Abstract
(1) Background: This study was aimed at determining the in vitro inhibitory effect of new natural substances obtained by minimal processing from shrimp wastes on fungi and oomycetes in the genera Alternaria, Colletotrichum, Fusarium, Penicillium, Plenodomus and Phytophthora; the effectiveness of the substance with the highest in vitro activity in preventing citrus and apple fruit rot incited by P. digitatum and P. expansum, respectively, was also evaluated. (2) Methods: The four tested substances, water-extract, EtOAc-extract, MetOH-extract and nitric-extract, were analyzed by HPLC-ESI-MS-TOF; in vitro preliminary tests were carried out to determine the minimal inhibitory/fungicidal concentrations (MIC and MFC, respectively) of the raw dry powder, EtOAc-extract, MetOH-extract and nitric-extract for each pathogen. (3) Results: in the agar-diffusion-assay, nitric-extract showed an inhibitory effect on all pathogens, at all concentrations tested (100, 75, 50 and 25%); the maximum activity was on Plenodomus tracheiphilus, C. gloeosporioides and Ph. nicotianae; the diameters of inhibition halos were directly proportional to the extract concentration; values of MIC and MFC of this extract for all pathogens ranged from 2 to 3.5%; the highest concentrations (50 to 100%) tested in vivo were effective in preventing citrus and apple fruit molds. (4) Conclusions: This study contributes to the search for natural and ecofriendly substances for the control of pre- and post-harvest plant pathogens.
1. Introduction
Plant pathogenic fungi are responsible for many serious diseases that affect agricultural productions both pre- and post-harvest. In this respect, the losses of products along the post-harvest chains (i.e., warehousing, transport and final distribution) determine strong impactful consequences, especially in agriculture-based-economy countries [1,2,3]. To minimize production losses and maintain crop sustainability, several strategies based on the application of different means, such as physical, chemical and biological, have been adopted over time [4,5]. Currently, one of the most consolidated and effective means for controlling fungal diseases is represented by chemical synthetic fungicides [4,6]. However, their use negatively affects both human health and the preservation of the environment. Moreover, the restricted number of active ingredients which are allowed for post-harvest treatments increases the risk of selection of fungicide resistant plant pathogens, with the consequent dramatic reduction of the efficacy of synthetic fungicides [7]. For these reasons, during past years, their application has been strictly limited by several governmental institutions worldwide [8,9].
In order to satisfy the growing request for high-quality and, at the same time, safe and eco-friendly products, throughout the past two decades, the research field strongly focused on the investigation of the potentialities of alternative means to synthetic fungicides to control plant diseases; these include antagonistic microorganisms or derivatives thereof, natural biostimulants [7,9,10], as well as natural antimicrobial compounds [11,12].
With the perspective of reducing environmental pollution and related consequences for human health, nowadays, the scientific research is also strongly focused on valorizing wastes, especially those largely generated by processing industries [13]. Within this framework, the shrimp market has stood out for considerable development, especially during the past few years. In this respect, it has been estimated that in 2020, the production of shrimp reached a total of 5.03 million tons around the globe, with an amount of waste ranging between 40–50% per ton of fresh product [14,15,16]. Therefore, the wastes generated by shrimp processing industries in food production are clearly undergoing a dramatic increase [17]. Shrimp wastes generated for production of human food are represented by heads, intestines, tails and shells [17], which are usually disposed by throwing into garbage heaps [18], ocean dumping, incineration and land filling [19]. Therefore, an inevitable increase in generated wastes could be determined by their non-use [20].
Shrimp are, overall, considered a high-value aquaculture product [17], not only because of the nutritional properties of the meat used for human consumption, but also for the composition of their wastes; in fact, their major constituents are proteins (35–50%), chitin (15–25%), calcium and phosphorus (10–15%), and other substances (such as amino acids, vitamins, carotenoids, astaxanthin, polyunsaturated fatty acids and other enzymes) [15,21,22,23]. For this reason, nowadays, the valorization of shrimp wastes is a consolidated practice.
Shrimp wastes as such have been used for feeding in veterinary practice and aquaculture [17] as well as in compost fertilizer [24,25]. Dried shrimp wastes are also used in animal feeding in mixtures with other agricultural raw materials; however, since drying processes are usually carried out directly along the beaches, these practices of the use of shrimp wastes favor additional pollution, especially in coastal areas [17]. A further strategy for the use of shrimp wastes includes both the extraction of bioactive molecules or the secondary chemically-mediated transformation of some parts of these into other bioactive compounds; one of these is the chitosan, the large-scale production of which is commonly carried out by alkaline deacetylation of the chitin extracted from shrimp shells [26]. Chitosan has several useful applications in various fields, including medicine, cosmetics, agriculture, paper and textile industries, biotechnologies and bioremediation of the environment (water treatment) [15,27]; however, the acid/alkaline-mediated industrial processes for its production from shrimp wastes have serious environmental consequences [17,18,26].
The aforementioned products arising from shrimp wastes represent, therefore, a precious asset in several fields of application; however, it is an accepted fact that their processing generates highly impactful new wastes, which in turn contribute to environmental pollution and, consequently, negatively affect human health.
The investigation of the potentialities of new products arising from a minimal and sustainable processing of shrimp wastes stands, therefore, as an essential challenge for scientific research. Considering that plant pathology is strongly focused on finding eco-friendly strategies for controlling plant pathogens and related diseases, the present study evaluated the effectiveness of new substances obtained by the minimal processing of shrimp wastes in the in vitro and in vivo control of major fungal and oomycete pathogens of the genera Alternaria, Colletotrichum, Fusarium, Penicillium, Plenodomus and Phytophthora.
2. Results
In this study, wastes from the shrimp species Parapenaeus longirostris were processed to obtain four substances: (i) “Water-extract”, (ii) “EtOAc-extract”, (iii) “MetOH-extract” and (iv) “Nitric-extract”. All these extracts were analyzed, to determine their composition in metabolites and phenolic compounds, by HPLC-ESI-MS/TOF. Then, the antifungal activity of the “dry-powder”, “EtOAc-extract”, “MetOH-extract” and “Nitric-extract” was preliminarily tested in vitro by an agar diffusion test toward several fungal and oomycete pathogens. “Dry-powder”, “EtOAc-extract” and “MetOH-extract” did not demonstrate any inhibitory effect in the mycelial growth of all pathogens under study (data not shown); therefore, they were not further tested. “Nitric-extract” was the only extract that negatively affected the mycelial growth of all pathogens; the diameter of the inhibition halos consequently observed at each concentration was, therefore, recorded at the end of the incubation period (see Figure 1a–o). The most effective substance resulting from the in vitro test was further investigated to determine its efficiency in terms of minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC). The in vivo effectiveness of the selected substance in the control of post-harvest infections of fruits by Penicillium spp. was finally tested.
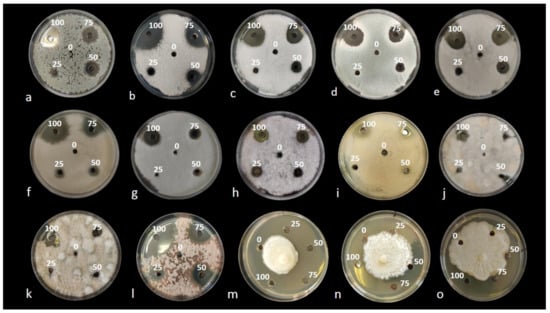
Figure 1.
Agar diffusion test. Inhibition halos determined by the Nitric-extract at different concentrations (0, 25, 50, 75, 100%), after 3 days of incubation at 25 °C on PDA: (a) Penicillium digitatum P1PP0; (b) P. commune CECT 20767; (c) P. expansum CECT 2278; (d) P. italicum CECT 20909; (e) Colletotrichum acutatum UW14; (f) C. karsti CAM; (g) C. gloeosporioides C2; (h) Fusarium proliferatum CBS 145950; (i) F. sacchari CBS 145949; (j) Alternaria arborescens 803; (k) A. alternata 646; (l) Plenodomus tracheiphilus Pt2. Inhibition halos at different concentrations after 15 days of incubation at 25 °C on PDA: (m) Phytophthora nicotianae T2.C-M1A; (n) Ph. nicotianae T3-B-K1A; (o) Ph. citrophthora Ax1Ar.
2.1. Metabolites and Phenolic Compounds Detected in Test Substances by HPLC-ESI-MS/TOF
The metabolites detected by HPLC-ESI-MS/TOF in the analyzed substances are presented, as a heat map, in Figure 2. Colors are based on the relative abundance (logarithmic scale) of the metabolites detected, where red represents high abundance and green represents low abundance. Overall, among all substances examined, the analysis evidenced the presence of a total of 54 metabolites already known in the literature. In particular, the “Water-extract” showed 50 metabolites, which is the highest number recovered; “EtOAc-extract” and “Nitric-extract” contained 36 and 35 metabolites, respectively; finally, only 25 metabolites were detected in the “MetOH-extract”. Some marked differences were observed among the substances; in particular, a higher abundance of free amino acids, such as phenyalanine, proline, serine, tyrosine and valine, was evidenced in the “Water-extract” and “MetOH-extract” over the “EtOAc-extract” and “Nitric-extract”. Really high relative abundances in some metabolites were also observed; in particular, 2-Hydroxyisocaproic acid, 3-(4-Hydroxyphenyl) propionic acid and 4-aminobenzoic acid in “MetOH-extract”, docosahexaenoic acid in “EtOAc-extract” and the phenylalanine in the “Water-extract” and “MetOH-extract”.
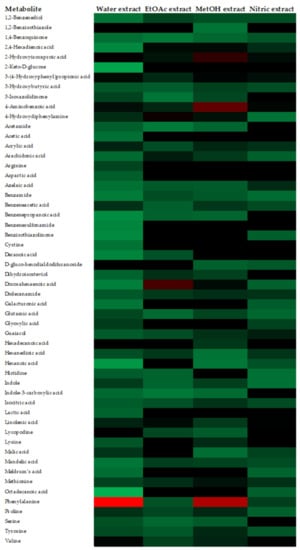
Figure 2.
Heat map representing the relative abundances of metabolites detected in different shrimp extracts.
The most important phenolic acids detected by HPLC-ESI-MS/TOF in the substances analyzed are presented in Table 1. Their abundance is expressed in mg/kg of each substance. The most abundant phenolic compound detected in all the analyzed substances was benzoic acid, whose amount ranged from a minimum of 0.87 mg/kg in “Nitric-extract” to a maximum of 3.57 mg/kg in “EtOAc-extract”. In order of abundance, vanillin (0.21–2.04 mg/kg) and syringic acid (0.16–1.21 mg/kg), which had the highest concentrations of “MetOH-extract”, were detected. The p-coumaric (4-hydroxycinnamic acid) acid was another phenolic compound recovered in all the substances; its abundance ranged from a minimum of 0.27 mg/kg in the “Water-extract” to a maximum of 0.88 mg/kg in the sample “Nitric-extract”. The “Nitric-extract” also reported the highest concentration of 1-2-Dihydroxybenzene (0.86 mg/kg). Few phenolic compounds were detected just in one substance; among these, the 3-(4-hydroxy-3-methoxyphenyl) propionic acid and ellagic acid were detected only in “Nitric-extract”, while sinapic acid only in the “Water-extract”.

Table 1.
Concentration of phenolic compounds detected in the tested substances (mean value ± standard deviation).
2.2. In Vitro Preliminary Tests
Results from the in vitro preliminary tests evidenced an inhibitory effect on the growth of the pathogens examined only for the waste shrimp extracted with nitric acid, named “Nitric-extract”. Additionally, none of the control solutions (each solvent used for the preparation of the respective extract) inhibited mycelial growth. In the agar diffusion test, “Nitric-extract” at concentrations of 100, 75 and 50% showed an inhibitory effect on all strains of fungal and oomycete pathogens, while at concentration of 25%, an inhibitory effect was still observed only on Ph. nicotianae T2.C-M1A, F. sacchari CBS 145949, A. alternata 646, P. digitatum P1PP0, P. commune CECT 20767, C. gloeosporioides C2, F. proliferatum CBS 145950, Pl. tracheiphilus Pt2 and Ph. nicotianae T3-B-K1A, in order of significance (Table 2 and Figure 2). The diameter of inhibition halos was directly proportional to the concentration of the extract (Table 2). Significant differences in the inhibitory effects of the extracts were noticed among fungal and oomycete species as well as between species of the same genus and even between strains of the same species (Table 2). At the maximum dose, which is 100% of the extract concentration, the highest inhibitory effect was on Pl. tracheiphilus Pt2; at 75% concentration, the highest inhibitory activity was on Pl. tracheiphilus Pt2 and Ph. nicotianae T2.C-M1A; at 50%, on Ph. nicotianae T2.C-M1A; and at the lowest dose (25% extract concentration), on Ph. nicotianae T2.C-M1A as well as on three typically post-harvest pathogens, i.e., F. sacchari CBS 145949, A. alternata 646 and P. digitatum P1PP0.

Table 2.
Inhibitory effect of different concentrations (from 25 to 100%) of shrimp nitric-extract on the mycelium growth of 12 fungal and three oomycete plant pathogens, determined with the agar diffusion test by measuring the diameter of the inhibition halo around the wells. The incubation period was three days for fungi and 15 days for oomycetes.
2.3. Determination of MIC and MFC
To further test the inhibitory activity of “Nitric-extract” on the growth of pathogens, the minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC) were determined, and results are summarized in Table 3. The values of both MIC and MFC for all pathogens were in the range 2–3.5%. In more detail, the highest values of MIC (3.5%) were recorded for P. expansum CECT 2278 and F. saccari CBS 145949, while the lowest (2%) were recorded for C. gloeosporioides C2, Ph. nicotianae T3-B-K1A and Pl. tracheiphilus Pt 2. Values of MFC were the same as MIC for the majority of the strains. Only for strains P. commune CECT 20767, A. alternata 646, Ph. nicotianae T3-B-K1A and Ph. citrophthora Ax1Ar, MFC was higher than MIC, indicating that for these four strains, MIC exerted only a fungistatic effect.

Table 3.
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) determined by the nitric-extract.
2.4. In Vivo Antifungal Activity
The antifungal activity of “Nitric-extract” was finally tested in vivo on citrus (oranges and lemons) and apple fruits artificially infected by P. digitatum and P. expansum, respectively. Results are summarized below.
2.4.1. Antifungal Activity on Oranges
Three days post inoculation with P. digitatum P1PP0 of oranges, all concentrations of “Nitric-extract” significantly reduced rot severity compared to the water control (treatment ID01) (Figure 3). However, except for “Nitric-extract” applied as such (ID02), each of the other concentrations was not statistically different from the respective control.
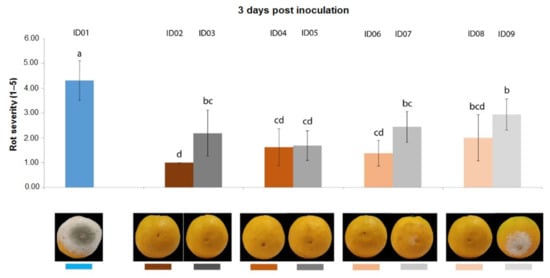
Figure 3.
Rot severity caused by Penicillium digitatum strain P1PP0 in orange (Citrus × sinensis) fruits cv. Valencia treated with water (ID01) or nitric-extract as such (ID02), 75% Nitric-extract (ID04), 50% Nitric-extract (ID06), 25% Nitric-extract (ID08) and respective controls (NaNO3 0.17 g/mL—ID03; NaNO3 0.17 g/mL diluted in sterile distilled water (sdw) at 75%—ID05; NaNO3 0.17 g/mL diluted in sdw at 50%—ID07; NaNO3 0.17 g/mL diluted in sdw at 25%—ID09) 3 days after inoculation. Values sharing the same letters are not statistically different according to Tukey’s HSD (honestly significant difference) test (p ≤ 0.05). Bars represent SD.
Five days after inoculation (Figure 4), all concentrations of “Nitric-extract” still demonstrated values of rot severity significantly lower than the water control (treatment ID01); however, in this case, only treatment with “Nitric-extract” at 25% (ID08) significantly differed from the respective control (ID09), although this difference was not statistically significant in comparison to the other control treatments (ID03, ID05, ID07).
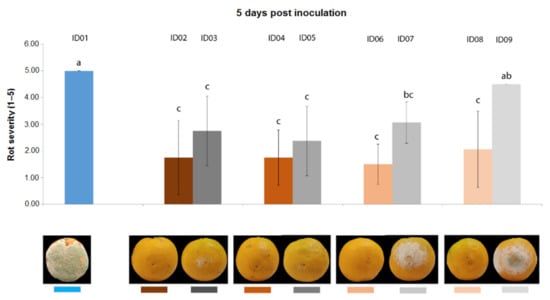
Figure 4.
Rot severity caused by Penicillium digitatum strain P1PP0 in orange (Citrus × sinensis) fruits cv. Valencia treated with water (ID01) or nitric-extract as such (ID02), 75% Nitric-extract (ID04), 50% Nitric-extract (ID06), 25% Nitric-extract (ID08) and respective controls (NaNO3 0.17 g/mL—ID03; NaNO3 0.17 g/mL diluted in sterile distilled water (sdw) at 75%—ID05; NaNO3 0.17 g/mL diluted in sdw at 50%—ID07; NaNO3 0.17 g/mL diluted in sdw at 25%—ID09) 5 days after inoculation. Values sharing the same letters are not statistically different according to Tukey’s HSD test (p ≤ 0.05). Bars represent SD.
2.4.2. Antifungal Activity on Lemons
Three days after inoculation of P. digitatum P1PP0 in lemons (Figure 5), all tested concentrations of “Nitric-extract” significantly reduced rot severity compared to the control. Additionally, among these, “Nitric-extract” as such (ID02) and “Nitric-extract” at 75% (ID02) significantly reduced rot severity compared to all other control solution (treatments ID03, ID05, ID07 and ID09). “Nitric-extract” as such (ID02) and “Nitric-extract” 75% (ID04) were also the only treatments that, five days after inoculation, still maintained significant effectiveness in the reduction of rot severity in lemons (Figure 6).
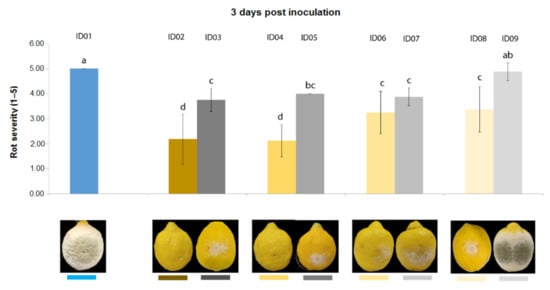
Figure 5.
Rot severity caused by Penicillium digitatum strain P1PP0 in lemon (Citrus × limon) fruits cv. Femminello Siracusano treated with water (ID01) or Nitric-extract as such (ID02), 75% Nitric-extract (ID04), 50% Nitric-extract (ID06), 25% Nitric-extract (ID08) and respective controls (NaNO3 0.17 g/mL—ID03; NaNO3 0.17 g/mL diluted in sterile distilled water (sdw) at 75%—ID05; NaNO3 0.17 g/mL diluted in sdw at 50%—ID07; NaNO3 0.17 g/mL diluted in sdw at 25%—ID09) 3 days after inoculation. Values sharing the same letters are not statistically different according to Tukey’s HSD test (p ≤ 0.05). Bars represent SD.
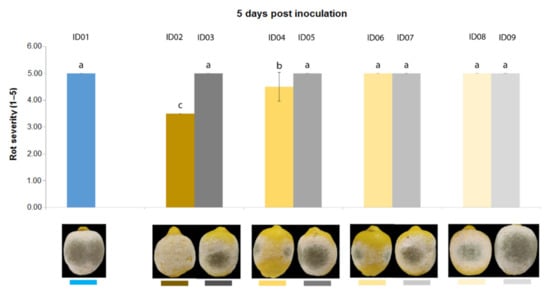
Figure 6.
Rot severity caused by Penicillium digitatum strain P1PP0 in lemon (Citrus × limon) fruits cv. Femminello Siracusano fruits treated with water (ID01) or Nitric-extract as such (ID02), 75% Nitric-extract (ID04), 50% Nitric-extract (ID06), 25% Nitric-extract (ID08) and respective controls (NaNO3 0.17 g/mL—ID03; NaNO3 0.17 g/mL diluted in sterile distilled water (sdw) at 75%—ID05; NaNO3 0.17 g/mL diluted in sdw at 50%—ID07; NaNO3 0.17 g/mL diluted in sdw at 25%—ID09) 5 days after inoculation. Values sharing the same letters are not statistically different according to Tukey’s HSD test (p ≤ 0.05). Bars represent SD.
2.4.3. Antifungal Activity on Apples
Results from the trial carried out on apple fruits inoculated with P. expansum CECT 2278 evidenced that, three days post inoculation (Figure 7), “Nitric-extract” as such (ID02), at 75% (ID04) and at 50% significantly reduced rot severity in comparison with any other treatment and controls. Five days post inoculation, only “Nitric-extract” as such (ID02) still significantly reduced rot severity (Figure 8).
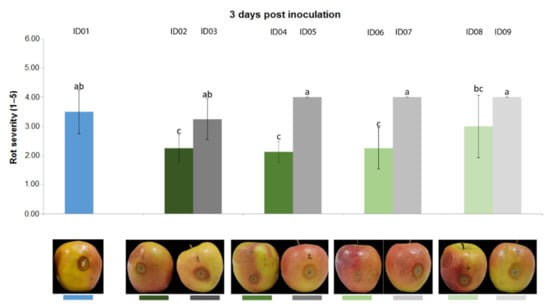
Figure 7.
Rot severity caused by Penicillium expansum strain CECT 2278 in apple (Malus domestica) fruits cv. Braeburn treated with water (ID01) or Nitric-extract as such (ID02), 75% Nitric-extract (ID04), 50% Nitric-extract (ID06), 25% Nitric-extract (ID08) and respective controls (NaNO3 0.17 g/mL—ID03; NaNO3 0.17 g/mL diluted in sterile distilled water (sdw) at 75%—ID05; NaNO3 0.17 g/mL diluted in sdw at 50%—ID07; NaNO3 0.17 g/mL diluted in sdw at 25%—ID09) 3 days after inoculation. Values sharing the same letters are not statistically different according to Tukey’s HSD test (p ≤ 0.05). Bars represent SD.
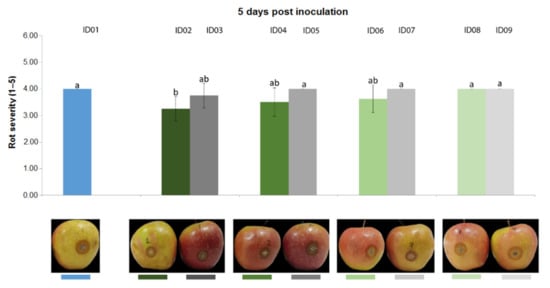
Figure 8.
Rot severity caused by Penicillium expansum strain CECT 2278 in apple (Malus domestica) fruits cv. Braeburn treated with water (ID01) or Nitric-extract as such (ID02), 75% Nitric-extract (ID04), 50% Nitric-extract (ID06), 25% Nitric-extract (ID08) and respective controls (NaNO3 0.17 g/mL—ID03; NaNO3 0.17 g/mL diluted in sterile distilled water (sdw) at 75%—ID05; NaNO3 0.17 g/mL diluted in sdw at 50%—ID07; NaNO3 0.17 g/mL diluted in sdw at 25%—ID09) 5 days after inoculation. Values sharing the same letters are not statistically different according to Tukey’s HSD test (p ≤ 0.05). Bars represent SD.
3. Discussion
This study evaluated, for the first time, the potentialities of minimally processed shrimp wastes in the in vitro inhibitory activity on fungal and oomycete plant pathogens, and their effectiveness in controlling post-harvest rots caused by Penicillium spp. in citrus and apple fruits. To this aim, wastes from the shrimp species Parapenaeus longirostris were dried and grounded to result in a “dry-powder”, which was further processed leading to four different extracts “Water-extract”, “EtOAc-extract”, “MetOH-extract” and “Nitric-extract”. Acid hydrolysis is mandatory for the mineralization of calcium-containing shrimp waste, and hydrolysis is commonly performed by hydrochloric, acetic, phosphoric, sulfuric, nitric and lactic acids. Nitric acid was selected as, among the above-mentioned acids, it has the slowest reaction kinetics [28], which allows for better digestion control. All these substances, “Water extract”, “EtOAc-extract”, “MetOH-extract” and “Nitric-extract, were analyzed to determine their composition in metabolites and phenolic compounds. Then, the “dry-powder”, “EtOAc-extract”, “MetOH-extract” and “Nitric-extract” were also preliminarily tested in vitro, in order to select the substance with the highest mycelial growth inhibitory activity. “Nitric-extract” was the most effective substance and was further investigated to determine its antifungal properties (in terms of MIC and MFC) and in vivo antifungal activity.
Results from the chemical analysis showed that all substances extracted from the shrimp waste were miscellaneous mixtures of a conspicuous number of metabolites and phenolic compounds. Interestingly, a high relative abundance of the 2-Hydroxyisocaproic, 3-(4-Hydroxyphenyl) propionic and 4-Aminobenzoic acids in “MetOH-extract”, and of docosahexaenoic acid in “EtOAc-extract” were reported. Various studies reported fungicidal activity for these molecules when tested as pure substances; 2-hydroxyisocaproic acid was effective against Candida and Aspergillus species [29]; 3-(4-Hydroxyphenyl) propionic acid contains the hydroxyl group, which has been reported as one of the substance responsible for the antifungal activity of Lactobacillus paracasei [30]. Moreover, the para-aminobenzoic acid showed antibiotic activity toward Staphylococcus aureus [31]; a Pseudomonas aeruginosa-bioconverted oil extract of docosahexaenoic acid was effective against the mycelial growth of several plant pathogens, including Botrytis cinerea, Colletotrichum capsici, Fusarium oxysporum, F. solani, Phytophthora capsici, Rhizoctonia solani and Sclerotinia sclerotiorum [32]. However, in the present study, two extracts, “MetOH-extract” and “EtOAc-extract”, containing a higher amount of the above-mentioned acids, showed no inhibitory activity on mycelial growth.
An additional interesting metabolite present in all substances was phenylalanine, which was also detected in high amount in “Water-extract” and “MetOH-extract”. A recent study [33] reported that post-harvest treatments of mango, avocado and citrus fruits with phenylalanine induced resistance against infections caused by Colletotrichum gloeosporioides, Lasiodiplodia theobromae and P. digitatum, respectively, although in vitro tests carried out in the same study evidenced no inhibitory effects toward the same pathogens. Therefore, although lacking of fungicidal action, the “Water-extract” and “MetOH-extract”, which showed a high amount of phenylalanine, could provide strong resistance induction properties to control post-harvest disease. It goes without saying that, since phenylalanine was also detected in “EtOAc-extract” and “Nitric-extract”, these samples could also have resistance induction properties, as demonstrated for other extracts of natural origin [34]. This possibility assumes a particular significance of the extract “Nitric-extract”, which was the only substance tested that demonstrated clear and strong in vitro antifungal activity as well as significant in vivo control of infective processes. Additional studies are, therefore, ongoing, to verify possible resistance induction properties of all the minimally processed shrimp wastes produced in this study. Quite interestingly, although the exoskeleton of shellfish is the main raw material for the extraction of chitosan, whose inhibitory activity on post-harvest fruit rots is well documented [35], this biopolymer was not present in the extracts examined in this study. As a consequence, it can be inferred that other substances are responsible for the antimycotic activity showed by the “Nitric-extract”.
With reference to composition in phenolic compounds, analyses evidenced the presence, in all tested substances, of molecules whose antimicrobial activity is supported by a wide range of literature [2,36,37,38,39,40,41,42,43,44]. Some of these compounds have been also applied as eco-friendly alternatives to synthetic fungicides [1,45]. Among the phenolic compounds, the molecules that recurred in all analyzed substances were the benzoic, caffeic and p-coumaric acids and the vanillin. Benzoic and caffeic acids have important preservative properties that determine the inhibition of fungal growth [43,46]. Vanillin (4-hydroxy-3-methoxybenzaldehyde) is considered one of the most important additives used in the food industry; it is characterized by effective inhibitory activity toward a wide range of microorganisms, thus causing a delay in the growth of yeasts and fungi [36,40]. The p-coumaric acid (4-hydroxycinnamic acid), which, in “Nitric-extract”, had the highest concentration, is the main phenolic acid contained in the peel of sweet oranges [44], and is well known for its efficacy in negatively affecting the growth of post-harvest pathogens, such as Monilinia fructicola, Botrytis cinerea and Alternaria alternata [2]. Interestingly, “Nitric-extract” also reported the highest concentration of catechol (1-2-dihydroxybenzene) and the exclusive presence of dihydroferulic (3-(4-hydroxy-3-methoxyphenyl) propionic acid) and ellagic acids. Catechol shows significant activity in the control of Fusarium oxysporum and Penicillium italicum [38]. Dihydroferulic acid significantly inhibits the in vitro growth of Saccharomyces cerevisiae, Aspergillus fumigatus and A. flavus [39]. Moreover, ellagic acid, which possesses well-documented antibacterial activity [37], shows extraordinary antifungal effects toward Botrytis cinerea [41], as well as a significant growth inhibition of several fungal species belonging to the genera Trichophyton and Candida [42]. Finally, phenolic compounds are hypothesized to be, at least in part, responsible for the strong broad-spectrum antifungal activity shown by a pomegranate peel extract [34].
Overall, unlike the “Water-extract”, “EtOAc-extract” and “MetOH-extract”, “Nitric-extract” results were characterized by p-coumaric acid and catechol, both present at high concentrations, and by the exclusive presence of the acids dihydroferulic and ellagic; these molecules could be, therefore, responsible for the antifungal activity of this extract. Synergetic action of some of the molecules detected in “Nitric-extract” also cannot be excluded. This effect has already been observed for the active components of extracts from different natural matrices. This is the case, for example, of pomegranate, whose high biological value is recognized as being the result of the synergistic chemical action of the total phytoconstituents of the fruit rather than of single extracted components [47,48,49].
The quantity and quality of the molecules that were active (individually or in synergy) in determining the in vitro antifungal activity of the tested substances could also be related to the extraction process. By comparing the compositions of the three extracts, namely, “EtOAc-extract”, “MetOH-extract” and “Nitric-extract”, the three applied extraction processes had different efficiencies. The choice of the best solvent for the extraction of precise bioactive components from a specific matrix is a crucial aspect for reaching the expected qualitative and quantitative yield of the desired molecules in the final extract [34]. Examples of this aspect are provided by studies carried out on pomegranate extracts; Al-Zoreky [50] observed that the 80% methanolic extract was richer in polyphenols compared to hot water and diethyl ether extracts and, therefore, led to higher antimicrobial activity against pathogenetic bacteria. Tayel et al. [51] found that, regardless the concentration of specific bioactive components, a methanolic pomegranate peel extract was more effective than ethanol and water extracts in controlling Penicillium digitatum. In view of these aspects, it is quite surprising that, among the extracts, only “Nitric-extract” provided in vitro antifungal efficacy and, at the same time, neither “EtOAc-extract” nor “MetOH-extract” resulted in an inhibitory effect on mycelial growth.
Results from the in vitro preliminary test together with those from MIC and MFC tests overall demonstrated that the pathogens mostly affected by “Nitric-extract” were Pl. tracheiphilus Pt 2, C. gloeosporioides C2 and Ph. Nicotianae—both tested isolates. Plenodomus tracheiphilus is the causal agent of ‘mal secco, one of the most destructive diseases affecting lemon trees [52]. Because of the vascular propagation of the pathogen in all aerial parts of the infected plant, the management of the disease is complicated [53]. It is commonly carried out by the pruning of diseased twigs, withered shoots and suckers, followed by the spraying of the canopy with copper-based fungicides, which can reduce the occurrence of new Pl. tracheiphilus-infections. However, many copper-based treatments are not cost effective in commercial lemon groves, and also represent a significant source of environmental pollution [53]. Another copper-susceptible pathogen is C. gloeosporioides, the causative agent of anthracnoses in several fruits and vegetables [54] as well as of twig and shoot dieback in citruses [55]. Phytophthora nicotianae is very likely the most widespread and destructive Phytophthora species worldwide, affecting a very wide host range of more than 255 plant species [8,56,57]. Control strategies may be different depending on the specific situation, although the pathogen is markedly sensitive to Metalaxyl and Fosetyl Al, fungicides which are commonly used for controlling plant diseases affecting roots, collars and stems [56]. Results from this study pose “Nitric-extract” as a promising alternative to the use of conventional fungicides in controlling not only Pl. tracheiphilus, C. gloeosporioides and Ph. nicotianae, but all pathogens tested in the present study. To this aim, further investigations are needed to evaluate the phytotoxicity, if any, of the extract, its attitude to systemic translocation, which is of particular relevance in the case of tracheomycoses, such as ‘mal secco’ caused by Pl. tracheiphilus, as well as the most effective method of application, e.g., by drenching, spraying or incorporation into fruit coatings, which also depends on the type of disease.
As a preliminary step towards the application of “Nitric-extract” to control plant diseases, its effectiveness was tested in vivo against molds caused by Penicillium species in orange, lemon and apple fruits, which are the most economically important post-harvest diseases affecting these fruits [58,59]. Post-harvest molds of citrus and apple fruits are traditionally controlled by the application of highly effective chemicals, such as imidazole and bendimidazole (thiabendazole) fungicides [60,61]. More recently, as a consequence of the selection of imidazole- and bendimidazole-resistant strains of Penicillium, several other synthetic fungicides, including azoxystrobin, fludioxonil, cyprodinil and pyrimethanil, have been proposed as alternatives for the chemical control of these post-harvest fruit diseases [7,60,61,62,63]. Like imidazoles and benzimidazoles, all these fungicides are effective at relatively low doses but are characterized by a high acute toxicity [64,65,66,67,68,69].
There is boundless literature evaluating the efficacy of alternative strategies to the use of conventional synthetic fungicides for the control of postharvest molds of Penicillium species [70,71,72,73,74,75,76,77,78]. A novelty in the present study is the in vivo control of Penicillium spp. using a natural substance that is derived from minimum waste treatment.
Overall, treatments with “Nitric-extract” at the highest concentrations were the most effective in positively affecting the reduction of rot severity in all tested fruits. Additionally, an interesting weak positive effect was also observed in all control treatments, including NaNO3 in water solution (ID03, ID05, ID07 and ID09), although, in vitro, they were not effective in inhibiting the mycelial growth of all pathogens included in this study. As already observed for other inorganic salts [74], it cannot be excluded that the in vivo effectiveness of NaNO3 was not the consequence of direct antifungal activity, but the possible result of the triggering of defense mechanisms in fruits. Further tests are ongoing to verify this hypothesis.
The results from the treatments with “Nitric-extract” demonstrated that three days post-treatment, “Nitric-extract” as such determined a significant reduction of rot severity over any other treatment in all fruits (oranges, lemons and apples). Additionally, “Nitric-extract” at 75% significantly reduced rot severity in lemon and apples over controls; “Nitric-extract” at 50% had a significant effect over controls only on apples; the concentration of 25% was as effective as the controls in all fruits. Five days post-treatment, “Nitric-extract” as such still maintained significant effects in reduction of rots only in lemons and apples; “Nitric-extract” at 75% demonstrated significant reduction of rot severity only in lemons; finally, “Nitric-extract” at 50% and at 25% were as effective as the controls in all tested fruits. Overall, the results showed an interesting performance of “Nitric-extract” in controlling postharvest mold caused by Penicillium spp., although the effective dose was much higher than that of traditional synthetic fungicides [7], and, as with other eco-friendly alternatives to synthetic fungicides [7,74], its use may not provide complete protection. A successful strategy for improving its efficacy or reducing fungicide residues from post-harvest fruit treatments could include the use of “Nitric-extract” in a mixture with conventional fungicides applied at a concentration lower than the standard dose, or by incorporating it in a fruit coating.
This study is part of a research program aimed at exploring the antifungal activity of extracts obtained from minimally processed shrimp wastes and their possible application in agriculture. The antifungal activity shown in vitro against a wide range of fungal and oomycete pathogens by the nitric extract appears promising and could be exploited in the context of new strategies for the management of plant diseases caused by these pathogens. In vivo preliminary results suggest a possible use of nitric extract for post-harvest treatments against citrus and apple molds caused by Penicillium species. To this aim, and to optimize the efficacy of treatments, next steps will be to define the methods and times of application. In this study, nitric extracts were applied to fruits 24 h after inoculation with the pathogen, indicating curative efficacy. However, an additional aspect that would merit further investigation is whether nitric extract, like other natural substances, is able to elicit plant defense mechanisms against infections by pathogens. In this case, the treatment of fruits with this extract might also have preventive efficacy against infections by molds. Regarding this, it cannot be ruled out that the other shrimp waste extracts, which, in preliminary in vitro tests did not show inhibitory activity on the mycelium growth, may also be effective in vivo acting as resistance elicitors. Last but not least, a prerequisite for the use of nitric extracts of shrimp waste to prevent post-harvest molds is to evaluate if the treatment leaves unpleasant odors on the fruits. A sensory analysis using an electron nose is planned to clarify this aspect. Although the effective dose of nitric shrimp waste extract is far higher than the label dose of synthetic fungicides used to control post-harvest fruit diseases, this extract, as a natural substance, could be an interesting alternative to traditional post-harvest chemical treatments, as it is more eco-friendly and far less toxic than synthetic fungicides.
4. Materials and Methods
4.1. Preparation of Shrimp Waste Substances
Around 5 kg of shrimp waste (cephalothorax, head and carapace) of the species Parapenaeus longirostris, common name deep-water rose shrimp, was collected in a local fish market in Catania (Italy), in February 2021. Shrimp waste was kept on ice until processed in the laboratory and firstly, washed with distilled water; then, dried in an oven at 30 °C for a week. The dried sample was powdered and homogenized. Then, 10 g of shrimp waste powder was packed in plastic food bags labelled “dry-powder” and stored at −20 °C until further use; 10 g of shrimp waste powder was processed to extraction (20 min long sonication by means of ArgoLab DU-100) with (i) 50 mL of ethyl acetate (EtOAc from Aldrich) or (ii) 50 mL of methanol (MetOH from Aldrich). Then, the supernatant was carefully collected and transferred into a clean beaker. The powder was then re-subjected to the described procedure for a total of three times. The 150 mL of supernatant were firstly filtrated and then evaporated under vacuum at the temperature of 40 °C until a crude extract was obtained. The crude extracts, representing the “EtOAc-extract” and “MetOH-extract”, were stored at −20 °C until further use. (iii) To obtain “Nitric-extract”, 20 mL of nitric acid (HNO3, 65% from Aldrich) was added to 5 g of shrimp waste powder; the mixture was then stirred for 1 h at 150 rpm and then the acid was neutralized by adding 80 mL of NaOH (0.10 g/mL). pH was verified to be around pH 5. To obtain the “water-extract”, 25 mL of water with 1% of acetic acid were added to 5 g of shrimp waste powder; the mixture was homogenized by vortexing and ultrasonication. The liquid extracts were then filtrated and stored at −20 °C until further use.
4.2. Analysis of Metabolites Present in Shrimp Waste Samples by HPLC-ESI-MS/TOF
The differential analysis of the metabolites contained in the four substances tested was carried out by HPLC-ESI-MS-TOF. Before the analysis, each sample was subjected to specific pretreatments. In particular, “EtOAc-extract” and “MetOH-extract” were dissolved in a methanol solution at 1% of acetic acid. Finally, “Water-extract” and “Nitric-extract” were mixed to acidified water. Each sample was finally filtered with 0.22 µm filter and then analyzed using an UPLC (1290 Infinity LC, Agilent Technologies, Santa Clara, CA, USA) coupled with a quadrupole time of flight mass spectrometer (Agilent 6546 LC/Q-TOF) operating in positive and negative ionization mode. Chromatographic separation was performed with an Agilent Zorbax RRHD SB-C18, 2.1 mm × 50 mm, 1.8 µm column. Mobile phase A was composed of Milli-Q water and acetonitrile was used for mobile phase B (both phases were acidified with 0.1% formic acid), with gradient elution, as follows: 0 min, 2% B; 22 min 95% B; 25 min, 5% B. The column was equilibrated for 3 min before every analysis. The flow rate was 0.4 mL/min, and 5 μL of sample was injected. Dual AJS ESI source conditions were as follows: gas temperature: 325 °C; gas flow: 10 L/min; nebulizer pressure: 40 psig; sheath gas temperature: 295 °C; sheath gas flow: 12 L/min; capillary voltage: 4000 V; nozzle voltage: 500 V; Fragmentor: 120 V; skimmer: 70 V; product ion scan range: 100–1500 Da; MS scan rate: 5 spectra/s; MS/MS scan rate: 3 spectra/s; maximum precursors per cycle: 2; and collision energy: 10, 20, 40 eV. The analysis of the metabolites was carried out in triplicate. Untargeted LC/Q-TOF based metabolomics approach was used to identify the metabolic profiling of shrimp waste extracts. Integration, data elaboration and identification of metabolites were managed using MassHunter Qualitative Analysis software B.08.00 and library PCDL Manager B.08.00.
4.3. Fungal and Oomycete Strains, Culture Conditions and Propagules Production
Fungal and oomycete strains were included in this study. Most of them had been previously characterized [7,8,55,79,80]. The complete list of strains tested in this study is as follows: four Penicillium spp. (P. digitatum P1PP0, P. commune CECT 20767, P. expansum CECT 2278 and P. italicum CECT 20909); three Phytophthora spp. (Ph. nicotianae strains T3-B-K1A and T2.C-M1A, Ph. citrophthora strain Ax1Ar); Plenodomus tracheiphilus strain Pt2; two Alternaria species (A. alternata strain 646, and A. arborescens strain 803); three Colletotrichum species (C. acutatum strains UW14, C. karsti strain CAM and C. gloeosporioides strain C2); two Fusarium species (F. proliferatum strain CBS 145950 and F. sacchari strain 145949). All strains were from the collection of the laboratory of Molecular Plant Pathology of the Di3A (University of Catania, Catania, Italy).
4.4. In Vitro Preliminary Screening for Selecting the Most Effective Extract
The antifungal activity of the “dry-powder”, “EtOAc-extract”, “MetOH-extract” and “Nitric-extract” were preliminarily checked in order to select, among them, the most promising one to be used in further tests.
For testing the effectiveness of the “dry-powder” in affecting mycelial growth, 16 g of shrimp waste powder were homogenized with 1 L of autoclaved PDA and poured in 90 mm Petri dishes. For each pathogen, a mycelial plug (diameter 3 mm) from a 7-day-old culture grown on PDA at 25 °C was transferred in the center of a “dry-powder”—amended PDA plate; control cultures of each pathogen, obtained by subcultures in “dry-powder”—non amended PDA plates, were included in the test. The plates were incubated at room temperature (20 ± 2 °C) for three days (for fungal pathogens) or for 15 days (for oomycete pathogens). At the end of the incubation period, no negative effects were observed in mycelial growth compared with controls for any of the pathogens. The “dry-powder” was not further tested.
The effect of “EtOAc-extract”, “MetOH-extract” and “Nitric-extract” on the mycelial growth of the pathogens was tested at different concentrations. To this purpose, “EtOAc-extract” and “MetOH-extract” were separately diluted in 1% dimethyl sulfoxide to obtain, for each substance, four solutions at the following concentrations 10, 25, 50 and 100 mg/mL; “Nitric-extract” was diluted in water to obtain the following concentrations: 25, 50, 75 and 100%.
“EtOAc-extract”, “MetOH-extract”, “Nitric-extract” and each fungal pathogen were tested separately in a 90 mm PDA plate as it follows: 500 µL of a suspension of conidia of the fungal pathogen (concentration 104 conidia/mL) were homogeneously spread on the surface of a PDA plate; by using a cork borer, five wells (diameter 3 mm, each) were then realized on the PDA plate; then, 60 µL of each concentration of the substance were pipetted into the respective well; the plates were finally incubated at 25 °C for three days. For the oomycete pathogens (Phytophthora spp.), the influence of “EtOAc-extract”, “MetOH-extract” and “Nitric-extract” was tested separately as follows: for each Phytophthora strain, a mycelial plug (diameter 3 mm) from a 7-day-old culture grown on PDA at 25 °C was transferred in the center of a PDA plate and surrounded by 5 wells at a distance of 3 cm from the plug; then, 60 µL of each concentration of the substance tested were pipetted into the respective well. The plates were then incubated at 25 °C for 15 days.
In all the experiments, the possible mycelial growth inhibitory activity induced by each solvent used for the preparation of the respective extract was verified by in vitro tests performed as described above. For all pathogens and substances at each concentration, all the tests were performed in triplicate.
4.5. Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) of Nitric-Extract
The minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC) are dilution end points of a substance which completely inhibits the growth or kills the fungi tested; both are widely used in routine tests of substances with antimicrobial activity [9,81]. The minimum inhibitory concentration (MIC), defined as the lowest concentration of the test substance that inhibits visible growth, was determined with a microdilution method. For each pathogen, in a 2.0 mL tube, 400 µL of “Nitric-extract” at specific concentrations were added to 400 µL of sterile PDB and to 200 µL of spores suspension (concentration 104 spores/mL) to obtain 10 serial dilutions (1 mL each) of the substance tested (final concentrations 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0%). Then, the tubes were incubated at 25 °C for 3 days.
After the incubation period, the MIC was the lowest concentration where no cloudiness was visible in the tubes, which means that no pathogen growth was observed. The determination of MFC was an additional step of the MIC test. The MFC is defined as the lowest concentration of a substance required to kill a fungal pathogen corresponding to no visible subculture growth on an unamended culture medium in environmental conditions favorable to the growth. In the present study, the evaluation of the MFC was carried out by transferring 10 μL from each of the wells where solution cloudiness was not observed into PDA medium. The inoculated plates were incubated at 25 °C for 3 days. The MFC for each pathogen was represented by the plated concentration that did not lead to any mycelial growth after the incubation period.
4.6. Evaluating the In Vivo Antifungal Activity of Nitric-Extract in Preventing Fruit Rots
The antifungal activity of the “Nitric-extract” was evaluated in vivo against infections caused by P. digitatum and P. expansum on citrus (oranges and lemons) and apple fruits, respectively.
4.6.1. Nitric-Extract Dilutions
For the test, “Nitric-extract” was tested in all fruits (orange, lemon and apple) as such (ID02) or as three serial dilutions in sterilized distilled water (sdw) (concentrations; 75%—ID04; 50%—ID06; 25%—ID08). In addition, ID03, ID05, ID07 and ID09 were the respective controls.
In this experiment, four control groups were considered: (i) water (ID01); (ii) a solution of nitric acid (HNO3, 65%) and sodium hydroxide (NaOH, 0.1 g/mL) at the ratio 1:4—they are the solvent and base used for the preparation of “Nitric-extract”, respectively, which leads to a water solution of NaNO3 at the concentration 0.002 mol/mL (0.17 g/mL); (iii) NaNO3 0.17 g/mL diluted in sdw at 75%—ID05; (iv) NaNO3 0.17 g/mL diluted in sdw at 50%—ID07; NaNO3 0.17 g/mL diluted in sdw at 25%—ID09 (Table 4).

Table 4.
List of treatments of the in vivo tests with shrimp powder extract (Nitric-extract). Control IDs were obtained by adding sterile distilled water (sdw) to NaNO3 (0.17 g/mL).
4.6.2. Fruits
All fruits used in this test came from organic crops. Citrus fruits were mature oranges (Citrus × sinensis) cv. Valencia and lemons (Citrus × limon) cv. Femminello Siracusano, while apples (Malus domestica) were of the cv. Braeburn. Before the tests, all fruits were preliminarily surface-disinfected by dipping in 1% NaClO (NaClO 0.5% for apples) for 2 min, rinsing under tap water and air-drying at room temperature (20 ± 2 °C).
4.6.3. Fungal Pathogens and Inoculum Preparation
The strains used in the trial were P. digitatum strain P1PP0 and P. expansum strain CECT 2278. For each strain, the inoculum was represented by a conidial suspension at the concentration 106 conidia/mL.
4.6.4. Inoculation
Surface-disinfected fruits (oranges, lemons and apples) were wounded with a 2 mm-diameter plastic tip at four points along the equatorial surface; then, 10 µL of conidial suspension (P. digitatum strain P1PP0 for citrus and P. expansum strain CECT2278 for apples) was pipetted into each wound. Inoculated fruits were incubated in a plastic container at 20 °C and 80% RH (relative humidity) for 24 h. For all fruit (oranges, lemons and apples), the treatment with “Nitric-extract” as such or as a dilution was carried out as follows: after the incubation period, at each inoculation point, 20 µL of the substance was placed into the wound; overall, 3 fruits per treatment were used. An additional control group, represented by 3 fruits wounded as above, received 20 µL of sterile distilled water (sdw) per wound. The experiment was repeated another two times, with similar results. Analysis of variance did not reveal any differences among the experiments (F not significant); therefore, only the results of a single experiment are reported here.
4.6.5. Evaluation of the Efficacy of the Nitric-Extract in Preventing Fruit Rot
The antifungal activity of “Nitric-extract”, as such, or as a dilution, was recorded at 3 and 5 days after inoculation and expressed as rot severity, rated according to empirical scales, from 1 to 5. This scale was different according to the fruit. For citrus fruits, the scale 25% was as follows: 1. absence of symptoms or signs of the pathogen; 2. slight presence of rot; 3. clear presence of rot and slight appearance of mycelium; 4. rot and clear presence of white mycelium; 5. clear presence of soft rot, white mycelium and sporulation. For apple fruits the scale was as follows: 1. absence of symptoms or signs of the pathogen; 2. slight presence of rot; 3. clear presence of rot and slight appearance of mycelium; 4. presence of rot, white mycelium and slight appearance of sporulation; 5. clear presence of soft rot, white mycelium and sporulation.
All data were subjected to one-way analysis of variance (ANOVA) using the R software (https://www.r-project.org/) (accessed on 9 November 2021). In order to normalize the distributions, data were transformed in square-root values, but untransformed values are reported in the respective graphs. Tukey’s HSD (honestly significant difference) post-hoc test was applied to evidence significant statistical differences (p ≤ 0.05).
Author Contributions
Conceptualization, S.O.C., N.T., G.M. (Giuseppe Meca), G.M. (Giovanni Marletta), A.P. and A.D.; methodology, S.O.C., N.T., G.M., S.E.b., F.L.S., E.I.R. and C.L.M.; software, F.L.S. and C.L.M.; validation, S.O.C., N.T., G.M. (Giuseppe Meca), G.M. (Giovanni Marletta), A.P. and A.D.; formal analysis, N.T., S.E.b. and C.L.M.; investigation, S.E.b., E.I.R., C.L.M. and F.L.S.; resources, S.O.C., A.P., N.T., G.M. (Giuseppe Meca) and G.M. (Giovanni Marletta); data curation, S.E.b. and F.L.S.; writing—original draft preparation, S.E.b. and F.L.S.; writing—review and editing, S.O.C., N.T., G.M. (Giuseppe Meca), A.P., G.M. (Giovanni Marletta) and A.D.; visualization, S.O.C.; supervision, A.D. and S.O.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the University of Catania, Italy “Investigation of phytopathological problems of the main Sicilian productive contexts and eco-sustainable defense strategies (MED-IT-ECO)” PiaCeRi-PIAno di inCEntivi per la Ricerca di Ateneo 2020–22 linea 2’’ (5A722192155) and the project “Smart and innovative packaging, postharvest rot management and shipping of organic citrus fruit (BiOrangePack)” under Partnership for Research and Innovation in the Mediterranea Area (PRIMA)-H2020 (E69C20000130001). F.L.S. was supported by a fellowship funded by the BiOrangePack project. This study is part of E.I.R. activity of a Ph.D. student in “Agricultural, Food and Environmental Science”, University of Catania; XXXVI Cycle. E.I.R. is supported by a grant funded by the National Operative Program (PON) “Research and Innovation” 2014–2020, line “Innovative doctorates with industrial characterization”) (CCI 2014IT16M2OP005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data can be shared upon reasonable request.
Acknowledgments
We are grateful to A. Davies for the English revision of the text.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Korukluoglu, M.; Sahan, Y.; Yigit, A. Antifungal Properties of Olive Leaf Extracts and Their Phenolic Compounds. J. Food Saf. 2008, 28, 76–87. [Google Scholar] [CrossRef]
- Hernández, A.; Ruiz-Moyano, S.; Galván, A.I.; Merchán, A.V.; Pérez Nevado, F.; Aranda, E.; Serradilla, M.J.; de Guía Córdoba, M.; Martín, A. Anti-fungal activity of phenolic sweet orange peel extract for controlling fungi responsible for post-harvest fruit decay. Fungal Biol. 2021, 125, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, J.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. Antifungal activity of double Schiff bases of chitosan derivatives bearing active halogeno-benzenes. Int. J. Biol. Macromol. 2021, 179, 292–298. [Google Scholar] [CrossRef]
- Meng, D.; Garba, B.; Ren, Y.; Yao, M.; Xia, X.; Li, M.; Wang, Y. Antifungal activity of chitosan against Aspergillus ochraceus and its possible mechanisms of action. Int. J. Biol. Macromol. 2020, 158, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Abade, A.; Ferreira, P.A.; de Barros Vidal, F. Plant Diseases recognition on images using Convolutional Neural Networks: A Systematic Review. Comput. Electron. Agric. 2021, 185, 106125. [Google Scholar] [CrossRef]
- Huang, F.-C.; Molnár, P.; Schwab, W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 2009, 60, 3011–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Spada, F.; Aloi, F.; Coniglione, M.; Pane, A.; Cacciola, S.O. Natural Biostimulants Elicit Plant Immune System in an Integrated Management Strategy of the Postharvest Green Mold of Orange Fruits Incited by Penicillium digitatum. Front. Plant Sci. 2021, 12, 684722. [Google Scholar] [CrossRef]
- La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O. Trichoderma Counteracts the Challenge of Phytophthora nicotianae Infections on Tomato by Modulating Plant Defense Mechanisms and the Expression of Crinkler, Necrosis-Inducing Phytophthora Protein 1, and Cellulose-Binding Elicitor Lectin Pathogenic Effectors. Front. Plant Sci. 2020, 11, 583539. [Google Scholar] [CrossRef]
- Stracquadanio, C.; Quiles, J.M.; Meca, G.; Cacciola, S.O. Antifungal Activity of Bioactive Metabolites Produced by Trichoderma asperellum and Trichoderma atroviride in Liquid Medium. JoF 2020, 6, 263. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wu, H.; Qin, G.; Meng, X. Chitosan disrupts Penicillium expansum and controls postharvest blue mold of jujube fruit. Food Control 2014, 41, 56–62. [Google Scholar] [CrossRef]
- Yang, R.; Miao, J.; Shen, Y.; Cai, N.; Wan, C.; Zou, L.; Chen, C.; Chen, J. Antifungal effect of cinnamaldehyde, eugenol and carvacrol nanoemulsion against Penicillium digitatum and application in postharvest preservation of citrus Fruit. LWT 2021, 141, 110924. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef]
- Prameela, K.; Venkatesh, K.; Immandi, S.B.; Kasturi, A.P.K.; Rama Krishna, C.; Murali Mohan, C. Next generation nutraceutical from shrimp waste: The convergence of applications with extraction methods. Food Chem. 2017, 237, 121–132. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in shrimp processing waste utilization: An industrial prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Taser, B.; Ozkan, H.; Adiguzel, A.; Orak, T.; Baltaci, M.O.; Taskin, M. Preparation of chitosan from waste shrimp shells fermented with Paenibacillus Jamilae BAT1. Int. J. Biol. Macromol. 2021, 183, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Kandra, P.; Challa, M.M.; Kalangi Padma Jyothi, H. Efficient use of shrimp waste: Present and future trends. Appl. Microbiol. Biotechnol. 2012, 93, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, N.; Wang, Z.; Zhao, T.; Sun, J.; Mao, X. Evaluation of a clean fermentation-organic acid method for processing Sshrimp waste from six major cultivated shrimp Sspecies in China. J. Clean. Prod. 2021, 294, 126135. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, D.; George, N.; Sharma, P.; Gupta, N. A process for complete biodegradation of shrimp waste by a novel marine isolate Paenibacillus sp. AD with simultaneous production of chitinase and chitin oligosaccharides. Int. J. Biol. Macromol. 2018, 109, 263–272. [Google Scholar] [CrossRef]
- Goossens, Y.; Schmidt, T.G.; Kuntscher, M. Evaluation of Food Waste Prevention Measures—The Use of Fish Products in the Food Service Sector. Sustainability 2020, 12, 6613. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Muskhazli, M.; Nor Azwady, A.A.; Ahmad, S.A.; Adli, A.A. Three dimensional optimisation for the enhancement of astaxanthin recovery from shrimp shell wastes by Aeromonas hydrophila. Biocatal. Agric. Biotechnol. 2020, 27, 101649. [Google Scholar] [CrossRef]
- Roy, V.C.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Chun, B.-S. Recovery and bio-potentialities of astaxanthin-rich oil from shrimp (Peneanus monodon) waste and mackerel (Scomberomous niphonius) skin using concurrent supercritical CO2 extraction. J. Supercrit. Fluids 2020, 159, 104773. [Google Scholar] [CrossRef]
- Shen, Q.; Song, G.; Wang, H.; Zhang, Y.; Cui, Y.; Xie, H.; Xue, J.; Wang, H. Isolation and lipidomics characterization of fatty acids and phospholipids in shrimp waste through GC/FID and HILIC-QTrap/MS. J. Food Compos. Anal. 2021, 95, 103668. [Google Scholar] [CrossRef]
- Pattanaik, S.S.; Sawant, P.B.; Xavier, K.A.M.; Dube, K.; Srivastava, P.P.; Dhanabalan, V.; Chadha, N.K. Characterization of carotenoprotein from different shrimp shell waste for possible use as supplementary nutritive feed ingredient in animal diets. Aquaculture 2020, 515, 734594. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; Lima, M.A.B.d.; Franco, L.d.O.; Campos-Takaki, G.M.d. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Eddya, M.; Tbib, B.; EL-Hami, K. A comparison of chitosan properties after extraction from shrimp shells by diluted and concentrated acids. Heliyon 2020, 6, e03486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Y.; Zhang, Z.; Cui, W.; Zhang, X.; Wang, S. Removing copper and cadmium from water and sediment by magnetic microspheres—MnFe2O4/chitosan prepared by waste shrimp shells. J. Environ. Chem. Eng. 2021, 9, 104647. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, C.; Wang, Q.; Zhao, Z.K. Efficient hydrolysis of chitosan in ionic liquids. Carbohydr. Polym. 2009, 78, 685–689. [Google Scholar] [CrossRef]
- Sakko, M.; Moore, C.; Novak-Frazer, L.; Rautemaa, V.; Sorsa, T.; Hietala, P.; Järvinen, A.; Bowyer, P.; Tjäderhane, L.; Rautemaa, R. 2-hydroxyisocaproic acid is fungicidal for Candida and Aspergillus species. Mycoses 2014, 57, 214–221. [Google Scholar] [CrossRef]
- Honoré, A.H.; Aunsbjerg, S.D.; Ebrahimi, P.; Thorsen, M.; Benfeldt, C.; Knøchel, S.; Skov, T. Metabolic footprinting for investigation of antifungal properties of Lactobacillus paracasei. Anal. Bioanal. Chem. 2016, 408, 83–96. [Google Scholar] [CrossRef]
- Krátký, M.; Konečná, K.; Janoušek, J.; Brablíková, M.; Janďourek, O.; Trejtnar, F.; Stolaříková, J.; Vinšová, J. 4-Aminobenzoic Acid Derivatives: Converting Folate Precursor to Antimicrobial and Cytotoxic Agents. Biomolecules 2019, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, V.K.; Kim, H.R.; Hou, C.T.; Kang, S.C. Microbial conversion and in vitro and in vivo antifungal assessment of bioconverted docosahexaenoic acid (bDHA) used against agricultural plant pathogenic fungi. J. Ind. Microbiol. Biotechnol. 2009, 36, 695–704. [Google Scholar] [CrossRef]
- Kumar Patel, M.; Maurer, D.; Feygenberg, O.; Ovadia, A.; Elad, Y.; Oren-Shamir, M.; Alkan, N. Phenylalanine: A Promising Inducer of Fruit Resistance to Postharvest Pathogens. Foods 2020, 9, 646. [Google Scholar] [CrossRef]
- Belgacem, I.; Pangallo, S.; Abdelfattah, A.; Romeo, F.V.; Cacciola, S.O.; Li Destri Nicosia, M.G.; Ballistreri, G.; Schena, L. Transcriptomic Analysis of Orange Fruit Treated with Pomegranate Peel Extract (PGE). Plants 2019, 8, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a Biopolymer with Triple Action on Postharvest Decay of Fruit and Vegetables: Eliciting, Antimicrobial and Film-Forming Properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Narbad, A. Structure−Function Analysis of the Vanillin Molecule and Its Antifungal Properties. J. Agric. Food Chem. 2005, 53, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-F.; Tsou, M.-F.; Lin, J.-G.; Ho, C.-C.; Liu, J.-Y.; Chuang, J.-Y.; Chung, J.-G. Ellagic Acid Inhibits Growth and Arylamine N-Acetyltransferase Activity and Gene Expression in Staphylococcus Aureus. In Vivo 2005, 19, 195–199. [Google Scholar]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial Activity of Catechol and Pyrogallol as Allelochemicals. Zeitschrift für Naturforschung C 2006, 61, 639–642. [Google Scholar] [CrossRef]
- Beck, J.J.; Kim, J.H.; Campbell, B.C.; Chou, S.-C. Fungicidal Activities of Dihydroferulic Acid Alkyl Ester Analogues. J. Nat. Prod. 2007, 70, 779–782. [Google Scholar] [CrossRef]
- Ngarmsak, M. Antifungal Activity of Vanillin on Fresh-Cut Tropical Fruits. Acta Hortic. 2007, 409–416. [Google Scholar] [CrossRef]
- Tao, S.; Zhang, S.; Tsao, R.; Charles, M.T.; Yang, R.; Khanizadeh, S. In vitro antifungal activity and mode of action of selected polyphenolic antioxidants on Botrytis Cinerea. Arch. Phytopathol. Plant Prot. 2010, 43, 1564–1578. [Google Scholar] [CrossRef]
- Li, B.; Lai, T.; Qin, G.; Tian, S. Ambient pH Stress Inhibits Spore Germination of Penicillium expansum by Impairing Protein Synthesis and Folding: A Proteomic-Based Study. J. Proteome Res. 2010, 9, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.-H.; Lee, Y.-S.; Cho, J.-Y.; Ahn, Y.-S.; Moon, J.-H.; Hyun, H.-N.; Cha, G.-S.; Kim, K.-Y. Isolation and characterization of metabolites from Bacillus Licheniformis MH48 with antifungal activity against plant pathogens. Microb. Pathog. 2017, 110, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. p-Coumaric acid induces antioxidant capacity and defense responses of sweet cherry fruit to fungal pathogens. Postharvest Biol. Technol. 2020, 169, 111297. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.S.; Dzuvor, C.K.O.; Zhang, W.; Sant’Ana, A.S.; Lorenzo, J.M. The role of phenolic compounds against Listeria monocytogenes in food. A review. Trends Food Sci. Technol. 2021, 110, 385–392. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.-M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Seeram, N.; Adams, L.; Henning, S.; Niu, Y.; Zhang, Y.; Nair, M.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the Antioxidant Activity of Pomegranate (Punica granatum) Peel and Seed Extracts Using in Vitro Models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Vora, A.; Londhe, V.; Pandita, N. Herbosomes enhance the in vivo antioxidant activity and bioavailability of punicalagins from standardized pomegranate extract. J. Funct. Foods 2015, 12, 540–548. [Google Scholar] [CrossRef]
- Al-Zoreky, N.S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009, 134, 244–248. [Google Scholar] [CrossRef]
- Tayel, A.A.; El-Baz, A.F.; Salem, M.F.; El-Hadary, M.H. Potential applications of pomegranate peel extract for the control of citrus green mould. J. Plant Dis. Prot. 2009, 116, 252–256. [Google Scholar] [CrossRef]
- Barash, I.; Pupkin, G.; Koren, L.; Ben-Hayyim, G.; Strobel, G.A. A low molecular weight phytotoxin produced by Phoma tracheiphila, the cause of mal secco disease in citrus. Physiol. Plant Pathol. 1981, 19, 17–29. [Google Scholar] [CrossRef]
- Migheli, Q.; Cacciola, S.O.; Balmas, V.; Pane, A.; Ezra, D.; di San Lio, G.M. Mal Secco Disease Caused by Phoma tracheiphila: A Potential Threat to Lemon Production Worldwide. Plant Dis. 2009, 93, 852–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, P.; Singh, R.; Punia, R. Studies on Collection, Isolation, Purification and Maintenance of Culture of Colletotrichum gloeosporioides. Int. J. Agric. Innov. Res. 2017, 6, 351–353. [Google Scholar]
- Riolo, M.; Aloi, F.; Pane, A.; Cara, M.; Cacciola, S.O. Twig and Shoot Dieback of Citrus, a New Disease Caused by Colletotrichum Species. Cells 2021, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Mammella, M.A.; Martin, F.N.; Cacciola, S.O.; Coffey, M.D.; Faedda, R.; Schena, L. Analyses of the Population Structure in a Global Collection of Phytophthora nicotianae Isolates Inferred from Mitochondrial and Nuclear DNA Sequences. Phytopathology 2013, 103, 610–622. [Google Scholar] [CrossRef] [Green Version]
- Biasi, A.; Martin, F.N.; Cacciola, S.O.; di San Lio, G.M.; Grünwald, N.J.; Schena, L. Genetic Analysis of Phytophthora nicotianae Populations from Different Hosts Using Microsatellite Markers. Phytopathology 2016, 106, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.; Zhang, J. Post-Harvest Citrus Diseases and Their Control. Outlook Pest Manag. 2004, 15, 29–35. [Google Scholar] [CrossRef]
- Errampalli, D. Penicillium expansum (Blue Mold). In Postharvest Decay; Elsevier: Amsterdam, The Netherlands, 2014; pp. 189–231. ISBN 978-0-12-411552-1. [Google Scholar]
- Eckert, J.W. The Chemical Control of Postharvest Diseases: Deciduous Fruits, Berries, Vegetables and Root/Tuber Crops. Ann. Rev. Phytopathol. 1988, 26, 433–469. [Google Scholar] [CrossRef]
- Davé, B.; Sales, M.; Walia, M. Resistance of different strains of Penicillium digitatum to imazalil treatment in California citrus packinghouses. Proc. Fla. State Hortic. Soc. 1989, 102, 178–179. [Google Scholar]
- Kanetis, L.; Förster, H.; Adaskaveg, J.E. Comparative Efficacy of the New Postharvest Fungicides Azoxystrobin, Fludioxonil, and Pyrimethanil for Managing Citrus Green Mold. Plant Dis. 2007, 91, 1502–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Errampalli, D.; Crnko, N. Control of blue mold caused by Penicillium expansum on apples “Empire” with fludioxonil and cyprodinil. Can. J. Plant Pathol. 2004, 26, 70–75. [Google Scholar] [CrossRef]
- Conclusion regarding the peer review of the pesticide risk assessment of the active substance fluoxastrobin. EFSA Sci. Rep. 2007, 102, 1–84. [CrossRef]
- Conclusion regarding the peer review of the pesticide risk assessment of the active substance cyprodinil. EFSA Sci. Rep. 2005, 51, 1–78. [CrossRef]
- Conclusion regarding the peer review of the pesticide risk assessment of the active substance pyrimethanil. EFSA Sci. Rep. 2006, 61, 1–70. [CrossRef] [Green Version]
- Conclusion on the peer review of the pesticide risk assessment of the active substance thiabendazole. EFSA J. 2014, 12, 1–57. [CrossRef] [Green Version]
- Conclusion on the peer review of the pesticide risk Assessment of the active substance azoxystrobin. EFSA J. 2010, 8, 1–110. [CrossRef]
- Conclusion on the peer review of the pesticide risk Assessment of the active substance imazalil. EFSA J. 2010, 8, 1–110. [CrossRef] [Green Version]
- Borrás, A.D.; Aguilar, R.V. Biological control of Penicillium digitatum by Trichoderma viride on postharvest citrus fruits. Int. J. Food Microbiol. 1990, 11, 179–183. [Google Scholar] [CrossRef]
- Del Río, J.A.; Arcas, M.C.; Benavente-García, O.; Ortuño, A. Citrus polymethoxylated flavones can confer resistance against Phytophthora citrophthora, Penicillium digitatum, and Geotrichum species. J. Agric. Food Chem. 1998, 46, 4423–4428. [Google Scholar] [CrossRef]
- Holmes, G.J.; Eckert, J.W. Sensitivity of Penicillium digitatum and P. italicum to Postharvest Citrus Fungicides in California. Phytopathology 1999, 89, 716–721. [Google Scholar] [CrossRef] [Green Version]
- Youssef, K.; Ligorio, A.; Sanzani, S.M.; Nigro, F.; Ippolito, A. Control of storage diseases of citrus by pre- and postharvest application of salts. Postharvest Biol. Technol. 2012, 72, 57–63. [Google Scholar] [CrossRef]
- Youssef, K.; Sanzani, S.M.; Ligorio, A.; Ippolito, A.; Terry, L.A. Sodium carbonate and bicarbonate treatments induce resistance to postharvest green mould on citrus fruit. Postharvest Biol. Technol. 2014, 87, 61–69. [Google Scholar] [CrossRef]
- Abo-Elnaga, H.I.G. Control of green mould of citrus by using Trichoderma harzianum, humic acid or garlic. Arch. Phytopathol. Plant Prot. 2013, 46, 1118–1126. [Google Scholar] [CrossRef]
- Aloui, H.; Licciardello, F.; Khwaldia, K.; Hamdi, M.; Restuccia, C. Physical properties and antifungal activity of bioactive films containing Wickerhamomyces anomalus killer yeast and their application for preservation of oranges and control of postharvest green mold caused by Penicillium digitatum. Int. J. Food Microbiol. 2015, 200, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Fallanaj, F.; Ippolito, A.; Ligorio, A.; Garganese, F.; Zavanella, C.; Sanzani, S.M. Electrolyzed sodium bicarbonate inhibits Penicillium digitatum and induces defence responses against green mould in citrus fruit. Postharvest Biol. Technol. 2016, 115, 18–29. [Google Scholar] [CrossRef]
- Pangallo, S.; Li Destri Nicosia, M.G.; Raphael, G.; Levin, E.; Ballistreri, G.; Cacciola, S.O.; Rapisarda, P.; Droby, S.; Schena, L. Elicitation of resistance responses in grapefruit and lemon fruits treated with a pomegranate peel extract. Plant Pathol. 2017, 66, 633–640. [Google Scholar] [CrossRef]
- Riolo, M.; Aloi, F.; La Spada, F.; Sciandrello, S.; Moricca, S.; Santilli, E.; Pane, A.; Cacciola, S.O. Diversity of Phytophthora Communities across Different Types of Mediterranean Vegetation in a Nature Reserve Area. Forests 2020, 11, 853. [Google Scholar] [CrossRef]
- Aloi, F.; Riolo, M.; Parlascino, R.; Pane, A.; Cacciola, S.O. Bot Gummosis of Lemon (Citrus × Limon) Caused by Neofusicoccum parvum. JoF 2021, 7, 294. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).