Contrasting Metabolic Fingerprints and Seed Protein Profiles of Cucurbita foetidissima and C. radicans Fruits from Feral Plants Sampled in Central Mexico
Abstract
:1. Introduction
2. Results
2.1. Morphological and Proximal Composition Variables
2.2. Neutral Lipids
2.3. Saponins/Cucurbitacins
2.4. Phenolics
2.5. Seed Proteins
2.6. Non-Structural Carbohydrates (NSCs) and Raffinose Family Oligosaccharides (RFOs)
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villaseñor, J.L.; Ortiz, E. Biodiversidad de las plantas con flores (División Magnoliophyta) en México. Rev. Mex. Biodivers. 2014, 85, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Granados-Sánchez, D.; López-Ríos, G.F. Un recurso forestal de zonas áridas: Calabacilla loca (Cucurbita foetidissima H.B.K.). Rev. Chapingo Ser. Cienc. For. Y Del Ambiente 1999, 5, 35–40. [Google Scholar]
- Gómez-González, A.; Rangel-Guerrero, J.M.; Morales-Flores, F.; Aquino-Pérez, G.; Santana-Garcia, M.A.; Silos-Espino, H. Diagnóstico de poblaciones silvestres de calabacilla loca en el altiplano central de México. Rev. Mex. Cienc. Agric. 2019, 10, 1517–1528. [Google Scholar] [CrossRef] [Green Version]
- Kates, H.R.; Soltis, P.S.; Soltis, D.E. Evolutionary and domestication history of Cucurbita (pumpkin and squash) species inferred from 44 nuclear loci. Mol. Phylogenet. Evol. 2017, 111, 98–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bemis, W.P.; Whitaker, T.W. The xerophytic Cucurbita of northwestern Mexico and southwestern United States. Madroño 1969, 20, 33–41. [Google Scholar]
- Lira-Saade, R.; Eguiarte-Fruns, L.E.; Montes-Hernandez, S. Proyecto Recopilación Y Análisis De La Información Existente De Las Especies De Los Géneros Cucurbita Y Sechium Que Crecen Y/O Se Cultivan En México; CONABIO: Mexico, D.F., Mexico, 2009; p. 107. [Google Scholar]
- Ríos-Santos, E.; González-Santos, R.; Cadena-Iñiguez, J.; Mera-Ovando, L. Distribution of cultivated species and wild relatives of squash (Cucurbita L.) in México. Agroproductividad 2018, 11, 21–27. [Google Scholar]
- Castellanos-Morales, G.; Paredes-Torres, L.M.; Gámez, N.; Hernández-Rosales, H.S.; Sánchez-de la Vega, G.; Barrera-Redondo, J.; Aguirre-Planter, E.; Vázquez-Lobo, A.; Montes-Hernández, S.; Lira-Saade, R.; et al. Historical biogeography and phylogeny of Cucurbita: Insights from ancestral area reconstruction and niche evolution. Mol. Phylogenetics Evol. 2018, 128, 38–54. [Google Scholar] [CrossRef]
- Lira-Saade, R. Cucurbitaceae. In Flora Del Bajío Y De Regiones Adyacentes, 1st ed.; Rzedowski, J., Calderón de Rzedowski, G., Eds.; Ed. 92; Instituto de Ecología A.C.: Pátzcuaro, Mexico, 2001; Volume 1, pp. 1–128. [Google Scholar]
- Schrot, J.; Weng, A.; Melzi, M.F. Ribosome-inactivating and related proteins. Toxins 2015, 7, 1556–1615. [Google Scholar] [CrossRef]
- Lira-Saade, R.; Rodríguez-Arevalo, I. Informe Final Del Proyecto DS002: Catálogo De La Familia Cucurbitaceae De México; Universidad Nacional Autónoma de México: Mexico, D.F., Mexico, 2006; p. 81. [Google Scholar]
- Khoury, C.K.; Carver, D.; Kates, H.R.; Achicanoy, H.A.; van Zonneveld, M.; Thomas, E.; Heinitz, C.; Jarret, R.; Labate, J.A.; Reitsma, K.; et al. Distributions, conservation status, and abiotic stress tolerance potential of wild cucurbits (Cucurbita L.). Plants People Planet 2019, 2, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Calderon de Rzedowski, G.; Rzedowski, J. Flora Fanerogámica Del Valle De México, 2nd ed.; CONABIO: Mexico, D.F., Mexico, 2001; pp. 1–983. [Google Scholar]
- Trombulak, S.C.; Frissell, C.A. Review of ecological effects of roads on terrestrial and aquatic communities. Biol. Conserv. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Hogan, L.; Bernis, W.P. Buffalo gourd and jojoba: Potential new crops for arid lands. Adv. Agron. 1983, 36, 317–349. [Google Scholar] [CrossRef]
- Berry, J.W.; Weber, C.W.; Dreher, M.L.; Bemis, W.P. Chemical composition of buffalo gourd, a potential food source. J. Food Sci. 1976, 41, 465–466. [Google Scholar] [CrossRef]
- DeVeaux, J.S.; Shultz, E.B. Development of buffalo gourd (Cucurbita foetidissima) as a semiarid land starch and oil crop. Econ. Bot. 1985, 39, 454–472. [Google Scholar] [CrossRef]
- El Bassam, N. Handbook of Bioenergy Crops: A Complete Reference to Species, Development and Applications, 1st ed.; Routledge: England, UK, 2010; pp. 1–572. [Google Scholar]
- Bemis, W.P.; Curtis, L.D.; Weber, C.W.; Berry, J. The feral buffalo gourd, Cucurbita foetidissima. Econ. Bot. 1978, 32, 87–95. [Google Scholar] [CrossRef]
- Dittmer, H.J.; Talley, B.P. Gross morphology of tap roots of desert Cucurbits. Bot. Gaz. 1964, 125, 121–126. [Google Scholar] [CrossRef]
- Mejía-Morales, C.; Universidad de Guadalajara, Zapopan, Jalisco, México. Personal communication, 2021.
- Hernández-Centeno, F.; López-De la Peña, H.Y.; Hernández-González, M.; Rodríguez-González, C.A.; Tirado-Gallegos, J.M.; Ríos-Velasco, C.; Zamudio-Flores, P.B. Physicochemical, thermal, rheological and morphological characteristics of flour and starch from a non-conventional source: Cucurbita foetidissima Kunth roots. J. Food Meas. Charact. 2020, 14, 1976–1985. [Google Scholar] [CrossRef]
- Ba-Amer, M.A.; Bemis, W.P. Fruit and seed development in Cucurbita foetidissima. Econ. Bot. 1968, 22, 297–299. [Google Scholar] [CrossRef]
- Scheerens, J.C.; Bemis, W.P.; Dreher, M.L.; Berry, J.W. Phenotypic variation in fruit and seed characteristics of buffalo gourd. J. Am. Oil Chem. Soc. 1978, 55, 523–525. [Google Scholar] [CrossRef]
- Lancaster, M.; Storey, R.; Bower, N.W. Nutritional evaluation of buffalo gourd: Elemental analysis of seed. Econ. Bot. 1983, 37, 306. [Google Scholar] [CrossRef]
- Hernández-Centeno, F.; López De la Peña, H.Y.; Guigón-López, C.; Hernández-González, M. Biolixiviación y su impacto en el rendimiento de aceite de semillas de Cucurbita foetidissima Kunth en dos métodos de extracción. Investig. Y Cienc. De La Univ. Autónoma De Aguascalientes 2018, 75, 13–19. [Google Scholar] [CrossRef]
- Andres, T.C. Cucurbita fraterna, the closest wild relative and progenitor of C. pepo. CGC Rep. 1987, 10, 69–71. [Google Scholar]
- Merrick, L.C. Systematics and evolution of a domesticated squash, Cucurbita argyrosperma, and its wild and weedy relatives. In Biology and Utilization of the Cucurbitaceae; Bates, D.M., Robinson, R.W., Jeffrey, C., Eds.; Cornell University Press: New York, NY, USA, 1990; pp. 77–95. [Google Scholar] [CrossRef]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Balkaya, A.; Özbakir, M.; Kurtar, E.S. The phenotypic diversity and fruit characterization of winter squash (Cucurbita maxima) populations from the Black sea region of Turkey. Afr. J. Biotechnol. 2010, 9, 152–162. [Google Scholar] [CrossRef]
- Vasconcellos, J.A.; Berry, J.W.; Weber, C.W.; Bemis, W.P.; Scheerens, J.C. The properties of Cucurbita foetidissima seed oil. J. Am. Oil Chem. Soc. 1980, 57, 310–313. [Google Scholar] [CrossRef]
- Rezig, L.; Chouaibi, M.; Msaada, K.; Hamdi, S. Chemical composition and profile characterisation of pumpkin (Cucurbita maxima) seed oil. Ind. Crop. Prod. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- Habib, A.; Biswas, S.; Siddique, A.H.; Manirujjaman, M.; Uddin, B.; Hasan, S.; Uddin, M.; Islam, M.; Hasan, M.; Rahman, M.; et al. Nutritional and lipid composition analysis of pumpkin seed (Cucurbita maxima Linn.). J. Nutr. Food Sci. 2015, 5, 1–6. [Google Scholar]
- Petkova, Z.Y.; Antova, G.A. Changes in the composition of pumpkin seeds (Cucurbita moschata) during development and maturation. Grasas Aceites 2015, 66, e058. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Sharifi-Rad, J.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; et al. Cucurbita Plants: From Farm to Industry. Appl. Sci. 2019, 9, 3387. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.D. The initial domestication of Cucurbita pepo in the Americas 10,000 years ago. Science 1997, 276, 932–934. [Google Scholar] [CrossRef] [Green Version]
- Shahani, H.S.; Dollear, F.G.; Markley, K.S.; Quinby, J.R. The Buffalo gourd: A potential oil seed crop of the Southwestern drylands. J. Am. Oil Chem. Soc. 1951, 28, 90–95. [Google Scholar] [CrossRef]
- Paris, H.S. Overview of the origins and history of the five major cucurbit crops: Issues for ancient DNA analysis of archaeological specimens. Veg. Hist. Archaeobotany 2016, 25, 405–414. [Google Scholar] [CrossRef]
- Zheng, Y.-H.; Alverson, A.J.; Wang, Q.-F.; Palmer, J.D. Chloroplast phylogeny of Cucurbita: Evolution of the domesticated and wild species. J. Syst. Evol. 2013, 51, 326–334. [Google Scholar] [CrossRef]
- Wang, Y.-H. Mapping and molecular breeding of monogenic traits, In Genetics, Genomics, and Breeding of Cucurbits. Genetics, Genomics, and Breeding of Crop Plants, 1st ed.; Wang, Y.-H., Behera, T., Kole, C., Eds.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Harlan, J.R.; de Wet, J.M.J. Toward a rational classification of cultivated plants. Taxon 1971, 20, 509–517. [Google Scholar] [CrossRef]
- Lira Saade, R.; Montes Hernandez, M. Cucurbits (Cucurbita spp.), In Neglected Crops: 1492 from a Different Perspective; Series No.2; Hernandez Bermejo, J., Leon, J., Eds.; FAO Plant Production and Protection: Rome, Italy, 1994; pp. 63–77. [Google Scholar]
- Pontifes, P.A.; García-Meneses, P.M.; Gómez-Aíza, L.; Monterroso-Rivas, A.I.; Caso-Chávez, M. Land use/land cover change and extreme climatic events in the arid and semi-arid ecoregions of Mexico. Atmósfera 2018, 31, 355–372. [Google Scholar] [CrossRef] [Green Version]
- Ríos, J.L.; Escandell, J.M.; Recio, M.C. New insights into the bioactivity of cucurbitacins. Stud. Nat. Prod. Chem. 2005, 32, 429–469. [Google Scholar] [CrossRef]
- Hara, I.; Ohmiya, M.; Matsubara, H. Pumpkin (Cucurbita sp.) seed globulin III. Comparison of subunit structures among seed globulins of various Cucurbita species and characterization of peptide components. Plant Cell Physiol. 1978, 19, 237–243. [Google Scholar] [CrossRef]
- Reilly, C.C.; O’Kennedy, B.T.; Titus, J.S.; Splittstoesser, W.E. The solubilization and degradation of pumpkin seed globulin during germination. Plant Cell Physiol. 1978, 19, 1235–1246. [Google Scholar] [CrossRef]
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945–956. [Google Scholar] [CrossRef] [Green Version]
- Rezig, L.; Chibani, F.; Chouaibi, M.; Dalgalarrondo, M.; Hessini, K.; Guéguen, J.; Hamdi, S. Pumpkin (Cucurbita maxima) seed proteins: Sequential extraction processing and fraction characterization. J. Agric. Food Chem. 2013, 61, 7715–7721. [Google Scholar] [CrossRef]
- Aragon-Cuevas, F.; Sanchez de la Vega, G.; Castellanos Morales, G.; Contreras, A.; Lira Saade, R. Cucurbita radicans, calabacilla (amended version of 2019 assessment). Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T109928871A173925627.en (accessed on 8 November 2021).
- Flores-Villamil, M.Á.; Méndez-Gallegos, S.d.J.; García-Herrera, E.J.; Amante-Orozco, A.; Gómez-González, A.; Cabral-Arellano, F.J.; Vasco-Leal, J.F. Wild plants of the center-north of Mexico with potential for oil production. Rev. Mex. Cienc. Agríc. 2018, 9, 1363–1376. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Castillo, F.L.M.; Mares-Mares, E.; Del Rincon Castro, M.C.; Ordoñez Acevedo, L.G.; León-Galván, M.F. Análisis proteómico preliminar de las proteínas de reserva de la semilla de chicayota (Cucurbita argyrosperma sororia). Investig. Desarro. Cienc. Tecnol. Aliment. 2016, 1, 430–435. [Google Scholar]
- Jacks, T.J.; Hensarling, T.P.; Yatsu, L.Y. Cucurbit seeds: I. Characterizations and uses of oils and proteins. A review. Econ. Bot. 1972, 26, 135–141. [Google Scholar] [CrossRef]
- Fedko, M.; Kmiecik, D.; Siger, A.; Kulczyński, B.; Przeor, M.; Kobus-Cisowska, J. Comparative characteristics of oil composition in seeds of 31 Cucurbita varieties. J. Food Meas. Charact. 2020, 14, 894–904. [Google Scholar] [CrossRef]
- Wiesman, Z.; Chapagain, B.P. Determination of fatty acid profiles and tags in vegetable oils by MALDI-TOF/MS fingerprinting. In Lipidomics, 1st ed.; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 2009; Volume 1, pp. 315–336. [Google Scholar] [CrossRef]
- Azevedo-Meleiro, C.H.; Rodriguez-Amaya, D.B. Qualitative and quantitative differences in carotenoid composition among Cucurbita moschata, Cucurbita maxima, and Cucurbita pepo. J. Agric. Food Chem. 2007, 55, 4027–4033. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Valdez-Arjona, L.P.; Ramírez-Mella, M. Pumpkin waste as livestock feed: Impact on nutrition and animal health and on quality of meat, milk, and egg. Animals 2019, 9, 769. [Google Scholar] [CrossRef] [Green Version]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.L.; You, C.X.; Li, Y.Y.; Hao, Y.J. Advances in biosynthesis, regulation, and function of apple cuticular wax. Front. Plant Sci. 2020, 11, 1165. [Google Scholar] [CrossRef]
- Perez Gutierrez, R.M. Review of Cucurbita pepo (Pumpkin) its phytochemistry and pharmacology. Med. Chem. 2016, 6, 12–21. [Google Scholar] [CrossRef]
- Matus, Z.; Molnár, P.; Szabó, L.G. Main carotenoids in pressed seeds (cucurbitae semen) of oil pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Acta Pharm. Hung. 1993, 63, 247–256. [Google Scholar]
- Goodman, L.A.; Berry, J.W. Phospholipids of three xerophytic cucurbit seed oils. J. Am. Oil Chem. Soc. 1986, 63, 98–100. [Google Scholar] [CrossRef]
- Salama, H.M.H. Alkaloids and flavonoids from the air-dried aerial parts of Citrullus colocynthis. J. Med. Plants Res. 2012, 6, 5150–5155. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.A.; Bauer, R.; Cagiotti, M.R.; Wagner, H. Foetidissimoside A, a new 3,28-bidesmosidic triterpenoid saponin, and cucurbitacins from Cucurbita foetidissima. Phytochemistry 1988, 27, 881–885. [Google Scholar] [CrossRef]
- Gaidi, G.; Marouf, A.; Hanquet, B.; Bauer, R.; Correia, M.; Chauffert, B.; Lacaille-Dubois, M.-A. A new major triterpene saponin from the roots of Cucurbita foetidissima. J. Nat. Prod. 2000, 63, 122–124. [Google Scholar] [CrossRef]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef]
- Fa-Sheng, L.; Jing, X.; De-Qiang, D.; Xiao-Feng, C.; Ting-Guo, K.; Xue, K.H. Structures of new phenolic glycosides from the seeds of Cucurbita moschata. Nat. Prod. Commun. 2009, 4, 511–512. [Google Scholar] [CrossRef] [Green Version]
- Bubueanu, C.; Pavaloiu, R. HPTLC chromatographic polyphenolic fingerprints of plant species from Eastern Europe. Malays. J. Med. Biol. Res. 2018, 5, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 1–384. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. HPTLC analysis, antioxidant and anti-gout activity of Indian plants. Iran. J. Pharm. Res. 2014, 13, 531–539. [Google Scholar]
- González, B.; Vogel, H.; Razmilic, I.; Wolfram, E. Polyphenol, anthocyanin and antioxidant content in different parts of maqui fruits (Aristotelia chilensis) during ripening and conservation treatments after harvest. Ind. Crop. Prod. 2015, 76, 158–165. [Google Scholar] [CrossRef]
- Ramírez-Briones, E.; Rodríguez-Macías, R.; Salcedo-Pérez, E.; Ramírez-Chávez, E.; Molina-Torres, J.; Tiessen, A.; Ordaz-Ortiz, J.; Martínez-Gallardo, N.; Délano-Frier, J.P.; Zañudo-Hernández, J. Seasonal changes in the metabolic profiles and biological activity in leaves of Diospyros digyna and D. rekoi “Zapote” trees. Plants 2019, 8, 449. [Google Scholar] [CrossRef] [Green Version]
- Medić-Ŝarić, M.; Jasprica, I.; Smolčić-Bubalo, A.; Mornar, A. Optimization of chromatographic conditions in thin layer chromatography of flavonoids and phenolic acids. Croat. Chem. Acta 2004, 77, 361–366. [Google Scholar]
- Eleiwa, N.Z.H.; Bakr, R.O.; Mohamed, S.A. Phytochemical and pharmacological screening of seeds and fruits pulp of Cucurbita moschata Duchesne cultivated in Egypt. Int. J. Pharmacogn. Phytochem. 2014, 29, 1226–1236. [Google Scholar] [CrossRef]
- Irshad, M.; Ahmad, I.; Mehdi, S.J.; Goel, H.C.; Rizvi, M.M.A. Antioxidant capacity and phenolic content of the aqueous extract of commonly consumed cucurbits. Int. J. Food Prop. 2014, 17, 179–186. [Google Scholar] [CrossRef]
- Sreeramulu, D.; Raghunath, M. Antioxidant activity and phenolic content of roots, tubers and vegetables commonly consumed in India. Food Res. Int. 2010, 43, 1017–1020. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Van Camp, J.; Trawka, A.; Verhé, R. Phenolic compounds and some quality parameters of pumpkin seed oil. Eur. J. Lipid Sci. Technol. 2010, 112, 208–217. [Google Scholar] [CrossRef]
- Jessica, G.G.; Mario, G.L.; Alejandro, Z.; Cesar, A.P.J.; Ivan, J.V.E.; Ruben, R.R.; Javier, A.F. Chemical characterization of a hypoglycemic extract from Cucurbita ficifolia Bouche that induces liver glycogen accumulation in diabetic mice. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 218–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peričin, D.; Krimer, V.; Trivić, S.; Radulović, L. The distribution of phenolic acids in pumpkin’s hull-less seed, skin, oil cake meal, dehulled kernel and hull. Food Chem. 2009, 113, 450–456. [Google Scholar] [CrossRef]
- Akomolafe, S.F. Effects of roasting on the phenolic phytochemicals and antioxidant activities of pumpkin seed. Vegetos 2021, 34, 505–514. [Google Scholar] [CrossRef]
- Fang, E.F.; Wong, J.H.; Lin, P.; Ng, T.B. Biochemical characterization of the RNA-hydrolytic activity of a pumpkin 2S albumin. FEBS Lett. 2010, 584, 4089–4096. [Google Scholar] [CrossRef] [Green Version]
- Tu, G.L.; Bui, T.H.N.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Comparison of enzymatic and ultrasonic extraction of albumin from defatted pumpkin (Cucurbita pepo) seed powder. Food Technol. Biotechnol. 2015, 53, 479–487. [Google Scholar] [CrossRef] [PubMed]
- King, R.D.; Onuora, J.O. Aspects of melon seed protein characteristics. Food Chem. 1984, 14, 65–77. [Google Scholar] [CrossRef]
- Goffman, F.D.; Alonso, A.P.; Schwender, J.; Shachar-Hill, Y.; Ohlrogge, J. Light enables a very high efficiency of carbon storage in developing embryos of rapeseed. Plant Physiol. 2005, 138, 2269–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Beisson, F.; Pollard, M.; Ohlrogge, J. Oil content of Arabidopsis seeds: The influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 2006, 67, 904–915. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; Bhandari, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.; Nayyar, H. Impact of heat stress during seed filling on seed quality and seed yield in lentil (Lens culinaris Medikus) genotypes. J. Sci. Food Agric. 2018, 98, 5134–5141. [Google Scholar] [CrossRef]
- Valdivia-Cea, W.; Bustamante, L.; Jara, J.; Fischer, S.; Holzapfel, E.; Wilckens, R. Effect of soil water availability on physiological parameters, yield, and seed quality in four quinoa genotypes (Chenopodium quinoa Willd.). Agronomy 2021, 11, 1012. [Google Scholar] [CrossRef]
- Teugwa, C.M.; Boudjeko, T.; Tchinda, B.T.; Mejiato, P.C.; Zofou, D. Anti-hyperglycaemic globulins from selected Cucurbitaceae seeds used as antidiabetic medicinal plants in Africa. BMC Complement. Altern. Med. 2013, 13, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hara-Nishimura, I.; Inoue, K.; Nishimura, M. A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett. 1991, 294, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.P.; Matta, N.K. Variation studies on seed storage proteins and phylogenetics of the genus Cucumis. Plant Syst. Evol. 2008, 275, 209–218. [Google Scholar] [CrossRef]
- Singh, N.P. Studies on Seed Storage Proteins of Some Important Cucurbits. Ph.D. Thesis, Kurukshetra University, Thanesar, India, 2006. [Google Scholar]
- Singh, N.P.; Matta, N.K. Levels of seed proteins in Citrullus and Praecitrullus accessions. Plant Syst. Evol. 2010, 290, 47–56. [Google Scholar] [CrossRef]
- Horax, R.; Hettiarachchy, N.; Over, K.; Chen, P.; Gbur, E. Extraction, fractionation and characterization of bitter melon seed proteins. J. Agric. Food Chem. 2010, 58, 1892–1897. [Google Scholar] [CrossRef]
- Handley, L.W.; Pharr, D.M.; Mcfeeters, R.F. Relationship between galactinol synthase activity and sugar composition of leaves and seeds of several crop species. J. Am. Soc. Hortic. Sci. 1983, 108, 600–605. [Google Scholar]
- Handley, L.W.; Pharr, D.M.; McFeeters, R.F. Carbohydrate changes during maturation of cucumber fruit: Implications for sugar metabolism and transport. Plant Physiol. 1983, 72, 498–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botha, F.C.; Small, J.G.C. Effect of water stress on the carbohydrate metabolism of Citrullus lanatus seeds during germination. Plant Physiol. 1985, 77, 79–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Adawy, T.A.; Taha, K.M. Characteristics and composition of watermelon, pumpkin, and paprika seed oils and flours. J. Agric. Food Chem. 2001, 49, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Yoko, T.; Ryoichi, M.; Kisei, I.; Kiyoko, Y. Changes in carbohydrate composition in pumpkins [Cucurbita maxima] (Kabocha) during fruit growth. J. Jpn. Soc. Hortic. Sci. 2001, 70, 656–658. [Google Scholar] [CrossRef]
- Irving, D.E.; Hurst, P.L.; Ragg, J.S. Changes in carbohydrates and carbohydrate metabolizing enzymes during the development, maturation, and ripening of buttercup squash (Cucurbita maxima D. “Delica”). J. Am. Soc. Hortic. Sci. 1997, 122, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Henderson, C.W.; Scheerens, J.C.; Berry, J.W. Antinutritional factors in Cucurbita seed meals. J. Agric. Food Chem. 1986, 34, 434–436. [Google Scholar] [CrossRef]
- Kuo, T.M.; VanMiddlesworth, J.F.; Wolf, W.J. Content of raffinose oligosaccharides and sucrose in various plant seeds. J. Agric. Food Chem. 1988, 36, 32–36. [Google Scholar] [CrossRef]
- Schaffer, A.A.; Aloni, B.; Fogelman, E. Sucrose metabolism and accumulation in developing fruit of Cucumis. Phytochemistry 1987, 26, 1883–1887. [Google Scholar] [CrossRef]
- Mitchell, D.E.; Madore, M.A. Patterns of assimilate production and translocation in muskmelon (Cucumis melo L.): II. Low temperature effects. Plant Physiol. 1992, 99, 966–971. [Google Scholar] [CrossRef] [Green Version]
- ElSayed, A.I.; Rafudeen, M.S.; Golldack, D. Physiological aspects of raffinose family oligosaccharides in plants: Protection against abiotic stress. Plant Biol. J. 2014, 16, 1–8. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Basak, P.; Majumder, A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015, 6, 656. [Google Scholar] [CrossRef] [Green Version]
- Miao, M.; Zhang, Z. Carbohydrate metabolism of cucurbits. In Handbook of Cucurbits: Growth, Cultural Practices, and Physiology, 1st ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; Volume 1, pp. 69–91. [Google Scholar]
- Li, T.; Zhang, Y.; Wang, D.; Liu, Y.; Dirk, L.M.A.; Goodman, J.; Downie, A.B.; Wang, J.; Wang, G.; Zhao, T. Regulation of seed vigor by manipulation of raffinose family oligosaccharides in maize and Arabidopsis thaliana. Mol. Plant 2017, 10, 1540–1555. [Google Scholar] [CrossRef] [Green Version]
- Vinson, C.C.; Mota, A.P.Z.; Porto, B.N.; Oliveira, T.N.; Sampaio, I.; Lacerda, A.L.; Danchin, E.G.J.; Guimaraes, P.M.; Williams, T.C.R.; Brasileiro, A.C.M. Characterization of raffinose metabolism genes uncovers a wild Arachis galactinol synthase conferring tolerance to abiotic stresses. Sci. Rep. 2020, 10, 15258. [Google Scholar] [CrossRef]
- Andres, T.C. ybridization of Cucurbita foetidissima with C. pedatifolia C. radicans, and C. ficifolia. CGC Rep. 1987, 10, 72–73. [Google Scholar]
- Neđeral, S.; Petrović, M.; Vincek, D.; Pukec, D.; Škevin, D.; Kraljić, K.; Obranović, M. Variance of quality parameters and fatty acid composition in pumpkin seed oil during three crop seasons. Ind. Crop. Prod. 2014, 60, 15–21. [Google Scholar] [CrossRef]
- De Deyn, G.B. Plant life history and above-belowground interactions: Missing links. Oikos 2017, 126, 497–507. [Google Scholar] [CrossRef]
- Shaar-Moshe, L.; Hayouka, R.; Roessner, U.; Peleg, Z. Phenotypic and metabolic plasticity shapes life-history strategies under combinations of abiotic stresses. Plant Direct 2019, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lira, R.; Téllez, O.; Dávila, P. The effects of climate change on the geographic distribution of Mexican wild relatives of domesticated Cucurbitaceae. Genet. Resour. Crop Evol. 2009, 56, 691703. [Google Scholar] [CrossRef]
- Pautasso, M.; Döring, T.F.; Garbelotto, M.; Pellis, L.; Jeger, M.J. Impacts of climate change on plant diseases-opinions and trends. Eur. J. Plant Pathol. 2012, 133, 295–313. [Google Scholar] [CrossRef] [Green Version]
- Kates, H.R. Pumpkins, squashes, and gourds. In North American Crop Wild Relatives; Greene, S.L., Williams, K.A., Khoury, C.K., Kantar, M.B., Marek, L.F., Eds.; Springer Nature: Cham, Switzerland, 2019; Volume 2, pp. 195–224. [Google Scholar] [CrossRef]
- Kurpis, J.; Serrato-Cruz, M.A.; Feria Arroyo, T.P. Modeling the effects of climate change on the distribution of Tagetes lucida Cav. (Asteraceae). Glob. Ecol. Conserv. 2019, 20, e00747. [Google Scholar] [CrossRef]
- Luna-Luna, M.; Barretero-Hernández, R. Guía Para El Establecimiento Del Pasto Llorón (Eragrostis curvula) En Los Altos De Jalisco, Folleto Para Productores N°2; Prometeo Editores, S.A. de C.V: Jalisco, Mexico, 2013; pp. 1–16. [Google Scholar]
- AOAC: Official Methods of Analysis (Volume 1), 15th ed.; AOAC: Rockville, MD, USA, 1990; pp. 1–771.
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins—An insight into medicinal leads from nature. Pharmacogn Rev. 2015, 9, 12–18. [Google Scholar] [PubMed] [Green Version]
- Jesionek, W.; Móricz, Á.M.; Ott, P.G.; Kocsis, B.; Horváth, G.; Choma, I.M. TLC-Direct Bioautography and LC/MS as complementary methods in identification of antibacterial agents in plant tinctures from the Asteraceae family. J. AOAC Int. 2015, 98, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ramírez-Pimentel, J.G.; Herrera-Herrera, A.; Aguirre-Mancilla, C.L.; Covarrubias-Prieto, J.; Iturriaga de la Fuente, G.; Raya-Pérez, J.C. Caracterización de las proteínas de reserva y contenido mineral de semilla de melón (Cucumis melo L.). Rev. Mex. Cienc. Agríc. 2016, 7, 1667–1678. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; MacKown, C.T.; Starks, P.J.; Kindiger, B.K. Rapid analysis of nonstructural carbohydrate components in grass forage using microplate enzymatic assays. Crop Sci. 2010, 50, 1537–1545. [Google Scholar] [CrossRef] [Green Version]
- Mellado-Mojica, E.; González de la Vara, L.E.; López, M.G. Fructan active enzymes (FAZY) activities and biosynthesis of fructooligosaccharides in the vacuoles of Agave tequilana Weber Blue variety plants of different age. Planta 2017, 245, 265–281. [Google Scholar] [CrossRef]
- Lay, J.; Liyanage, R.; Durham, B.; Brooks, J. Rapid characterization of edible oils by direct matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis using triacylglycerols. Rapid Commun. Mass Spectrom. 2006, 20, 952–958. [Google Scholar] [CrossRef]
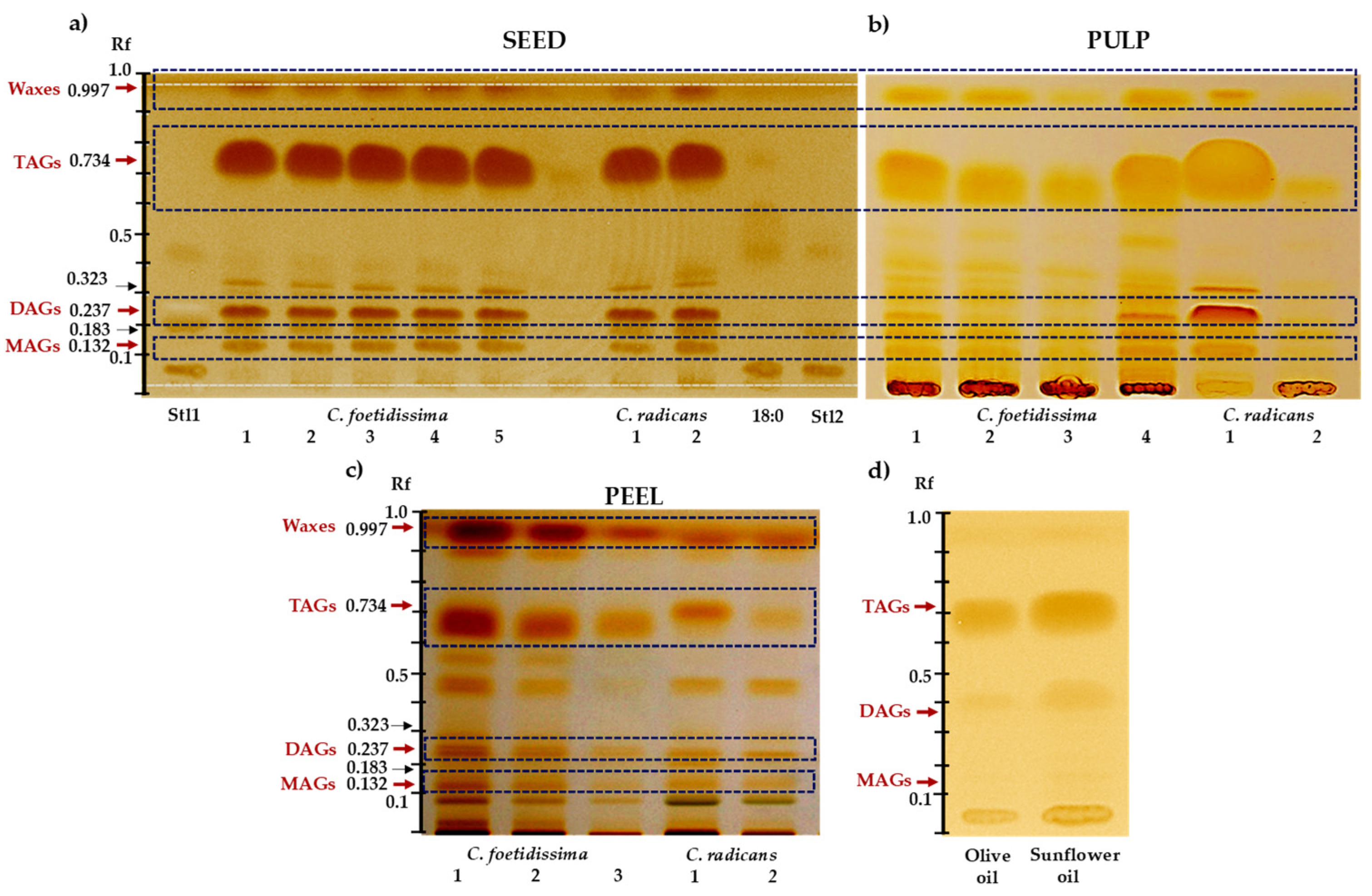
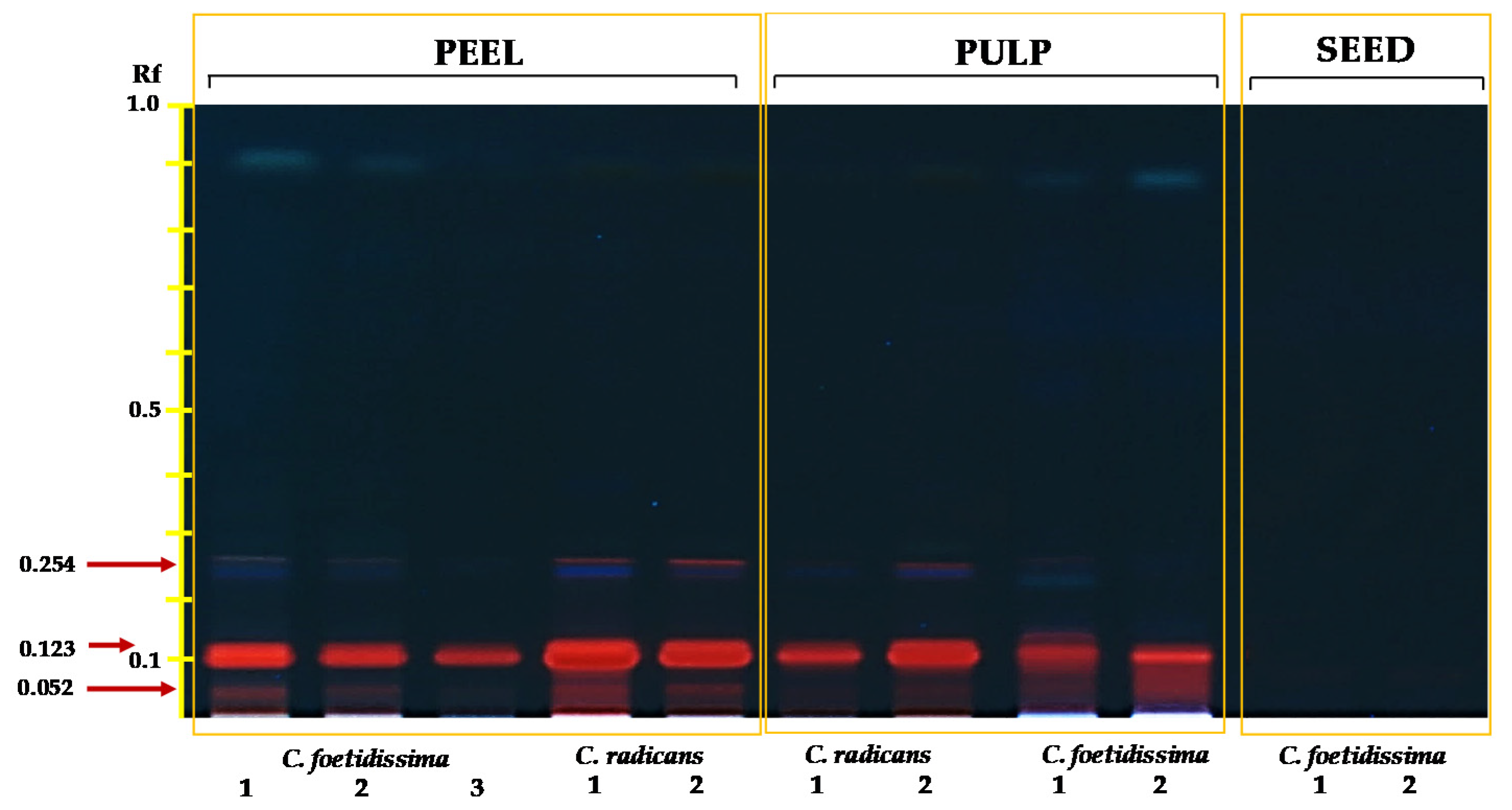

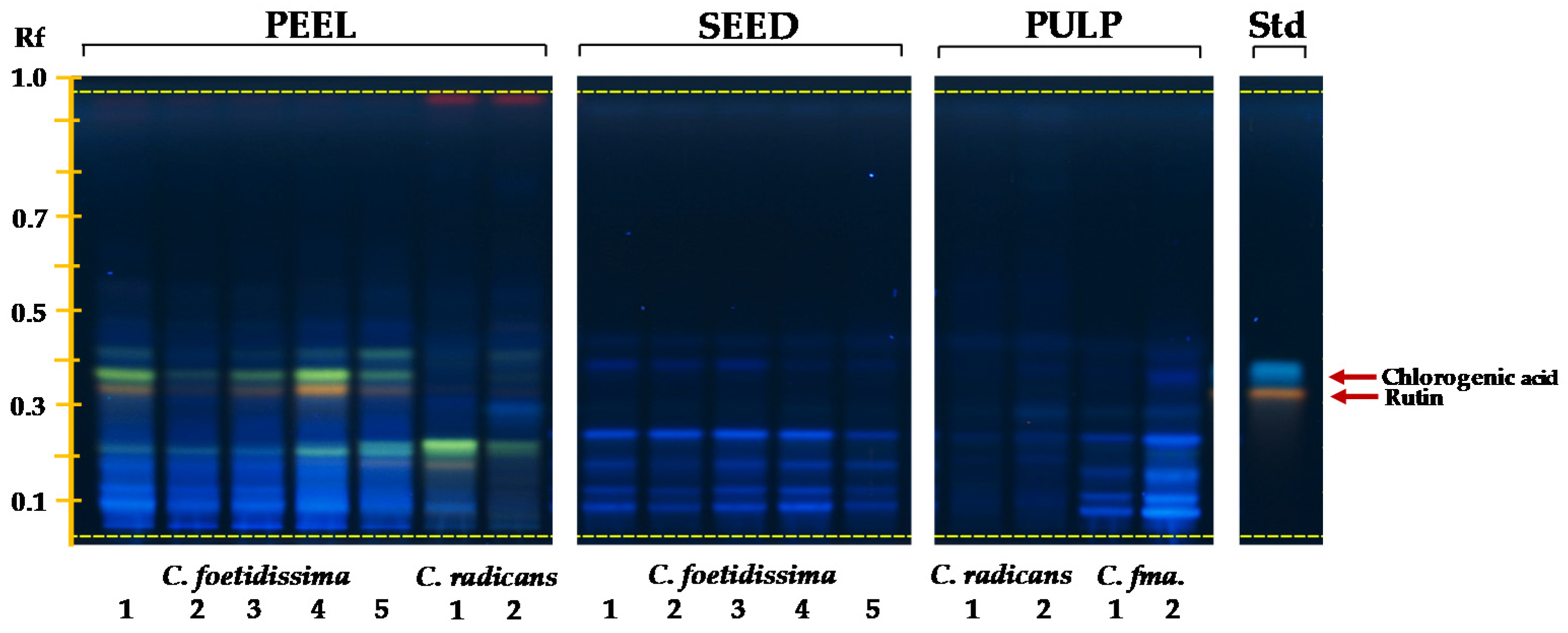
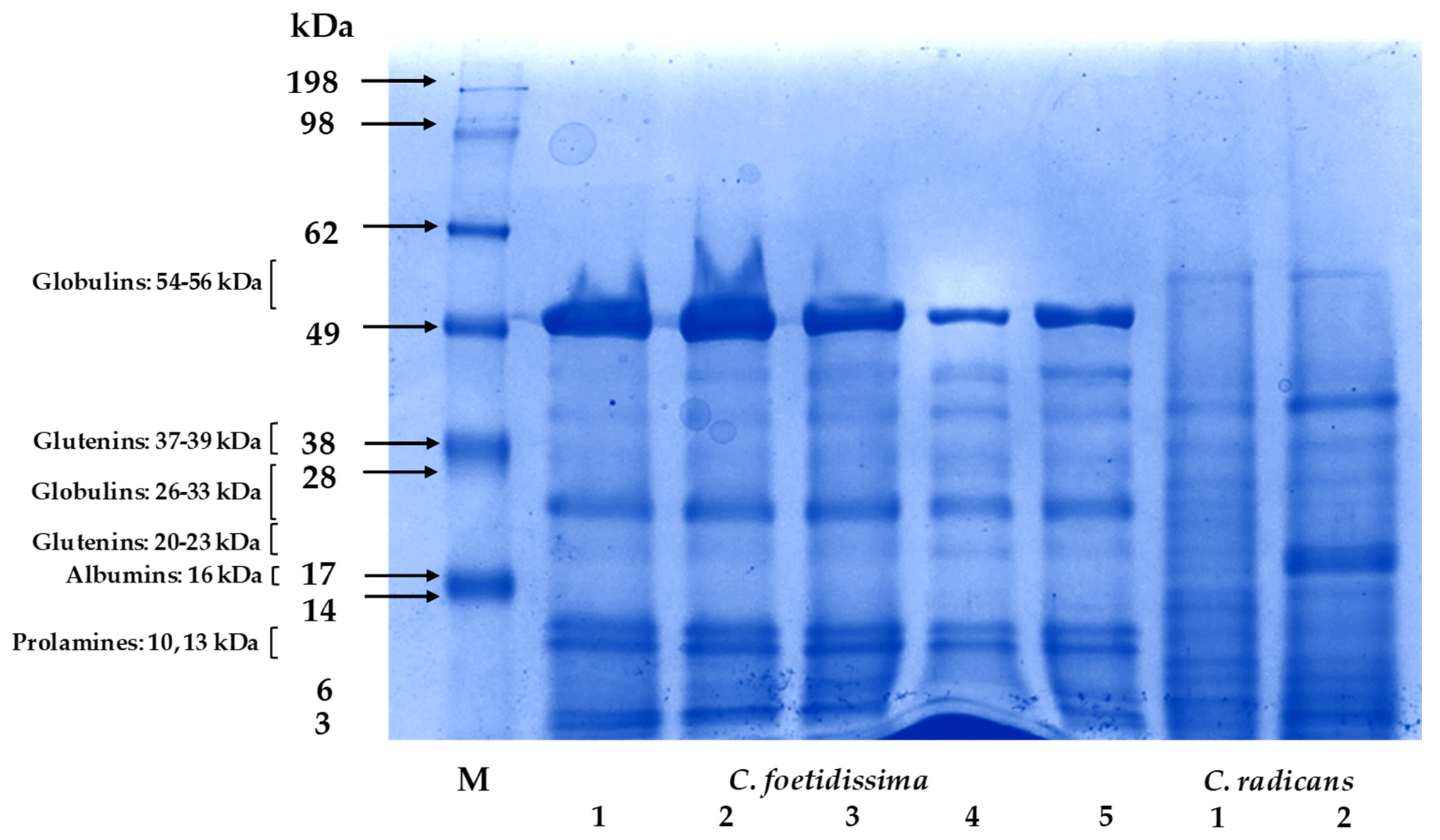
| FFW 3 | ED | LD | SW | TSN | W100S | PuFW | PeFW | |
|---|---|---|---|---|---|---|---|---|
| C. radicans1 | 90.3 ± 19.8 | 6.4 ± 0.4 | 5.5 ± 0.3 | 7.0 ± 2.2 | 93 ± 30.9 | 7.0 ± 1.8 * | 55.6 ± 10.2 | 23.9 ± 2.9 |
| C. foetidissima1 | 148.8 ± 21.4 *,2 | 6.7 ± 0.3 | 6.7 ± 0.2 * | 16.5 ± 1.1 * | 280 ± 18.5 * | 6.1 ± 0.9 | 80.2 ± 12.3 * | 35.1 ± 9.5 * |
| PEEL | SEED | PULP | ||||
|---|---|---|---|---|---|---|
| % | C. radicans1 | C. foetidissima1 | C. radicans | C. foetidissima | C. radicans | C. foetidissima |
| Dry Matter | 95.3 ± 3.1 | 95.6 ± 3.8 | 95.9 ± 2.8 | 96.6 ± 2.2 | 91.4 ± 4.7 | 93.2 ± 5.1 |
| Ash (minerals) | 6.1 ± 1.0 | 7.0 ± 0.8 | 5.2 ± 0.7 | 4.4 ± 0.7 | 16.1 ± 0.6 *,4 | 15.9 ± 1.0 |
| Crude Protein | 9.8 ± 4.0 | 6.6 ± 1.3 | 33.1 ± 3.0 * | 30.6 ± 2.9 | 18.2 ± 2.4 | 13.4 ± 4.3 |
| Total Lipids | 2.17 ± 2.0 | 2.5 ± 2.5 | 28.7 ± 4.1 | 32.3 ± 1.3 * | 3.3 ± 4.3 | 0.89 ± 0.5 |
| Total Fiber | 42.1 ± 3.8 | 48.4 ± 5.5 * | 21.8 ± 1.9 | 26.5 ± 1.8 | 19.7 ± 3.9 | 19.0 ± 1.7 |
| N-free Extract 2 | 34.2 ± 10.8 * | 31.0 ± 3.2 | 8.6 ± 6.0 | 4.1 ± 4.7 | 34.0 ± 10.8 | 44.1 ± 2.9 * |
| ADF 3 | 51.9 ± 3.8 | 55.9 ± 8.1 * | 36.6 ± 4.8 | 38.3 ± 5.4 * | 29.0 ± 6.0 | 29.9 ± 4.3 |
| Lignin | 16.5 ± 2.3 | 18.7 ± 4.2 * | 15.3 ± 2.0 | 17.8 ± 5.3 * | 4.5 ± 3.8 | 2.6 ± 0.7 |
| Cellulose 2 | 35.4 ± 2.0 | 37.2 ± 4.1 * | 21.3 ± 3.3 | 20.2 ± 1.1 | 24.5 ± 3.6 | 27.3 ± 4.3 |
| Standards (m/z) | ID | C. radicans1 | ID | C. foetidissima1 | ID |
|---|---|---|---|---|---|
| 550.673 | Matrix peak? | ||||
| 551.251 | NI | ||||
| 567.247 | NI 2 | ||||
| 599.827 | PO (DAG) 3 | 599.84 | PO (DAG) | ||
| 601.6 | PS | ||||
| 638.671 | LaLaLa | ||||
| 657.841 | NI | ||||
| 661.611 | LaLaLa (Na+) | ||||
| 696.713 | |||||
| 745.708 | MMM (Na+) | ||||
| 759.067 | NI | ||||
| 761.681 | MMM (K+) | ||||
| 783.097 | NI | ||||
| 805.096 | NI | ||||
| 829.8 | PPP (Na+) | ||||
| 878.341 | PLL (TAG) | 878.353 | PLL | ||
| 880.348 | POL | 880.368 | POL | ||
| 892.243 | NI | ||||
| 894.325 | NI | 894.339 | NI | ||
| 895.756 | LnLnLn (Na+) | 896.323 | LnLnLn | ||
| 901.806 | LLL (Na+) | 902.374 | LLL | 902.374 | LLL |
| 904.382 | LLO | 904.394 | LLO | ||
| 906.394 | OOL | 906.42 | OOL | ||
| 907.855 | OOO (Na+) | ||||
| 908.43 | OOS | ||||
| 911.755 | SOS-SSO (Na+) | ||||
| 913.897 | SSS (Na+) | ||||
| 916.35 | NI | ||||
| 917.794 | LLL (K+) | ||||
| 918.36 | NI | 918.317 | NI | ||
| 920.367 | NI | 920.38 | NI | ||
| 922.373 | NI | 922.4 | NI | ||
| 934.36 | NI | ||||
| 943.742 | NI |
| Cucurbitacin Glycosides | Rf Value 2 Range | Non-Glycosylated Cucurbitacins | Rf Value Range |
|---|---|---|---|
| Cucurbitacin-L-glucoside Cucurbitacin-I-glucoside | 0.14–0.18 | Cucurbitacin D Cucurbitacin C Cucurbitacin A Cucurbitacin L | 0.58–0.69 |
| Cucurbitacin-B-glucoside Cucurbitacin-E-glucoside 23, 24 dihydrocucurbitacin E-glucoside | 0.26–0.30 | ||
| Cucurbitacin I Cucurbitacin B 23, 24-dihydrocucurbitacin B 23, 24-dihydrocucurbitacin E Cucurbitacin E | 0.79–0.95 |
| SEED | PULP | PEEL | ||||
|---|---|---|---|---|---|---|
| mg/g (FWB) 2 | C. foetidissima1 | C. radicans | C. foetidissima | C. radicans | C. foetidissima | C. radicans |
| Glucose | 1.97 ± 0.01 **,3 | 1.4 ± 0.02 | 20.7 ± 0.2 ** | 17.8 ± 0.2 | 19.6 ± 0.2 ** | 7.18 ± 0.1 |
| Fructose | 1.98 ± 0.03 ** | 1.32 ± 0.03 | 12.8 ± 0.08 | 14.9 ± 0.04 ** | 12.4 ± 0.2 ** | 10.1 ± 0.13 |
| Sucrose | 8.28 ± 0.08 | 8.04 ± 0.08 | 5.16 ± 0.2 ** | 2.63 ± 0.08 | 2.97 ± 0.05 | 3.11 ± 0.07 |
| Starch | 0.33 ± 0.04 * | 0.14 ± 0.02 | 5.57 ± 0.13 ** | 4.29 ± 0.2 | 3.12 ± 0.14 ** | 0.58 ± 0.05 |
| SEED | PULP | PEEL | ||||
|---|---|---|---|---|---|---|
| mg/g (DWB) 2 | C. foetidissima1 | C. radicans | C. foetidissima | C. radicans | C. foetidissima | C. radicans |
| Myo-inositol | 1.02 ± 0.01 | 1.31 ± 0.04 ** | 3.52 ± 0.16 | 4.48 ± 0.48 | 2.38 ± 0.02 | 5.46 ± 0.08 ** |
| Galactinol | 0.84 ± 0.02 * | 0.67 ± 0.02 | 2.99 ± 0.31 | 2.41 ± 0.39 | 3.36 ± 0.15 | 3.17 ± 0.12 |
| NI-RFO 4 | 0.40 ± 0.02 | 0.43 ± 0.04 | 1.93 ± 0.27 * | 0.90 ± 0.18 | 1.21 ± 0.09 | 1.18 ± 0.03 |
| Raffinose | 1.85 ± 0.06 *,3 | 1.54 ± 0.05 | 0.10 ± 0.02 | 0.06 ± 0.01 | 0.35 ± 0.01 ** | 0.11 ± 0.01 |
| Staquiose | 4.73 ± 0.1 * | 3.74 ± 0.21 | 0.62 ± 0.07 | 0.75 ± 0.1 | 0.19 ± 0.01 | 0.55 ± 0.08 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía-Morales, C.; Rodríguez-Macías, R.; Salcedo-Pérez, E.; Zamora-Natera, J.F.; Rodríguez-Zaragoza, F.A.; Molina-Torres, J.; Délano-Frier, J.P.; Zañudo-Hernández, J. Contrasting Metabolic Fingerprints and Seed Protein Profiles of Cucurbita foetidissima and C. radicans Fruits from Feral Plants Sampled in Central Mexico. Plants 2021, 10, 2451. https://doi.org/10.3390/plants10112451
Mejía-Morales C, Rodríguez-Macías R, Salcedo-Pérez E, Zamora-Natera JF, Rodríguez-Zaragoza FA, Molina-Torres J, Délano-Frier JP, Zañudo-Hernández J. Contrasting Metabolic Fingerprints and Seed Protein Profiles of Cucurbita foetidissima and C. radicans Fruits from Feral Plants Sampled in Central Mexico. Plants. 2021; 10(11):2451. https://doi.org/10.3390/plants10112451
Chicago/Turabian StyleMejía-Morales, Claudia, Ramón Rodríguez-Macías, Eduardo Salcedo-Pérez, Juan Francisco Zamora-Natera, Fabián Alejandro Rodríguez-Zaragoza, Jorge Molina-Torres, John Paul Délano-Frier, and Julia Zañudo-Hernández. 2021. "Contrasting Metabolic Fingerprints and Seed Protein Profiles of Cucurbita foetidissima and C. radicans Fruits from Feral Plants Sampled in Central Mexico" Plants 10, no. 11: 2451. https://doi.org/10.3390/plants10112451
APA StyleMejía-Morales, C., Rodríguez-Macías, R., Salcedo-Pérez, E., Zamora-Natera, J. F., Rodríguez-Zaragoza, F. A., Molina-Torres, J., Délano-Frier, J. P., & Zañudo-Hernández, J. (2021). Contrasting Metabolic Fingerprints and Seed Protein Profiles of Cucurbita foetidissima and C. radicans Fruits from Feral Plants Sampled in Central Mexico. Plants, 10(11), 2451. https://doi.org/10.3390/plants10112451







