Metabolites of Prickly Rose: Chemodiversity and Digestive-Enzyme-Inhibiting Potential of Rosa acicularis and the Main Ellagitannin Rugosin D
Abstract
1. Introduction
2. Results and Discussion
2.1. Metabolites of Rosa acicularis: Distribution of Phytochemicals in Organs
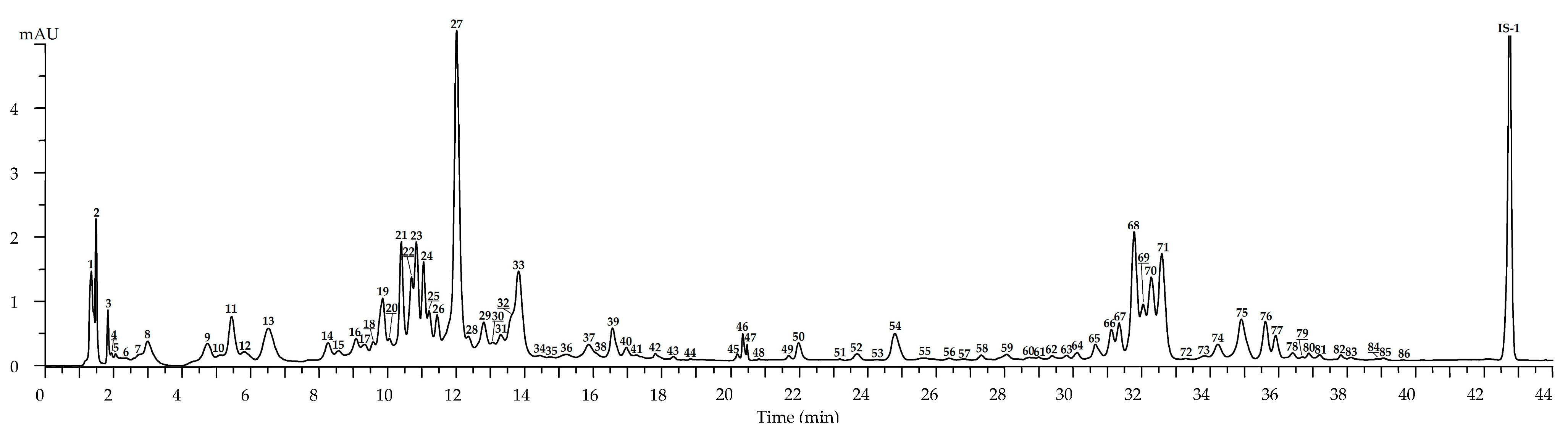
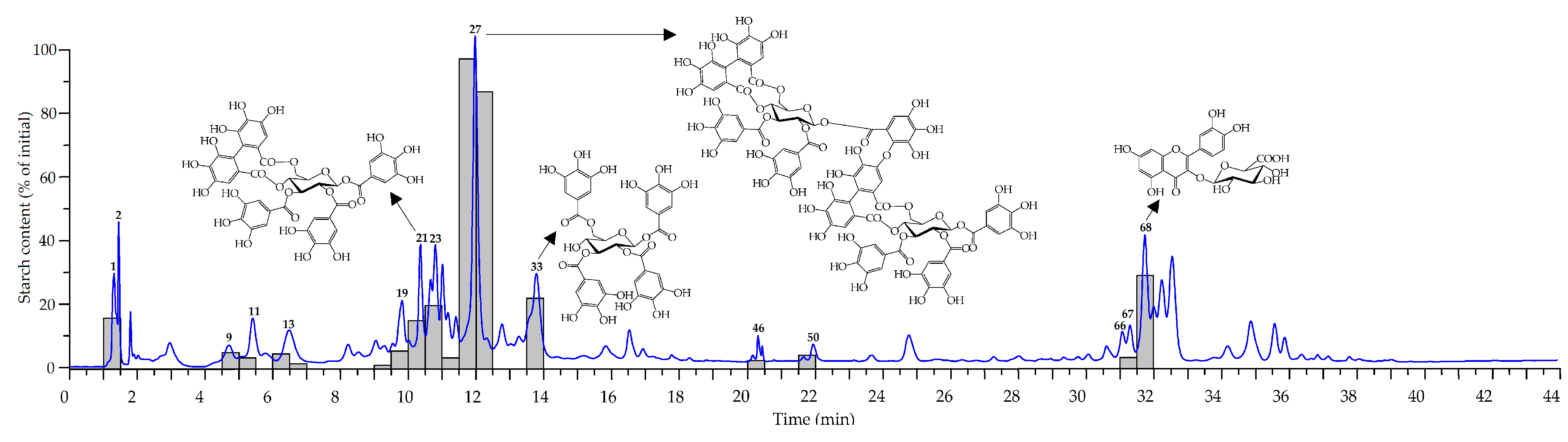
2.2. Metabolites of Rosa acicularis: LC-MS Profile and Organ-Specific Distribution
2.2.1. Leaves
2.2.2. Flowers
2.2.3. Roots
2.2.4. Fruits
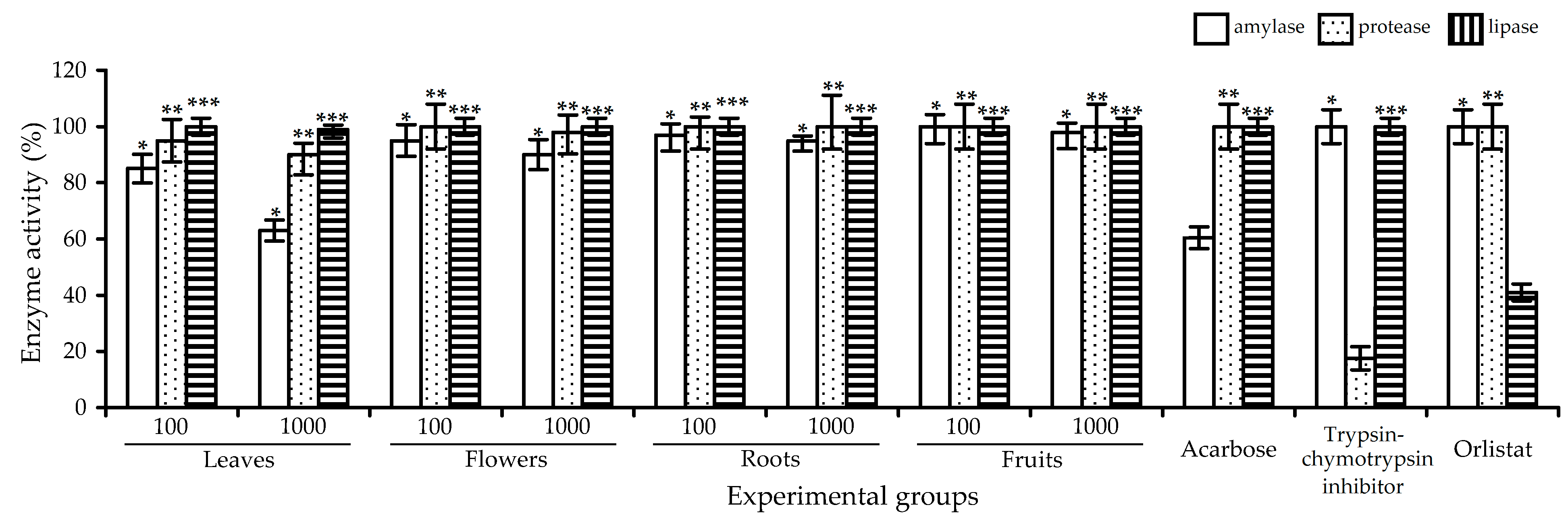
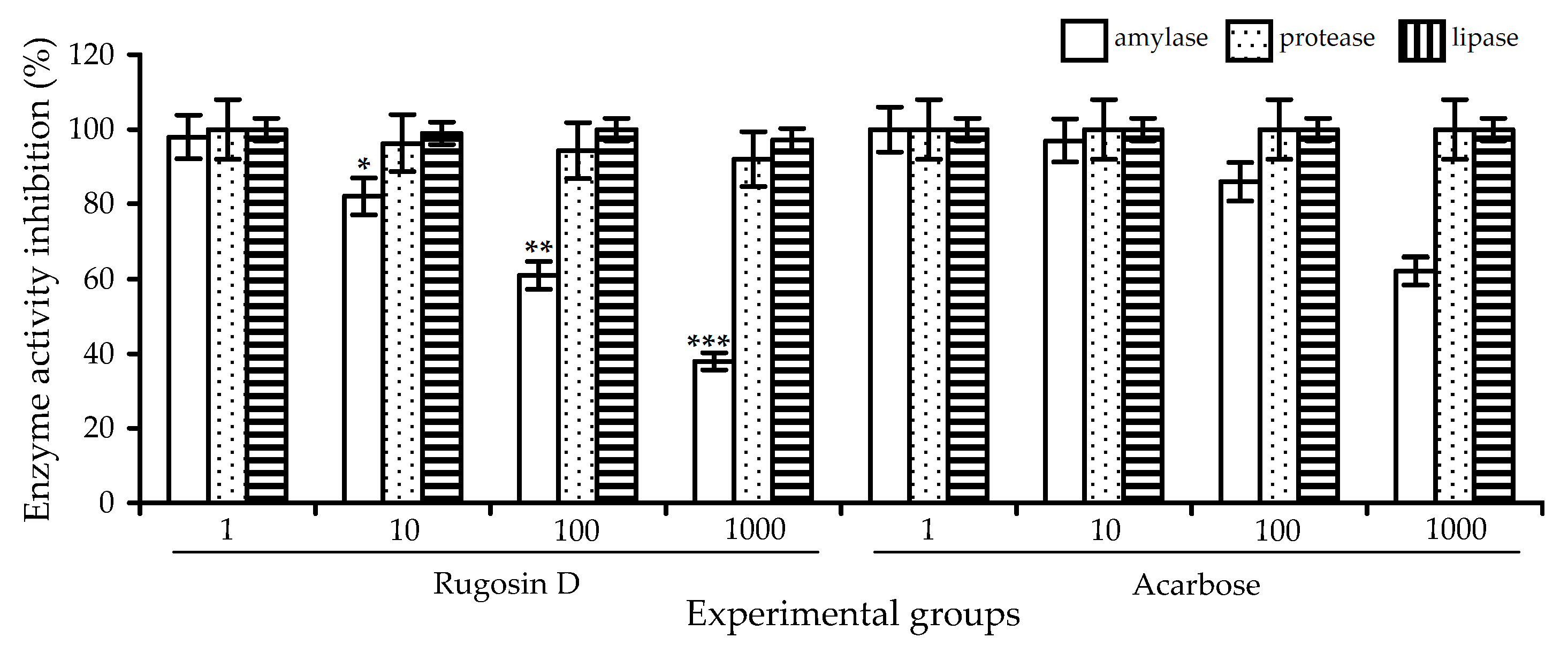
2.3. Digestive-Enzyme-Inhibiting Potential of R. acicularis Extracts and Rugosin D
3. Materials and Methods
3.1. Plant Material and Chemicals
3.2. Chemical Composition Analysis
3.3. Plant Extracts Preparation
3.4. High-Performance Liquid Chromatography with Photodiode Array Detection and Electrospray Ionization Triple Quadrupole Mass Spectrometric Detection (HPLC-PDA-ESI-tQ-MS) Metabolite Profiling
3.5. HPLC-ESI-tQ-MS Metabolite Quantification
3.6. Simulated Gastrointestinal Digestion
3.7. Quantification of Gastrointestinal Digestion Markers by HPLC-DAD
3.8. HPLC Microfractionation with Post-Column Pancreatic α-Amylase Inhibition
3.9. α-Amylase Inhibitory Activity
3.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruneau, A.; Starr, J.R.; Joly, S. Phylogenetic relationships in the genus Rosa: New evidence from chloroplast DNA sequences and an appraisal of current knowledge. Syst. Bot. 2007, 32, 366–378. [Google Scholar] [CrossRef]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, traditional uses and pharmacological profile of rose hip: A review. Curr. Pharm. Des. 2018, 24, 4101–4124. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Flora of Siberia; Malyschev, L.I., Ed.; CRC Press: Boca Raton, FL, USA, 2004; Volume 8, pp. 124–129. [Google Scholar] [CrossRef]
- Anonymous. Plant Recourses of USSR; Sokolov, P.D., Ed.; Nauka: Leningrad, Russia, 1987; Volume 3, pp. 74–86. [Google Scholar]
- Lewis, W.H. A monograph of the genus Rosa in North America. I. R. acicularis. Brittonia 1959, 11, 1–24. [Google Scholar] [CrossRef]
- Shishmareva, T.M.; Shishmarev, V.M. Resources of Medicinal Plants of Transbaikalia; BSC SB RAS: Ulan-Ude, Russia, 2017; pp. 107–112. [Google Scholar]
- Nikitin, G.I. Wild Fruits and Berries of Sakhalin and Kuriles; Nauka: Yuzhno-Sakhalinsk, Russia, 1957; pp. 83–87. [Google Scholar]
- Shreter, A.I. The Medical Flora of Soviet Far East; Nauka: Moscow, Russia, 1975; pp. 282–285. [Google Scholar]
- Makarov, A.A. Bioactive Compounds in Plants of Yakutia; SO RAN: Yakutsk, Russia, 1989; pp. 69–71. [Google Scholar]
- Makarov, A.A. Plant Medical Remedies of Yakut Traditional Medicine; YaGU: Yakutsk, Russia, 1974; pp. 52–57. [Google Scholar]
- Sanchzhai-Chzhamso, D. Vaiduria Onbo. The Blue Beryl; Dashiev, D.B.T., Aseeva, T.A., Eds.; Vostochnaya Literatura: Moscow, Russia, 2014; pp. 282–283. [Google Scholar]
- Batorova, S.M.; Yakovlev, G.P.; Aseeva, T.A. Reference-Book of Traditional Tibetan Medicine Herb; Nauka: Novosibirsk, Russia, 2013; pp. 211–212. [Google Scholar]
- Aseeva, T.A. Digestive Diseases: Symptomatology and Treatment (by Materials of Tibetan Medical Books); Nauka: Novosibirsk, Russia, 2016; pp. 109–143. [Google Scholar]
- Lu, J.; Wang, C. Medicinal components and pharmacological effects of Rosa rugosa. Rec. Nat. Prod. 2018, 12, 535–543. [Google Scholar] [CrossRef]
- Patel, S. Rose hip as an underutilized functional food: Evidence-based review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Enkhtuya, E.; Kashiwagi, T.; Shimamura, T.; Ukeda, H.; Tseye-Oidov, O. Screening study on antioxidant activity of plants grown wildly in Mongolia. Food Sci. Technol. Res. 2014, 20, 891–897. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takahashi, T.; Takano, F.; Fushiya, S.; Batkhuu, J.; Sanchir, C.; Yoshizaki, F. Survey of the influence of Mongolian plants on lipase activity in mouse plasma and gastrointestinal tube. Nat. Med. 2004, 58, 204–208. [Google Scholar]
- Park, J.C.; Kim, S.C.; Choi, M.R.; Song, S.H.; Yoo, E.J.; Kim, S.H.; Miyashiro, H.; Hattori, M. Anti-HIV protease activity from Rosa family plant extracts and rosamultin from Rosa rugosa. J. Med. Food 2005, 8, 107–109. [Google Scholar] [CrossRef]
- Gonchig, E.; Erdenebat, S.; Togtoo, O.; Bataa, S.; Gendaram, O.; Young, S.K.; Shi, Y.R. Antimicrobial activity of Mongolian medicinal plants. Nat. Prod. Sci. 2008, 14, 32–36. [Google Scholar]
- Afanasyeva, L.V.; Ayushina, T.A. Features of microelements accumulation in Rosa acicularis plants. Khimiya Rastitel’nogo Syr’ya 2019, 3, 197–204. [Google Scholar] [CrossRef]
- Zhao, M.-Y.; Zhao, Y.-H. Analysis and comparison of essential oil composition between Rosa acicularis ‘Luhe’ and Lindl. Modern Food Sci. Technol. 2019, 35, 241–248. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Shi, S.-S.; Zhang, L.-G. Bioactive compounds extracted by different solvents from Rosa acicularis ’Luhe’ leaves and their antioxidant activity. Modern Food Sci. Technol. 2019, 35, 159–166. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Olennikov, D.N. Phenolome of Asian agrimony tea (Agrimonia asiatica Juz., Rosaceae): LC-MS profile, α-glucosidase inhibitory potential and stability. Foods 2020, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Vasilieva, A.G.; Fedorov, I.A. LC-MS profile, gastrointestinal and gut microbiota stability and antioxidant activity of Rhodiola rosea herb metabolites: A comparative study with subterranean organs. Antioxidants 2020, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Vasil’eva, A.G.; Gadimli, A.I.; Isaev, J.I.; Vennos, C. Caffeoylquinic acids and flavonoids of fringed sagewort (Artemisia frigida Willd.): HPLC-DAD-ESI-QQQ-MS profile, HPLC-DAD quantification, in vitro digestion stability, and antioxidant capacity. Antioxidants 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Gadimli, A.I.; Isaev, J.I.; Kashchenko, N.I.; Prokopyev, A.S.; Katayeva, T.N.; Chirikova, N.K.; Vennos, C. Caucasian Gentiana species: Untargeted LC-MS metabolic profiling, antioxidant and digestive enzyme inhibiting activity of six plants. Metabolites 2019, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Nikolaev, V.M.; Kim, S.-W.; Vennos, C. Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the Siberian species and their inhibitory potential against α-amylase and α-glucosidase. Front. Pharmacol. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Kashchenko, N.I.; Chirikova, N.K.; Olennikov, D.N. Acylated flavonoids from Spiraea genus as inhibitors of α-amylase. Russ. J. Bioorg. Chem. 2018, 44, 768–778. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Chirikova, N.K.; Olennikov, D.N. Agrimoniin, an active ellagitannin from Comarum palustre herb with anti-α-glucosidase and antidiabetic potential in streptozotocin-induced diabetic rats. Molecules 2017, 22, 73. [Google Scholar] [CrossRef]
- Bitis, L.; Sen, A.; Ozsoy, N.; Birteksoz-Tan, S.; Kultur, S.; Melikoglu, G. Flavonoids and biological activities of various extracts from Rosa sempervirens leaves. Biotechnol. Biotechnol. Equip. 2017, 31, 299–303. [Google Scholar] [CrossRef]
- Nowak, R.; Gawlik-Dziki, U. Polyphenols of Rosa, L. leaves extracts and their radical scavenging activity. Z. Naturforsch. C 2007, 62, 32–38. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total polyphenol content and antioxidant capacity of rosehips of some Rosa species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Noreikienė, I.; Zymonė, K.; Juodytė, R.; Žvikas, V.; Janulis, V. Composition and antioxidant activity of phenolic compounds in fruit of the genus Rosa L. Antioxidants 2021, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Nowacka-Jechalke, N.; Masłyk, M.; Martyna, A.; Pietrzak, W.; Kubiński, K.; Załuski, D.; Nowak, R. Polysaccharide-rich fractions from Rosa rugosa Thunb.—Composition and chemopreventive potential. Molecules 2019, 24, 1354. [Google Scholar] [CrossRef] [PubMed]
- Oprica, L.; Bucsa, C.; Zamfirache, M.M. Ascorbic acid content of rose hip fruit depending on altitude. Iran J. Public Health 2015, 44, 138–139. [Google Scholar]
- Roman, I.; Stănilă, A.; Stănilă, S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem. Centr. J. 2013, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.C.; Rumpunen, K.; Johansson, E.; Olsson, M.E. Carotenoid content and composition in rose hips (Rosa spp.) during ripening, determination of suitable maturity marker and implications for health promoting food products. Food Chem. 2011, 128, 689–696. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Nikolaev, V.M.; Chirikova, N.K. Sagan Dalya tea, a new “old” probable adaptogenic drug: Metabolic characterization and bioactivity potentials of Rhododendron adamsii leaves. Antioxidants 2021, 10, 863. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kirillina, C.S.; Chirikova, N.K. Water-soluble melanoidin pigment as a new antioxidant component of fermented willowherb leaves (Epilobium angustifolium). Antioxidants 2021, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Vennos, C. New ellagic acid glycosides from Punica granatum. Chem. Nat. Comp. 2019, 55, 878–882. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Vasilieva, A.G.; Chirikova, N.K. Fragaria viridis fruit metabolites: Variation of LC-MS profile and antioxidant potential during ripening and storage. Pharmaceuticals 2020, 13, 262. [Google Scholar] [CrossRef]
- Bijttebier, S.; Van der Auwera, A.; Voorspoels, S.; Noten, B.; Hermans, N.; Pieters, L.; Apers, S. A first step in the quest for the active constituents in Filipendula ulmaria (meadowsweet): Comprehensive phytochemical identification by liquid chromatography coupled to quadrupole-orbitrap mass spectrometry. Planta Med. 2016, 82, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-J.; Wang, Z.-B.; Mi, Y.-Y.; Gao, M.-J.; Lv, J.-N.; Meng, Y.-H.; Yang, B.-Y.; Kuang, H.-X. UHPLC-MS/MS determination, pharmacokinetic, and bioavailability study of taxifolin in rat plasma after oral administration of its nanodispersion. Molecules 2016, 21, 494. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N. Synanthropic plants as an underestimated source of bioactive phytochemicals: A case of Galeopsis bifida (Lamiaceae). Plants 2020, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Wubshet, S.G.; Moresco, H.H.; Tahtah, Y.; Brighente, I.M.C.; Staerk, D. High-resolution bioactivity profiling combined with HPLC-HRMS-SPE-NMR: α-Glucosidase inhibitors and acetylated ellagic acid rhamnosides from Myrcia palustris DC. (Myrtaceae). Phytochemistry 2015, 116, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Iritani, K.; Yonemori, S.; Oyama, Y.; Takeda, Y. Isolation and antioxidant activity of galloyl flavonol glycosides from the seashore plant. Pemphis Acidula. Biosci. Biotechnol. Biochem. 2001, 65, 1302–1309. [Google Scholar] [CrossRef]
- Skaltsa, H.; Verykokidou, E.; Harvala, C.; Karabourniotis, G.; Manetasi, Y. UV-B protective potential and flavonoid content of leaf hairs of Quercus ilex. Phytochemistry 1994, 37, 987–990. [Google Scholar] [CrossRef]
- Zhao, M.; Sui, X.; Wang, Y.; Xu, Z.; Yu, X.; Han, X. Component analysis of anthocyanins in petals at different flowering stages of three Rosa rugosa hybrid cultivars. Adv. Biosci. Biotechnol. 2019, 10, 1–11. [Google Scholar] [CrossRef][Green Version]
- Agarwal, C.; Veluri, R.; Kaur, M.; Chou, S.-C.; Thompson, J.A.; Agarwal, R. Fractionation of high molecular weight tannins in grape seed extract and identification of procyanidin B2-3,3′-di-O-gallate as a major active constituent causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis 2007, 28, 1478–1484. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Iwasaki, M.; Kubo, M.; Orime, T.; Yoshizaki, M.; Naruhashi, N. Hydrolysable tannins as chemotaxonomic markers in the Rosaceae. Phytochemistry 1992, 31, 3091–3096. [Google Scholar] [CrossRef]
- Fecka, I. Qualitative and quantitative determination of hydrolysable tannins and other polyphenols in herbal products from meadowsweet and dog rose. Phytochem. Anal. 2009, 20, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-Z.; Xing, J.; Sun, M.; Zhan, Z.-Q.; Corke, H. Phenolic antioxidants (hydrolyzable tannins, flavonols, and anthocyanins) identified by LC-ESI-MS and MALDI-QIT-TOF MS from Rosa chinensis flowers. J. Agric. Food Chem. 2005, 53, 9940–9948. [Google Scholar] [CrossRef] [PubMed]
- Cendrowski, A.; Scibisz, I.; Kieliszek, M.; Kolniak-Ostek, J.; Mitek, M. UPLC-PDA-Q/TOF-MS Profile of polyphenolic compounds of liqueurs from rose petals (Rosa rugosa). Molecules 2017, 22, 1832. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.-P.; Ossipov, V.; Haukioja, E.; Pihlaja, K. Seasonal variation in the content of hydrolysable tannins in leaves of Betula pubescens. Phytochemistry 2001, 57, 15–22. [Google Scholar] [CrossRef]
- Cunja, V.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Compound identification of selected rose species and cultivars: An insight to petal and leaf phenolic profiles. J. Am. Soc. Hort. Sci. 2014, 139, 157–166. [Google Scholar] [CrossRef]
- Budzianowski, J. Six flavonol glucuronides from Tulipa gesneriana. Phytochemistry 1991, 30, 1679–1682. [Google Scholar] [CrossRef]
- Olennikov, D.N. Flavonol glycosides from leaves of Allium microdictyon. Chem. Nat. Comp. 2020, 56, 1035–1039. [Google Scholar] [CrossRef]
- Saleh, A.M.N. Flavonol glycosides of Euphorbia retusa and E. sanctae-catharinae. Phytochemistry 1985, 24, 371–372. [Google Scholar] [CrossRef]
- Urushibara, S.; Kitayama, Y.; Watanabe, T.; Okuno, T.; Watarai, A.; Matsumoto, T. New flavonol glycosides, major determinants inducing the green fluorescence in the guard cells of Allium cepa. Tetrahedr. Lett. 1992, 33, 1213–1216. [Google Scholar] [CrossRef]
- Markham, U.R. Techniques of Flavonoid Identification; Academic Press: London, UK, 1982; pp. 36–51. [Google Scholar]
- Zadorozhnii, A.M.; Zapesochnaya, G.G.; Pervykh, L.N.; Shchavlinskii, A.N.; Kovtun, L.S.; Svanidze, N.V. Investigation of the herb Aerva lanata. 1-O-Acylglycosides of flavonoids. Pharm. Chem. J. 1986, 20, 855–858. [Google Scholar]
- Jungblut, T.P.; Schnitzler, J.-P.; Heller, W.; Szymczak, W.; Metzger, J.W. Structures of UV-B induced sunscreen pigments of the Scots pine (Pinus sylvestris L.). Angew. Chem. 1995, 34, 312–314. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Markham, K.R. (Eds.) Flavonoids: Chemistry, Biochemistry, and Applications; CRC Press: Boca Raton, FL, USA, 2006; pp. 917–1002. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Olennikov, D.N.; Chirikova, N.K. Metabolites of Siberian raspberries: LC-MS profile, seasonal variation, antioxidant activity and thermal stability of Rubus matsumuranus phenolome. Plants 2021, 10, 2317. [Google Scholar] [CrossRef]
- Mikanagi, Y.; Yokoi, M.; Ueda, Y.; Saito, N. Flower flavonol and anthocyanin distribution in subgenus Rosa. Biochem. Syst. Ecol. 1995, 23, 183–200. [Google Scholar] [CrossRef]
- Park, J.C.; Ito, H.; Yoshida, T. 1H-NMR assignment of HIV protease inhibitor, procyanidin B3 isolated from Rosa rugosa. Nat. Prod. Sci. 2003, 9, 49–51. [Google Scholar]
- Zagorac, D.Č.D.; Akšić, M.M.F.; Glavnik, V.; Gašić, U.M.; Vovk, I.; Tešić, Ž.L.; Natić, M.M. Establishing the chromatographic fingerprints of flavan-3-ols and proanthocyanidins from rose hip (Rosa sp.) species. J. Sep. Sci. 2020, 43, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Medveckienė, B.; Kulaitienė, J.; Levickienė, D.; Hallmann, E. The effect of ripening stages on the accumulation of carotenoids, polyphenols and vitamin C in rosehip species/cultivars. Appl. Sci. 2021, 11, 6761. [Google Scholar] [CrossRef]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Nađpal, J.D.; Lesjak, M.M.; Šibul, F.S.; Anačkov, G.T.; Četojević-Simin, D.D.; Mimica-Dukić, N.M.; Beara, I.N. Comparative study of biological activities and phytochemical composition of two rose hips and their preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 2016, 192, 907–914. [Google Scholar] [CrossRef]
- Yang, Y.; Lian, G.; Yu, B. Naturally occurring polyphenolic glucosidase inhibitors. Israel J. Chem. 2015, 55, 268–284. [Google Scholar] [CrossRef]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharmaceut. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef]
- Gholamhoseinian, A.; Fallah, H.; Sharifi far, F. Inhibitory effect of methanol extract of Rosa damascena Mill. flowers on α-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine 2009, 16, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Asghari, B.; Salehi, P.; Farimani, M.M.; Ebrahimi, S.N. α-Glucosidase inhibitors from fruits of Rosa canina L. Rec. Nat. Prod. 2015, 9, 276–283. [Google Scholar]
- Zhu, J.; Zhang, B.; Wang, B.; Li, C.; Fu, X.; Huang, Q. In-vitro inhibitory effects of flavonoids in Rosa roxburghii and R. sterilis fruits on α-glucosidase: Effect of stomach digestion on flavonoids alone and in combination with acarbose. J. Funct. Foods 2019, 54, 13–21. [Google Scholar] [CrossRef]
- Jemaa, H.B.; Jemia, A.B.; Khlifi, S.; Ahmed, H.B.; Slama, F.B.; Benzarti, A.; Aouidet, A. Antioxidant activity and α-amylase inhibitory potential of Rosa canina L. Afr. J. Tradit. Compl. Altern. Med. 2017, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Ochir, S.; Nishizawa, M.; Park, B.J.; Ishii, K.; Kanazawa, T.; Funaki, M.; Yamagishi, T. Inhibitory effects of Rosa gallica on the digestive enzymes. J. Nat. Med. 2010, 64, 275–280. [Google Scholar] [CrossRef]
- Farzaei, F.; Morovati, M.R.; Farjadmand, F.; Farzaei, M.H. A mechanistic review on medicinal plants used for diabetes mellitus in Traditional Persian Medicine. J. Evid. Based Compl. Altern. Med. 2017, 22, 944–955. [Google Scholar] [CrossRef]
- Bindu, J.; Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotech. 2019, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Naveen, Y.P.; Urooj, A.; Byrappa, K. A review on medicinal plants evaluated for anti-diabetic potential in clinical trials: Present status and future perspective. J. Herbal Med. 2021, 28, 100436. [Google Scholar] [CrossRef]
- Ochir, S.; Park, B.; Nishizawa, M.; Kanazawa, T.; Funaki, M.; Yamagishi, T. Simultaneous determination of hydrolysable tannins in the petals of Rosa rugosa and allied plants. J. Nat. Med. 2010, 64, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tanaka, T.; Zhang, Y.-J.; Yang, C.-R.; Kouno, I. Rubusuaviins A–F, monomeric and oligomeric ellagitannins from chinese sweet tea and their α-amylase inhibitory activity. Chem. Pharm. Bull. 2007, 55, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Juśkiewicz, J.; Jurgonski, A.; Kołodziejczyk, K.; Kosmala, M.; Milala, J.; Zduńczyk, Z.; Fotschki, B.; Żary-Sikorska, E. Blood glucose lowering efficacy of strawberry extracts rich in ellagitannins with different degree of polymerization in rats. Pol. J. Food Nutr. Sci. 2016, 66, 109–117. [Google Scholar] [CrossRef]
- Adamczyk, B.; Salminen, J.-P.; Smolander, A.; Kitunen, V. Precipitation of proteins by tannins: Effects of concentration, protein/tannin ratio and pH. Int. J. Food Sci. Technol. 2012, 47, 875–878. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Rice, M.E.; Ritchard, N.T. Mechanisms of protein precipitation for two tannins, pentagalloyl glucose and epicatechin16(4→8) catechin (procyanidin). J. Agricult. Food Chem. 1998, 46, 2590–2595. [Google Scholar] [CrossRef]
- Olennikov, D.N. Ellagitannins and other phenolic compounds from Comarum palustre. Chem. Nat. Comp. 2016, 52, 619–621. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kruglova, M.Y. New quercetin glucoside and other phenolic compounds from Filipendula genus. Chem. Nat. Comp. 2013, 49, 524–529. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Spireasalicin, a new acylated glycoside of quercetin from Spiraea salicicfolia. Chem. Nat. Comp. 2017, 53, 1038–1044. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. New compounds from flowers of Phlojodicarpus sibiricus. Chem. Nat. Comp. 2020, 56, 628–632. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. New C,O-glycosylflavones from the genus Silene. Chem. Nat. Comp. 2020, 56, 1026–1034. [Google Scholar] [CrossRef]
- Olennikov, D.N. New flavonoids from Artemisia frigida. Chem. Nat. Comp. 2020, 56, 623–627. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. New acylated flavone-O-glycosides and iridoids from the genus Veronica. Chem. Nat. Comp. 2021, 57, 436–444. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. New compounds from Siberian Gentiana species. II. Xanthone and C,O-glycosylflavone. Chem. Nat. Comp. 2021, 57, 681–684. [Google Scholar] [CrossRef]
- Chirikova, N.K.; Olennikov, D.N.; Tankhaeva, L.M. Quantitative determination of flavonoid content in the aerial part of Baical scullcap (Scutellaria baicalensis Georgi). Russ. J. Bioorg. Chem. 2010, 36, 915–922. [Google Scholar] [CrossRef]
- Damien Dorman, H.J.; Shikov, A.N.; Pozharitskaya, O.N.; Hiltunen, R. Antioxidant and pro-oxidant evaluation of a Potentilla alba L. rhizome extract. Chem. Biodiv. 2011, 8, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agricult. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M. Quantitative determination of phenolic compounds in Mentha piperita leaves. Chem. Nat. Comp. 2010, 46, 22–27. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef]
- Wilson, T.C.; Hagerman, A.E. Quantitative determination of ellagic acid. J. Agric. Food Chem. 1990, 38, 1678–1683. [Google Scholar] [CrossRef]
- Inoue, K.H.; Hagerman, A.E. Determination of gallotannin with rhodanine. Anal. Biochem. 1988, 169, 363–369. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Samuelsen, A.B. Quantitative analysis of polysaccharides from Plantago major leaves using the Dreywood method. Chem. Nat. Comp. 2006, 42, 265–268. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Gornostai, T.G.; Selyutina, I.Y.; Zilfikarov, I.N. Effect of low temperature cultivation on the phytochemical profile and bioactivity of Arctic plants: A case of Dracocephalum palmatum. Int. J. Mol. Sci. 2017, 18, 2579. [Google Scholar] [CrossRef] [PubMed]
- Rajković, M.B.; Novaković, I.D.; Petrović, A. Determination of titratable acidity in white wine. J. Agricult. Sci. 2007, 52, 169–184. [Google Scholar] [CrossRef]
- Iverson, S.J.; Lang, S.L.C.; Cooper, M.H. Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 2001, 36, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I. Componential profile and amylase inhibiting activity of phenolic compounds from Calendula officinalis L. leaves. Sci. World J. 2014, 2014, 654193. [Google Scholar] [CrossRef] [PubMed]

| Phytochemical Group | Leaves (n = 53) 3 | Flowers (n = 42) 3 | Roots (n = 6) 4 | Fruits (n = 104) 5 |
|---|---|---|---|---|
| Phenolic compounds | ||||
| Flavonols | 41.48 (3.31) | 67.39 (4.98) | 0.11 (0.00) | <0.01 |
| Dihydroflavonols | 0.52 (0.04) | 0.73 (0.06) | <0.01 | <0.01 |
| Catechins | 27.37 (2.46) | 28.86 (2.40) | 43.04 (1.24) | 0.76 (0.10) |
| Hydroxycinnamates | 1.47 (0.08) | 0.20 (0.02) | <0.01 | <0.01 |
| Proanthocyanidins | 8.22 (0.57) | 16.56 (1.82) | 26.04 (1.04) | 1.05 (0.14) |
| Anthocyanins | <0.01 | 5.34 (0.64) | <0.01 | <0.01 |
| Ellagitannins | 73.69 (6.63) | 33.14 (3.64) | 12.83 (0.64) | <0.01 |
| Gallotannins | 21.23 (2.01) | 8.53 (0.51) | 3.16 (0.06) | 1.22 (0.15) |
| Total phenolic compounds | 173.98 | 160.75 | 85.18 | 3.03 |
| Non-phenolic compounds | ||||
| Water-soluble polysaccharides | 41.88 (3.35) | 65.14 (5.86) | 21.76 (0.65) | 58.39 (4.08) |
| Ascorbic acid | 5.37 (0.42) | 1.14 (0.07) | <0.01 | 56.12 (6.17) |
| Titratable acids | 8.23 (0.74) | 11.27 (1.01) | 2.03 (0.08) | 38.26 (3.07) |
| Carotenoids | 0.43 (0.01) | <0.01 | <0.01 | 2.33 (0.21) |
| Lipids | 11.07 (1.28) | 5.67 (0.45) | 2.53 (0.07) | 65.12 (7.16) |
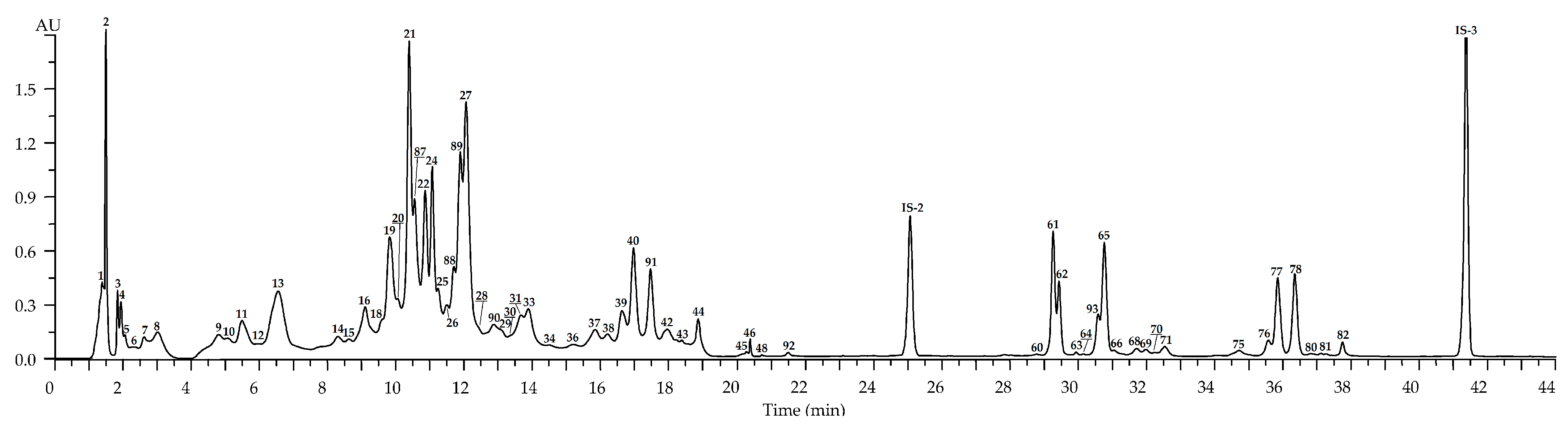
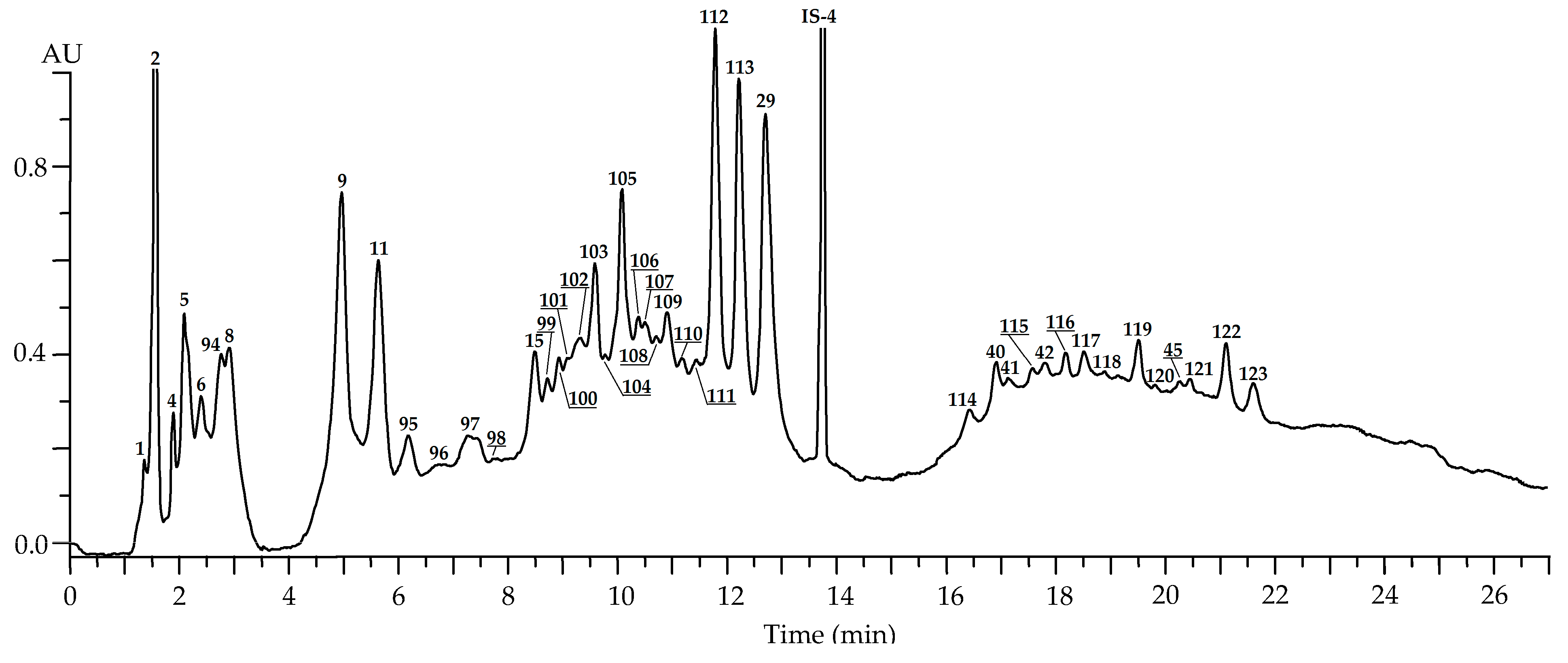
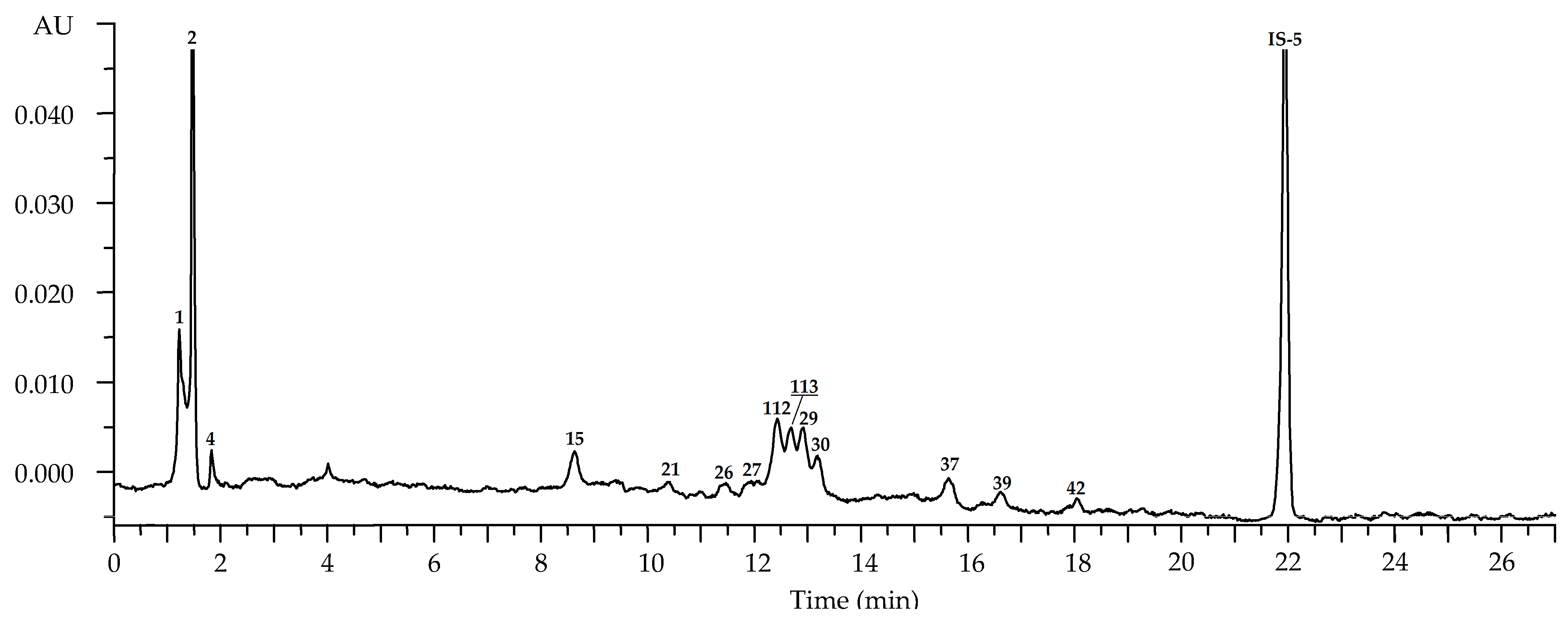
| No | tR, min | Compound a (Ref.) | [M-H]−, m/z b | MS/MS, m/z | Content, mg/g of Dry Plant Weight ± S.D. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Leaves: May (n = 35) c | Leaves: July (n = 81) c | Leaves: September (n = 57) c | Flowers: July (n = 45) c | Roots: September (n = 12) c | |||||
| 1 | 1.26 | 1-O-Galloyl glucose S (glucogallin) [24] | 331 | 169 | 0.93 (0.11) | 1.58 (0.14) | 0.24 (0.01) | 0.43 (0.03) | 0.23 (0.03) |
| 2 | 1.48 | Gallic acid S [24] | 169 | 1.04 (0.09) | 3.68 (0.44) | 10.39 (1.45) | 2.27 (0.25) | 4.27 (0.59) | |
| 3 | 1.79 | 1-O-Caffeoylquinic acid S [38] | 353 | 191, 179, 173 and 135 | 0.36 (0.02) | 0.53 (0.04) | Traces | 0.14 (0.01) | n.d. |
| 4 | 1.95 | Galloyl-hexahydroxydiphenoyl-di-O-hexoside L [39] | 795 | 633, 481, 463 and 301 | Traces | Traces | Traces | 0.10 (0.10) | 0.29 (0.03) |
| 5 | 2.11 | Galloyl-hexahydroxydiphenoyl-di-O-hexoside L [39] | 795 | 633, 481, 463 and 301 | Traces | Traces | Traces | Traces | 0.37 (0.04) |
| 6 | 2.41 | Ellagic acid tri-O-hexoside L [39,40] | 787 | 625, 463 and 301 | Traces | Traces | Traces | Traces | 1.26 (0.14) |
| 7 | 2.73 | Caffeic acid O-hexoside L [38] | 341 | 179, 165 | Traces | Traces | Traces | Traces | n.d. |
| 8 | 2.98 | 2-Pyrone-4,6-dicarboxylic acid S [41] | 183 | 139, 111 | 1.44 (0.12) | 2.50 (0.34) | 1.86 (0.22) | 1.43 (0.15) | 0.95 (0.11) |
| 9 | 4.71 | Epigallocatechin S [24] | 305 | 179, 137 | 2.34 (0.22) | 5.39 (0.54) | 3.77 (0.40) | 4.73 (0.42) | 20.45 (1.84) |
| 10 | 5.08 | 5-O-Caffeoylquinic acid S [38] | 353 | 191, 179 and 165 | Traces | 0.28 (0.03) | Traces | Traces | n.d. |
| 11 | 5.48 | Catechin S [24] | 289 | 205, 137 | 12.56 (1.01) | 18.63 (2.60) | 16.24 (1.78) | 19.26 (2.11) | 17.22 (1.89) |
| 12 | 5.83 | Di-O-galloyl hexose L [24] | 483 | 331, 169 and 125 | 0.04 (0.00) | 0.64 (0.81) | Traces | Traces | n.d. |
| 13 | 6.58 | 1,6-Di-O-galloyl glucose S [24] | 483 | 331, 169 and 125 | 1.20 (0.14) | 4.26 (0.53) | 0.92 (0.10) | 4.98 (0.45) | n.d. |
| 14 | 8.27 | Tellimagrandin I1 S [41,42] | 785, 392 * | 301 | 0.12 (0.01) | 0.89 (0.09) | 0.05 (0.00) | 0.09 (0.00) | n.d. |
| 15 | 8.53 | Epicatechin S [24] | 289 | 205, 137 | 0.89 (0.93) | 1.29 (0.09) | 0.46 (0.52) | Traces | 8.23 (0.74) |
| 16 | 9.08 | Rugosin B1 S [41,42] | 953, 476 * | 785, 597 and 301 | 0.08 (0.00) | 0.40 (0.04) | 0.02 (0.00) | 0.76 (0.06) | n.d. |
| 17 | 9.38 | Tri-O-galloyl hexose L [24] | 635 | 483, 331, 169 and 125 | Traces | 0.44 (0.05) | Traces | n.d. | n.d. |
| 18 | 9.59 | 1,3,6-Tri-O-galloyl glucose S [24] | 635 | 483, 331, 169 and 125 | Traces | 1.29 (0.11) | Traces | Traces | n.d. |
| 19 | 9.82 | Tellimagrandin I2 S [41,42] | 785, 392 * | 301 | 0.65 (0.04) | 2.93 (0.33) | 0.59 (0.04) | 2.14 (0.19) | n.d. |
| 20 | 10.02 | Tri-O-galloyl hexose L [24] | 635 | 483, 331, 169 and 125 | 0.26 (0.02) | 2.35 (0.28) | 0.14 (0.01) | Traces | n.d. |
| 21 | 10.48 | Tellimagrandin II1 S [41,42] | 937, 468 * | 301 | 2.96 (0.34) | 8.98 (0.95) | 4.22 (0.51) | 6.03 (0.58) | n.d. |
| 22 | 10.72 | Rugosin B2 S [41,42] | 953, 476 * | 785, 597 and 301 | 0.82 (0.08) | 3.86 (0.42) | 1.59 (0.17) | 2.37 (0.19) | n.d. |
| 23 | 10.88 | Tellimagrandin II isomer S [41,42] | 937, 468 * | 301 | 2.50 (0.22) | 8.29 (0.91) | 5.26 (0.73) | n.d. | n.d. |
| 24 | 11.01 | Rugosin E1 S [41,42] | 860 * | 937, 785, 597 and 301 | 0.73 (0.06) | 1.89 (0.26) | 0.56 (0.06) | 2.85 (0.40) | n.d. |
| 25 | 11.10 | Rugosin E2 S [41,42] | 860 * | 937, 785, 597 and 301 | 0.50 (0.05) | 1.12 (0.14) | 0.27 (0.03) | 0.39 (0.03) | n.d. |
| 26 | 11.48 | Rugosin A S [41,42] | 1105, 552 * | 301 | 0.28 (0.02) | 0.53 (0.06) | 0.10 (0.01) | 0.11 (0.01) | n.d. |
| 27 | 12.03 | Rugosin D S [41,42] | 936 *, 623 ** | 917, 851, 765, 749 and 301 | 18.35 (1.46) | 41.15 (4.93) | 32.67 (3.25) | 4.06 (0.44) | n.d. |
| 28 | 12.42 | 1-O-Ellagoyl glucose S [39,40] | 463 | 301 | Traces | 0.62 (0.05) | Traces | Traces | n.d. |
| 29 | 12.78 | Ellagic acid S [39,40] | 301 | 0.25 (0.03) | 2.37 (0.33) | 4.29 (0.58) | Traces | 4.25 (0.51) | |
| 30 | 13.05 | Ellagic acid methyl ester O-hexoside L [39,40] | 477 | 315, 301 | Traces | 0.36 (0.02) | Traces | Traces | n.d. |
| 31 | 13.42 | Ellagic acid methyl ester O-hexoside L [39,40] | 477 | 315, 301 | Traces | 0.45 (0.05) | Traces | 0.62 (0.05) | n.d. |
| 32 | 13.52 | Tetra-O-galloyl hexose L [24] | 787 | 635, 483, 331 and 169 | 7.33 (0.87) d | 10.54 (1.14) d | 4.27 (0.51) d | n.d. | n.d. |
| 33 | 13.82 | 1,2,3,6-Tetra-O-galloyl glucose S [24] | 787 | 635, 483, 331 and 169 | 0.97 (0.10) | n.d. | |||
| 34 | 14.48 | Epicatechin/catechin trimer L [24] | 865 | 577, 451, 407 and 287 | Traces | Traces | Traces | Traces | n.d. |
| 35 | 14.67 | Epicatechin/catechin trimer L [24] | 865 | 577, 451, 407 and 287 | Traces | Traces | Traces | n.d. | n.d. |
| 36 | 15.21 | Ellagic acid dimethyl ester O-hexoside L [39,40] | 491 | 329, 301 | Traces | Traces | Traces | Traces | n.d. |
| 37 | 15.82 | Epicatechin/catechin trimer L [24] | 865 | 577, 451, 407 and 287 | Traces | 2.14 (0.22) | Traces | 0.35 (0.02) | n.d. |
| 38 | 16.04 | Epicatechin/catechin trimer L [24] | 865 | 577, 451, 407 and 287 | Traces | Traces | Traces | 0.29 (0.02) | n.d. |
| 39 | 16.54 | Di-ellagoyl methyl ester O-hexoside L [39,40] | 761 | 477, 315 and 301 | 0.26 (0.03) | 0.94 (0.11) | 0.14 (0.01) | 2.45 (0.29) | n.d. |
| 40 | 16.97 | Epicatechin/catechin tetramer L [24] | 1153 | 863, 577, 575, 451, 407 and 287 | Traces | 1.95 (0.22) | 0.11 (0.01) | 8.76 (0.96) | 0.73 (0.06) |
| 41 | 17.09 | Epicatechin/catechin tetramer L [24] | 1153 | 863, 577, 451, 407 and 287 | Traces | Traces | Traces | n.d. | 0.07 (0.01) |
| 42 | 17.81 | Epicatechin/catechin tetramer L [24] | 1153 | 863, 577, 451, 407 and 287 | Traces | 0.26 (0.02) | Traces | 1.14 (0.10) | 0.05 (0.00) |
| 43 | 18.42 | Di-ellagoyl dimethyl ester O-hexoside L [39,40] | 775 | 477, 315 and 301 | Traces | Traces | Traces | Traces | n.d. |
| 44 | 18.80 | Ellagic acid trimethyl ester O-hexoside L [39,40] | 505 | 343, 301 | Traces | Traces | Traces | 1.54 (0.18) | n.d. |
| 45 | 20.14 | Epicatechin/catechin tetramer L [24] | 1153 | 863, 577, 451, 407 and 287 | Traces | Traces | Traces | Traces | Traces |
| 46 | 20.38 | Penta-O-galloyl hexose L [24] | 939 | 787, 635, 483, 331 and 169 | Traces | 1.80 (0.22) | Traces | 0.77 (0.08) | n.d. |
| 47 | 20.45 | 1,2,3,4,6-Penta-O-galloyl glucose S [24] | 939 | 787, 635, 483, 331 and 169 | Traces | 0.53 (0.06) | Traces | n.d. | n.d. |
| 48 | 20.73 | Hexa-O-galloyl hexose L [24] | 1091 | 939, 787, 635, 483, 331 and 169 | Traces | Traces | Traces | Traces | n.d. |
| 49 | 21.59 | Hexa-O-galloyl hexose L [24] | 1091 | 939, 787, 635, 483, 331, 169 | Traces | Traces | Traces | n.d. | n.d. |
| 50 | 21.98 | Hexa-O-galloyl hexose L [24] | 1091 | 939, 787, 635, 483, 331 and 169 | Traces | 2.99 (0.33) | 0.84 (0.73) | n.d. | n.d. |
| 51 | 23.11 | Dihydroquercetin di-O-hexuronoside-di-O-hexoside L [43] | 979 | 817, 655, 479 and 303 | Traces | Traces | Traces | n.d. | n.d. |
| 52 | 23.76 | Quercetin di-O-hexuronoside-tri-O-hexoside L [39,44,45] | 1139 | 977, 815, 653, 477 and 301 | Traces | 0.12 (0.01) | Traces | n.d. | n.d. |
| 53 | 24.27 | Dihydroquercetin O-hexuronoside-di-O-hexoside L [43] | 803 | 641, 479 and 303 | Traces | Traces | Traces | n.d. | n.d. |
| 54 | 24.80 | Quercetin O-hexuronoside-tri-O-hexoside L [39,44,45] | 963 | 801, 639, 477 and 301 | 0.64 (0.05) | 1.52 (0.12) | 0.21 (0.2) | n.d. | n.d. |
| 55 | 25.63 | Dihydroquercetin O-hexuronoside-O-hexoside L [43] | 641 | 479, 303 | Traces | Traces | Traces | n.d. | n.d. |
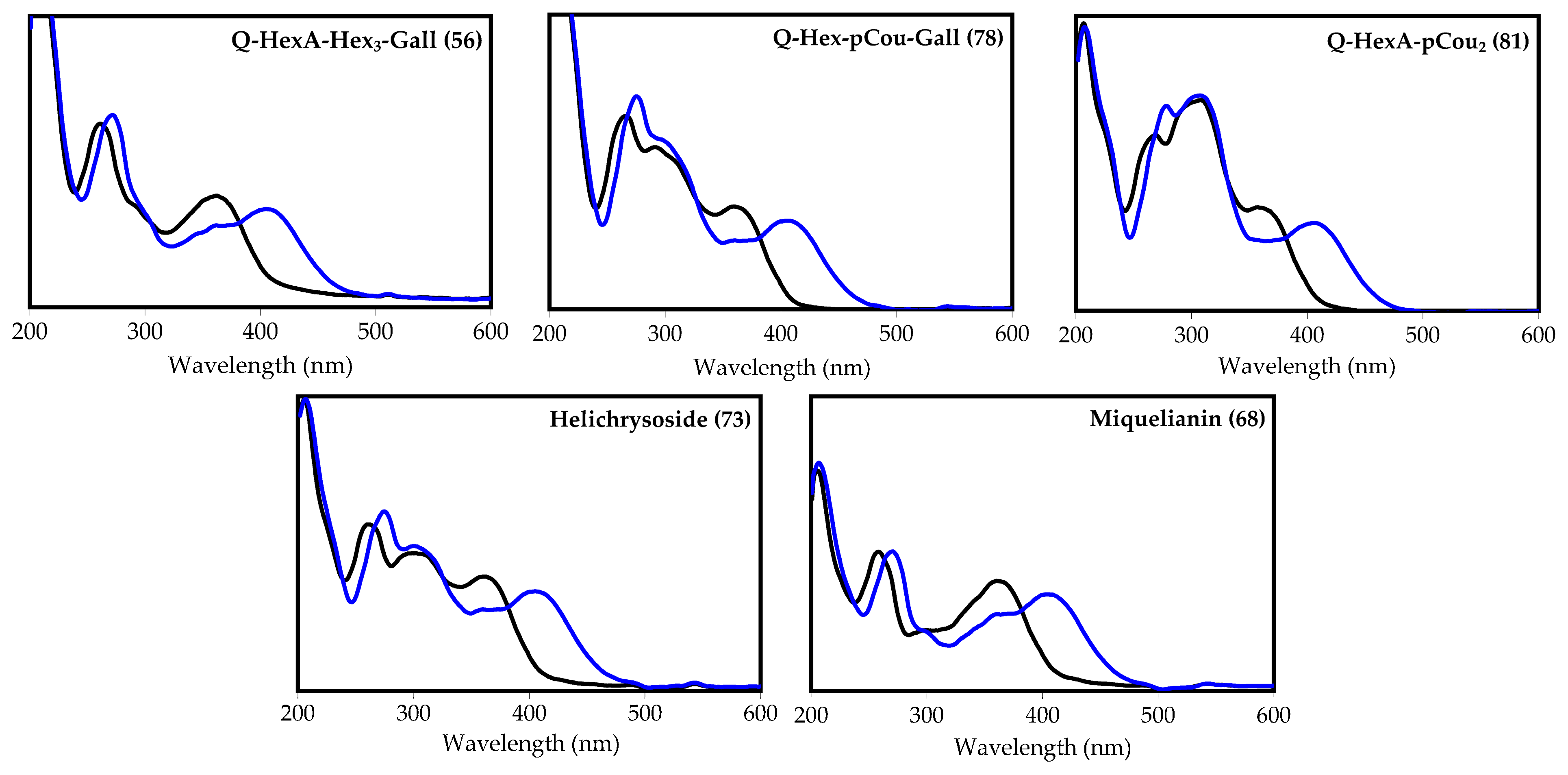
| 56 | 28.44 | Quercetin O-hexuronoside-tri-O-hexoside-O-gallate L [39,44,45,46] | 1115 | 963, 801, 639, 477 and 301 | Traces | Traces | Traces | n.d. | n.d. |
| 57 | 26.78 | Quercetin di-O-hexuronoside-di-O-hexoside L [39,44,45] | 977 | 815, 653, 477 and 301 | Traces | Traces | Traces | n.d. | n.d. |
| 58 | 27.31 | Kaempferol O-hexuronoside-tri-O-hexoside L [39,44,45] | 947 | 785, 623, 461 and 285 | Traces | Traces | Traces | n.d. | n.d. |
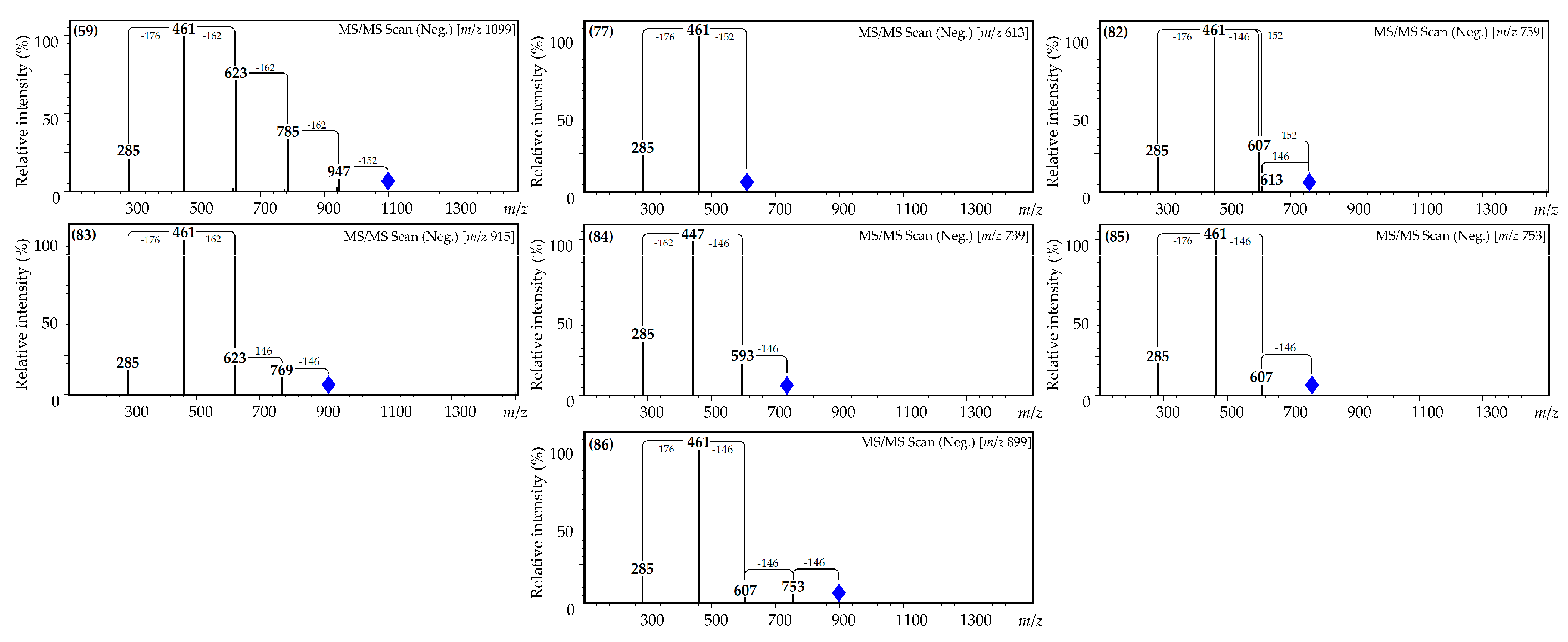
| 59 | 28.02 | Kaempferol O-hexuronoside-tri-O-hexoside-O-gallate L [39,44,45,46] | 1099 | 947, 785, 623, 461 and 285 | Traces | Traces | Traces | n.d. | n.d. |
| 60 | 28.76 | Kaempferol di-O-hexuronoside-di-O-hexoside L [39,44,45] | 961 | 799, 637, 461 and 285 | Traces | Traces | Traces | Traces | n.d. |
| 61 | 29.01 | Quercetin O-hexuronoside-di-O-hexoside L [39,44] | 801 | 639, 477 and 301 | Traces | Traces | Traces | 16.78 (2.01) | n.d. |
| 62 | 29.44 | Kaempferol O-hexuronoside-di-O-hexoside L [39,44,45] | 785 | 623, 461 and 285 | Traces | Traces | Traces | 7.37 (0.74) | n.d. |
| 63 | 29.81 | Dihydroquercetin O-hexoside L [43] | 465 | 303 | Traces | Traces | Traces | Traces | n.d. |
| 64 | 30.10 | Dihydroquercetin O-hexuronoside L [43] | 479 | 303 | 0.22 (0.03) | 0.35 (0.03) | 0.39 (0.04) | Traces | n.d. |
| 65 | 30.71 | Quercetin-O-rutinoside S (rutin) [39,44,45] | 609 | 463, 301 | 0.10 (0.01) | 0.28 (0.03) | 0.06 (0.00) | 15.25 (1.67) | n.d. |
| 66 | 31.09 | Quercetin O-hexuronoside-O-hexoside L [39,44,45] | 639 | 477, 301 | 0.16 (0.01) | 0.88 (0.09) | 0.12 (0.01) | Traces | n.d. |
| 67 | 31.42 | Quercetin O-hexuronoside-O-pentoside L [39,44,45] | 609 | 477, 301 | 0.52 (0.04) | 3.04 (0.28) | 0.83 (0.09) | n.d. | n.d. |
| 68 | 31.83 | Quercetin-3-O-glucuronide S (miquelianin) [24,39,44] | 477 | 301 | 5.31 (0.42) | 10.08 (1.22) | 9.35 (0.94) | 0.21 (0.02) | n.d. |
| 69 | 32.02 | Kaempferol O-hexuronoside-O-hexoside L [39,44] | 623 | 461, 285 | 1.11 (0.09) | 3.16 (0.37) | 0.51 (0.05) | Traces | n.d. |
| 70 | 32.40 | Kaempferol O-hexuronoside-O-pentoside L [39,44] | 593 | 461, 285 | 1.57 (0.12) | 5.38 (0.51) | 2.21 (0.25) | Traces | n.d. |
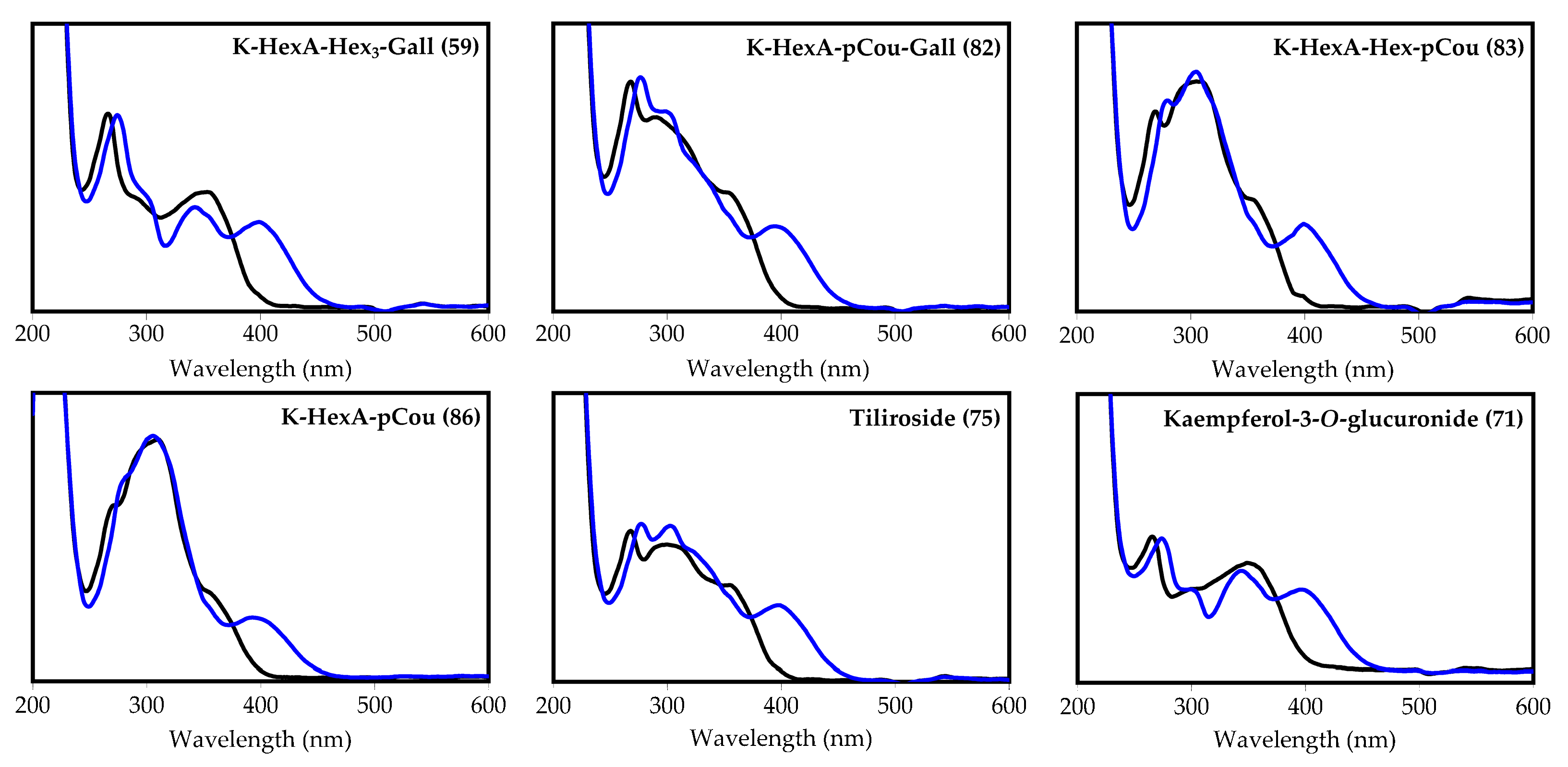
| 71 | 32.62 | Kaempferol-3-O-glucuronide S [24,39,44] | 461 | 285 | 3.61 (0.28) | 7.63 (0.79) | 3.37 (0.34) | 0.12 (0.01) | n.d. |
| 72 | 33.24 | Quercetin-3-O-rhamnoside S (quercitrin) [24,39,44] | 447 | 301 | Traces | Traces | Traces | n.d. | n.d. |
| 73 | 33.76 | Quercetin-3-O-(6″-O-p-coumaroyl)-glucoside S (helichrysoside) [24,39,44] | 609 | 463, 301 | Traces | Traces | Traces | n.d. | n.d. |
| 74 | 34.22 | Kaempferol-3-O-arabinoside S (juglanin) [24,39,44] | 417 | 285 | 0.22 (0.02) | 0.40 (0.04) | Traces | n.d. | n.d. |
| 75 | 34.97 | Kaempferol-3-O-(6″-O-p-coumaroyl)-glucoside S (tiliroside) [24,39,44] | 593 | 447, 285 | 1.73 (0.16) | 2.14 (0.23) | 0.47 (0.04) | 0.31 (0.02) | n.d. |
| 76 | 35.63 | Kaempferol-3-O-(6″-O-galloyl)-glucoside S [39,44,45,46] | 599 | 447, 285 | 0.12 (0.01) | 5.03 (0.52) | Traces | 0.98 (0.09) | n.d. |
| 77 | 35.96 | Kaempferol O-hexuronoside-O-gallate L [39,44,45,46] | 613 | 461, 285 | 0.04 (0.00) | 0.35 (0.03) | Traces | 10.95 (1.28) | n.d. |
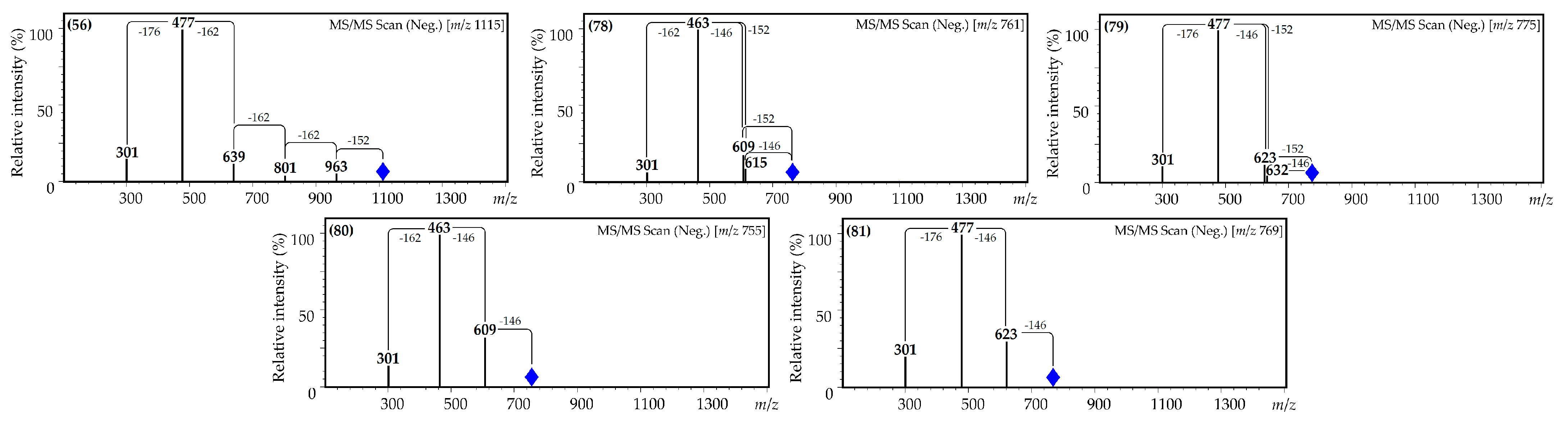
| 78 | 36.47 | Quercetin O-hexoside-O-p-coumarate-O-gallate L [39,44,45,46] | 761 | 615, 609, 463 and 301 | Traces | 0.15 (0.01) | Traces | 11.19 (1.01) | n.d. |
| 79 | 36.69 | Quercetin O-hexuronoside-O-p-coumarate-O-gallate L [39,44,45,46,47] | 775 | 632, 623, 477 and 301 | Traces | Traces | Traces | n.d. | n.d. |
| 80 | 36.97 | Quercetin O-hexoside-di-O-p-coumarate L [24,39,44,47] | 755 | 609, 463 and 301 | Traces | Traces | Traces | Traces | n.d. |
| 81 | 37.15 | Quercetin O-hexuronoside-di-O-p-coumarate L [24,39,44,47] | 769 | 623, 477 and 301 | Traces | Traces | Traces | Traces | n.d. |
| 82 | 37.90 | Kaempferol O-hexuronoside-O-p-coumarate-O-gallate L [39,44,45,46,47] | 759 | 613, 607, 461 and 285 | Traces | Traces | Traces | 0.35 (0.03) | n.d. |
| 83 | 38.09 | Kaempferol O-hexuronoside-O-hexoside-di-O-p-coumarate L [24,39,44,47] | 915 | 769, 623, 461 and 285 | Traces | Traces | Traces | n.d. | n.d. |
| 84 | 38.93 | Kaempferol O-hexoside-di-O-p-coumarate L [24,39,44,47] | 739 | 593, 447 and 285 | Traces | Traces | Traces | n.d. | n.d. |
| 85 | 39.05 | Kaempferol O-hexuronoside-di-O-p-coumarate L [24,39,44,47] | 753 | 607, 461 and 285 | Traces | Traces | Traces | n.d. | n.d. |
| 86 | 39.58 | Kaempferol O-hexuronoside-tri-O-p-coumarate L [24,39,44,47] | 899 | 753, 607, 461 and 285 | Traces | Traces | Traces | n.d. | n.d. |
| 87 | 10.53 | Hexahydroxydiphenoyl-tri-O-galloyl-hexose (tellimagrandin II isomer) L [41,42] | 937, 468 * | 301 | n.d. | n.d. | n.d. | 1.95 (0.20) | n.d. |
| 88 | 11.80 | Valoneoyl-tri-O-galloyl-hexose (rugosin A isomer) L [41,42] | 1105, 552 * | 301 | n.d. | n.d. | n.d. | 0.52 (0.04) | n.d. |
| 89 | 11.98 | Hexahydroxydiphenoyl-valoneoyl-tetra-O-galloyl- di-O-hexose (rugosin D isomer) L [41,42] | 936 *, 623 ** | 917, 851, 765, 749 and 301 | n.d. | n.d. | n.d. | 2.73 (0.38) | n.d. |
| 90 | 12.81 | Ellagic acid O-hexoside L [39,40] | 463 | 301 | n.d. | n.d. | n.d. | Traces | n.d. |
| 91 | 17.49 | Epicatechin/catechin tetramer L [24] | 1153 | 863, 577, 575, 451, 407 and 287 | n.d. | n.d. | n.d. | 7.53 (1.05) | n.d. |
| 92 | 21.50 | Hexa-O-galloyl hexoside L [24] | 1091 | 939, 787, 635, 483, 331 and 169 | n.d. | n.d. | n.d. | Traces | n.d. |
| 93 | 31.12 | Peonidin 3,5-di-O-glucoside S [48] | 625 e | 463, 301 e | n.d. | n.d. | n.d. | 3.21 (0.35) | n.d. |
| 94 | 2.84 | Ellagic acid tri-O-hexoside L [39,40] | 787 | 625, 463 and 301 | n.d. | n.d. | n.d. | n.d. | 1.63 (0.19) |
| 95 | 6.18 | Epicatechin/catechin dimer O-hexoside L [24] | 739 | 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | 1.14 (0.14) |
| 96 | 6.57 | Epicatechin/catechin dimer O-hexoside L [24] | 739 | 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | Traces |
| 97 | 7.29 | Procyanidin B3 S [24] | 577 | 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | 1.29 (0.15) |
| 98 | 7.71 | Procyanidin B1 S [24] | 577 | 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | Traces |
| 99 | 8.52 | Epicatechin/catechin dimer O-hexoside L [24] | 739 | 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | Traces |
| 100 | 8.82 | Procyanidin B4 S [24] | 577 | 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | 0.11 (0.01) |
| 101 | 9.01 | Epicatechin/catechin dimer O-hexoside L [24] | 739 | 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | Traces |
| 102 | 9.11 | Epicatechin/catechin dimer O-hexoside L [24] | 739 | 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | 0.10 (0.01) |
| 103 | 9.47 | Epicatechin/catechin dimer O-hexoside L [24] | 739 | 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | 3.84 (0.42) |
| 104 | 9.58 | Epicatechin/catechin dimer O-hexoside L [24] | 739 | 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | Traces |
| 105 | 10.00 | Procyanidin B2 S [24] | 577 | 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | 7.62 (0.91) |
| 106 | 10.25 | Procyanidin B2 3-O-gallate S [49] | 729 | 577, 559, 541, 441 and 289 | n.d. | n.d. | n.d. | n.d. | Traces |
| 107 | 10.41 | Procyanidin B2 3″-O-gallate S [49] | 729 | 577, 559, 541, 441 and 289 | n.d. | n.d. | n.d. | n.d. | Traces |
| 108 | 10.52 | Epicatechin/catechin dimer O-gallate L [49] | 729 | 577, 541, 441 and 289 | n.d. | n.d. | n.d. | n.d. | Traces |
| 109 | 10.78 | Procyanidin B2 3,3″-di-O-gallate S [49] | 881 | 729, 577, 559, 541, 441 and 289 | n.d. | n.d. | n.d. | n.d. | 0.96 (0.10) |
| 110 | 11.09 | 1-O-Ellagoyl-gentiobiose (amritoside) S [39,40] | 625 | 463, 301 | n.d. | n.d. | n.d. | n.d. | Traces |
| 111 | 11.41 | Epicatechin gallate S [24] | 441 | n.d. | n.d. | n.d. | n.d. | 0.14 (0.01) | |
| 112 | 11.72 | Ellagic acid di-O-desoxyhexoside L [39,40] | 593 | 447, 301 | n.d. | n.d. | n.d. | n.d. | 3.10 (0.43) |
| 113 | 12.05 | Ellagic acid 4-O-rhamnoside (eschweilenol C) S [39,40] | 447 | 301 | n.d. | n.d. | n.d. | n.d. | 3.91 (0.46) |
| 114 | 16.41 | Epicatechin/catechin tetramer L [24] | 1153 | 863, 577, 575, 451, 407, 287 | n.d. | n.d. | n.d. | n.d. | 0.31 (0.02) |
| 115 | 17.51 | Epicatechin/catechin tetramer L [24] | 1153 | 863, 577, 575, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | Traces |
| 116 | 18.10 | Epicatechin/catechin tetramer L [24] | 1153 | 863, 577, 575, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | 0.40 (0.04) |
| 117 | 18.48 | Epicatechin/catechin tetramer L [24] | 1153 | 863, 577, 575, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | 0.46 (0.04) |
| 118 | 18.97 | Epicatechin/catechin pentamer L [24] | 1441 | 1153, 863, 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | Traces |
| 119 | 19.50 | Epicatechin/catechin pentamer L [24] | 1441 | 1153, 863, 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | 1.26 (0.17) |
| 120 | 19.72 | Epicatechin/catechin pentamer L [24] | 1441 | 1153, 863, 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | Traces |
| 121 | 20.41 | Epicatechin/catechin pentamer L [24] | 1441 | 1153, 863, 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | Traces |
| 122 | 21.01 | Epicatechin/catechin pentamer L [24] | 1441 | 1153, 863, 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | 1.87 (0.26) |
| 123 | 21.52 | Epicatechin/catechin pentamer L [24] | 1441 | 1153, 863, 577, 451, 407 and 287 | n.d. | n.d. | n.d. | n.d. | 1.59 (0.22) |
| Total phenolic content, | 71.24 | 178.09 | 106.41 | 148.48 | 88.10 | ||||
| incl. ellagic acid and hexosides | 0.51 | 4.74 | 4.43 | 4.61 | 14.15 | ||||
| ellagitannins | 26.99 | 70.04 | 45.33 | 24.10 | 0.66 | ||||
| gallotannins | 10.80 | 30.10 | 16.80 | 9.42 | 4.50 | ||||
| catechins | 15.79 | 25.04 | 20.47 | 23.99 | 46.04 | ||||
| catechin oligomers | Traces | 4.35 | Traces | 18.07 | 21.80 | ||||
| hydroxycinnamates | 0.36 | 0.81 | Traces | 0.14 | n.d. | ||||
| flavonoids, | 15.35 | 40.51 | 17.52 | 66.72 | n.d. | ||||
| incl. quercetin glycosides | 6.73 | 16.07 | 10.57 | 43.43 | n.d. | ||||
| kaempferol glycosides | 8.40 | 24.09 | 6.56 | 20.08 | n.d. | ||||
| dihydroquercetin glycosides | 0.22 | 0.35 | 0.39 | Traces | n.d. | ||||
| anthocyanins | n.d. | n.d. | n.d. | 3.21 | Traces | ||||
| various compounds | 1.44 | 2.50 | 1.86 | 1.43 | 0.95 | ||||
| Compound | Porcine Pancreas α-Amylase | Human Saliva α-Amylase | Human Pancreas α-Amylase |
|---|---|---|---|
| Rugosin D | 32.09 ± 1.21 | 67.59 ± 4.12 * | 30.84 ± 1.23 * |
| Acarbose | 35.67 ± 1.42 | 82.33 ± 3.54 | 56.39 ± 2.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olennikov, D.N.; Chemposov, V.V.; Chirikova, N.K. Metabolites of Prickly Rose: Chemodiversity and Digestive-Enzyme-Inhibiting Potential of Rosa acicularis and the Main Ellagitannin Rugosin D. Plants 2021, 10, 2525. https://doi.org/10.3390/plants10112525
Olennikov DN, Chemposov VV, Chirikova NK. Metabolites of Prickly Rose: Chemodiversity and Digestive-Enzyme-Inhibiting Potential of Rosa acicularis and the Main Ellagitannin Rugosin D. Plants. 2021; 10(11):2525. https://doi.org/10.3390/plants10112525
Chicago/Turabian StyleOlennikov, Daniil N., Vladimir V. Chemposov, and Nadezhda K. Chirikova. 2021. "Metabolites of Prickly Rose: Chemodiversity and Digestive-Enzyme-Inhibiting Potential of Rosa acicularis and the Main Ellagitannin Rugosin D" Plants 10, no. 11: 2525. https://doi.org/10.3390/plants10112525
APA StyleOlennikov, D. N., Chemposov, V. V., & Chirikova, N. K. (2021). Metabolites of Prickly Rose: Chemodiversity and Digestive-Enzyme-Inhibiting Potential of Rosa acicularis and the Main Ellagitannin Rugosin D. Plants, 10(11), 2525. https://doi.org/10.3390/plants10112525







