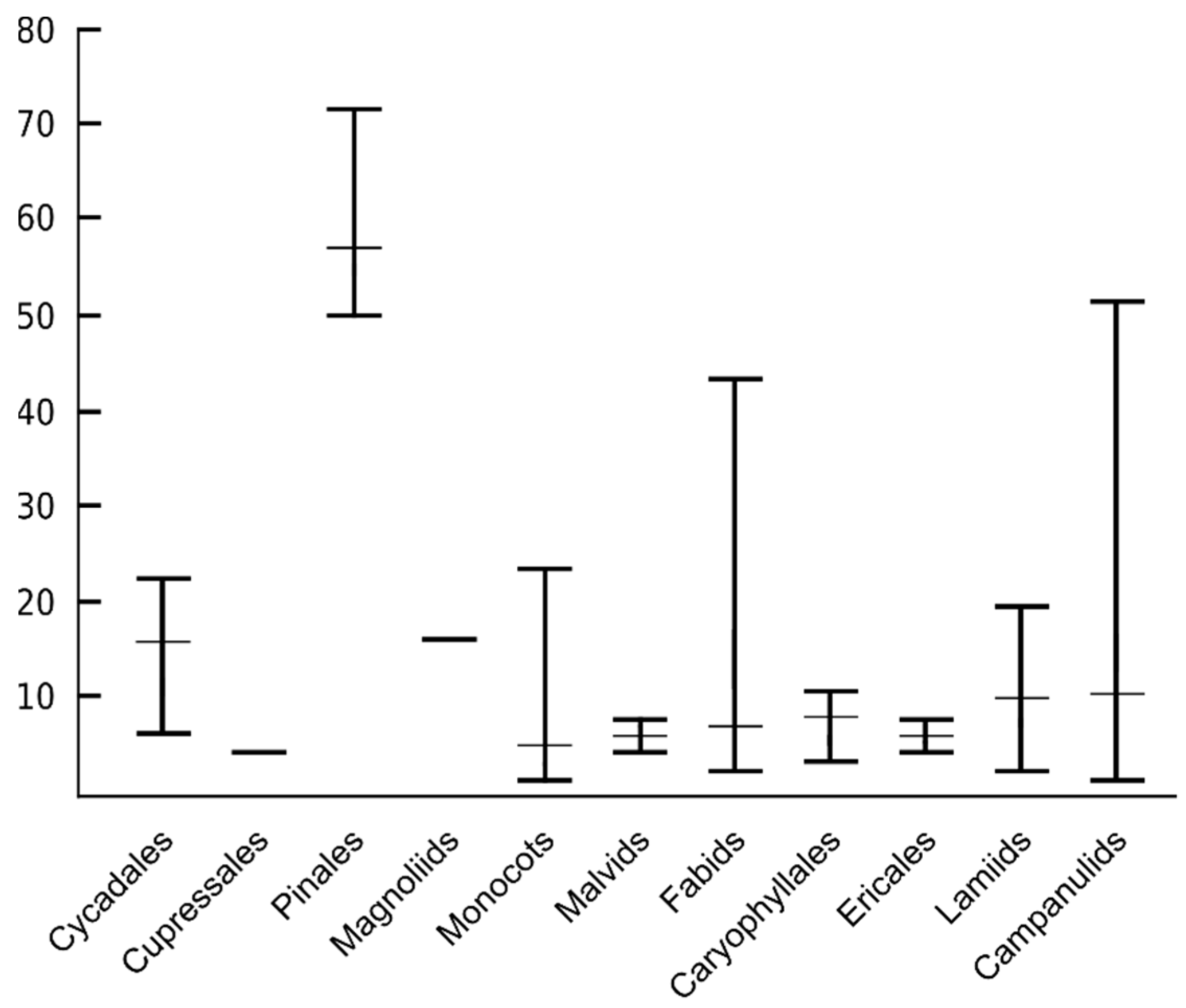
6. The Number of ITR Sites Greatly Varies among Congeneric Species and Higher Taxonomic Units
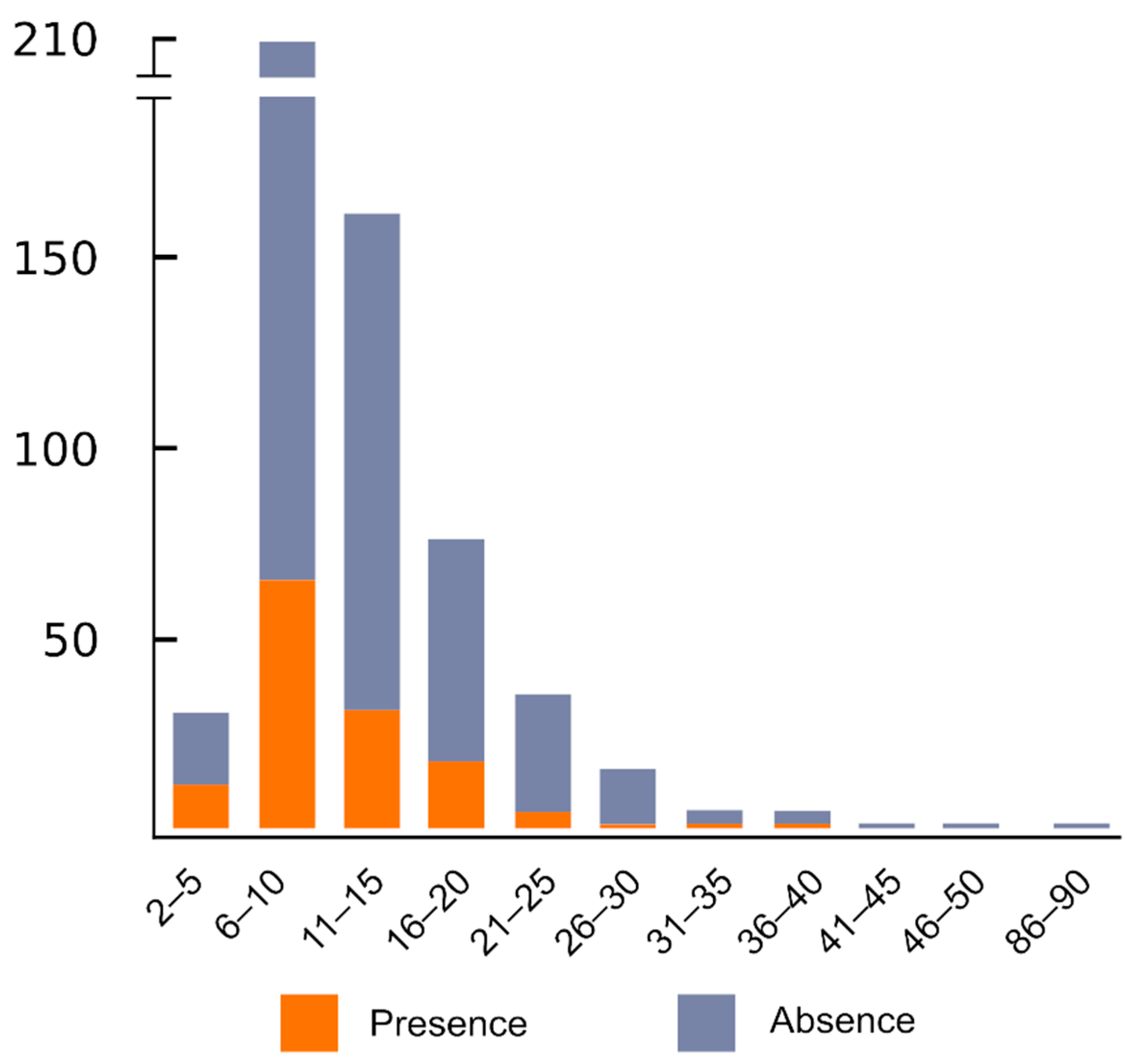
7. Variable Presence and Location of ITR Sites Occur within Species
8. The Presence of ITRs and the Number of Sites Is not Significantly Related to Number of Chromosomes
9. Origin of ITRs
10. Data Analysis
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, A.D.; Beilstein, M.A.; Shippen, D.E. Plant telomeres and telomerase. In Molecular Biology; Howell, S.H., Ed.; Springer: New York, NY, USA, 2014; pp. 25–49. [Google Scholar]
- Lin, K.W.; Yan, J. Endings in the middle: Current knowledge of interstitial telomeric sequences. Mutat. Res. 2008, 658, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, A.Y.; Greenwell, P.W.; Dominska, M.; Shishkin, A.A.; Kim, J.C.; Petes, T.D.; Mirkin, S.M. Genome rearrangements caused by interstitial telomeric sequences in yeast. Proc. Natl. Acad. Sci. USA 2013, 110, 19866–19871. [Google Scholar] [CrossRef]
- Ocalewicz, K. Telomeres in fishes. Cytogenet. Genome Res. 2013, 141, 114–125. [Google Scholar] [CrossRef]
- Bolzán, A.D. Interstitial telomeric sequences in vertebrate chromosomes: Origin, function, instability and evolution. Mutat. Res./Rev. Mutat. Res. 2017, 773, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.V.; Bennett, S.T.; Parokonny, A.S.; Kenton, A.; Callimassia, M.A.; Bennett, M.D. Comparison of plant telomere locations using a PCR-generated synthetic probe. Ann. Bot. 1993, 72, 239–247. [Google Scholar] [CrossRef]
- Fuchs, J.; Brandes, A.; Schubert, I. Telomere sequence localization and karyotype evolution in higher plants. Plant Syst. Evol. 1995, 196, 227–241. [Google Scholar] [CrossRef]
- Tek, A.; Jiang, J. The centromeric regions of potato chromosomes contain megabase-sized tandem arrays of telomere-similar sequence. Chromosoma 2004, 113, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Gaspin, C.; Rami, J.F.; Lescure, B. Distribution of short interstitial telomere motifs in two plant genomes: Putative origin and function. BMC Plant Biol. 2010, 10, 1–12. [Google Scholar] [CrossRef]
- Mandáková, T.; Gloss, A.D.; Whiteman, N.K.; Lysak, M.A. How diploidization turned a tetraploid into a pseudotriploid. Am. J. Bot. 2016, 103, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Schwarzacher, T.; Heslop-Harrison, J.S. In situ hybridization to plant telomeres using synthetic oligomers. Genome 1991, 34, 317–323. [Google Scholar] [CrossRef]
- Waminal, N.E.; Pellerin, R.J.; Kang, S.H.; Kim, H.H. Chromosomal mapping of tandem repeats revealed massive chromosomal rearrangements and insights into Senna tora dysploidy. Front. Plant Sci. 2021, 12, 154. [Google Scholar] [CrossRef]
- Menke, M.; Fuchs, J.; Schubert, I. A comparison of sequence resolution on plant chromosomes: PRINS versus FISH. Theor. Appl. Genet. 1998, 97, 1314–1320. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, B.S. Current status and the future of fluorescence in situ hybridization (FISH) in plant genome research. Genome 2006, 49, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, D.M.; Bass, H.W. A historical and modern perspective on plant cytogenetics. Brief. Funct. Genom. 2010, 9, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Waminal, N.E.; Pellerin, R.J.; Kim, N.S.; Jayakodi, M.; Park, J.Y.; Yang, T.J.; Kim, H.H. Rapid and efficient FISH using pre-labeled oligomer probes. Sci. Rep. 2018, 8, 1–10. [Google Scholar]
- Thomas, H.M.; Williams, K.; Harper, J.A. Labelling telomeres of cereals, grasses and clover by primed in situ DNA labelling. Chromosome Res. 1996, 4, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Emadzade, K.; Jang, T.S.; Macas, J.; Kovařík, A.; Novák, P.; Parker, J.; Weiss-Schneeweiss, H. Differential amplification of satellite PaB6 in chromosomally hypervariable Prospero autumnale complex (Hyacinthaceae). Ann. Bot. 2014, 114, 1597–1608. [Google Scholar] [CrossRef]
- Cuadrado, Á.; Golczyk, H.; Jouve, N. A novel, simple and rapid nondenaturing FISH (ND-FISH) technique for the detection of plant telomeres. Potential used and possible target structures detected. Chromosome Res. 2009, 17, 755–762. [Google Scholar] [CrossRef]
- Lou, Q.; Iovene, M.; Spooner, D.M.; Buell, C.R.; Jiang, J. Evolution of chromosome 6 of Solanum species revealed by comparative fluorescence in situ hybridization mapping. Chromosoma 2010, 119, 435–442. [Google Scholar] [CrossRef]
- Majerová, E.; Fojtová, M.; Mandáková, T.; Fajkus, J. Methylation of plant telomeric DNA: What do the results say? Plant Mol. Biol. 2011, 77, 533–536. [Google Scholar] [CrossRef]
- Majerová, E.; Mandáková, T.; Vu, G.T.; Fajkus, J.; Lysak, M.A.; Fojtová, M. Chromatin features of plant telomeric sequences at terminal vs. internal positions. Front. Plant Sci. 2014, 5, 593. [Google Scholar] [CrossRef]
- Sýkorová, E.; Fajkus, J.; Mezníková, M.; Lim, K.Y.; Neplechová, K.; Blattner, F.R.; Leitch, A.R. Minisatellite telomeres occur in the family Alliaceae but are lost in Allium. Am. J. Bot. 2006, 93, 814–823. [Google Scholar] [CrossRef]
- Fajkus, P.; Peška, V.; Sitová, Z.; Fulnečková, J.; Dvořáčková, M.; Gogela, R.; Sýkorová, E.; Fajkus, J. Allium telomeres unmasked: The unusual telomeric sequence (CTCGGTTATGGG)n is synthesized by telomerase. Plant J. 2016, 85, 337–347. [Google Scholar] [CrossRef]
- Peška, V.; Fajkus, P.; Fojtová, M.; Dvořáčková, M.; Hapala, J.; Dvořáček, V.; Polanská, P.; Leitch, A.R.; Sýkorová, E.; Fajkus, J. Characterisation of an unusual telomere motif (TTTTTTAGGG)n in the plant Cestrum elegans (Solanaceae), a species with a large genome. Plant J. 2015, 82, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Sýkorová, E.; Lim, K.Y.; Chase, M.W.; Knapp, S.; Leitch, I.J.; Leitch, A.R.; Fajkus, J. The absence of Arabidopsis-type telomeres in Cestrum and closely related genera Vestia and Sessea (Solanaceae): First evidence from eudicots. Plant J. 2003, 34, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Cao, H.X.; Jovtchev, G.; Neumann, P.; Novák, P.; Fojtová, M.; Vu, G.T.H.; Macas, J.; Fajkus, J.; Schubert, I.; et al. Centromere and telomere sequence alterations reflect the rapid genome evolution within the carnivorous plant genus Genlisea. Plant J. 2015, 84, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Katsiotis, A.; Hagidimitriou, M.; Heslop-Harrison, J.S. The close relationship between the A and B genomes in Avena L. (Poaceae) determined by molecular cytogenetic analysis of total genomic, tandemly and dispersed repetitive DNA sequences. Ann. Bot. 1997, 79, 103–109. [Google Scholar] [CrossRef]
- Hizume, M.; Shibata, F.; Matsusaki, Y.; Kondo, T. Chromosomal localization of telomere sequence repeats in five gymnosperm species. Chromosome Sci. 2000, 4, 39–42. [Google Scholar]
- Hizume, M.; Kurose, N.; Shibata, F.; Kondo, K. Molecular cytogenetic studies on sex chromosomes and proximal heterochromatin containing telomere-like sequence in Cycas revoluta. Chromosome Sci. 1998, 2, 63–72. [Google Scholar]
- Shibata, F.; Hizume, M. Survey of Arabidopsis-and human-type telomere repeats in plants using fluorescence in situ hybridisation. Cytologia 2011, 76, 353–360. [Google Scholar] [CrossRef]
- Hizume, M.; Shibata, F.; Matsusaki, Y.; Garajova, Z. Chromosome identification and comparative karyotypic analyses of four Pinus species. Theor. Appl. Genet. 2002, 105, 491–497. [Google Scholar] [CrossRef]
- Shibata, F.; Matsusaki, Y.; Hizume, M. AT-rich sequences containing Arabidopsis-type telomere sequence and their chromosomal distribution in Pinus densiflora. Theor. Appl. Genet. 2005, 110, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Doudrick, R.L.; Heslop-Harrison, J.S.; Schmidt, T. The contribution of short repeats of low sequence complexity to large conifer genomes. Theor. Appl. Genet. 2000, 101, 7–14. [Google Scholar] [CrossRef]
- Islam-Faridi, M.N.; Nelson, C.D.; Kubisiak, T.L. Reference karyotype and cytomolecular map for loblolly pine (Pinus taeda L.). Genome 2007, 50, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.G.; Friesen, N.; Heslop-Harrison, J.S. Molecular cytogenetic analysis of Podocarpus and comparison with other gymnosperm species. Ann. Bot. 2002, 89, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Tagashira, N. Regions in situ-hybridized by the Arabidopsis-type telomere sequence repeats in Zamia chromosomes. Chromosome Sci. 1998, 2, 87–89. [Google Scholar]
- Kondo, K.; Tagashira, N.; Abd El-Twab, M.H.; Hoshi, Y.; Kokubugata, G.; Honda, Y.; Khaung, K.K. Structural differences of chromosomes in plants detected by fluorescence in situ hybridization using probes of rDNA, Arabidopsis-type telomere sequence repeats and pCrT7-4. In Some Aspects of Chromosome Structure and Functions; Sobti, R.C., Obe, G., Athwal, R.S., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 27–35. [Google Scholar]
- Abd El-Twab, M.H.; Kondo, K. FISH physical mapping of 5S rDNA and telomere sequence repeats identified a peculiar chromosome mapping and mutation in Leucanthemella linearis and Nipponanthemum nipponicum in Chrysanthemum sensu lato. Chromosom. Bot. 2007, 2, 11–17. [Google Scholar] [CrossRef]
- Amosova, A.V.; Bolsheva, N.L.; Samatadze, T.E.; Twardovska, M.O.; Zoshchuk, S.A.; Andreev, I.O.; Badaeva, E.D.; Kunakh, V.A.; Muravenko, O.V. Molecular cytogenetic analysis of Deschampsia antarctica Desv. (Poaceae), Maritime Antarctic. PLoS ONE 2015, 10, e0138878. [Google Scholar] [CrossRef]
- Begum, R.; Alam, S.S.; Menzel, G.; Schmidt, T. Comparative molecular cytogenetics of major repetitive sequence families of three Dendrobium species (Orchidaceae) from Bangladesh. Ann. Bot. 2009, 104, 863–872. [Google Scholar] [CrossRef]
- Begum, R.; Zakrzewski, F.; Menzel, G.; Weber, B.; Alam, S.S.; Schmidt, T. Comparative molecular cytogenetic analyses of a major tandemly repeated DNA family and retrotransposon sequences in cultivated jute Corchorus species (Malvaceae). Ann. Bot. 2013, 112, 123–134. [Google Scholar] [CrossRef]
- Bolsheva, N.L.; Zelenin, A.V.; Nosova, I.V.; Amosova, A.V.; Samatadze, T.E.; Yurkevich, O.Y.; Melnikova, N.V.; Zelenina, D.A.; Volkov, A.A.; Muravenko, O.V. The diversity of karyotypes and genomes within section Syllinum of the genus Linum (Linaceae) revealed by molecular cytogenetic markers and RAPD analysis. PLoS ONE 2015, 10, e0122015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chacón, J.; Sousa, A.; Baeza, C.M.; Renner, S.S. Ribosomal DNA distribution and a genus-wide phylogeny reveal patterns of chromosomal evolution in Alstroemeria (Alstroemeriaceae). Am. J. Bot. 2012, 99, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Navrátilová, A.; Neumann, P.; Macas, J. Karyotype analysis of four Vicia species using in situ hybridization with repetitive sequences. Ann. Bot. 2003, 91, 921–926. [Google Scholar] [CrossRef]
- Hanmoto, H.; Kataoka, R.; Ohmido, N.; Yonezawa, Y. Interstitial telomere-like repeats in the Haplopappus gracilis (Asteraceae) genome revealed by fluorescence in situ hybridization. Cytologia 2007, 72, 483–488. [Google Scholar] [CrossRef][Green Version]
- Cuadrado, Á.; Carmona, A.; Jouve, N. Chromosomal characterization of the three subgenomes in the polyploids of Hordeum murinum L.: New insight into the evolution of this complex. PLoS ONE 2013, 8, e81385. [Google Scholar] [CrossRef]
- Da Silva, C.R.; González-Elizondo, M.S.; Vanzela, A.L. Reduction of chromosome number in Eleocharis subarticulata (Cyperaceae) by multiple translocations. Bot. J. Linn. Soc. 2005, 149, 457–464. [Google Scholar] [CrossRef]
- Deng, H.; Tang, G.; Xu, N.; Gao, Z.; Lin, L.; Liang, D.; Xia, H.; Deng, Q.; Wang, J.; Cai, Z.; et al. Integrated karyotypes of diploid and tetraploid Carrizo Citrange (Citrus sinensis L. Osbeck × Poncirus trifoliata L. Raf.) as determined by sequential multicolor fluorescence in situ hybridization with tandemly repeated DNA sequences. Front Plant Sci. 2020, 11, 569. [Google Scholar] [CrossRef]
- Du, P.; Li, L.N.; Zhang, Z.X.; Liu, H.; Qin, L.; Huang, B.Y.; Dong, W.; Tang, F.; Qi, Z.; Zhang, X.Y. Chromosome painting of telomeric repeats reveals new evidence for genome evolution in peanut. J. Integr. Agric. 2016, 15, 2488–2496. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Tia, L.; Chen, L.; Yu, W. Identification of peanut (Arachis hypogaea) chromosomes using a fluorescence in situ hybridization system reveals multiple hybridization events during tetraploid peanut formation. New Phytol. 2016, 211, 1424–1439. [Google Scholar] [CrossRef]
- Falistocco, E. Insight into the chromosome structure of the cultivated tetraploid alfalfa (Medicago sativa subsp. sativa L.) by a combined use of GISH and FISH techniques. Plants 2020, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Dechyeva, D.; Schmidt, T. Molecular organization of terminal repetitive DNA in Beta species. Chromosome Res. 2006, 14, 881–897. [Google Scholar] [CrossRef]
- Zatloukalová, P.; Hřibová, E.; Kubaláková, M.; Suchánková, P.; Šimková, H.; Adoración, C.; Kahl, G.; Millán, T.; Doležel, J. Integration of genetic and physical maps of the chickpea (Cicer arietinum L.) genome using flow-sorted chromosomes. Chromosome Res. 2011, 19, 729–739. [Google Scholar] [CrossRef]
- Gortner, G.; Nenno, M.; Weising, K.; Zink, D.; Nagl, W.; Kahl, G. Chromosomal localization and distribution of simple sequence repeats and the Arabidopsis-type telomere sequence in the genome of Cicer arietinum L. Chromosome Res. 1998, 6, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Grabowska-Joachimiak, A.; Mosiolek, M.; Lech, A.; Góralski, G. C-Banding/DAPI and in situ hybridization reflect karyotype structure and sex chromosome differentiation in Humulus japonicus Siebold & Zucc. Cytogenet. Genome Res. 2011, 132, 203–211. [Google Scholar] [PubMed]
- Grabowska-Joachimiak, A.; Kula, A.; Gernand-Kliefoth, D.; Joachimiak, A.J. Karyotype structure and chromosome fragility in the grass Phleum echinatum Host. Protoplasma 2015, 252, 301–306. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, J.; Torres, G.A.; Zhang, H.; Jiang, J.; Xie, C. Interstitial telomeric repeats are enriched in the centromeres of chromosomes in Solanum species. Chromosome Res. 2013, 21, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, C.; Li, J.; Yang, S.; Wang, Y.; Li, Z.; Chen, J.; Lou, Q. Chromosomal structures and repetitive sequences divergence in Cucumis species revealed by comparative cytogenetic mapping. BMC Genom. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Koo, D.H.; Hong, C.P.; Batley, J.; Chung, Y.S.; Edwards, D.; Bang, J.W.; Hur, Y.; Lim, Y.P. Rapid divergence of repetitive DNAs in Brassica relatives. Genomics 2011, 97, 173–185. [Google Scholar] [CrossRef]
- Kim, E.S.; Bolshev, N.L.; Samatadze, T.E.; Nosov, N.N.; Nosova, I.V.; Zelenin, A.V.; Punina, E.O.; Muravenko, O.V.; Rodionov, A.V. The unique genome of two-chromosome grasses Zingeria and Colpodium, its origin, and evolution. Russ. J. Genet. 2009, 45, 1329–1337. [Google Scholar] [CrossRef]
- Kirov, I.V.; Van Laere, K.; Van Roy, N.; Khrustaleva, L.I. Towards a FISH-based karyotype of Rosa L. (Rosaceae). Comp. Cytogenet. 2016, 10, 543. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Furuta, T. Region in situ-hybridized by the Arabidopsis-type telomere sequence repeats in Drosera chromosomes. Chromosome Sci. 1999, 3, 63–67. [Google Scholar]
- Kono, Y.; Hoshi, Y.; Setoguchi, H.; Yokota, M.; Oginuma, K. Distribution patterns rDNAs and telomeres and chromosomal rearrangement between two cytotypes of Lysimachia mauritiana L. (Primulaceae). Caryologia 2011, 64, 91–98. [Google Scholar] [CrossRef]
- Kono, Y.; Peng, C.I.; Hoshi, Y.; Yokota, M.; Setoguchi, H.; Lum, S.K.; Oginuma, K. Intraspecific karyotype polymorphism and chromosomal evolution of Lysimachia mauritiana (Primulaceae) in the Ryukyu archipelago of Japan and Taiwan. Cytologia 2019, 84, 93–103. [Google Scholar] [CrossRef]
- Lan, T.Y.; Liu, B.; Dong, F.P.; Chen, R.Y.; Li, X.L.; Chen, C.B. Multicolor FISH analysis of rDNA and telomere on spinach. Front Agric. China 2008, 29, 1405–1408. [Google Scholar] [CrossRef]
- Li, J.; He, S.; Zhang, L.; Hu, Y.; Yang, F.; Ma, L.; Huang, J.; Li, L. Telomere and 45S rDNA sequences are structurally linked on the chromosomes in Chrysanthemum segetum L. Protoplasma 2012, 249, 207–215. [Google Scholar] [CrossRef]
- Luo, X.; Chen, J. Physical map of FISH 5S rDNA and (AG3T3)3 signals displays Chimonanthus campanulatus R.H. Chang & C.S. Ding chromosomes, reproduces its metaphase dynamics and distinguishes its chromosomes. Genes 2019, 10, 904. [Google Scholar]
- Maravilla, A.J.; Rosato, M.; Álvarez, I.; Nieto Feliner, G.; Rosselló, J.A. Interstitial Arabidopsis-type telomeric repeats in Asteraceae. Plants 2021, submitted. [Google Scholar]
- Mlinarec, J.; Skuhala, A.; Jurković, A.; Malenica, N.; McCann, J.; Weiss-Schneeweiss, H.; Bohanec, B.; Besendorfer, V. The repetitive DNA composition in the natural pesticide producer Tanacetum cinerariifolium: Interindividual variation of subtelomeric tandem repeats. Front. Plant Sci. 2019, 10, 613. [Google Scholar] [CrossRef]
- Moscone, E.A.; Samuel, R.; Schwarzacher, T.; Schweizer, D.; Pedrosa-Harand, A. Complex rearrangements are involved in Cephalanthera (Orchidaceae) chromosome evolution. Chromosome Res. 2007, 15, 931–943. [Google Scholar] [CrossRef]
- Németh, A.V.; Dudits, D.; Molnár-Láng, M.; Linc, G. Molecular cytogenetic characterisation of Salix viminalis L. using repetitive DNA sequences. J. Appl. Genet. 2013, 54, 265–269. [Google Scholar] [CrossRef][Green Version]
- Nenno, M.; Zink, D.; Nagl, W. The Arabidopsis telomere sequence is highly abundant in the genome of Phaseolus acutifolius and preferentially located in the centromeres. Rep. Bean Improv. Coop. Nat. Dry Bean Counc. Res. Conf. Ann. Rep. 1998, 41, 103–104. [Google Scholar]
- Nguyen, T.H.; Waminal, N.E.; Lee, D.S.; Pellerin, R.J.; Ta, T.D.; Campomayor, N.B.; Kang, B.Y.; Kim, H.H. Comparative triple-color FISH mapping in eleven Senna species using rDNA and telomeric repeat probes. Hortic. Environ. Biotechnol. 2021, 62, 927–935. [Google Scholar] [CrossRef]
- Pedrosa, A.; Sandal, N.; Stougaard, J.; Schweizer, D.; Bachmair, A. Chromosomal map of the model legume Lotus japonicus. Genetics 2002, 161, 1661–1672. [Google Scholar] [CrossRef]
- Pellerin, R.J.; Waminal, N.E.; Kim, H.H. Triple-color FISH karyotype analysis of four Korean wild Cucurbitaceae species. Hortic. Sci. Technol. 2018, 36, 98–107. [Google Scholar]
- Pellerin, R.J.; Waminal, N.E.; Kim, H.H. FISH mapping of rDNA and telomeric repeats in 10 Senna species. Hortic. Environ. Biotechnol. 2019, 60, 253–260. [Google Scholar] [CrossRef]
- Raskina, O.; Barber, J.C.; Nevo, E.; Belyayev, A. Repetitive DNA and chromosomal rearrangements: Speciation-related events in plant genomes. Cytogenet. Genome Res. 2008, 120, 351–357. [Google Scholar] [CrossRef]
- Uchida, W.; Matsunaga, S.; Sugiyama, R.; Kawano, S. Interstitial telomere-like repeats in the Arabidopsis thaliana genome. Genes Genet. Syst. 2002, 77, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Uchida, W.; Matsunaga, S.; Sugiyama, R.; Shibata, F.; Kazama, Y.; Miyazawa, Y.; Hizume, M.; Kawano, S. Distribution of interstitial telomere-like repeats and their adjacent sequences in a dioecious plant, Silene latifolia. Chromosoma 2002, 111, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Rockinger, A.; Sousa, A.; Carvalho, F.A.; Renner, S.S. Chromosome number reduction in the sister clade of Carica papaya with concomitant genome size doubling. Am. J. Bot. 2016, 103, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Rosato, M.; Álvarez, I.; Nieto Feliner, G.; Rosselló, J.A. Inter-and intraspecific hypervariability in interstitial telomeric-like repeats (TTTAGGG)n in Anacyclus (Asteraceae). Ann. Bot. 2018, 122, 387–395. [Google Scholar] [CrossRef]
- Sevilleno, S.S.; Ju, Y.H.; Kim, J.S.; Mancia, F.H.; Byeon, E.J.; Cabahug, R.A.; Hwang, Y.J. Cytogenetic analysis of Bienertia sinuspersici Akhani as the first step in genome sequencing. Genes Genom. 2020, 42, 337–345. [Google Scholar] [CrossRef]
- Sousa, A.; Renner, S.S. Interstitial telomere-like repeats in the monocot family Araceae. Bot. J. Linn. Soc. 2015, 177, 15–26. [Google Scholar] [CrossRef]
- Sousa, A.; Cusimano, N.; Renner, S.S. Combining FISH and model-based predictions to understand chromosome evolution in Typhonium (Araceae). Ann. Bot. 2014, 113, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.; Vanzela, A.L.; Crosa, O.; Guerra, M. Interstitial telomeric sites and Robertsonian translocations in species of Ipheion and Nothoscordum (Amaryllidaceae). Genetica 2016, 144, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sýkorová, E.; Lim, K.Y.; Fajkus, J.; Leitch, A.R. The signature of the Cestrum genome suggests an evolutionary response to the loss of (TTTAGGG)n telomeres. Chromosoma 2003, 112, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, E.V.; Vasconcelos, S.; Ribeiro, T.; Benko-Iseppon, A.M.; Brasileiro-Vidal, A.C. Karyotype heterogeneity in Philodendron s.l. (Araceae) revealed by chromosome mapping of rDNA loci. PLoS ONE 2018, 13, e0207318. [Google Scholar] [CrossRef]
- Waminal, N.E.; Pellerin, R.J.; Jang, W.; Kim, H.H.; Yang, T.J. Characterization of chromosome-specific microsatellite repeats and telomere repeats based on low coverage whole genome sequence reads in Panax ginseng. Plant Breed. Biotechnol. 2018, 6, 74–81. [Google Scholar] [CrossRef]
- Waminal, N.E.; Yang, T.J.; In, J.G.; Kim, H.H. Five-color fluorescence in situ hybridization system for karyotyping of Panax ginseng. Hortic. Environ. Biotechnol. 2020, 61, 869–877. [Google Scholar] [CrossRef]
- Weiss-Schneeweiss, H.; Riha, K.; Jang, C.G.; Puizina, J.; Scherthan, H.; Schweizer, D. Chromosome termini of the monocot plant Othocallis siberica are maintained by telomerase, which specifically synthesises vertebrate-type telomere sequences. Plant J. 2004, 37, 484–493. [Google Scholar] [CrossRef]
- Deng, H.; Xiang, S.; Guo, Q.; Jin, W.; Cai, Z.; Liang, G. Molecular cytogenetic analysis of genome-specific repetitive elements in Citrus clementina Hort. ex Tan. and its taxonomic implications. BMC Plant Biol. 2019, 19, 77. [Google Scholar] [CrossRef]
- Deng, H.; Cai, Z.; Xiang, S.; Guo, Q.; Huang, W.; Liang, G. Karyotype analysis of diploid and spontaneously occurring tetraploid blood orange [Citrus sinensis (L.) Osbeck] using multicolor FISH with repetitive DNA sequences as probes. Front. Plant Sci. 2019, 10, 331. [Google Scholar] [CrossRef]
- Prieto, P.; Martín, A.; Cabrera, A. Chromosomal distribution of telomeric and telomeric-associated sequences in Hordeum chilense by in situ hybridization. Hereditas 2004, 141, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, M.R.; Kotseruba, V.; Cremonini, R. Methylated-rich regions and tandem repeat arrays along the chromosome complement of Colpodium versicolor (Stev.) Schmalh. Protoplasma 2009, 237, 13. [Google Scholar] [CrossRef]
- Heckmann, S.; Schroeder-Reiter, E.; Kumke, K.; Ma, L.; Nagaki, K.; Murata, M.; Wanner, G.; Houben, A. Holocentric chromosomes of Luzula elegans are characterized by a longitudinal centromere groove, chromosome bending, and a terminal nucleolus organizer region. Cytogenet. Genome Res. 2011, 134, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Maximiano Da Silva, C.M.; González-Elizondo, M.S.; Laforga Vanzela, A.L. Chromosome reduction in Eleocharis maculosa (Cyperaceae). Cytogenet Genome Res. 2008, 122, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Yagi, K.; Matsuda, M.; Matoba, H.; Tagashira, N.; Pląder, W.; Malepszy, S.; Nagano, K.; Morikawa, A. A comparative study of the three cucumber cultivars using fluorescent staining and fluorescence in situ hybridization. Cytologia 2011, 76, 3–10. [Google Scholar] [CrossRef]
- Pellerin, R.J.; Waminal, N.E.; Belandres, H.R.; Kim, H.H. Karyotypes of three exotic cucurbit species based on triple-color FISH analysis. Korean J. Hortic. Sci. Technol. 2018, 36, 417–425. [Google Scholar]
- Alexandrov, O.S.; Divashuk, M.G.; Yakovin, N.A.; Karlov, G.I. Sex chromosome differentiation in Humulus japonicus Siebold & Zuccarini, 1846 (Cannabaceae) revealed by fluorescence in situ hybridization of subtelomeric repeat. Comp. Cytogenet. 2012, 6, 239. [Google Scholar]
- Divashuk, M.G.; Alexandrov, O.S.; Kroupin, P.Y.; Karlov, G.I. Molecular cytogenetic mapping of Humulus lupulus sex chromosomes. Cytogenet Genome Res. 2011, 134, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Hasterok, R.; Ksiazczyk, T.; Wolny, E.; Maluszynska, J. FISH and GISH analysis of Brassica genomes. Acta Biol. Cracov. Bot. 2005, 47, 185–192. [Google Scholar]
- Adams, S.P.; Hartman, T.P.V.; Lim, K.Y.; Chase, M.W.; Bennett, M.D.; Leitch, I.J.; Leitch, A.R. Loss and recovery of Arabidopsis–type telomere repeat sequences 5′–(TTTAGGG)n–3′ in the evolution of a major radiation of flowering plants. Proc. R. Soc. Lond. B. Biol. Sci. 2001, 268, 1541–1546. [Google Scholar] [CrossRef]
- Jacobs, G.; Dechyeva, D.; Wenke, T.; Weber, B.; Schmidt, T. A BAC library of Beta vulgaris L. for the targeted isolation of centromeric DNA and molecular cytogenetics of Beta species. Genetica 2009, 135, 157–167. [Google Scholar] [CrossRef]
- Puizina, J.; Sviben, T.; Krajačić-Sokol, I.; Zoldoš-Pećnik, V.; Siljak-Yakovlev, S.; Papeš, D.; Besendorfer, V. Cytogenetic and molecular characterization of the Abies alba genome and its relationship with other members of the Pinaceae. Plant Biol. 2008, 10, 256–267. [Google Scholar] [CrossRef]
- Lubaretz, O.; Fuch, J.; Ahne, R.; Meister, A.; Schubert, I. Karyotyping of three Pinaceae species via fluorescent in situ hybridization and computer-aided chromosome analysis. Theor. Appl. Genet. 1996, 92, 411–416. [Google Scholar] [CrossRef]
- de la Herrán, R.; Cuñado, N.; Navajas-Pérez, R.; Santos, J.L.; Rejón, C.R.; Garrido-Ramos, M.A.; Rejón, M.R. The controversial telomeres of lily plants. Cytogenet. Genome Res. 2005, 109, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Sýkorová, E.; Lim, K.Y.; Kunická, Z.; Chase, M.W.; Bennett, M.D.; Fajkus, J.; Leitch, A.R. Telomere variability in the monocotyledonous plant order Asparagales. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 1893–1904. [Google Scholar] [CrossRef]
- Monkheang, P.; Chaveerach, A.; Sudmoon, R.; Tanee, T. Karyotypic features including organizations of the 5S, 45S rDNA loci and telomeres of Scadoxus multiflorus (Amaryllidaceae). Comp. Cytogenet. 2016, 10, 637. [Google Scholar]
- Báez, M.; Souza, G.; Guerra, M. Genome size and cytomolecular diversification in two species of the South African endemic genus Tulbaghia L. (Allioideae, Amaryllidaceae). S. Afr. J. Bot. 2020, 130, 407–413. [Google Scholar] [CrossRef]
- Falistocco, E.; Ferradini, N. Advances in the cytogenetics of Annonaceae, the case of Annona cherimola L. Genome 2020, 63, 357–364. [Google Scholar] [CrossRef]
- Nowicka, A.; Grzebelus, E.; Grzebelus, D. Precise karyotyping of carrot mitotic chromosomes using multicolour-FISH with repetitive DNA. Biol. Plant. 2016, 60, 25–36. [Google Scholar] [CrossRef]
- Zhou, H.C.; Pellerin, R.J.; Waminal, N.E.; Yang, T.J.; Kim, H.H. Pre-labelled oligo probe-FISH karyotype analyses of four Araliaceae species using rDNA and telomeric repeat. Genes Genom. 2019, 41, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Castilho, A.; Vershinin, A.; Heslop-Harrison, J.S. Repetitive DNA and the chromosomes in the genome of oil palm (Elaeis guineensis). Ann. Bot. 2000, 85, 837–844. [Google Scholar] [CrossRef]
- Zaki, N.M. The genome landscape of Elaeis guineensis: Development and Utility of Chromosome-Specific Cytogenetic Markers. Ph.D. Thesis, University of Leicester, Leicester, UK, April 2019. [Google Scholar]
- Pereira, T.N.S.; Neto, M.F.; de Souza, M.M.; da Costa Geronimo, I.G.; de Melo, C.A.F.; Pereira, M.G. Cytological characterization of Brazilian green dwarf coconut (Cocos nucifera L.) via meiosis and conventional and differential karyotyping. Cytologia 2017, 82, 167–174. [Google Scholar] [CrossRef]
- Robert, M.L.; Lim, K.Y.; Hanson, L.; Sanchez-Teyer, F.; Bennett, M.D.; Leitch, A.R.; Leitch, I.J. Wild and agronomically important Agave species (Asparagaceae) show proportional increases in chromosome number, genome size, and genetic markers with increasing ploidy. Bot. J. Linn. Soc. 2008, 158, 215–222. [Google Scholar] [CrossRef]
- Puizina, J.; Weiss-Schneeweiss, H.; Pedrosa-Harand, A.; Kamenjarin, J.; Trinajstić, I.; Riha, K.; Schweizer, D. Karyotype analysis in Hyacinthella dalmatica (Hyacinthaceae) reveals vertebrate-type telomere repeats at the chromosome ends. Genome 2003, 46, 1070–1076. [Google Scholar] [CrossRef]
- Weiss, H.; Scherthan, H. Aloe spp.–plants with vertebrate-like telomeric sequences. Chromosome Res. 2002, 10, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Twab, M.H.; Kondo, K. Physical mapping of 5S, 45S, Arabidopsis-type telomere sequence repeats and AT-rich regions in Achillea millefolium showing intrachromosomal variation by FISH and DAPI. Chromosom. Bot. 2009, 4, 37–45. [Google Scholar] [CrossRef]
- Borgen, L.; Leitch, I.; Santos-Guerra, A. Genome organization in diploid hybrid species of Argyranthemum (Asteraceae) in the Canary Islands. Bot. J. Linn. Soc. 2003, 141, 491–501. [Google Scholar] [CrossRef][Green Version]
- García, S.; Garnatje, T.; Pellicer, J.; McArthur, E.D.; Siljak-Yakovlev, S.; Vallés, J. Ribosomal DNA, heterochromatin, and correlation with genome size in diploid and polyploid North American endemic sagebrushes (Artemisia, Asteraceae). Genome 2009, 52, 1012–1024. [Google Scholar] [CrossRef]
- Matoba, H.; Uchiyama, H. Physical mapping of 5S rDNA, 18S rDNA and telomere sequences in three species of the genus Artemisia (Asteraceae) with distinct basic chromosome numbers. Cytologia 2009, 74, 115–123. [Google Scholar] [CrossRef]
- Houben, A.; Thompson, N.; Ahne, R.; Leach, C.R.; Verlin, D.; Timmis, J.N. A monophyletic origin of the B chromosomes of Brachycome dichromosomatica (Asteraceae). Plant Syst. Evol. 1999, 219, 127–135. [Google Scholar] [CrossRef]
- Mancia, F.H.; Ju, Y.H.; Lim, K.B.; Kim, J.S.; Nam, S.Y.; Hwang, Y.J. Cytogenetic mapping of Carthamus tinctorius L. with tandemly repeated DNA sequences by fluorescence in situ hybridization. Korean J. Plant Res. 2017, 30, 654–661. [Google Scholar]
- Dydak, M.; Kolano, B.; Nowak, T.; Siwinska, D.; Maluszynska, J. Cytogenetic studies of three European species of Centaurea L. (Asteraceae). Hereditas 2009, 146, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Cuyacot, A.R.; Won, S.Y.; Park, S.K.; Sohn, S.H.; Lee, J.; Kim, J.S.; Kim, H.H.; Lim, K.M.; Hwang, Y.J. The chromosomal distribution of repetitive DNA sequences in Chrysanthemum boreale revealed a characterization in its genome. Sci. Hortic. 2016, 198, 438–444. [Google Scholar] [CrossRef]
- Abd El-Twab, M.H.; Kondo, K. FISH physical mapping of 5S, 45S and Arabidopsis-type telomere sequence repeats in Chrysanthemum zawadskii showing intra-chromosomal variation and complexity in nature. Chromosom. Bot. 2006, 1, 1–5. [Google Scholar] [CrossRef]
- Cuyacot, A.R.; Lim, K.B.; Kim, H.H.; Hwang, Y.J. Chromosomal characterization based on repetitive DNA distribution in a tetraploid cytotype of Chrysanthemum zawadskii. Hortic. Environ. Biotechnol 2017, 58, 488–494. [Google Scholar] [CrossRef]
- Jamilena, M.; Rejón, C.R.; Rejón, M.R. A molecular analysis of the origin of the Crepis capillaris B chromosome. J. Cell Sci. 1994, 107, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Matoba, H.; Mizutani, T.; Nagano, K.; Hoshi, Y.; Uchiyama, H. Chromosomal study of lettuce and its allied species (Lactuca spp.; Asteraceae) by means of karyotype analysis and fluorescence in situ hybridization. Hereditas 2007, 144, 235–243. [Google Scholar] [CrossRef]
- Pires, J.C.; Lim, K.Y.; Kovarík, A.; Matyásek, R.; Boyd, A.; Leitch, A.R.; Bennett, N.D.; Soltis, P.S.; Soltis, D.E. Molecular cytogenetic analysis of recently evolved Tragopogon (Asteraceae) allopolyploids reveal a karyotype that is additive of the diploid progenitors. Am. J. Bot. 2004, 91, 1022–1035. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X. First report of bicolour FISH of Berberis diaphana and B. soulieana reveals interspecific differences and co-localization of (AGGGTTT)3 and rDNA 5S in B. diaphana. Hereditas 2019, 156, 1–8. [Google Scholar] [CrossRef]
- Mandáková, T.; Marhold, K.; Lysak, M.A. The widespread crucifer species Cardamine flexuosa is an allotetraploid with a conserved subgenomic structure. New Phytol. 2014, 201, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Mandáková, T.; Heenan, P.B.; Lysak, M.A. Island species radiation and karyotypic stasis in Pachycladon allopolyploids. BMC Evol. Biol. 2010, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, R.J.; Waminal, N.E.; Kim, J.Y.; Um, Y.; Kim, H.H. Fluorescence in situ hybridization karyotype analysis of seven Platycodon grandiflorum (Jacq.) A. DC. cultivars. Korean. J. Hortic. Sci. Technol. 2017, 35, 784–792. [Google Scholar]
- Vanzela, A.L.L.; Cuadrado, Á.; Vieira, A.O.S.; Jouve, N. Genome characterization and relationships between two species of the genus Lobelia (Campanulaceae) determined by repeated DNA sequences. Plant Syst. Evol. 1999, 214, 211–218. [Google Scholar] [CrossRef]
- Divashuk, M.G.; Alexandrov, O.S.; Razumova, O.V.; Kirov, I.V.; Karlov, G.I. Molecular cytogenetic characterization of the dioecious Cannabis sativa with an XY chromosome sex determination system. PLoS ONE 2014, 9, e85118. [Google Scholar]
- Iovene, M.; Yu, Q.; Ming, R.; Jiang, J. Evidence for emergence of sex-determining gene(s) in a centromeric region in Vasconcellea parviflora. Genetics 2015, 199, 413–421. [Google Scholar] [CrossRef]
- Riha, K.; Fajkus, J.; Siroky, J.; Vyskot, B. Developmental control of telomere lengths and telomerase activity in plants. Plant Cell 1998, 10, 1691–1698. [Google Scholar] [CrossRef]
- Sousa, A.; Fuchs, J.; Renner, S.S. Cytogenetic comparison of heteromorphic and homomorphic sex chromosomes in Coccinia (Cucurbitaceae) points to sex chromosome turnover. Chromosome Res. 2017, 25, 191–200. [Google Scholar] [CrossRef]
- Vanzela, A.L.; Cuadrado, A.; Guerra, M. Localization of 45S rDNA and telomeric sites on holocentric chromosomes of Rhynchospora tenuis Link (Cyperaceae). Genet. Mol. Biol. 2003, 26, 199–201. [Google Scholar] [CrossRef][Green Version]
- Leitch, A.R.; Lim, K.Y.; Leitch, I.J.; O’Neill, M.; Chye, M.; Low, F. Molecular cytogenetic studies in rubber, Hevea brasiliensis Muell. Arg. (Euphorbiaceae). Genome 1998, 41, 464–467. [Google Scholar] [CrossRef][Green Version]
- Galasso, I.; Schmidt, T.; Pignone, D. Identification of Lens culinaris ssp. culinaris chromosomes by physical mapping of repetitive DNA sequences. Chromosome Res. 2001, 9, 199–209. [Google Scholar] [CrossRef]
- Hajdera, I.; Siwinska, D.; Hasterok, R.; Maluszynska, J. Molecular cytogenetic analysis of genome structure in Lupinus angustifolius and Lupinus cosentinii. Theor. Appl. Genet. 2003, 107, 988–996. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Naganowska, B.; Wolko, B. Karyotyping of the narrow-leafed lupin (Lupinus angustifolius L.) by using FISH, PRINS and computer measurements of chromosomes. J. Appl. Genet. 2009, 50, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Fonsêca, A.; Ferraz, M.E.; Pedrosa-Harand, A. Speeding up chromosome evolution in Phaseolus: Multiple rearrangements associated with a one-step descending dysploidy. Chromosoma 2016, 125, 413–421. [Google Scholar] [CrossRef]
- Rawlins, D.J.; Highett, M.I.; Shaw, P.J. Localization of telomeres in plant interphase nuclei by in situ hybridization and 3D confocal microscopy. Chromosoma 1991, 100, 424–431. [Google Scholar] [CrossRef]
- Macas, J.; Neumann, P.; Navrátilová, A. Repetitive DNA in the pea (Pisum sativum L.) genome: Comprehensive characterization using 454 sequencing and comparison to soybean and Medicago truncatula. BMC Genom. 2007, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.M.; Kim, H.H. Chromosome karyotyping of Senna covesii and S. floribunda based on triple–color FISH mapping of rDNAs and telomeric repeats. Plant Breed. Biotechnol. 2018, 6, 51–56. [Google Scholar] [CrossRef]
- Galasso, I.; Schmidt, T.; Pignone, D.; Heslop-Harrison, J.S. The molecular cytogenetics of Vigna unguiculata (L.) Walp: The physical organization and characterization of 18S-5.8S-25S rRNA genes, 5S rRNA genes, telomere-like sequences, and a family of centromeric repetitive DNA sequences. Theor. Appl. Genet. 1995, 91, 928–935. [Google Scholar] [CrossRef]
- Jankowska, M.; Fuchs, J.; Klocke, E.; Fojtová, M.; Polanská, P.; Fajkus, J.; Schubert, V.; Houben, A. Holokinetic centromeres and efficient telomere healing enable rapid karyotype evolution. Chromosoma 2015, 124, 519–528. [Google Scholar] [CrossRef]
- Xie, S.; Marasek-Ciolakowska, A.; Ramanna, M.S.; Arens, P.; Visser, R.G.; van Tuyl, J.M. Characterization of B chromosomes in Lilium hybrids through GISH and FISH. Plant Syst. Evol. 2014, 300, 1771–1777. [Google Scholar] [CrossRef]
- Lombello, R.A.; Forni-Martins, E.R. Cytogenetics and evolutionary analysis of Lophanthera, an Amazonian arboreal Malpighiaceae. Cytologia 2002, 67, 41–45. [Google Scholar] [CrossRef]
- Islam-Faridi, N.; Sakhanokho, H.F.; Nelson, C.D. New chromosome number and cyto-molecular characterization of the African Baobab (Adansonia digitata L.)—“The Tree of Life”. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Ling, J.; Cheng, H.; Liu, F.; Song, G.L.; Wang, C.Y.; Li, S.H.; Zhang, X.D.; Wang, Y.H.; Wang, K.B. The cloning and fluorescence in situ hybridization analysis of cotton telomere sequence. J. Integr. Agric. 2012, 11, 1417–1423. [Google Scholar] [CrossRef]
- Osuji, J.O.; Crouch, J.; Harrison, G.; Heslop-Harrison, J.S. Molecular cytogenetics of Musa species, cultivars and hybrids: Location of 18S-5.8 S-25S and 5S rDNA and telomere-like sequences. Ann. Bot. 1998, 82, 243–248. [Google Scholar] [CrossRef]
- Luo, X.; Liu, J. Fluorescence in situ hybridization (FISH) analysis of the locations of the oligonucleotides 5S rDNA, (AGGGTTT)3, and (TTG)6 in three genera of Oleaceae and their phylogenetic framework. Genes 2019, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Golczyk, H.; Massouh, A.; Greiner, S. Translocations of chromosome end-segments and facultative heterochromatin promote meiotic ring formation in evening primroses. Plant Cell 2014, 26, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Park, E.J.; Kim, H.H. Analysis of chromosome composition of Gastrodia elata Blume by fluorescent in situ hybridization using rDNA and telomeric repeat probes. Korean J. Med. Crop Sci. 2018, 26, 113–118. [Google Scholar] [CrossRef]
- de Melo, C.A.F.; Souza, M.M.; Silva, G.S. Karyotype analysis by FISH and GISH techniques on artificial backcrossed interspecific hybrids involving Passiflora sublanceolata (Killip) MacDougal (Passifloraceae). Euphytica 2017, 213, 161. [Google Scholar] [CrossRef]
- Dhar, M.K.; Kaul, S.; Friebe, B.; Gill, B.S. Chromosome identification in Plantago ovata Forsk. through C-banding and FISH. Curr. Sci. 2002, 83, 150–152. [Google Scholar]
- Shams, I.; Raskina, O. Supernumerary B chromosomes and plant genome changes: A snapshot of wild populations of Aegilops speltoides Tausch (Poaceae, Triticeae). Int. J. Mol. Sci. 2020, 21, 3768. [Google Scholar] [CrossRef]
- Santos, F.C.; Guyot, R.; do Valle, C.B.; Chiari, L.; Techio, V.H.; Heslop-Harrison, P.; Vanzela, A.L.L. Chromosomal distribution and evolution of abundant retrotransposons in plants: Gypsy elements in diploid and polyploid Brachiaria forage grasses. Chromosome Res. 2015, 23, 571–582. [Google Scholar] [CrossRef]
- Rocha, L.C.; de Oliveira Bustamante, F.; Silveira, R.A.D.; Torres, G.A.; Mittelmann, A.; Techio, V.H. Functional repetitive sequences and fragile sites in chromosomes of Lolium perenne L. Protoplasma 2015, 252, 451–460. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, B.S.; Wang, G.L.; Ronald, P.C.; Ward, D.C. Metaphase and interphase fluorescence in situ hybridization mapping of the rice genome with bacterial artificial chromosomes. Proc. Natl. Acad. Sci. USA 1995, 92, 4487–4491. [Google Scholar] [CrossRef]
- Cheng, Z.; Stupar, R.M.; Gu, M.; Jiang, J. A tandemly repeated DNA sequence is associated with both knob-like heterochromatin and a highly decondensed structure in the meiotic pachytene chromosomes of rice. Chromosoma 2001, 110, 24–31. [Google Scholar] [CrossRef]
- Manzanero, S.; Puertas, M.J. Rye terminal neocentromeres: Characterisation of the underlying DNA and chromatin structure. Chromosoma 2003, 111, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.E.; Kota, R.S.; Gill, B.S.; Endo, T.R. Distribution of telomeric repeats and their role in the healing of broken chromosome ends in wheat. Genome 1992, 35, 844–848. [Google Scholar] [CrossRef]
- Mlinarec, J.; Papeš, D.A.; Besendorfer, V. Ribosomal, telomeric and heterochromatin sequences localization in the karyotype of Anemone hortensis. Bot. J. Linn. Soc. 2006, 150, 177–186. [Google Scholar] [CrossRef]
- Schuster, M.; Fuchs, J.; Schubert, I. Cytogenetics in fruit breeding-localization of ribosomal RNA genes on chromosomes of apple (Malus × domestica Borkh.). Theor. Appl. Genet. 1997, 94, 322–324. [Google Scholar] [CrossRef]
- Yu, C.; Deng, X.; Chen, C. Chromosomal characterization of a potential model mini-Citrus (Fortunella hindsii). Tree Genet. Genomes 2019, 15, 73. [Google Scholar] [CrossRef]
- Lan, H.; Chen, C.L.; Miao, Y.; Yu, C.X.; Guo, W.W.; Xu, Q.; Deng, X.X. Fragile sites of ‘Valencia’ sweet orange (Citrus sinensis) chromosomes are related with active 45S rDNA. PLoS ONE 2016, 11, e0151512. [Google Scholar] [CrossRef]
- Xin, H.; Zhang, T.; Han, Y.; Wu, Y.; Shi, J.; Xi, M.; Jiang, J. Chromosome painting and comparative physical mapping of the sex chromosomes in Populus tomentosa and Populus deltoides. Chromosoma 2018, 127, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Islam-Faridi, M.N.; Nelson, C.D.; DiFazio, S.P.; Gunter, L.E.; Tuskan, G.A. Cytogenetic analysis of Populus trichocarpa–ribosomal DNA, telomere repeat sequence, and marker-selected BACs. Cytogenet. Genome Res. 2009, 125, 74–80. [Google Scholar] [CrossRef]
- Datson, P.M.; Murray, B.G. Ribosomal DNA locus evolution in Nemesia: Transposition rather than structural rearrangement as the key mechanism? Chromosome Res. 2006, 14, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Waminal, N.E.; Kim, H.H. In silico mining and FISH mapping of a chromosome-specific satellite DNA in Capsicum annuum L. Genes Genom. 2019, 41, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Moscone, E.A.; Scaldaferro, M.A.; Grabiele, M.; Cecchini, N.M.; Sánchez García, Y.; Jarret, R.; Daviña, J.R.; Ducasse, D.A.; Barboza, G.E.; Ehrendorfer, F. The evolution of chili peppers (Capsicum-Solanaceae): A cytogenetic perspective. Acta Hortic. 2007, 745, 137–169. [Google Scholar] [CrossRef]
- Ganal, M.W.; Lapitan, N.L.; Tanksley, S.D. Macrostructure of the tomato telomeres. Plant Cell 1991, 3, 87–94. [Google Scholar]
- Parokonny, A.S.; Kenton, A.Y.; Gleba, Y.Y.; Bennett, M.D. Genome reorganization in Nicotiana asymmetric somatic hybrids analysed by in situ hybridization. Plant J. 1992, 2, 863–874. [Google Scholar] [CrossRef]
- Peška, V.; Matl, M.; Mandakova, T.; Vitales, D.; Fajkus, P.; Fajkus, J.; García, S. Human-like telomeres in Zostera marina reveal a mode of transition from the plant to the human telomeric sequences. J. Exp. Bot. 2020, 71, 5786–5793. [Google Scholar] [CrossRef]
- Cuadrado, Á.; de Bustos, A.; Jouve, N. On the allopolyploid origin and genome structure of the closely related species Hordeum secalinum and Hordeum capense inferred by molecular karyotyping. Ann. Bot. 2017, 120, 245–255. [Google Scholar]
- Rocha, L.C.; Mittelmann, A.; Houben, A.; Techio, V.H. Fragile sites of 45S rDNA of Lolium multiflorum are not hotspots for chromosomal breakages induced by X-ray. Mol. Biol. Rep. 2016, 43, 659–665. [Google Scholar] [CrossRef]
- Maluszynska, J.; Heslop-Harrison, J.S. Localization of tandemly repeated DNA sequences in Arabidopsis thaliana. Plant J. 1991, 1, 159–166. [Google Scholar] [CrossRef]
- Fonsêca, A.; Pedrosa-Harand, A. Karyotype stability in the genus Phaseolus evidenced by the comparative mapping of the wild species Phaseolus microcarpus. Genome 2013, 56, 335–343. [Google Scholar] [CrossRef]
- Vitales, D.; D’Ambrosio, U.; Gálvez, F.; Kovařík, A.; García, S. Third release of the plant rDNA database with updated content and information on telomere composition and sequenced plant genomes. Plant Syst. Evol. 2017, 303, 1115–1121. [Google Scholar] [CrossRef]
- Angiosperm Phylogeny Website. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 9 September 2021).
- Cole, T.C.H.; Bachelier, J.B.; Hilger, H.H. Tracheophyte phylogeny poster—Vascular plants: Systematics and characteristics. Peer J. Prepr. 2019, 7, e2614v3. [Google Scholar]
- Cole, T.C.H.; Hilger, H.H.; Stevens, P. Angiosperm phylogeny poster (APP)—Flowering plant systematics. Peer J. Prepr. 2019, 7, e2320v6. [Google Scholar]
- Clark, J.W.; Donoghue, P.C. Whole-genome duplication and plant macroevolution. Trends Plant Sci. 2018, 23, 933–945. [Google Scholar] [CrossRef] [PubMed]
- De Bodt, S.; Maere, S.; Van de Peer, Y. Genome duplication and the origin of angiosperms. Trends Ecol. Evol. 2005, 20, 591–597. [Google Scholar] [CrossRef]
- Landis, J.B.; Soltis, D.E.; Li, Z.; Marx, H.E.; Barker, M.S.; Tank, D.C.; Soltis, P.S. Impact of whole-genome duplication events on diversification rates in angiosperms. Am. J. Bot. 2018, 105, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M. Chromosome numbers in plant cytotaxonomy: Concepts and implications. Cytogenet. Genome Res. 2008, 120, 339–350. [Google Scholar] [CrossRef]
- Schubert, I.; Lysak, M.A. Interpretation of karyotype evolution should consider chromosome structural constraints. Trends Genet. 2011, 27, 207–216. [Google Scholar] [CrossRef]
- Wang, X.; Jin, D.; Wang, Z.; Guo, H.; Zhang, L.; Wang, L.; Li, J.; Paterson, A.H. Telomere-centric genome repatterning determines recurring chromosome number reductions during the evolution of eukaryotes. New Phytol. 2015, 205, 378–389. [Google Scholar] [CrossRef]
- Murat, F.; Xu, J.H.; Tannier, E.; Abrouk, M.; Guilhot, N.; Pont, C.; Messing, J.; Salse, J. Ancestral grass karyotype reconstruction unravels new mechanisms of genome shuffling as a source of plant evolution. Genome Res. 2010, 20, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Lysak, M.A.; Mandáková, T.; Schranz, M.E. Comparative paleogenomics of crucifers: Ancestral genomic blocks revisited. Curr. Opin. Plant Biol. 2016, 30, 108–115. [Google Scholar] [CrossRef]
- Schweizer, D.; Loidl, J. A model for heterochromatin dispersion and the evolution of C-band patterns. Chromosome Today 1987, 9, 61–74. [Google Scholar]
- Charlesworth, B.; Sniegowski, P.; Stephan, W. The evolutionary dynamics of the repetitive DNA of eukaryotes. Nature 1994, 371, 215–220. [Google Scholar] [CrossRef]
- Cohen, S.; Houben, A.; Segal, D. Extrachromosomal circular DNA derived from tandemly repeated genomic sequences in plants. Plant J. 2008, 53, 1027–1034. [Google Scholar] [CrossRef]
- Navrátilová, A.; Koblížková, A.; Macas, J. Survey of extrachromosomal circular DNA derived from plant satellite repeats. BMC Plant Biol. 2008, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Roa, F.; Guerra, M. Distribution of 45S rDNA sites in chromosomes of plants: Structural and evolutionary implications. BMC Evol. Biol. 2012, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Available online: http://www.theplantlist.org/ (accessed on 1 January 2021).

| Seed Plants (%) | Gymnosperms (%) | Angiosperms (%) | |
|---|---|---|---|
| c | 21.5 | 28.1 | 20.1 |
| p | 29.0 | 18.8 | 31.2 |
| i | 49.5 | 53.1 | 48.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maravilla, A.J.; Rosato, M.; Rosselló, J.A. Interstitial Telomeric-like Repeats (ITR) in Seed Plants as Assessed by Molecular Cytogenetic Techniques: A Review. Plants 2021, 10, 2541. https://doi.org/10.3390/plants10112541
Maravilla AJ, Rosato M, Rosselló JA. Interstitial Telomeric-like Repeats (ITR) in Seed Plants as Assessed by Molecular Cytogenetic Techniques: A Review. Plants. 2021; 10(11):2541. https://doi.org/10.3390/plants10112541
Chicago/Turabian StyleMaravilla, Alexis J., Marcela Rosato, and Josep A. Rosselló. 2021. "Interstitial Telomeric-like Repeats (ITR) in Seed Plants as Assessed by Molecular Cytogenetic Techniques: A Review" Plants 10, no. 11: 2541. https://doi.org/10.3390/plants10112541
APA StyleMaravilla, A. J., Rosato, M., & Rosselló, J. A. (2021). Interstitial Telomeric-like Repeats (ITR) in Seed Plants as Assessed by Molecular Cytogenetic Techniques: A Review. Plants, 10(11), 2541. https://doi.org/10.3390/plants10112541






