Effect of Two Kinds of Fertilizers on Growth and Rhizosphere Soil Properties of Bayberry with Decline Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Measurement of Vegetative Growth Parameters
2.3. Measurement of Fruit Economic Characters
2.4. Soil Sample Collection and Physical and Chemical Property Measurements
2.5. Soil Genome Sequencing
2.6. Gas Chromatography-Mass Spectrometry (GC-MS) Metabolomics Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. The Effects of Compound/Bio-Organic Fertilizer on Vegetative Growth and Fruit Quality
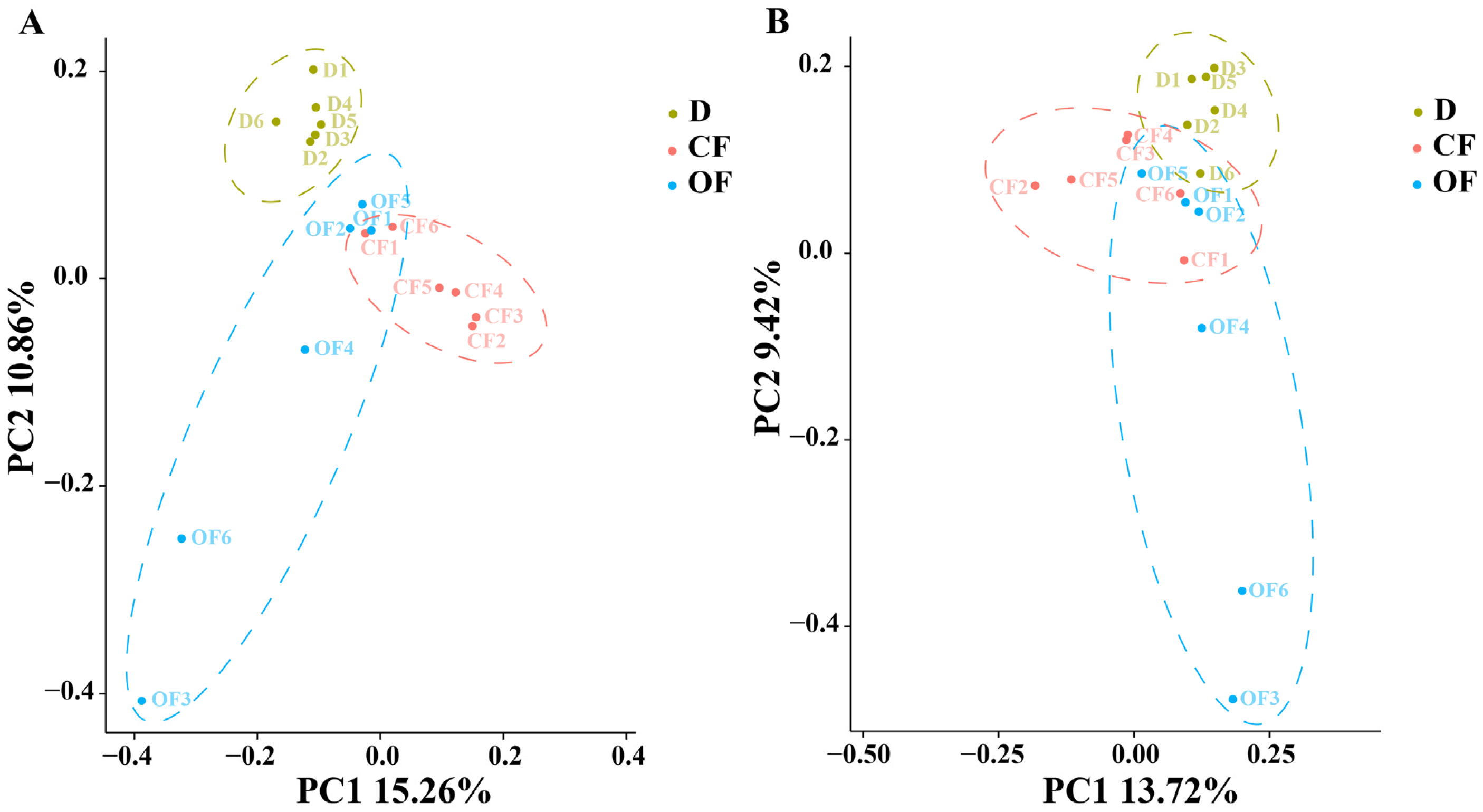
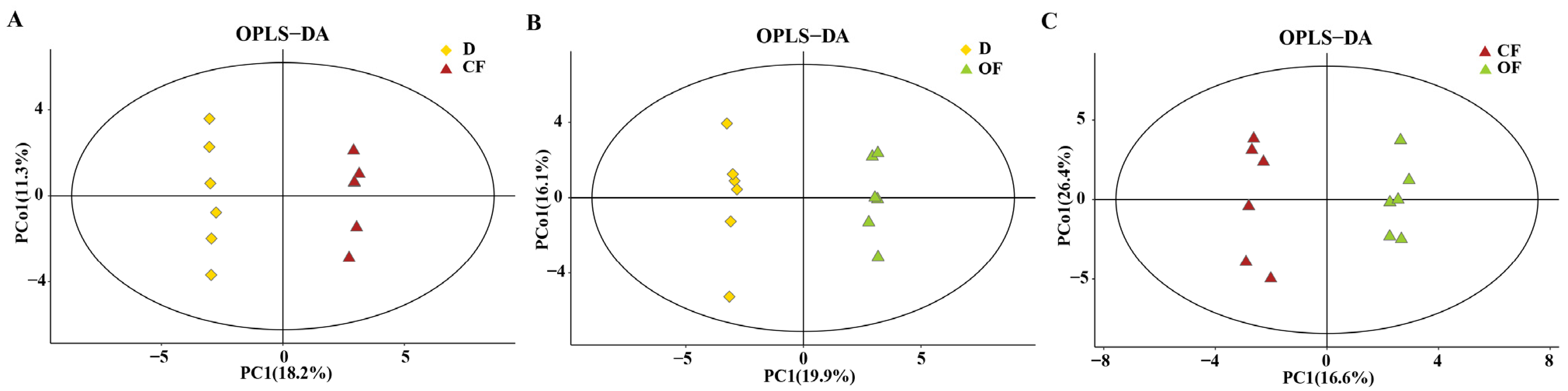
3.2. The Effect of Compound/Bio-Organic Fertilizer in Microbial Community Diversity
3.3. The Effect of Compound/Bio-Organic Fertilizer in Soil Microbial Community Structure
3.4. The Effect of Compound/Bio-Organic Fertilizer on the Soil Nutrient Status
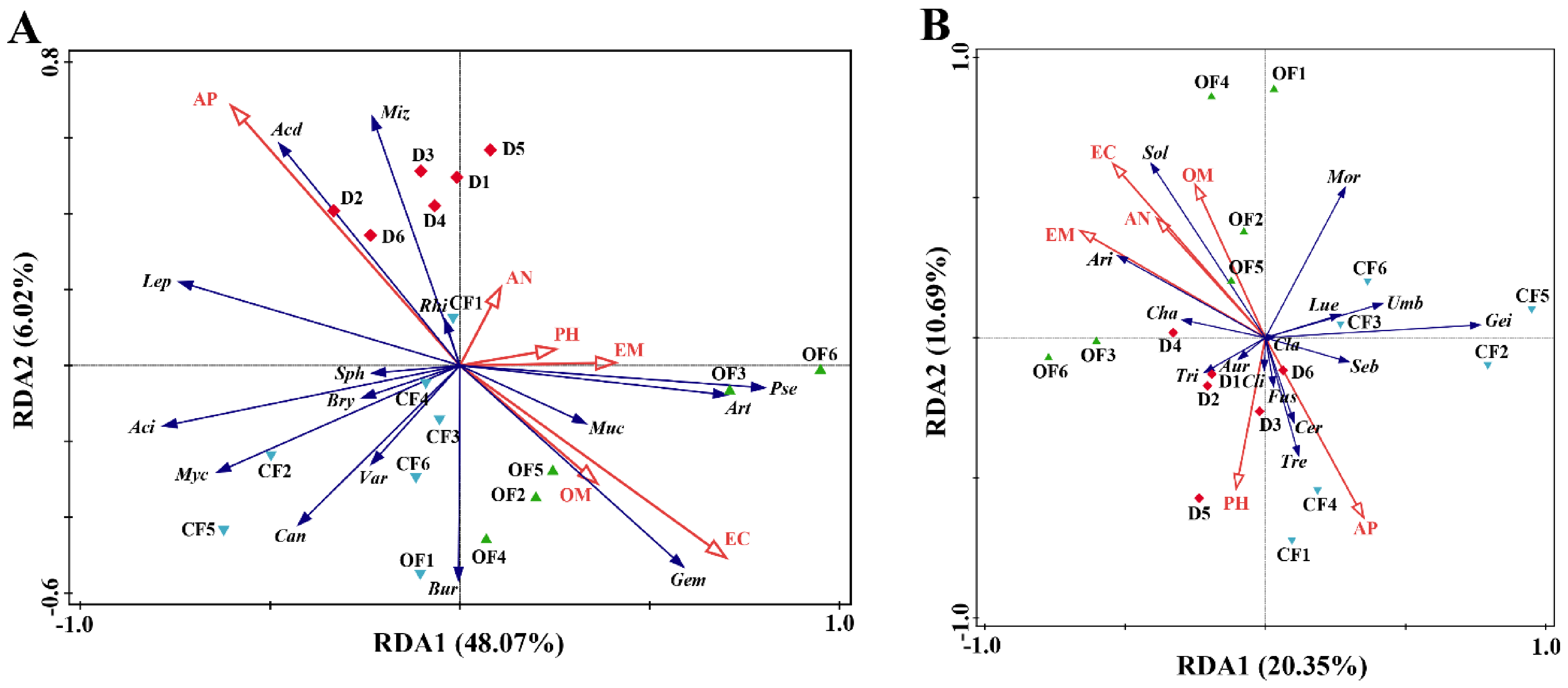
3.5. The Effect of Compound/Bio-Organic Fertilizer on RDA of Soil Properties and Microbial Communities
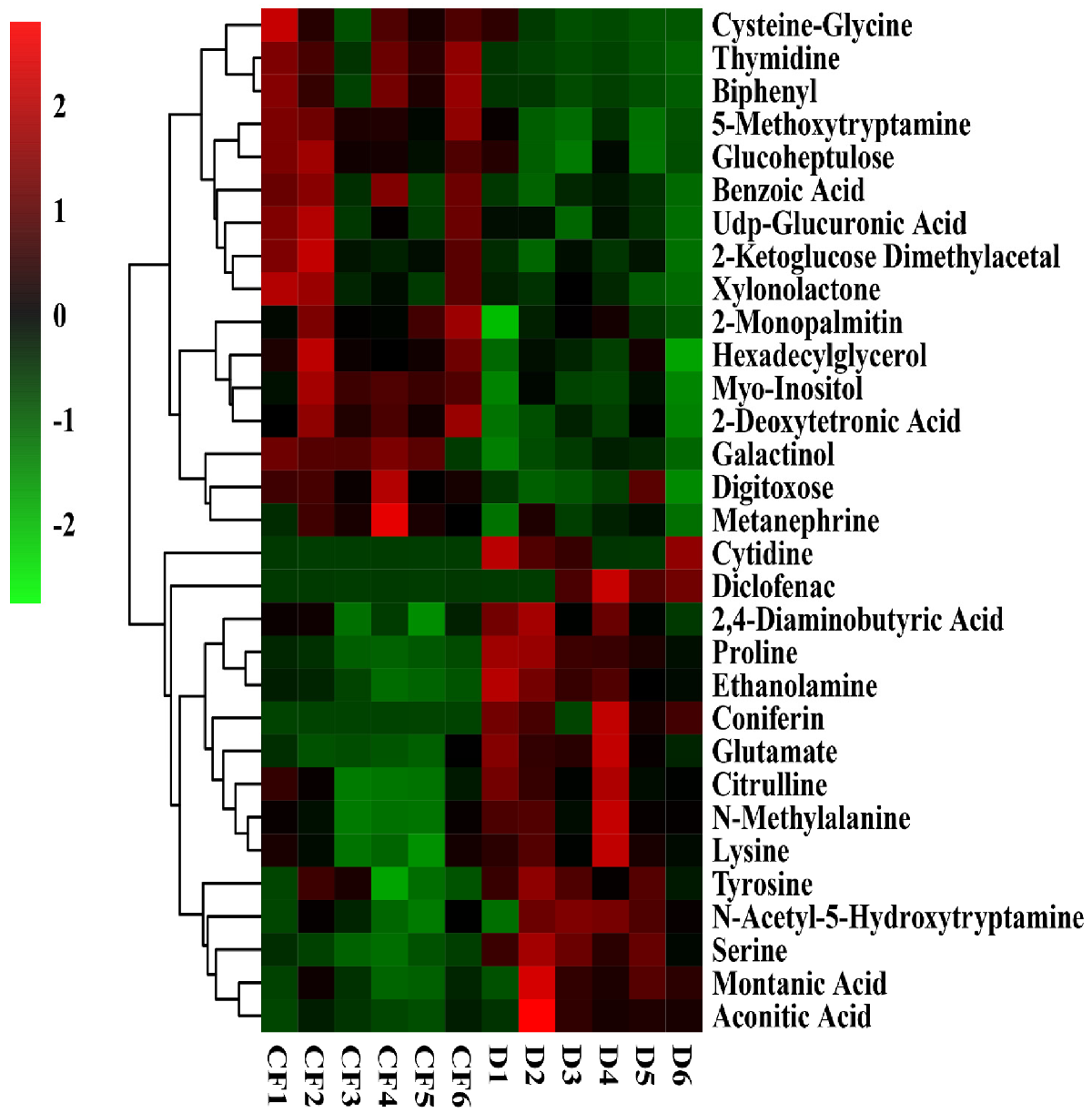
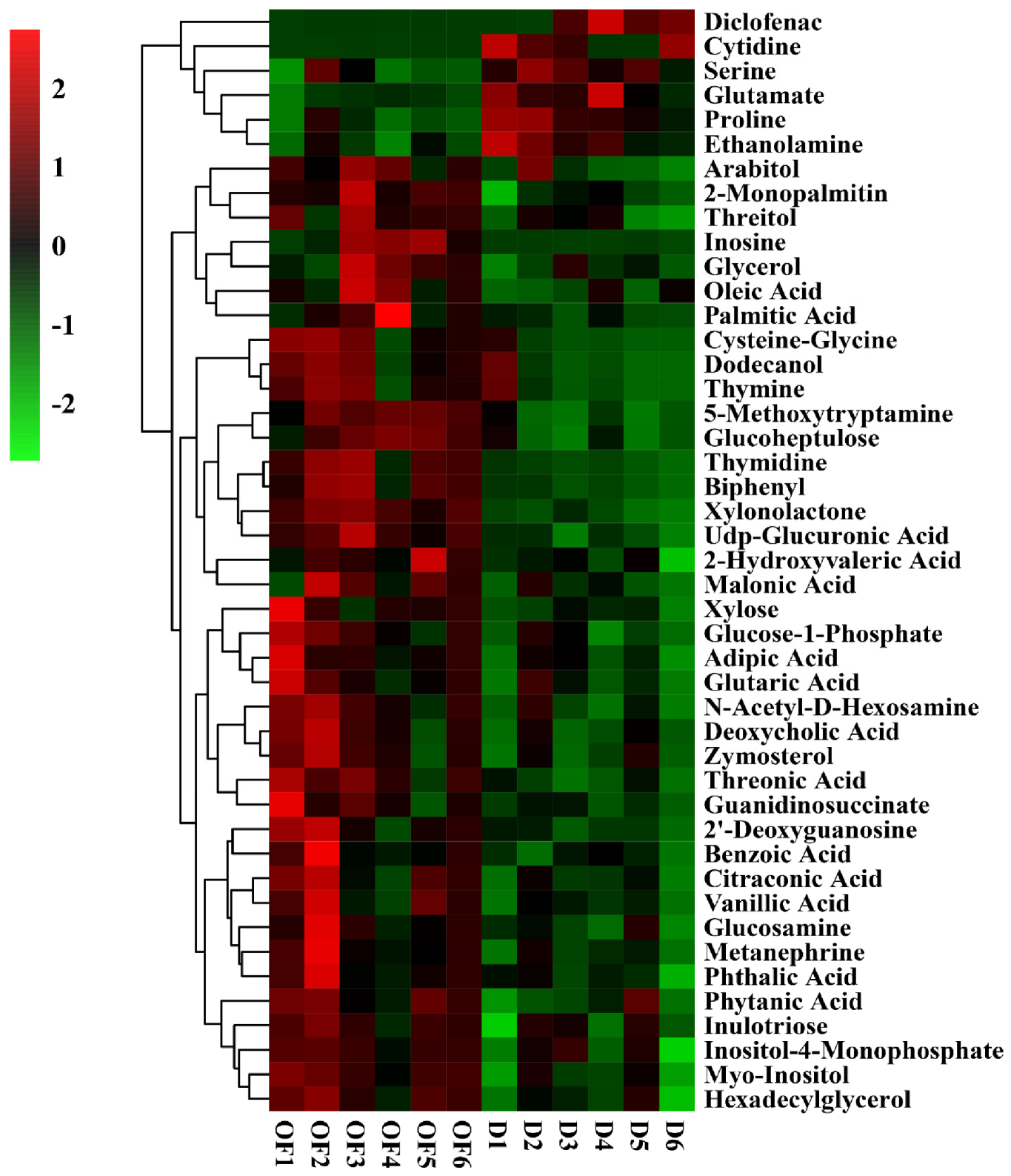
3.6. The Effect of Compound/Bio-Organic Fertilizer on Rhizosphere Soil Metabolomics
3.7. The Effect of Compound/Bio-Organic Fertilizer on the Metabolic Pathways
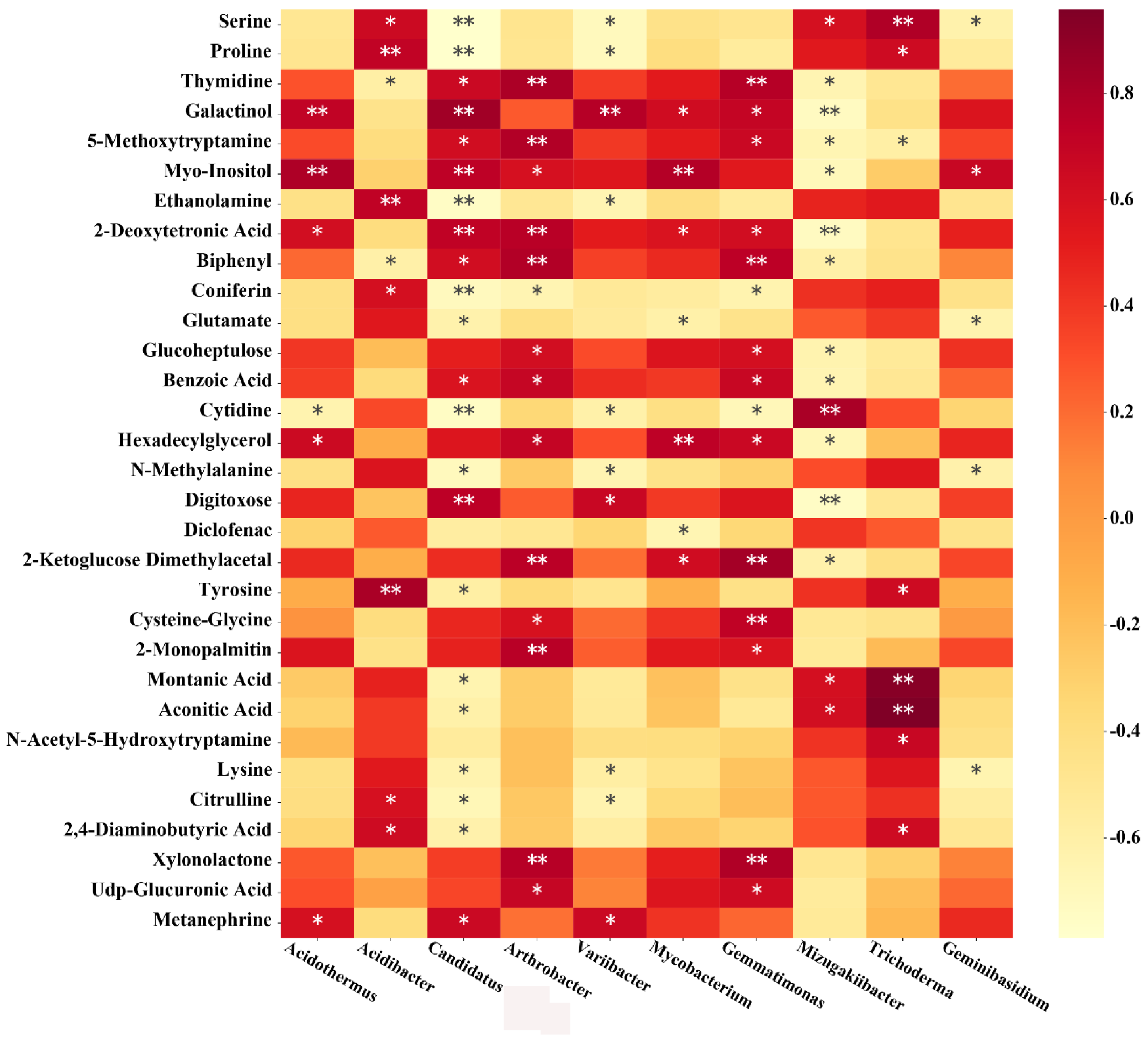
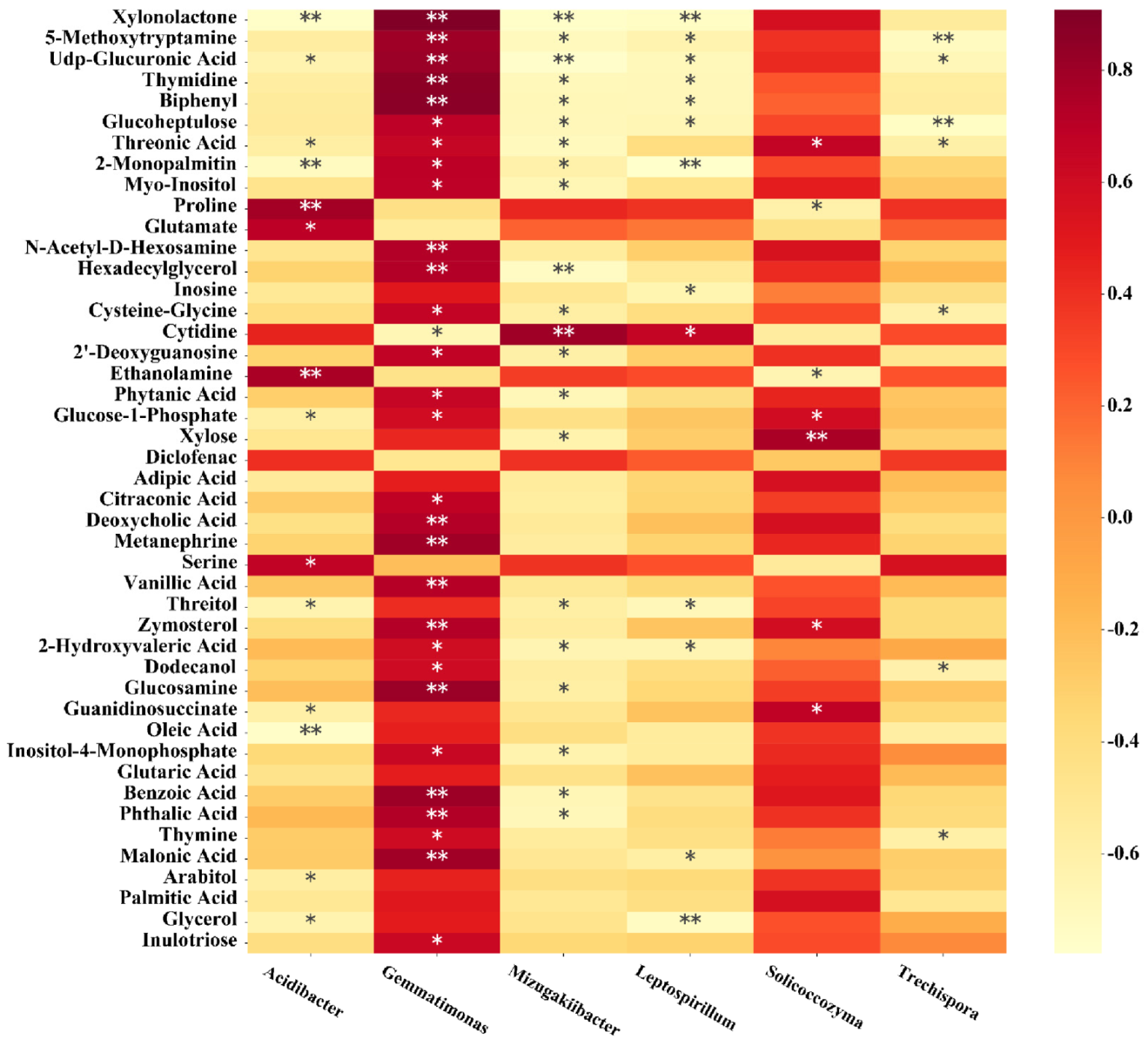
3.8. The Correlation of Soil Microorganisms with Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, H.Y.; Yu, H.Y.; Zhang, S.W.; Liang, S.M.; Zheng, X.L.; Zhang, S.J.; Yao, P.; Zheng, H.K.; Qi, X.J. Genome sequencing provides insights into the evolution and antioxidant activity of Chinese bayberry. BMC Genom. 2019, 20, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Huang, H.Z.; Xu, C.J.; Li, X.; Chen, K.S. Biological activities of extracts from Chinese bayberry (Myrica rubra Sieb. et Zucc.): A review. Plant Foods Hum. Nutr. 2013, 68, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.Y.; Wang, H.Y.; Qi, X.J.; Yu, Z.P.; Zheng, X.L.; Zhang, S.W.; Wang, Z.S.; Zhang, M.C.; Ahmed, T.; Li, B. The damage caused by decline disease in bayberry plants through changes in soil properties, rhizosphere microbial community structure and metabolites. Plants 2021, 10, 2083. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.Y.; Wu, C.W.; Liang, S.M.; Wang, K.Q.; Zhang, S.W.; Zheng, X.L.; Qi, X.J. The symptoms and nutritional status of decline diseased bayberry. Zhejiang Agric. Sci. 2020, 61, 93–98. (In Chinese) [Google Scholar]
- Blanco-Canqui, H.; Schlegel, A.J. Implications of inorganic fertilizer application of irrigated corn on soil properties: Lessons learned after 50 years. J. Environ. Qual. 2013, 42, 861–871. [Google Scholar] [CrossRef]
- Shang, L.R.; Wan, L.Q.; Zhou, X.X.; Li, S.; Li, X.L. Effects of organic fertilizer on soil nutrient status, enzyme activity, and bacterial community diversity in Leymus chinensis steppe in Inner Mongolia, China. PLoS ONE 2020, 15, e0240559. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.Q.; Zhang, P.Y.; Ye, J.P.; Liang, J.S.; Nabi, M.; Zhou, Z.Y.; Tao, X.; Chen, N.; Sun, K.; et al. Biological nutrient removal and recovery from solid and liquid livestock manure: Recent advance and perspective. Bioresour. Technol. 2020, 301, 122823. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Yang, F.; Yaoyao, E.; Raza, W.; Huang, Q.; Shen, Q. Application of bioorganic fertilizer significantly increased apple yields and shaped bacterial community structure in orchard soil. Microb. Ecol. 2017, 73, 404–416. [Google Scholar] [CrossRef]
- Li, C.; Yan, K.; Tang, L.; Jia, Z.; Li, Y. Change in deep soil microbial communities due to long-term fertilization. Soil Biol. Biochem. 2014, 75, 264–272. [Google Scholar] [CrossRef]
- Yu, W.; Guibing, Z.; Liyan, S.; Shanyun, W.; Chengqing, Y. Manure fertilization alters the population of ammonia-oxidizing bacteria rather than ammonia-oxidizing archaea in a paddy soil. J. Basic Microbiol. 2014, 54, 190–197. [Google Scholar]
- Wang, Z.T.; Geng, Y.B.; Liang, T. Optimization of reduced chemical fertilizer use in tea gardens based on the assessment of related environmental and economic benefits. Sci. Total Environ. 2020, 713, 136439. [Google Scholar] [CrossRef]
- Wu, L.N.; Jiang, Y.; Zhao, F.Y.; He, X.F.; Liu, H.F.; Yu, K. Increased organic fertilizer application and reduced chemical fertilizer application affect the soil properties and bacterial communities of grape rhizosphere soil. Sci. Rep. 2020, 10, 9568. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, D.Y.; Ling, N.; Chen, F.D.; Fang, W.M.; Shen, Q.R. Bio-organic fertilizer application significantly reduces the Fusarium oxysporum population and alters the composition of fungi communities of watermelon Fusarium wilt rhizosphere soil. Biol. Fert. Soils 2014, 50, 765–774. [Google Scholar] [CrossRef]
- Zhang, H.; Hua, Z.W.; Liang, W.Z.; Niu, Q.H.; Wang, X. The prevention of bio-organic fertilizer fermented from cow manure compost by Bacillus sp. XG-1 on watermelon continuous cropping barrier. Int. J. Environ. Res. Public Health 2020, 17, 5714. [Google Scholar] [CrossRef]
- Huang, N.; Wang, W.; Yao, Y.; Zhu, F.; Wang, W.; Chang, X. The influence of different concentrations of bio-organic fertilizer on cucumber Fusarium wilt and soil microflora alterations. PLoS ONE 2017, 12, e0171490. [Google Scholar] [CrossRef]
- Tao, C.Y.; Li, R.; Xiong, W.; Shen, Z.Z.; Liu, S.S.; Wang, B.B.; Ruan, Y.Z.; Geisen, S.; Shen, Q.R.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef]
- Tang, H.M.; Xiao, X.P.; Xu, Y.L.; Li, C.; Cheng, K.K.; Pan, X.C.; Li, W. Utilization of carbon sources in the rice rhizosphere and nonrhizosphere soils with different long-term fertilization management. J. Basic Microbiol. 2019, 59, 621–631. [Google Scholar] [CrossRef]
- Lucie, M.; Jakub, R.; Marketa, P.; Tomas, M.; Ondrej, U. Effects of secondary plant metabolites on microbial populations: Changes in community structure and metabolic activity in contaminated environments. Int. J. Mol. Sci. 2016, 17, 1205. [Google Scholar]
- Wang, Y.C.; Wu, B.D.; Jiang, K. Allelopathic effects of Canada goldenrod leaf extracts on the seed germination and seedling growth of lettuce reinforced under salt stress. Ecotoxicology 2019, 28, 103–116. [Google Scholar] [CrossRef]
- Zhou, X.A.; Wu, F.Z. P-Coumaric acid influenced cucumber rhizosphere soil microbial communities and the growth of Fusarium oxysporum f.sp. cucumerinum Owen. PLoS ONE 2012, 7, e48288. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tao, Y.; Zhang, H.; Wang, L.; Sun, G.Q.; Sun, X.; Erinle, K.O.; Feng, C.C.; Song, Q.X.; Li, M. Effect of di-n-butyl phthalate on root physiology and rhizosphere microbial community of cucumber seedings. J. Hazard. Mater. 2015, 289, 9–17. [Google Scholar] [CrossRef]
- Zhao, L.J.; Zhang, H.L.; Jason, C.W.; Chen, X.Q.; Li, H.B.; Qu, X.L.; Rong, J. Metabolomics reveals that engineered nanomaterial exposure in soil alters both soil rhizosphere metabolite profiles and maize metabolic pathways. Environ. Sci. Nano 2019, 6, 1716. [Google Scholar] [CrossRef]
- Tian, L.Y.; Shen, J.P.; Sun, G.X.; Wang, B.; Ji, R.; Zhao, L.J. Foliar application of SiO2 nanoparticles alters soil metabolite profiles and microbial community composition in the pakchoi (Brassica chinensis L.) rhizosphere grown in contaminated mine soil. Environ. Sci. Technol. 2020, 54, 13137–13146. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Ghimire, B.; Yu, C.Y.; Chung, I.M. Allelopathic and autotoxic effects of medicago sativa-derived allelochemicals. Plants 2019, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Lei, D.Y.; Jin, L.; He, Y.; Yang, Q.Q. Promising fungicides from allelochemicals: Synthesis of umbelliferone derivatives and their structure-activity relationships. Molecules 2018, 23, 3002. [Google Scholar] [CrossRef] [Green Version]
- Shibl, A.A.; Isaac, A.; Ochsenkuhn, M.A.; Cardenas, A.; Fei, C.; Behringerc, G.; Arnoux, M.; Drou, N.; Santos, M.P.; Gunsalus, K.C.; et al. Diatom modulation of select bacteria through use of two unique secondary metabolites. Proc. Natl. Acad. Sci. USA 2020, 117, 27445–27455. [Google Scholar] [CrossRef]
- Neal, A.L.; Ahmad, S.; Gordon-Weeks, R.; Ton, J. Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS ONE 2012, 7, e35498. [Google Scholar] [CrossRef] [Green Version]
- Sogi, D.S.; Siddiq, M.; Dolan, K.D. Total phenolics, carotenoids and antioxidant properties of tommy atkin mango cubes as affected by drying techniques. LWT Food Sci. Technol. 2015, 62, 564–568. [Google Scholar] [CrossRef]
- Jiang, Y.; Nie, W.J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef]
- Qu, B.P.; Liu, Y.X.; Sun, X.Y.; Li, S.Y.; Wang, S.Y.; Xiong, K.Y.; Yun, B.H.; Zhang, H. Effect of various mulches on soil physico-chemical properties and tree growth (Sophora japonica) in urban tree pits. PLoS ONE 2019, 14, e0210777. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.C.; Zhou, J.; Ongena, M.; Liu, W.Z.; Wei, D.; Zhao, B.; Guan, D.W.; Jiang, X.; Li, J. Effect of long-term fertilization strategies on bacterial community composition in a 35-year field experiment of Chinese mollisols. AMB Express 2018, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baran, A.; Mierzwa-Hersztek, M.; Gondek, K.; Tarnawski, M.; Szara, M.; Gorczyca, O.; Koniarz, T. The influence of the quantity and quality of sediment organic matter on the potential mobility and toxicity of trace elements in bottom sediment. Environ. Geochem. Health 2019, 41, 2893–2910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.Y.; Wen, C.Q.; Qin, Y.J.; Yin, H.Q.; Tu, Q.C.; Nostrand, J.D.V.; Yuan, T.; Yuan, M.T.; Deng, Y.; Zhou, J.Z. Phasing amplicon sequencing on Illumina Miseq for robust environmental microbial community analysis. BMC Microbiol. 2015, 15, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.K.; Chandra, J.; Retuerto, M.; Sikaroodi, M.; Brown, R.E.; Jurevic, R.; Salata, R.A.; Lederman, M.M.; Gillevet, P.M.; Ghannoum, M.A. Oral mycobiome analysis of HIV-infected patients: Identification of pichia as an antagonist of opportunistic fungi. PLoS Pathog. 2014, 10, e1003996. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina Caporaso sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Reyon, D.; Tsai, S.Q.; Khayter, C.; Foden, J.A.; Sander, J.D.; Joung, J.K. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotech. 2012, 30, 460–465. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Chao, J.; Bunge, J. Estimatin the number of species in a stochastic abundance model. Biometrics 2002, 58, 531–539. [Google Scholar] [CrossRef]
- Hill, T.C.; Walsh, K.A.; Harris, J.A.; Moffett, B.F. Using ecological diversity measures with bacterial communities. FEMS Microbiol. Ecol. 2003, 43, 1–11. [Google Scholar] [CrossRef]
- Li, X.P.; Liu, C.L.; Zhao, H.; Gao, F.; Ji, G.N.; Hu, F.; Li, H.X. Consistent improvements in soil biochemical properties and crop yields by organic fertilization for above-ground (rapeseed) and below-ground (sweet potato) crops. J. Agric. Sci. 2018, 156, 1186–1195. [Google Scholar] [CrossRef]
- Lin, W.W.; Lin, M.H.; Zhou, H.Y.; Wu, H.M.; Li, Z.W.; Lin, W.X. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE 2019, 14, e0217018. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Yu, Y.N.; Gao, R.W.; Wang, H.; Zhang, J.; Li, R.; Long, X.H.; Shen, Q.R.; Chen, W.; Cai, F. High-throughput absolute quantification sequencing reveals the effect of different fertilizer applications on bacterial community in a tomato cultivated coastal saline soil. Sci. Total Environ. 2019, 687, 601–609. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Q.; Feng, Z.Y.; Liu, Z.D.; Li, H.Y.; Sun, Y.F.; Liu, C.S.; Lai, H.X. Long-term organic fertilization improves the productivity of kiwifruit (Actinidia chinensis Planch.) through increasing rhizosphere microbial diversity and network complexity. Appl. Soil Ecol. 2020, 147, 103426. [Google Scholar] [CrossRef]
- Qiao, C.C.; Penton, C.R.; Xiong, W.; Liu, C.; Wang, R.F.; Liu, Z.Y.; Xu, X.; Li, R.; Shen, Q. Reshaping the rhizosphere microbiome by bio-organic amendment to enhance crop yield in a maize-cabbage rotation system. Appl. Soil Ecol. 2019, 142, 136–146. [Google Scholar] [CrossRef]
- Jiang, X.M.; Zhang, F.H.; Li, J.H.; Fan, H.; Cheng, Z.B.; Wang, K.Y. Effects of bio-organic fertilizer on soil microbiome against Verticillium dahliae. Int. J. Agric. Biol. 2016, 18, 923–931. [Google Scholar]
- Gao, M.; Zhou, J.J.; Wang, E.T.; Chen, Q.; Xu, J.; Sun, J.G. Multiphasic characterization of a plant growth promoting bacterial strain, Burkholderia sp 7016 and its effect on tomato growth in the field. J. Int. Agric. 2015, 14, 1855–1863. [Google Scholar] [CrossRef] [Green Version]
- Maqsood, A.; Shahid, M.; Hussain, S.; Mahmood, F.; Basit, F. Root colonizing Burkholderia sp. AQ12 enhanced rice growth and upregulated tillering-responsive genes in rice. Appl. Soil Ecol. 2020, 157, 103769. [Google Scholar] [CrossRef]
- Borrell, A.N.; Shi, Y.; Gan, Y.; Bainard, L.D.; Germida, J.J.; Hamel, C. Fungal diversity associates with pluses and its influence on the subsequent wheat crop in the Canadian prairies. Plant Soil 2017, 414, 13–31. [Google Scholar] [CrossRef]
- Sallam, N.M.A.; Eraky, A.M.I.; Sallam, A. Effect of Trichoderma spp. on Fusarium wilt disease of tomato. Mol. Biol. Rep. 2019, 46, 4463–4470. [Google Scholar] [CrossRef]
- Li, X.Q.; Su, Y.; Ahmed, T.; Ren, H.Y.; Javed, M.R.; Yao, Y.L.; An, Q.L.; Yan, J.L.; Li, B. Effects of different organic fertilizers on improving soil from newly reclaimed land to crop soil. Agriculture 2021, 11, 560. [Google Scholar] [CrossRef]
- Patra, B.; Ray, S.; Richter, A.; Majumder, A.L. Enhanced salt tolerance of transgenic tobacco plants by co-expression of PcINO1 and McIMT1 is accompanied by increased level of myo-inositol and methylated inositol. Protoplasma 2010, 245, 143–152. [Google Scholar] [CrossRef]
- Pierattini, E.C.; Francini, A.; Huber, C.; Sebastiani, L.; Schröder, P. Poplar and diclofenac pollution: A focus on physiology, oxidative stress and uptake in plant organs. Sci. Total Environ. 2018, 15, 944–952. [Google Scholar] [CrossRef]
- Li, R.Y.; Pang, Z.Q.; Zhou, Y.M.; Fallah, N.; Hu, C.H.; Lin, W.X.; Yuan, Z.N. Metagenomic analysis exploring taxonomic and functional diversity of soil microbial communities in sugarcane fields applied with organic fertilizer. Biomed Res. Int. 2020, 2020, 9381506. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, X.F.; Chaparro, J.M.; Badri, D.V.; Guo, J. Root and bacterial secretions regulate the interaction between plants and PGPR leading to distinct plant growth promotion effects. Plant Soil 2015, 401, 259–272. [Google Scholar] [CrossRef]
- Baccolini, C.; Witte, C.P. AMP and GMP catabolism in Arabidopsis converge on xanthosine, which is degraded by a nucleoside hydrolase heterocomplex. Plant Cell 2019, 31, 734–751. [Google Scholar] [CrossRef]
- Huang, X.F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.F.; Shen, Q.R.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, X.; Huang, G.; Shi, W.; Yang, X.; Jiang, Q.; Jia, Y.; Yang, X.; Jiang, H. Hepatopancreatic metabolomics shedding light on the mechanism underlying unsynchronized growth in giant freshwater prawn, Macrobrachium rosenbergii. PLoS ONE 2020, 15, e0243778. [Google Scholar] [CrossRef]
- Adams, Z.P.; Ehlting, J.; Edwards, R. The regulatory role of shikimate in plant phenylalanine metabolism. J. Theor. Biol. 2019, 462, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Wong-Ng, J.; Celani, A.; Vergassola, M. Exploring the function of bacterial chemotaxis. Curr. Opin. Microbiol. 2018, 45, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Zhao, Y.; He, L.; Ding, J.; Li, B.; Mu, W.; Liu, F. Comparison of transcriptome profiles of the fungus Botrytis cinerea and insect pest Bradysia odoriphaga in response to benzothiazole. Front. Microbiol. 2020, 11, 1043. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, S.; Otto, S.B.; Swinkels, A.; Bode, B.; van Wezel, G.P.; Rozen, D.E. Spatial structure increases the benefits of antibiotic production in Streptomyces. Evolution 2020, 74, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Coelho, A.I.; Berry, G.T.; Rubio-Gozalbo, M.E. Galactose metabolism and health. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 422–427. [Google Scholar] [CrossRef]
- Tiwari, K.; Dubey, V.K. Fresh insights into the pyrimidine metabolism in the trypanosomatids. Parasit Vectors 2018, 11, 87. [Google Scholar] [CrossRef]



| Parameters | Value | Parameters | Value |
|---|---|---|---|
| Twig length (cm) | Twig diameter (mm) | ||
| D | 13.22 ± 0.54 c | D | 2.80 ± 0.13 c |
| CF | 17.58 ± 0.55 b | CF | 3.41 ± 0.08 b |
| OF | 31.16 ± 0.40 a | OF | 3.59 ± 0.18 a |
| Leaf length (mm) | Leaf width (mm) | ||
| D | 99.50 ± 3.85 a | D | 27.18 ± 0.41 c |
| CF | 99.11 ± 5.98 a | CF | 28.11 ± 0.34 b |
| OF | 97.31 ± 7.62 a | OF | 30.80 ± 0.63 a |
| Leaf thickness (mm) | Photosynthesis rate (mg CO2·∙10 cm−2∙h−1) | ||
| D | 4.05 ± 0.92 b | D | 1.86 ± 0.28 c |
| CF | 4.65 ± 0.18 a | CF | 3.48 ± 0.21 b |
| OF | 4.85 ± 0.30 a | OF | 5.18 ± 0.32 a |
| Parameters | Value | Parameters | Value |
|---|---|---|---|
| Single fruit weight (g) | Soluble solids (%) | ||
| D | 11.03 ± 0.51 c | D | 8.91 ± 0.29 c |
| CF | 14.15 ± 0.30 b | CF | 10.01 ± 0.26 b |
| OF | 17.32 ± 1.06 a | OF | 12.34 ± 0.13 a |
| Vitamin C (mg/100 g) | Titratable acid (%) | ||
| D | 4.73 ± 0.17 c | D | 1.29 ± 0.11 a |
| CF | 6.57 ± 0.58 b | CF | 0.92 ± 0.07 b |
| OF | 8.06 ± 2.04 a | OF | 0.71 ± 0.07 c |
| Physical and Chemical Properties | D | CF | OF |
|---|---|---|---|
| pH | 4.22 ± 0.01 a | 4.36 ± 0.37 a | 4.34 ± 0.21 a |
| Organic matter (%) | 2.50 ± 0.86 b | 2.37 ± 0.13 b | 3.16 ± 0.35 a |
| Alkali-hydrolyzable nitrogen (mg/kg) | 106.11 ± 3.30 a | 93.12 ± 4.27 b | 105.15 ± 3.40 a |
| Available phosphorus (mg/kg) | 27.72 ± 0.31 a | 25.31 ± 0.74 a | 14.45 ± 0.45 b |
| Exchangeable calcium (mg/kg) | 315.16 ± 3.95 b | 285.61 ± 2.37 c | 763.66 ± 18.08 a |
| Exchangeable magnesium (mg/kg) | 31.98 ± 0.35 b | 28.90 ± 2.80 c | 36.05 ± 2.57 a |
| Soil Environment | Contribution at Bacterial Genus Level (%) | Contribution at Fungal Genus Level (%) |
|---|---|---|
| pH | 3.9 | 6.0 |
| Organic matter | 7.3 | 6.6 |
| Alkali-hydrolyzable nitrogen | 3.2 | 8.5 |
| Available phosphorus | 20.6 | 8.6 |
| Exchangeable calcium | 25.8 | 11.0 |
| Exchangeable magnesium | 10.2 | 12.3 |
| No. | Metabolite Name | D | CF | OF |
|---|---|---|---|---|
| 1 | Cysteine-Glycine | 1.71 ± 0.18 b | 5.97 ± 1.11 a | 6.41 ± 1.95 a |
| 2 | Thymidine | 1.27 ± 0.13 b | 3.50 ± 0.19 a | 3.21 ± 0.32 a |
| 3 | Biphenyl | 0.59 ± 0.04 c | 1.97 ± 0.12 a | 1.64 ± 0.16 b |
| 4 | 5-Methoxytryptamine | 38.13 ± 3.79 b | 82.28 ± 7.21 a | 83.54 ±7.26 a |
| 5 | Glucoheptulose | 3.90 ± 0.51 c | 7.18± 0.61 b | 8.02± 0.44 a |
| 6 | Benzoic Acid | 2.45 ± 0.20 c | 4.08 ±0.39 a | 3.44 ± 0.33 b |
| 7 | Udp-Glucuronic Acid | 0.32 ± 0.05 c | 0.44 ± 0.03 b | 0.53 ± 0.06 a |
| 8 | Xylonolactone | 1.28 ± 0.14 c | 1.67 ± 0.08 b | 2.08 ±0.17 a |
| 9 | 2-Monopalmitin | 1.83 ± 0.14 c | 2.71 ± 0.21 b | 3.07 ± 0.32 a |
| 10 | Hexadecylglycerol | 3.87 ± 0.40 b | 6.66 ±0.58 a | 6.23 ± 0.77 a |
| 11 | Myo-Inositol | 15.64 ± 1.43 c | 27.05± 2.25 a | 23.04 ± 2.22 b |
| 12 | Metanephrine | 0.79 ± 0.05 c | 1.49 ± 0.20 b | 1.65 ± 0.13 a |
| 13 | Cytidine | 18.39 ± 1.39 a | 0.60 ± 0.02 c | 0.76 ± 0.10 b |
| 14 | Diclofenac | 130.89 ± 12.65 a | 5.58 ± 0.32 b | 5.80 ± 0.51 b |
| 15 | Proline | 9.36 ± 0.73 a | 2.01 ± 0.15 c | 2.99 ± 0.24 b |
| 16 | Ethanolamine | 15.57 ± 1.28 a | 4.31 ± 0.38 c | 7.46 ± 0.58 b |
| 17 | Glutamate | 0.37 ± 0.04 a | 0.14 ± 0.02 b | 0.15 ± 0.02 b |
| 18 | Serine | 2.25 ± 0.03 a | 0.61 ± 0.03 c | 1.04 ± 0.16 b |
| Metabolite Name | Relative Content | Metabolite Name | Relative Content |
|---|---|---|---|
| 2-Ketoglucose imethylacetal | N-Methylalanine | ||
| D | 0.16 ± 0.05 | D | 2.55 ± 0.26 |
| CF | 0.30 * ±0.01 | CF | 1.47 # ±0.14 |
| 2-Deoxytetronic Acid | Lysine | ||
| D | 2.52 ± 0.23 | D | 0.54 ± 0.10 |
| CF | 4.55 * ± 0.45 | CF | 0.37 # ± 0.03 |
| Galactinol | Tyrosine | ||
| D | 1.18 ± 0.10 | D | 1.91 ± 0.12 |
| CF | 2.88 * ± 0.21 | CF | 1.04 # ±0.09 |
| Digitoxose | N-Acetyl-5-Hydroxytryptamine | ||
| D | 3.30± 0.29 | D | 1.42 ± 0.15 |
| CF | 4.76 * ± 0.34 | CF | 0.83 # ± 0.06 |
| 2,4-Diaminobutyric Acid | Montanic Acid | ||
| D | 0.93 ± 0.09 | D | 3.21 ± 0.47 |
| CF | 0.66 # ± 0.05 | CF | 1.30 # ± 0.15 |
| Coniferin | Aconitic Acid | ||
| D | 8.53 ± 0.84 | D | 2.68 ± 0.18 |
| CF | 0.25 # ± 0.03 | CF | 1.30 # ± 0.15 |
| Citrulline | |||
| D | 5.24 ± 0.46 | ||
| CF | 2.97 # ± 0.25 |
| Metabolite Name | Relative Content | Metabolite Name | Relative Content |
|---|---|---|---|
| Arabitol | N-Acetyl-D-Hexosamine | ||
| D | 7.98 ± 0.66 | D | 0.33 ± 0.01 |
| OF | 14.56 * ± 1.80 | OF | 0.56 *± 0.02 |
| Threitol | Deoxycholic Acid | ||
| D | 0.42 ± 0.04 | D | 0.45 ± 0.04 |
| OF | 0.57 * ± 0.05 | OF | 0.93 * ± 0.09 |
| Inosine | Zymosterol | ||
| D | 0.65 ± 0.05 | D | 0.46 ± 0.03 |
| OF | 3.19 * ±0.30 | OF | 0.89 * ± 0.05 |
| Glycerol | Threonic Acid | ||
| D | 195.86 ± 18.98 | D | 0.86 ± 0.04 |
| OF | 264.19 * ± 21.08 | OF | 1.34 * ± 0.14 |
| Oleic Acid | Guanidinosuccinate | ||
| D | 1.97 ± 0.02 | D | 0.36 ± 0.02 |
| OF | 3.18 * ± 0.02 | OF | 0.78 * ± 0.08 |
| Palmitic Acid | 2’-Deoxyguanosine | ||
| D | 10.39 ± 1.02 | D | 0.25± 0.02 |
| OF | 18.20 * ± 1.48 | OF | 0.46 * ±0.05 |
| Dodecanol | Citraconic Acid | ||
| D | 1.87 ± 0.12 | D | 5.12 ± 0.41 |
| OF | 4.71 * ± 0.36 | OF | 7.57 * ± 0.72 |
| Thymine | Vanillic Acid | ||
| D | 1.46 ± 0.12 | D | 3.69 ± 0.33 |
| OF | 3.47 * ± 0.36 | OF | 5.78 * ± 0.65 |
| 2-Hydroxyvaleric Acid | Glucosamine | ||
| D | 2.79 ± 0.26 | D | 0.70 ± 0.08 |
| OF | 3.54 * ± 0.29 | OF | 1.04 * ± 0.06 |
| Malonic Acid | Phthalic Acid | ||
| D | 0.21 ± 0.03 | D | 1.70 ± 0.20 |
| OF | 0.26 * ± 0.02 | OF | 2.24 * ± 0.20 |
| Xylose | Phytanic Acid | ||
| D | 0.79 ± 0.08 | D | 0.58 ± 0.05 |
| OF | 1.10 * ± 0.13 | OF | 1.08 * ± 0.13 |
| Glucose-1-Phosphate | Inulotriose | ||
| D | 0.35 ± 0.01 | D | 0.56 ± 0.05 |
| OF | 0.56 * ± 0.03 | OF | 0.80 * ± 0.01 |
| Adipic Acid | Inositol-4-Monophosphate | ||
| D | 1.42 ± 0.15 | D | 4.81 ± 0.40 |
| OF | 2.12 * ± 0.15 | OF | 6.40 * ± 0.49 |
| Glutaric Acid | |||
| D | 2.56 ± 0.16 | ||
| OF | 3.67 * ± 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Wang, H.; Yu, Z.; Zhang, S.; Qi, X.; Sun, L.; Wang, Z.; Zhang, M.; Ahmed, T.; Li, B. Effect of Two Kinds of Fertilizers on Growth and Rhizosphere Soil Properties of Bayberry with Decline Disease. Plants 2021, 10, 2386. https://doi.org/10.3390/plants10112386
Ren H, Wang H, Yu Z, Zhang S, Qi X, Sun L, Wang Z, Zhang M, Ahmed T, Li B. Effect of Two Kinds of Fertilizers on Growth and Rhizosphere Soil Properties of Bayberry with Decline Disease. Plants. 2021; 10(11):2386. https://doi.org/10.3390/plants10112386
Chicago/Turabian StyleRen, Haiying, Hongyan Wang, Zheping Yu, Shuwen Zhang, Xingjiang Qi, Li Sun, Zhenshuo Wang, Muchen Zhang, Temoor Ahmed, and Bin Li. 2021. "Effect of Two Kinds of Fertilizers on Growth and Rhizosphere Soil Properties of Bayberry with Decline Disease" Plants 10, no. 11: 2386. https://doi.org/10.3390/plants10112386
APA StyleRen, H., Wang, H., Yu, Z., Zhang, S., Qi, X., Sun, L., Wang, Z., Zhang, M., Ahmed, T., & Li, B. (2021). Effect of Two Kinds of Fertilizers on Growth and Rhizosphere Soil Properties of Bayberry with Decline Disease. Plants, 10(11), 2386. https://doi.org/10.3390/plants10112386







