Contrasting Metabolic Fingerprints and Seed Protein Profiles of Cucurbita foetidissima and C. radicans Fruits from Feral Plants Sampled in Central Mexico
Abstract
1. Introduction
2. Results
2.1. Morphological and Proximal Composition Variables
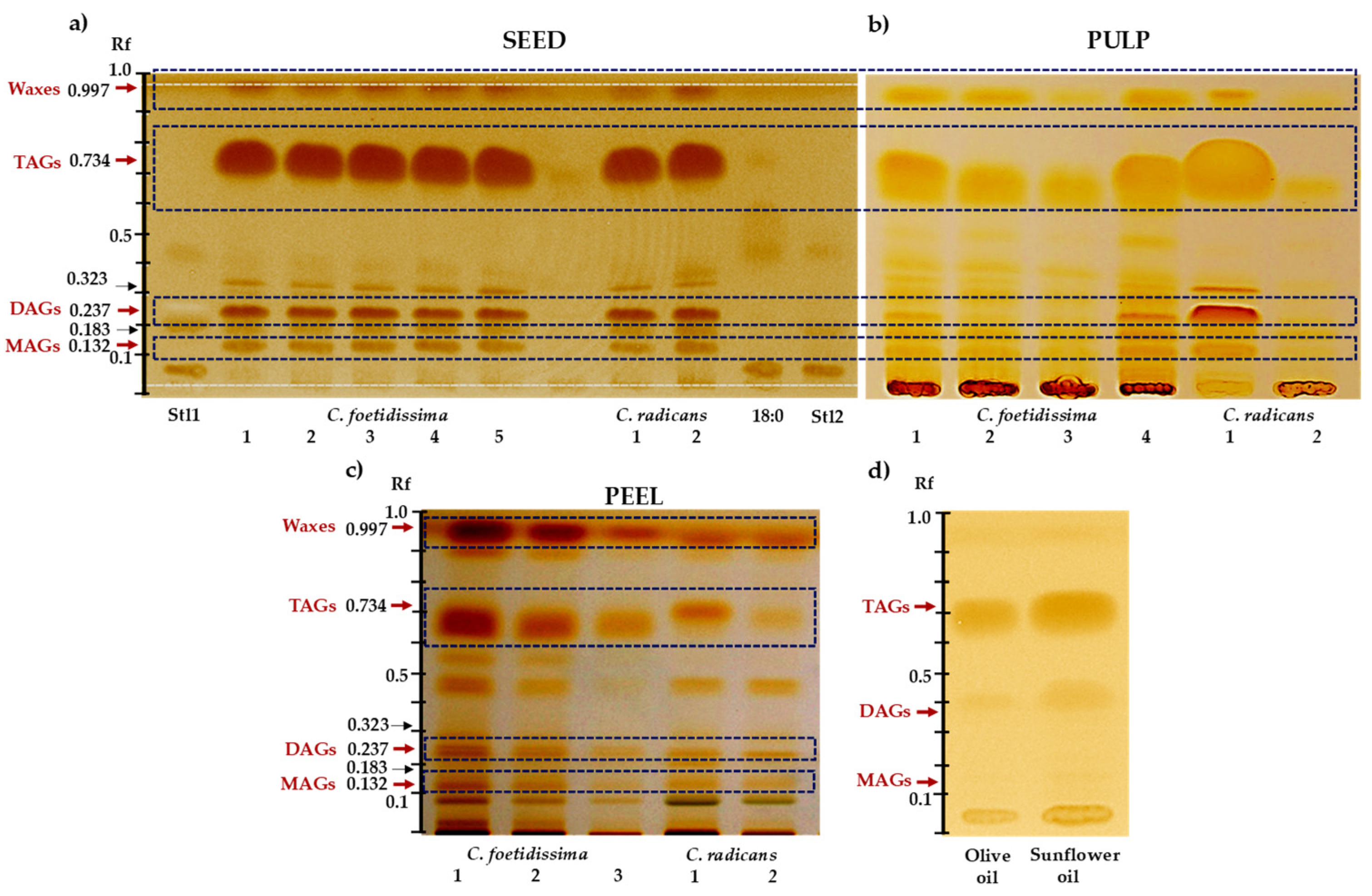
2.2. Neutral Lipids
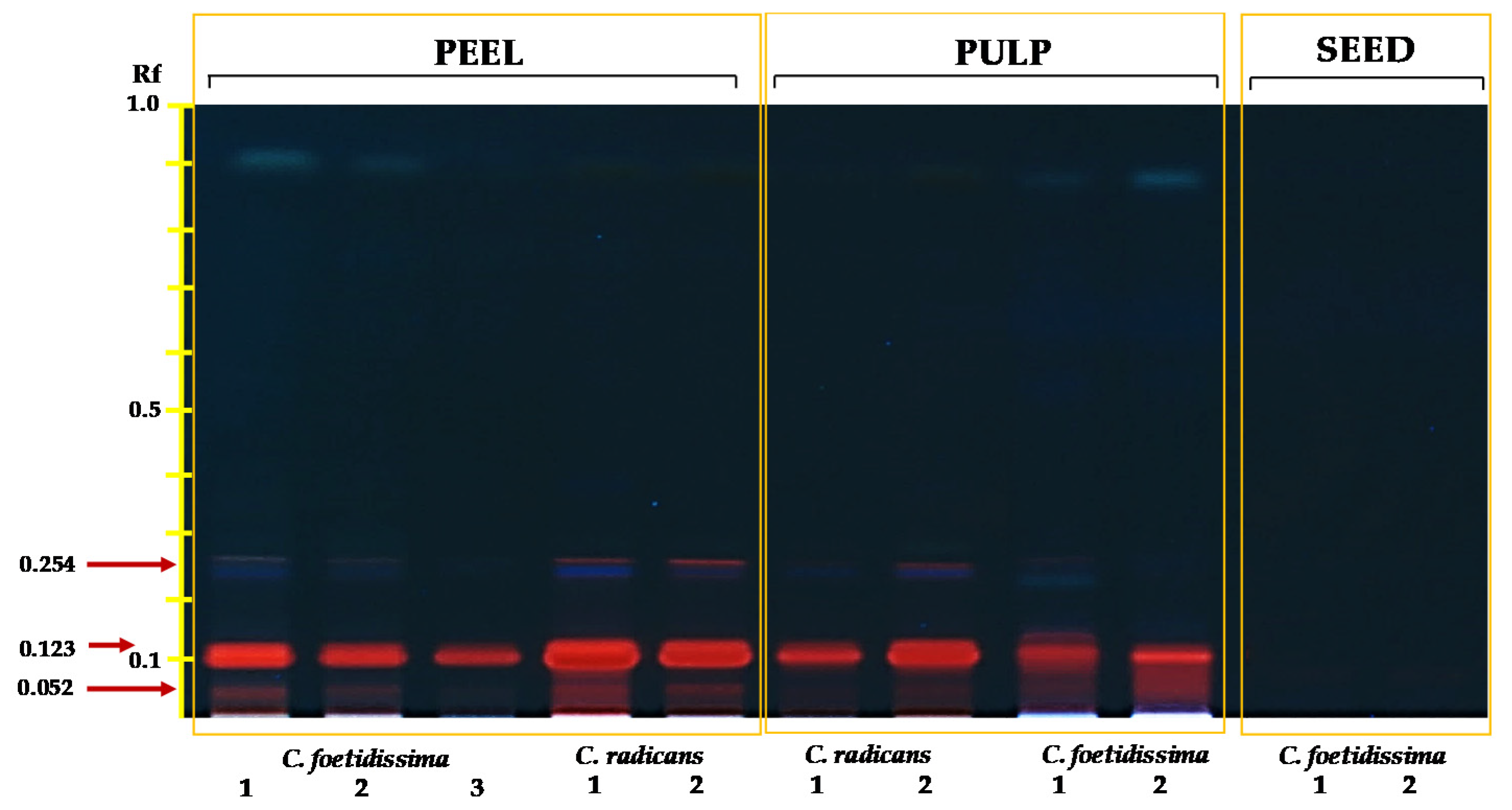
2.3. Saponins/Cucurbitacins
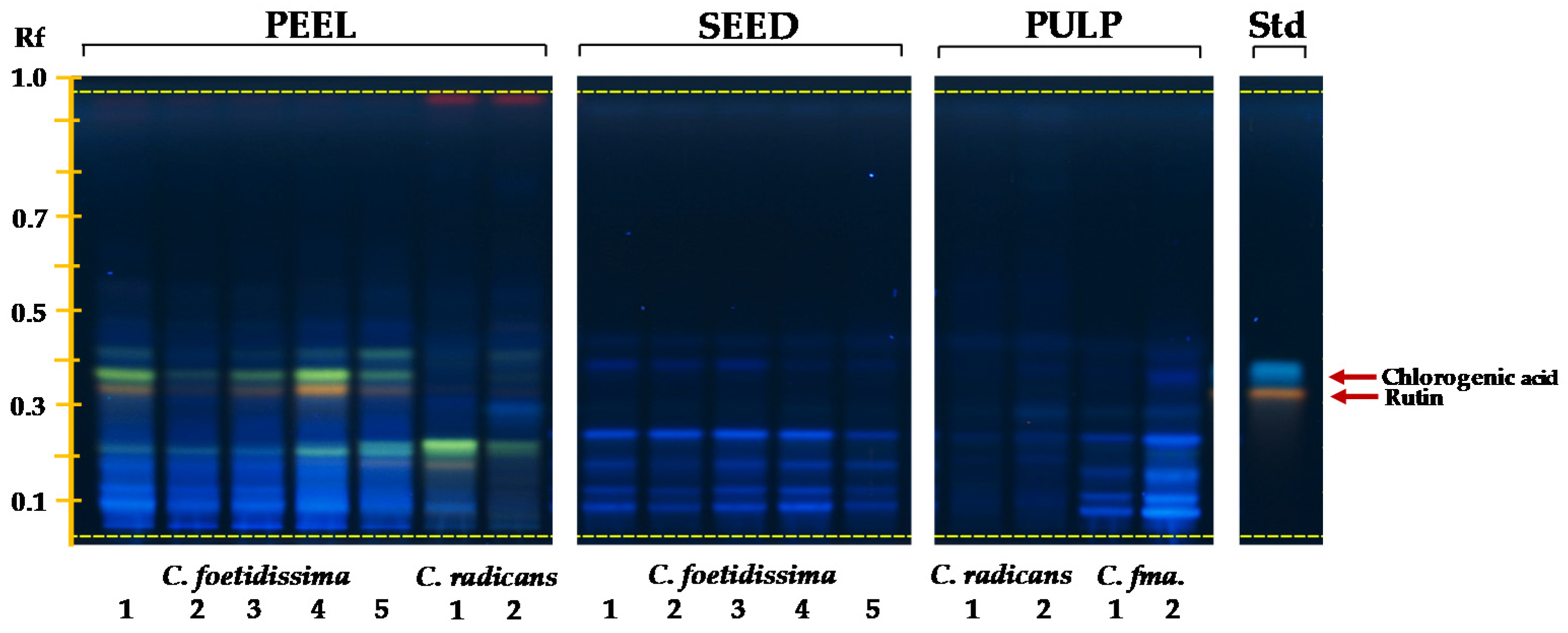
2.4. Phenolics
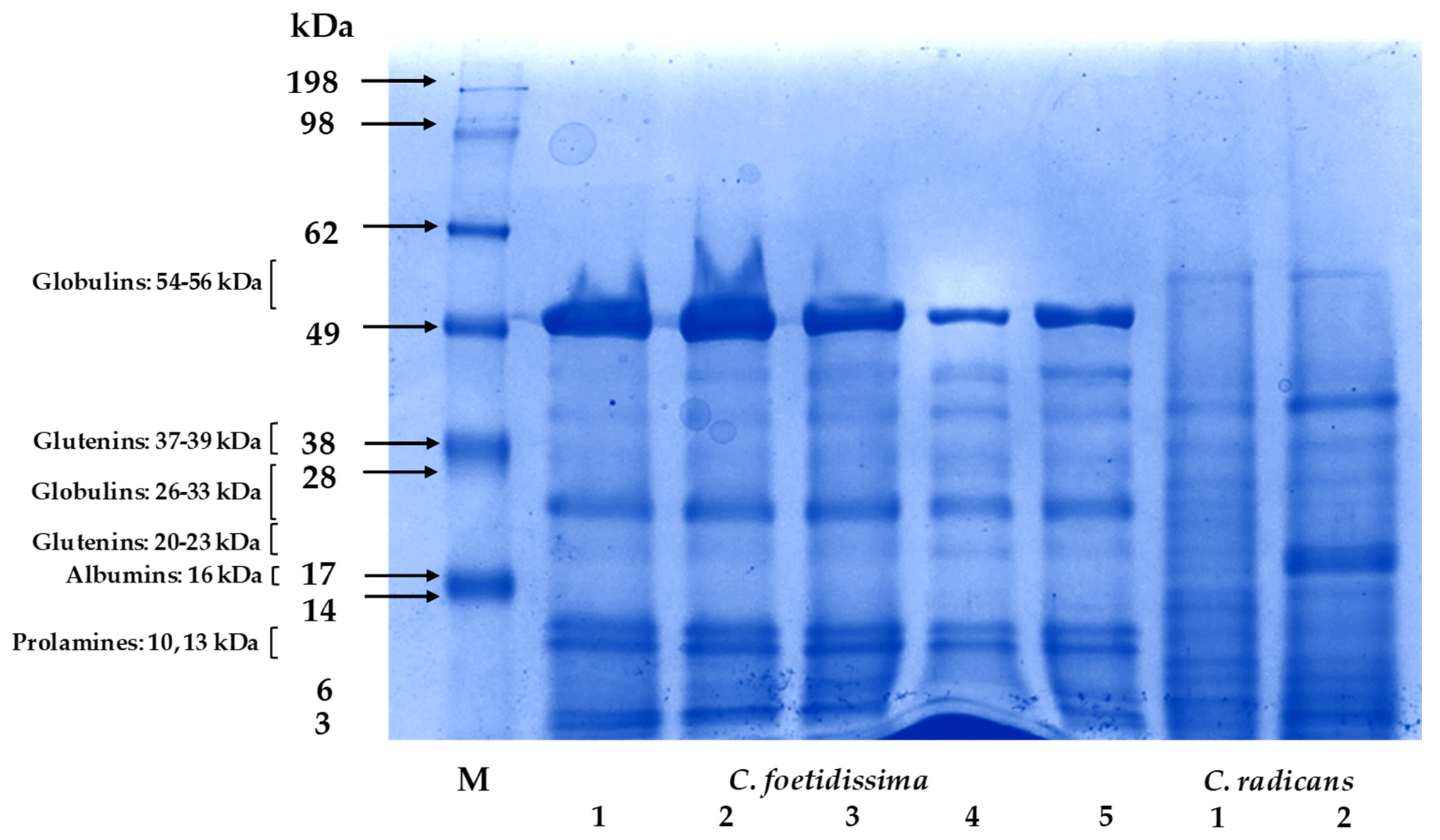
2.5. Seed Proteins
2.6. Non-Structural Carbohydrates (NSCs) and Raffinose Family Oligosaccharides (RFOs)
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villaseñor, J.L.; Ortiz, E. Biodiversidad de las plantas con flores (División Magnoliophyta) en México. Rev. Mex. Biodivers. 2014, 85, 134–142. [Google Scholar] [CrossRef]
- Granados-Sánchez, D.; López-Ríos, G.F. Un recurso forestal de zonas áridas: Calabacilla loca (Cucurbita foetidissima H.B.K.). Rev. Chapingo Ser. Cienc. For. Y Del Ambiente 1999, 5, 35–40. [Google Scholar]
- Gómez-González, A.; Rangel-Guerrero, J.M.; Morales-Flores, F.; Aquino-Pérez, G.; Santana-Garcia, M.A.; Silos-Espino, H. Diagnóstico de poblaciones silvestres de calabacilla loca en el altiplano central de México. Rev. Mex. Cienc. Agric. 2019, 10, 1517–1528. [Google Scholar] [CrossRef]
- Kates, H.R.; Soltis, P.S.; Soltis, D.E. Evolutionary and domestication history of Cucurbita (pumpkin and squash) species inferred from 44 nuclear loci. Mol. Phylogenet. Evol. 2017, 111, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Bemis, W.P.; Whitaker, T.W. The xerophytic Cucurbita of northwestern Mexico and southwestern United States. Madroño 1969, 20, 33–41. [Google Scholar]
- Lira-Saade, R.; Eguiarte-Fruns, L.E.; Montes-Hernandez, S. Proyecto Recopilación Y Análisis De La Información Existente De Las Especies De Los Géneros Cucurbita Y Sechium Que Crecen Y/O Se Cultivan En México; CONABIO: Mexico, D.F., Mexico, 2009; p. 107. [Google Scholar]
- Ríos-Santos, E.; González-Santos, R.; Cadena-Iñiguez, J.; Mera-Ovando, L. Distribution of cultivated species and wild relatives of squash (Cucurbita L.) in México. Agroproductividad 2018, 11, 21–27. [Google Scholar]
- Castellanos-Morales, G.; Paredes-Torres, L.M.; Gámez, N.; Hernández-Rosales, H.S.; Sánchez-de la Vega, G.; Barrera-Redondo, J.; Aguirre-Planter, E.; Vázquez-Lobo, A.; Montes-Hernández, S.; Lira-Saade, R.; et al. Historical biogeography and phylogeny of Cucurbita: Insights from ancestral area reconstruction and niche evolution. Mol. Phylogenetics Evol. 2018, 128, 38–54. [Google Scholar] [CrossRef]
- Lira-Saade, R. Cucurbitaceae. In Flora Del Bajío Y De Regiones Adyacentes, 1st ed.; Rzedowski, J., Calderón de Rzedowski, G., Eds.; Ed. 92; Instituto de Ecología A.C.: Pátzcuaro, Mexico, 2001; Volume 1, pp. 1–128. [Google Scholar]
- Schrot, J.; Weng, A.; Melzi, M.F. Ribosome-inactivating and related proteins. Toxins 2015, 7, 1556–1615. [Google Scholar] [CrossRef]
- Lira-Saade, R.; Rodríguez-Arevalo, I. Informe Final Del Proyecto DS002: Catálogo De La Familia Cucurbitaceae De México; Universidad Nacional Autónoma de México: Mexico, D.F., Mexico, 2006; p. 81. [Google Scholar]
- Khoury, C.K.; Carver, D.; Kates, H.R.; Achicanoy, H.A.; van Zonneveld, M.; Thomas, E.; Heinitz, C.; Jarret, R.; Labate, J.A.; Reitsma, K.; et al. Distributions, conservation status, and abiotic stress tolerance potential of wild cucurbits (Cucurbita L.). Plants People Planet 2019, 2, 269–283. [Google Scholar] [CrossRef]
- Calderon de Rzedowski, G.; Rzedowski, J. Flora Fanerogámica Del Valle De México, 2nd ed.; CONABIO: Mexico, D.F., Mexico, 2001; pp. 1–983. [Google Scholar]
- Trombulak, S.C.; Frissell, C.A. Review of ecological effects of roads on terrestrial and aquatic communities. Biol. Conserv. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Hogan, L.; Bernis, W.P. Buffalo gourd and jojoba: Potential new crops for arid lands. Adv. Agron. 1983, 36, 317–349. [Google Scholar] [CrossRef]
- Berry, J.W.; Weber, C.W.; Dreher, M.L.; Bemis, W.P. Chemical composition of buffalo gourd, a potential food source. J. Food Sci. 1976, 41, 465–466. [Google Scholar] [CrossRef]
- DeVeaux, J.S.; Shultz, E.B. Development of buffalo gourd (Cucurbita foetidissima) as a semiarid land starch and oil crop. Econ. Bot. 1985, 39, 454–472. [Google Scholar] [CrossRef]
- El Bassam, N. Handbook of Bioenergy Crops: A Complete Reference to Species, Development and Applications, 1st ed.; Routledge: England, UK, 2010; pp. 1–572. [Google Scholar]
- Bemis, W.P.; Curtis, L.D.; Weber, C.W.; Berry, J. The feral buffalo gourd, Cucurbita foetidissima. Econ. Bot. 1978, 32, 87–95. [Google Scholar] [CrossRef]
- Dittmer, H.J.; Talley, B.P. Gross morphology of tap roots of desert Cucurbits. Bot. Gaz. 1964, 125, 121–126. [Google Scholar] [CrossRef]
- Mejía-Morales, C. (Universidad de Guadalajara, Zapopan, Jalisco, México). Personal communication, 2021.
- Hernández-Centeno, F.; López-De la Peña, H.Y.; Hernández-González, M.; Rodríguez-González, C.A.; Tirado-Gallegos, J.M.; Ríos-Velasco, C.; Zamudio-Flores, P.B. Physicochemical, thermal, rheological and morphological characteristics of flour and starch from a non-conventional source: Cucurbita foetidissima Kunth roots. J. Food Meas. Charact. 2020, 14, 1976–1985. [Google Scholar] [CrossRef]
- Ba-Amer, M.A.; Bemis, W.P. Fruit and seed development in Cucurbita foetidissima. Econ. Bot. 1968, 22, 297–299. [Google Scholar] [CrossRef]
- Scheerens, J.C.; Bemis, W.P.; Dreher, M.L.; Berry, J.W. Phenotypic variation in fruit and seed characteristics of buffalo gourd. J. Am. Oil Chem. Soc. 1978, 55, 523–525. [Google Scholar] [CrossRef]
- Lancaster, M.; Storey, R.; Bower, N.W. Nutritional evaluation of buffalo gourd: Elemental analysis of seed. Econ. Bot. 1983, 37, 306. [Google Scholar] [CrossRef]
- Hernández-Centeno, F.; López De la Peña, H.Y.; Guigón-López, C.; Hernández-González, M. Biolixiviación y su impacto en el rendimiento de aceite de semillas de Cucurbita foetidissima Kunth en dos métodos de extracción. Investig. Y Cienc. De La Univ. Autónoma De Aguascalientes 2018, 75, 13–19. [Google Scholar] [CrossRef]
- Andres, T.C. Cucurbita fraterna, the closest wild relative and progenitor of C. pepo. CGC Rep. 1987, 10, 69–71. [Google Scholar]
- Merrick, L.C. Systematics and evolution of a domesticated squash, Cucurbita argyrosperma, and its wild and weedy relatives. In Biology and Utilization of the Cucurbitaceae; Bates, D.M., Robinson, R.W., Jeffrey, C., Eds.; Cornell University Press: New York, NY, USA, 1990; pp. 77–95. [Google Scholar] [CrossRef]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Balkaya, A.; Özbakir, M.; Kurtar, E.S. The phenotypic diversity and fruit characterization of winter squash (Cucurbita maxima) populations from the Black sea region of Turkey. Afr. J. Biotechnol. 2010, 9, 152–162. [Google Scholar] [CrossRef]
- Vasconcellos, J.A.; Berry, J.W.; Weber, C.W.; Bemis, W.P.; Scheerens, J.C. The properties of Cucurbita foetidissima seed oil. J. Am. Oil Chem. Soc. 1980, 57, 310–313. [Google Scholar] [CrossRef]
- Rezig, L.; Chouaibi, M.; Msaada, K.; Hamdi, S. Chemical composition and profile characterisation of pumpkin (Cucurbita maxima) seed oil. Ind. Crop. Prod. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- Habib, A.; Biswas, S.; Siddique, A.H.; Manirujjaman, M.; Uddin, B.; Hasan, S.; Uddin, M.; Islam, M.; Hasan, M.; Rahman, M.; et al. Nutritional and lipid composition analysis of pumpkin seed (Cucurbita maxima Linn.). J. Nutr. Food Sci. 2015, 5, 1–6. [Google Scholar]
- Petkova, Z.Y.; Antova, G.A. Changes in the composition of pumpkin seeds (Cucurbita moschata) during development and maturation. Grasas Aceites 2015, 66, e058. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; et al. Cucurbita Plants: From Farm to Industry. Appl. Sci. 2019, 9, 3387. [Google Scholar] [CrossRef]
- Smith, B.D. The initial domestication of Cucurbita pepo in the Americas 10,000 years ago. Science 1997, 276, 932–934. [Google Scholar] [CrossRef]
- Shahani, H.S.; Dollear, F.G.; Markley, K.S.; Quinby, J.R. The Buffalo gourd: A potential oil seed crop of the Southwestern drylands. J. Am. Oil Chem. Soc. 1951, 28, 90–95. [Google Scholar] [CrossRef]
- Paris, H.S. Overview of the origins and history of the five major cucurbit crops: Issues for ancient DNA analysis of archaeological specimens. Veg. Hist. Archaeobotany 2016, 25, 405–414. [Google Scholar] [CrossRef]
- Zheng, Y.-H.; Alverson, A.J.; Wang, Q.-F.; Palmer, J.D. Chloroplast phylogeny of Cucurbita: Evolution of the domesticated and wild species. J. Syst. Evol. 2013, 51, 326–334. [Google Scholar] [CrossRef]
- Wang, Y.-H. Mapping and molecular breeding of monogenic traits, In Genetics, Genomics, and Breeding of Cucurbits. Genetics, Genomics, and Breeding of Crop Plants, 1st ed.; Wang, Y.-H., Behera, T., Kole, C., Eds.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Harlan, J.R.; de Wet, J.M.J. Toward a rational classification of cultivated plants. Taxon 1971, 20, 509–517. [Google Scholar] [CrossRef]
- Lira Saade, R.; Montes Hernandez, M. Cucurbits (Cucurbita spp.), In Neglected Crops: 1492 from a Different Perspective; Series No.2; Hernandez Bermejo, J., Leon, J., Eds.; FAO Plant Production and Protection: Rome, Italy, 1994; pp. 63–77. [Google Scholar]
- Pontifes, P.A.; García-Meneses, P.M.; Gómez-Aíza, L.; Monterroso-Rivas, A.I.; Caso-Chávez, M. Land use/land cover change and extreme climatic events in the arid and semi-arid ecoregions of Mexico. Atmósfera 2018, 31, 355–372. [Google Scholar] [CrossRef]
- Ríos, J.L.; Escandell, J.M.; Recio, M.C. New insights into the bioactivity of cucurbitacins. Stud. Nat. Prod. Chem. 2005, 32, 429–469. [Google Scholar] [CrossRef]
- Hara, I.; Ohmiya, M.; Matsubara, H. Pumpkin (Cucurbita sp.) seed globulin III. Comparison of subunit structures among seed globulins of various Cucurbita species and characterization of peptide components. Plant Cell Physiol. 1978, 19, 237–243. [Google Scholar] [CrossRef]
- Reilly, C.C.; O’Kennedy, B.T.; Titus, J.S.; Splittstoesser, W.E. The solubilization and degradation of pumpkin seed globulin during germination. Plant Cell Physiol. 1978, 19, 1235–1246. [Google Scholar] [CrossRef]
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945–956. [Google Scholar] [CrossRef]
- Rezig, L.; Chibani, F.; Chouaibi, M.; Dalgalarrondo, M.; Hessini, K.; Guéguen, J.; Hamdi, S. Pumpkin (Cucurbita maxima) seed proteins: Sequential extraction processing and fraction characterization. J. Agric. Food Chem. 2013, 61, 7715–7721. [Google Scholar] [CrossRef]
- Aragon-Cuevas, F.; Sanchez de la Vega, G.; Castellanos Morales, G.; Contreras, A.; Lira Saade, R. Cucurbita radicans, calabacilla (amended version of 2019 assessment). Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T109928871A173925627.en (accessed on 8 November 2021).
- Flores-Villamil, M.Á.; Méndez-Gallegos, S.d.J.; García-Herrera, E.J.; Amante-Orozco, A.; Gómez-González, A.; Cabral-Arellano, F.J.; Vasco-Leal, J.F. Wild plants of the center-north of Mexico with potential for oil production. Rev. Mex. Cienc. Agríc. 2018, 9, 1363–1376. [Google Scholar] [CrossRef][Green Version]
- Herrera-Castillo, F.L.M.; Mares-Mares, E.; Del Rincon Castro, M.C.; Ordoñez Acevedo, L.G.; León-Galván, M.F. Análisis proteómico preliminar de las proteínas de reserva de la semilla de chicayota (Cucurbita argyrosperma sororia). Investig. Desarro. Cienc. Tecnol. Aliment. 2016, 1, 430–435. [Google Scholar]
- Jacks, T.J.; Hensarling, T.P.; Yatsu, L.Y. Cucurbit seeds: I. Characterizations and uses of oils and proteins. A review. Econ. Bot. 1972, 26, 135–141. [Google Scholar] [CrossRef]
- Fedko, M.; Kmiecik, D.; Siger, A.; Kulczyński, B.; Przeor, M.; Kobus-Cisowska, J. Comparative characteristics of oil composition in seeds of 31 Cucurbita varieties. J. Food Meas. Charact. 2020, 14, 894–904. [Google Scholar] [CrossRef]
- Wiesman, Z.; Chapagain, B.P. Determination of fatty acid profiles and tags in vegetable oils by MALDI-TOF/MS fingerprinting. In Lipidomics, 1st ed.; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 2009; Volume 1, pp. 315–336. [Google Scholar] [CrossRef]
- Azevedo-Meleiro, C.H.; Rodriguez-Amaya, D.B. Qualitative and quantitative differences in carotenoid composition among Cucurbita moschata, Cucurbita maxima, and Cucurbita pepo. J. Agric. Food Chem. 2007, 55, 4027–4033. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef]
- Valdez-Arjona, L.P.; Ramírez-Mella, M. Pumpkin waste as livestock feed: Impact on nutrition and animal health and on quality of meat, milk, and egg. Animals 2019, 9, 769. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef]
- Zhang, Y.L.; You, C.X.; Li, Y.Y.; Hao, Y.J. Advances in biosynthesis, regulation, and function of apple cuticular wax. Front. Plant Sci. 2020, 11, 1165. [Google Scholar] [CrossRef]
- Perez Gutierrez, R.M. Review of Cucurbita pepo (Pumpkin) its phytochemistry and pharmacology. Med. Chem. 2016, 6, 12–21. [Google Scholar] [CrossRef]
- Matus, Z.; Molnár, P.; Szabó, L.G. Main carotenoids in pressed seeds (cucurbitae semen) of oil pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Acta Pharm. Hung. 1993, 63, 247–256. [Google Scholar]
- Goodman, L.A.; Berry, J.W. Phospholipids of three xerophytic cucurbit seed oils. J. Am. Oil Chem. Soc. 1986, 63, 98–100. [Google Scholar] [CrossRef]
- Salama, H.M.H. Alkaloids and flavonoids from the air-dried aerial parts of Citrullus colocynthis. J. Med. Plants Res. 2012, 6, 5150–5155. [Google Scholar] [CrossRef]
- Dubois, M.A.; Bauer, R.; Cagiotti, M.R.; Wagner, H. Foetidissimoside A, a new 3,28-bidesmosidic triterpenoid saponin, and cucurbitacins from Cucurbita foetidissima. Phytochemistry 1988, 27, 881–885. [Google Scholar] [CrossRef]
- Gaidi, G.; Marouf, A.; Hanquet, B.; Bauer, R.; Correia, M.; Chauffert, B.; Lacaille-Dubois, M.-A. A new major triterpene saponin from the roots of Cucurbita foetidissima. J. Nat. Prod. 2000, 63, 122–124. [Google Scholar] [CrossRef]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef]
- Fa-Sheng, L.; Jing, X.; De-Qiang, D.; Xiao-Feng, C.; Ting-Guo, K.; Xue, K.H. Structures of new phenolic glycosides from the seeds of Cucurbita moschata. Nat. Prod. Commun. 2009, 4, 511–512. [Google Scholar] [CrossRef]
- Bubueanu, C.; Pavaloiu, R. HPTLC chromatographic polyphenolic fingerprints of plant species from Eastern Europe. Malays. J. Med. Biol. Res. 2018, 5, 41–44. [Google Scholar] [CrossRef][Green Version]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 1–384. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. HPTLC analysis, antioxidant and anti-gout activity of Indian plants. Iran. J. Pharm. Res. 2014, 13, 531–539. [Google Scholar]
- González, B.; Vogel, H.; Razmilic, I.; Wolfram, E. Polyphenol, anthocyanin and antioxidant content in different parts of maqui fruits (Aristotelia chilensis) during ripening and conservation treatments after harvest. Ind. Crop. Prod. 2015, 76, 158–165. [Google Scholar] [CrossRef]
- Ramírez-Briones, E.; Rodríguez-Macías, R.; Salcedo-Pérez, E.; Ramírez-Chávez, E.; Molina-Torres, J.; Tiessen, A.; Ordaz-Ortiz, J.; Martínez-Gallardo, N.; Délano-Frier, J.P.; Zañudo-Hernández, J. Seasonal changes in the metabolic profiles and biological activity in leaves of Diospyros digyna and D. rekoi “Zapote” trees. Plants 2019, 8, 449. [Google Scholar] [CrossRef]
- Medić-Ŝarić, M.; Jasprica, I.; Smolčić-Bubalo, A.; Mornar, A. Optimization of chromatographic conditions in thin layer chromatography of flavonoids and phenolic acids. Croat. Chem. Acta 2004, 77, 361–366. [Google Scholar]
- Eleiwa, N.Z.H.; Bakr, R.O.; Mohamed, S.A. Phytochemical and pharmacological screening of seeds and fruits pulp of Cucurbita moschata Duchesne cultivated in Egypt. Int. J. Pharmacogn. Phytochem. 2014, 29, 1226–1236. [Google Scholar] [CrossRef]
- Irshad, M.; Ahmad, I.; Mehdi, S.J.; Goel, H.C.; Rizvi, M.M.A. Antioxidant capacity and phenolic content of the aqueous extract of commonly consumed cucurbits. Int. J. Food Prop. 2014, 17, 179–186. [Google Scholar] [CrossRef]
- Sreeramulu, D.; Raghunath, M. Antioxidant activity and phenolic content of roots, tubers and vegetables commonly consumed in India. Food Res. Int. 2010, 43, 1017–1020. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Van Camp, J.; Trawka, A.; Verhé, R. Phenolic compounds and some quality parameters of pumpkin seed oil. Eur. J. Lipid Sci. Technol. 2010, 112, 208–217. [Google Scholar] [CrossRef]
- Jessica, G.G.; Mario, G.L.; Alejandro, Z.; Cesar, A.P.J.; Ivan, J.V.E.; Ruben, R.R.; Javier, A.F. Chemical characterization of a hypoglycemic extract from Cucurbita ficifolia Bouche that induces liver glycogen accumulation in diabetic mice. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 218–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peričin, D.; Krimer, V.; Trivić, S.; Radulović, L. The distribution of phenolic acids in pumpkin’s hull-less seed, skin, oil cake meal, dehulled kernel and hull. Food Chem. 2009, 113, 450–456. [Google Scholar] [CrossRef]
- Akomolafe, S.F. Effects of roasting on the phenolic phytochemicals and antioxidant activities of pumpkin seed. Vegetos 2021, 34, 505–514. [Google Scholar] [CrossRef]
- Fang, E.F.; Wong, J.H.; Lin, P.; Ng, T.B. Biochemical characterization of the RNA-hydrolytic activity of a pumpkin 2S albumin. FEBS Lett. 2010, 584, 4089–4096. [Google Scholar] [CrossRef]
- Tu, G.L.; Bui, T.H.N.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Comparison of enzymatic and ultrasonic extraction of albumin from defatted pumpkin (Cucurbita pepo) seed powder. Food Technol. Biotechnol. 2015, 53, 479–487. [Google Scholar] [CrossRef] [PubMed]
- King, R.D.; Onuora, J.O. Aspects of melon seed protein characteristics. Food Chem. 1984, 14, 65–77. [Google Scholar] [CrossRef]
- Goffman, F.D.; Alonso, A.P.; Schwender, J.; Shachar-Hill, Y.; Ohlrogge, J. Light enables a very high efficiency of carbon storage in developing embryos of rapeseed. Plant Physiol. 2005, 138, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Beisson, F.; Pollard, M.; Ohlrogge, J. Oil content of Arabidopsis seeds: The influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 2006, 67, 904–915. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; Bhandari, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.; Nayyar, H. Impact of heat stress during seed filling on seed quality and seed yield in lentil (Lens culinaris Medikus) genotypes. J. Sci. Food Agric. 2018, 98, 5134–5141. [Google Scholar] [CrossRef]
- Valdivia-Cea, W.; Bustamante, L.; Jara, J.; Fischer, S.; Holzapfel, E.; Wilckens, R. Effect of soil water availability on physiological parameters, yield, and seed quality in four quinoa genotypes (Chenopodium quinoa Willd.). Agronomy 2021, 11, 1012. [Google Scholar] [CrossRef]
- Teugwa, C.M.; Boudjeko, T.; Tchinda, B.T.; Mejiato, P.C.; Zofou, D. Anti-hyperglycaemic globulins from selected Cucurbitaceae seeds used as antidiabetic medicinal plants in Africa. BMC Complement. Altern. Med. 2013, 13, 1–8. [Google Scholar] [CrossRef]
- Hara-Nishimura, I.; Inoue, K.; Nishimura, M. A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett. 1991, 294, 89–93. [Google Scholar] [CrossRef]
- Singh, N.P.; Matta, N.K. Variation studies on seed storage proteins and phylogenetics of the genus Cucumis. Plant Syst. Evol. 2008, 275, 209–218. [Google Scholar] [CrossRef]
- Singh, N.P. Studies on Seed Storage Proteins of Some Important Cucurbits. Ph.D. Thesis, Kurukshetra University, Thanesar, India, 2006. [Google Scholar]
- Singh, N.P.; Matta, N.K. Levels of seed proteins in Citrullus and Praecitrullus accessions. Plant Syst. Evol. 2010, 290, 47–56. [Google Scholar] [CrossRef]
- Horax, R.; Hettiarachchy, N.; Over, K.; Chen, P.; Gbur, E. Extraction, fractionation and characterization of bitter melon seed proteins. J. Agric. Food Chem. 2010, 58, 1892–1897. [Google Scholar] [CrossRef]
- Handley, L.W.; Pharr, D.M.; Mcfeeters, R.F. Relationship between galactinol synthase activity and sugar composition of leaves and seeds of several crop species. J. Am. Soc. Hortic. Sci. 1983, 108, 600–605. [Google Scholar]
- Handley, L.W.; Pharr, D.M.; McFeeters, R.F. Carbohydrate changes during maturation of cucumber fruit: Implications for sugar metabolism and transport. Plant Physiol. 1983, 72, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Botha, F.C.; Small, J.G.C. Effect of water stress on the carbohydrate metabolism of Citrullus lanatus seeds during germination. Plant Physiol. 1985, 77, 79–82. [Google Scholar] [CrossRef] [PubMed]
- El-Adawy, T.A.; Taha, K.M. Characteristics and composition of watermelon, pumpkin, and paprika seed oils and flours. J. Agric. Food Chem. 2001, 49, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Yoko, T.; Ryoichi, M.; Kisei, I.; Kiyoko, Y. Changes in carbohydrate composition in pumpkins [Cucurbita maxima] (Kabocha) during fruit growth. J. Jpn. Soc. Hortic. Sci. 2001, 70, 656–658. [Google Scholar] [CrossRef]
- Irving, D.E.; Hurst, P.L.; Ragg, J.S. Changes in carbohydrates and carbohydrate metabolizing enzymes during the development, maturation, and ripening of buttercup squash (Cucurbita maxima D. “Delica”). J. Am. Soc. Hortic. Sci. 1997, 122, 310–314. [Google Scholar] [CrossRef]
- Henderson, C.W.; Scheerens, J.C.; Berry, J.W. Antinutritional factors in Cucurbita seed meals. J. Agric. Food Chem. 1986, 34, 434–436. [Google Scholar] [CrossRef]
- Kuo, T.M.; VanMiddlesworth, J.F.; Wolf, W.J. Content of raffinose oligosaccharides and sucrose in various plant seeds. J. Agric. Food Chem. 1988, 36, 32–36. [Google Scholar] [CrossRef]
- Schaffer, A.A.; Aloni, B.; Fogelman, E. Sucrose metabolism and accumulation in developing fruit of Cucumis. Phytochemistry 1987, 26, 1883–1887. [Google Scholar] [CrossRef]
- Mitchell, D.E.; Madore, M.A. Patterns of assimilate production and translocation in muskmelon (Cucumis melo L.): II. Low temperature effects. Plant Physiol. 1992, 99, 966–971. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Rafudeen, M.S.; Golldack, D. Physiological aspects of raffinose family oligosaccharides in plants: Protection against abiotic stress. Plant Biol. J. 2014, 16, 1–8. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Basak, P.; Majumder, A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015, 6, 656. [Google Scholar] [CrossRef]
- Miao, M.; Zhang, Z. Carbohydrate metabolism of cucurbits. In Handbook of Cucurbits: Growth, Cultural Practices, and Physiology, 1st ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; Volume 1, pp. 69–91. [Google Scholar]
- Li, T.; Zhang, Y.; Wang, D.; Liu, Y.; Dirk, L.M.A.; Goodman, J.; Downie, A.B.; Wang, J.; Wang, G.; Zhao, T. Regulation of seed vigor by manipulation of raffinose family oligosaccharides in maize and Arabidopsis thaliana. Mol. Plant 2017, 10, 1540–1555. [Google Scholar] [CrossRef]
- Vinson, C.C.; Mota, A.P.Z.; Porto, B.N.; Oliveira, T.N.; Sampaio, I.; Lacerda, A.L.; Danchin, E.G.J.; Guimaraes, P.M.; Williams, T.C.R.; Brasileiro, A.C.M. Characterization of raffinose metabolism genes uncovers a wild Arachis galactinol synthase conferring tolerance to abiotic stresses. Sci. Rep. 2020, 10, 15258. [Google Scholar] [CrossRef]
- Andres, T.C. ybridization of Cucurbita foetidissima with C. pedatifolia C. radicans, and C. ficifolia. CGC Rep. 1987, 10, 72–73. [Google Scholar]
- Neđeral, S.; Petrović, M.; Vincek, D.; Pukec, D.; Škevin, D.; Kraljić, K.; Obranović, M. Variance of quality parameters and fatty acid composition in pumpkin seed oil during three crop seasons. Ind. Crop. Prod. 2014, 60, 15–21. [Google Scholar] [CrossRef]
- De Deyn, G.B. Plant life history and above-belowground interactions: Missing links. Oikos 2017, 126, 497–507. [Google Scholar] [CrossRef]
- Shaar-Moshe, L.; Hayouka, R.; Roessner, U.; Peleg, Z. Phenotypic and metabolic plasticity shapes life-history strategies under combinations of abiotic stresses. Plant Direct 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Lira, R.; Téllez, O.; Dávila, P. The effects of climate change on the geographic distribution of Mexican wild relatives of domesticated Cucurbitaceae. Genet. Resour. Crop Evol. 2009, 56, 691703. [Google Scholar] [CrossRef]
- Pautasso, M.; Döring, T.F.; Garbelotto, M.; Pellis, L.; Jeger, M.J. Impacts of climate change on plant diseases-opinions and trends. Eur. J. Plant Pathol. 2012, 133, 295–313. [Google Scholar] [CrossRef]
- Kates, H.R. Pumpkins, squashes, and gourds. In North American Crop Wild Relatives; Greene, S.L., Williams, K.A., Khoury, C.K., Kantar, M.B., Marek, L.F., Eds.; Springer Nature: Cham, Switzerland, 2019; Volume 2, pp. 195–224. [Google Scholar] [CrossRef]
- Kurpis, J.; Serrato-Cruz, M.A.; Feria Arroyo, T.P. Modeling the effects of climate change on the distribution of Tagetes lucida Cav. (Asteraceae). Glob. Ecol. Conserv. 2019, 20, e00747. [Google Scholar] [CrossRef]
- Luna-Luna, M.; Barretero-Hernández, R. Guía Para El Establecimiento Del Pasto Llorón (Eragrostis curvula) En Los Altos De Jalisco, Folleto Para Productores N°2; Prometeo Editores, S.A. de C.V: Jalisco, Mexico, 2013; pp. 1–16. [Google Scholar]
- AOAC: Official Methods of Analysis (Volume 1), 15th ed.; AOAC: Rockville, MD, USA, 1990; pp. 1–771.
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins—An insight into medicinal leads from nature. Pharmacogn Rev. 2015, 9, 12–18. [Google Scholar] [PubMed]
- Jesionek, W.; Móricz, Á.M.; Ott, P.G.; Kocsis, B.; Horváth, G.; Choma, I.M. TLC-Direct Bioautography and LC/MS as complementary methods in identification of antibacterial agents in plant tinctures from the Asteraceae family. J. AOAC Int. 2015, 98, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ramírez-Pimentel, J.G.; Herrera-Herrera, A.; Aguirre-Mancilla, C.L.; Covarrubias-Prieto, J.; Iturriaga de la Fuente, G.; Raya-Pérez, J.C. Caracterización de las proteínas de reserva y contenido mineral de semilla de melón (Cucumis melo L.). Rev. Mex. Cienc. Agríc. 2016, 7, 1667–1678. [Google Scholar] [CrossRef][Green Version]
- Zhao, D.; MacKown, C.T.; Starks, P.J.; Kindiger, B.K. Rapid analysis of nonstructural carbohydrate components in grass forage using microplate enzymatic assays. Crop Sci. 2010, 50, 1537–1545. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; González de la Vara, L.E.; López, M.G. Fructan active enzymes (FAZY) activities and biosynthesis of fructooligosaccharides in the vacuoles of Agave tequilana Weber Blue variety plants of different age. Planta 2017, 245, 265–281. [Google Scholar] [CrossRef]
- Lay, J.; Liyanage, R.; Durham, B.; Brooks, J. Rapid characterization of edible oils by direct matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis using triacylglycerols. Rapid Commun. Mass Spectrom. 2006, 20, 952–958. [Google Scholar] [CrossRef]

| FFW 3 | ED | LD | SW | TSN | W100S | PuFW | PeFW | |
|---|---|---|---|---|---|---|---|---|
| C. radicans1 | 90.3 ± 19.8 | 6.4 ± 0.4 | 5.5 ± 0.3 | 7.0 ± 2.2 | 93 ± 30.9 | 7.0 ± 1.8 * | 55.6 ± 10.2 | 23.9 ± 2.9 |
| C. foetidissima1 | 148.8 ± 21.4 *,2 | 6.7 ± 0.3 | 6.7 ± 0.2 * | 16.5 ± 1.1 * | 280 ± 18.5 * | 6.1 ± 0.9 | 80.2 ± 12.3 * | 35.1 ± 9.5 * |
| PEEL | SEED | PULP | ||||
|---|---|---|---|---|---|---|
| % | C. radicans1 | C. foetidissima1 | C. radicans | C. foetidissima | C. radicans | C. foetidissima |
| Dry Matter | 95.3 ± 3.1 | 95.6 ± 3.8 | 95.9 ± 2.8 | 96.6 ± 2.2 | 91.4 ± 4.7 | 93.2 ± 5.1 |
| Ash (minerals) | 6.1 ± 1.0 | 7.0 ± 0.8 | 5.2 ± 0.7 | 4.4 ± 0.7 | 16.1 ± 0.6 *,4 | 15.9 ± 1.0 |
| Crude Protein | 9.8 ± 4.0 | 6.6 ± 1.3 | 33.1 ± 3.0 * | 30.6 ± 2.9 | 18.2 ± 2.4 | 13.4 ± 4.3 |
| Total Lipids | 2.17 ± 2.0 | 2.5 ± 2.5 | 28.7 ± 4.1 | 32.3 ± 1.3 * | 3.3 ± 4.3 | 0.89 ± 0.5 |
| Total Fiber | 42.1 ± 3.8 | 48.4 ± 5.5 * | 21.8 ± 1.9 | 26.5 ± 1.8 | 19.7 ± 3.9 | 19.0 ± 1.7 |
| N-free Extract 2 | 34.2 ± 10.8 * | 31.0 ± 3.2 | 8.6 ± 6.0 | 4.1 ± 4.7 | 34.0 ± 10.8 | 44.1 ± 2.9 * |
| ADF 3 | 51.9 ± 3.8 | 55.9 ± 8.1 * | 36.6 ± 4.8 | 38.3 ± 5.4 * | 29.0 ± 6.0 | 29.9 ± 4.3 |
| Lignin | 16.5 ± 2.3 | 18.7 ± 4.2 * | 15.3 ± 2.0 | 17.8 ± 5.3 * | 4.5 ± 3.8 | 2.6 ± 0.7 |
| Cellulose 2 | 35.4 ± 2.0 | 37.2 ± 4.1 * | 21.3 ± 3.3 | 20.2 ± 1.1 | 24.5 ± 3.6 | 27.3 ± 4.3 |
| Standards (m/z) | ID | C. radicans1 | ID | C. foetidissima1 | ID |
|---|---|---|---|---|---|
| 550.673 | Matrix peak? | ||||
| 551.251 | NI | ||||
| 567.247 | NI 2 | ||||
| 599.827 | PO (DAG) 3 | 599.84 | PO (DAG) | ||
| 601.6 | PS | ||||
| 638.671 | LaLaLa | ||||
| 657.841 | NI | ||||
| 661.611 | LaLaLa (Na+) | ||||
| 696.713 | |||||
| 745.708 | MMM (Na+) | ||||
| 759.067 | NI | ||||
| 761.681 | MMM (K+) | ||||
| 783.097 | NI | ||||
| 805.096 | NI | ||||
| 829.8 | PPP (Na+) | ||||
| 878.341 | PLL (TAG) | 878.353 | PLL | ||
| 880.348 | POL | 880.368 | POL | ||
| 892.243 | NI | ||||
| 894.325 | NI | 894.339 | NI | ||
| 895.756 | LnLnLn (Na+) | 896.323 | LnLnLn | ||
| 901.806 | LLL (Na+) | 902.374 | LLL | 902.374 | LLL |
| 904.382 | LLO | 904.394 | LLO | ||
| 906.394 | OOL | 906.42 | OOL | ||
| 907.855 | OOO (Na+) | ||||
| 908.43 | OOS | ||||
| 911.755 | SOS-SSO (Na+) | ||||
| 913.897 | SSS (Na+) | ||||
| 916.35 | NI | ||||
| 917.794 | LLL (K+) | ||||
| 918.36 | NI | 918.317 | NI | ||
| 920.367 | NI | 920.38 | NI | ||
| 922.373 | NI | 922.4 | NI | ||
| 934.36 | NI | ||||
| 943.742 | NI |
| Cucurbitacin Glycosides | Rf Value 2 Range | Non-Glycosylated Cucurbitacins | Rf Value Range |
|---|---|---|---|
| Cucurbitacin-L-glucoside Cucurbitacin-I-glucoside | 0.14–0.18 | Cucurbitacin D Cucurbitacin C Cucurbitacin A Cucurbitacin L | 0.58–0.69 |
| Cucurbitacin-B-glucoside Cucurbitacin-E-glucoside 23, 24 dihydrocucurbitacin E-glucoside | 0.26–0.30 | ||
| Cucurbitacin I Cucurbitacin B 23, 24-dihydrocucurbitacin B 23, 24-dihydrocucurbitacin E Cucurbitacin E | 0.79–0.95 |
| SEED | PULP | PEEL | ||||
|---|---|---|---|---|---|---|
| mg/g (FWB) 2 | C. foetidissima1 | C. radicans | C. foetidissima | C. radicans | C. foetidissima | C. radicans |
| Glucose | 1.97 ± 0.01 **,3 | 1.4 ± 0.02 | 20.7 ± 0.2 ** | 17.8 ± 0.2 | 19.6 ± 0.2 ** | 7.18 ± 0.1 |
| Fructose | 1.98 ± 0.03 ** | 1.32 ± 0.03 | 12.8 ± 0.08 | 14.9 ± 0.04 ** | 12.4 ± 0.2 ** | 10.1 ± 0.13 |
| Sucrose | 8.28 ± 0.08 | 8.04 ± 0.08 | 5.16 ± 0.2 ** | 2.63 ± 0.08 | 2.97 ± 0.05 | 3.11 ± 0.07 |
| Starch | 0.33 ± 0.04 * | 0.14 ± 0.02 | 5.57 ± 0.13 ** | 4.29 ± 0.2 | 3.12 ± 0.14 ** | 0.58 ± 0.05 |
| SEED | PULP | PEEL | ||||
|---|---|---|---|---|---|---|
| mg/g (DWB) 2 | C. foetidissima1 | C. radicans | C. foetidissima | C. radicans | C. foetidissima | C. radicans |
| Myo-inositol | 1.02 ± 0.01 | 1.31 ± 0.04 ** | 3.52 ± 0.16 | 4.48 ± 0.48 | 2.38 ± 0.02 | 5.46 ± 0.08 ** |
| Galactinol | 0.84 ± 0.02 * | 0.67 ± 0.02 | 2.99 ± 0.31 | 2.41 ± 0.39 | 3.36 ± 0.15 | 3.17 ± 0.12 |
| NI-RFO 4 | 0.40 ± 0.02 | 0.43 ± 0.04 | 1.93 ± 0.27 * | 0.90 ± 0.18 | 1.21 ± 0.09 | 1.18 ± 0.03 |
| Raffinose | 1.85 ± 0.06 *,3 | 1.54 ± 0.05 | 0.10 ± 0.02 | 0.06 ± 0.01 | 0.35 ± 0.01 ** | 0.11 ± 0.01 |
| Staquiose | 4.73 ± 0.1 * | 3.74 ± 0.21 | 0.62 ± 0.07 | 0.75 ± 0.1 | 0.19 ± 0.01 | 0.55 ± 0.08 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía-Morales, C.; Rodríguez-Macías, R.; Salcedo-Pérez, E.; Zamora-Natera, J.F.; Rodríguez-Zaragoza, F.A.; Molina-Torres, J.; Délano-Frier, J.P.; Zañudo-Hernández, J. Contrasting Metabolic Fingerprints and Seed Protein Profiles of Cucurbita foetidissima and C. radicans Fruits from Feral Plants Sampled in Central Mexico. Plants 2021, 10, 2451. https://doi.org/10.3390/plants10112451
Mejía-Morales C, Rodríguez-Macías R, Salcedo-Pérez E, Zamora-Natera JF, Rodríguez-Zaragoza FA, Molina-Torres J, Délano-Frier JP, Zañudo-Hernández J. Contrasting Metabolic Fingerprints and Seed Protein Profiles of Cucurbita foetidissima and C. radicans Fruits from Feral Plants Sampled in Central Mexico. Plants. 2021; 10(11):2451. https://doi.org/10.3390/plants10112451
Chicago/Turabian StyleMejía-Morales, Claudia, Ramón Rodríguez-Macías, Eduardo Salcedo-Pérez, Juan Francisco Zamora-Natera, Fabián Alejandro Rodríguez-Zaragoza, Jorge Molina-Torres, John Paul Délano-Frier, and Julia Zañudo-Hernández. 2021. "Contrasting Metabolic Fingerprints and Seed Protein Profiles of Cucurbita foetidissima and C. radicans Fruits from Feral Plants Sampled in Central Mexico" Plants 10, no. 11: 2451. https://doi.org/10.3390/plants10112451
APA StyleMejía-Morales, C., Rodríguez-Macías, R., Salcedo-Pérez, E., Zamora-Natera, J. F., Rodríguez-Zaragoza, F. A., Molina-Torres, J., Délano-Frier, J. P., & Zañudo-Hernández, J. (2021). Contrasting Metabolic Fingerprints and Seed Protein Profiles of Cucurbita foetidissima and C. radicans Fruits from Feral Plants Sampled in Central Mexico. Plants, 10(11), 2451. https://doi.org/10.3390/plants10112451







