Uncovering Research Trends of Phycobiliproteins Using Bibliometric Approach
Abstract
1. Introduction
2. Bibliometric Analysis
2.1. Methodology
2.1.1. Data Collection
2.1.2. Refinement of the Search Results
2.1.3. Bibliometric Analysis
2.2. General Characteristics of Research Publications
2.2.1. Publication Types and Languages
2.2.2. Subject Categories of Publications
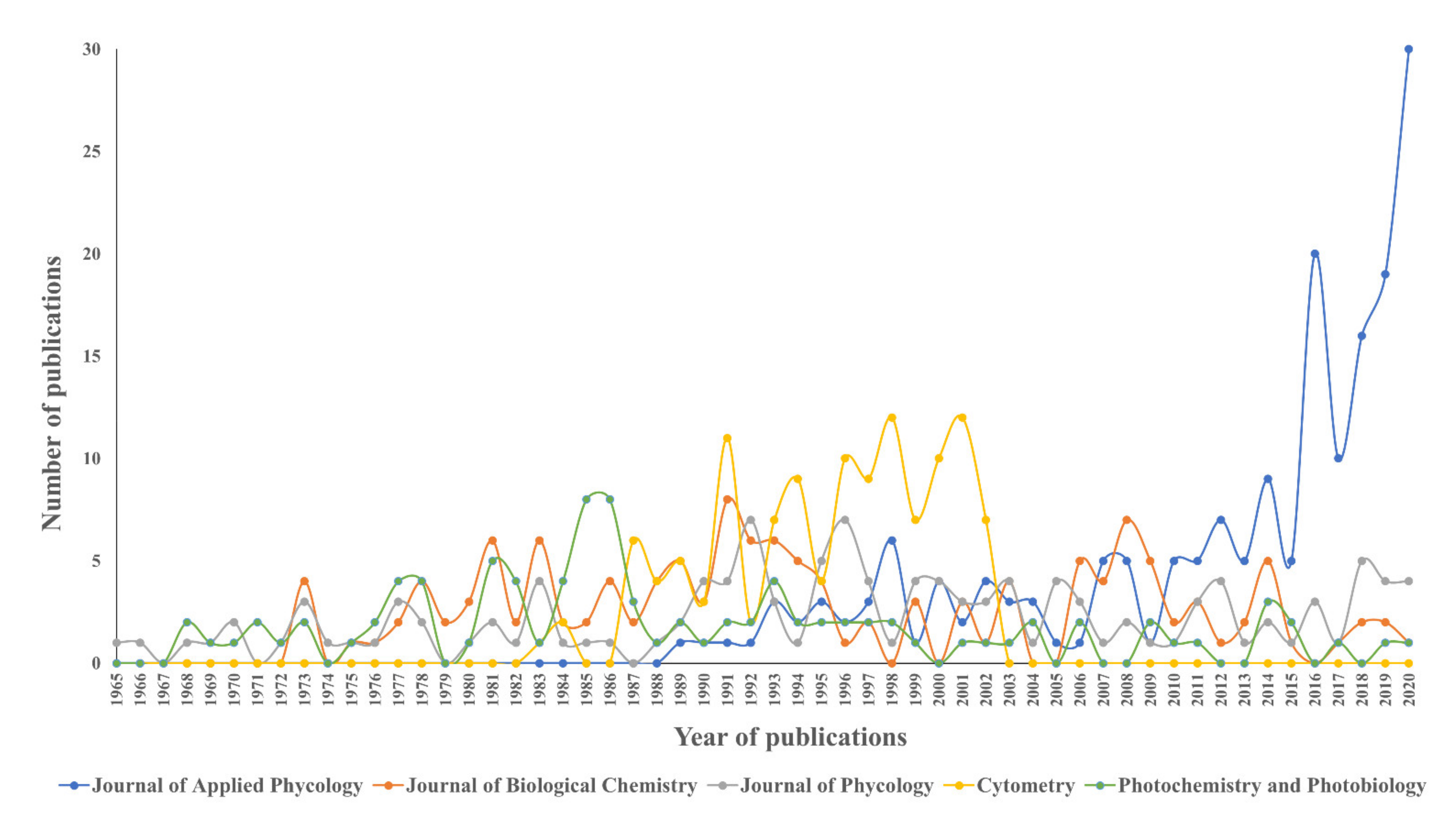
2.2.3. Sources of Publications
2.3. Specific Performance of Research Publications
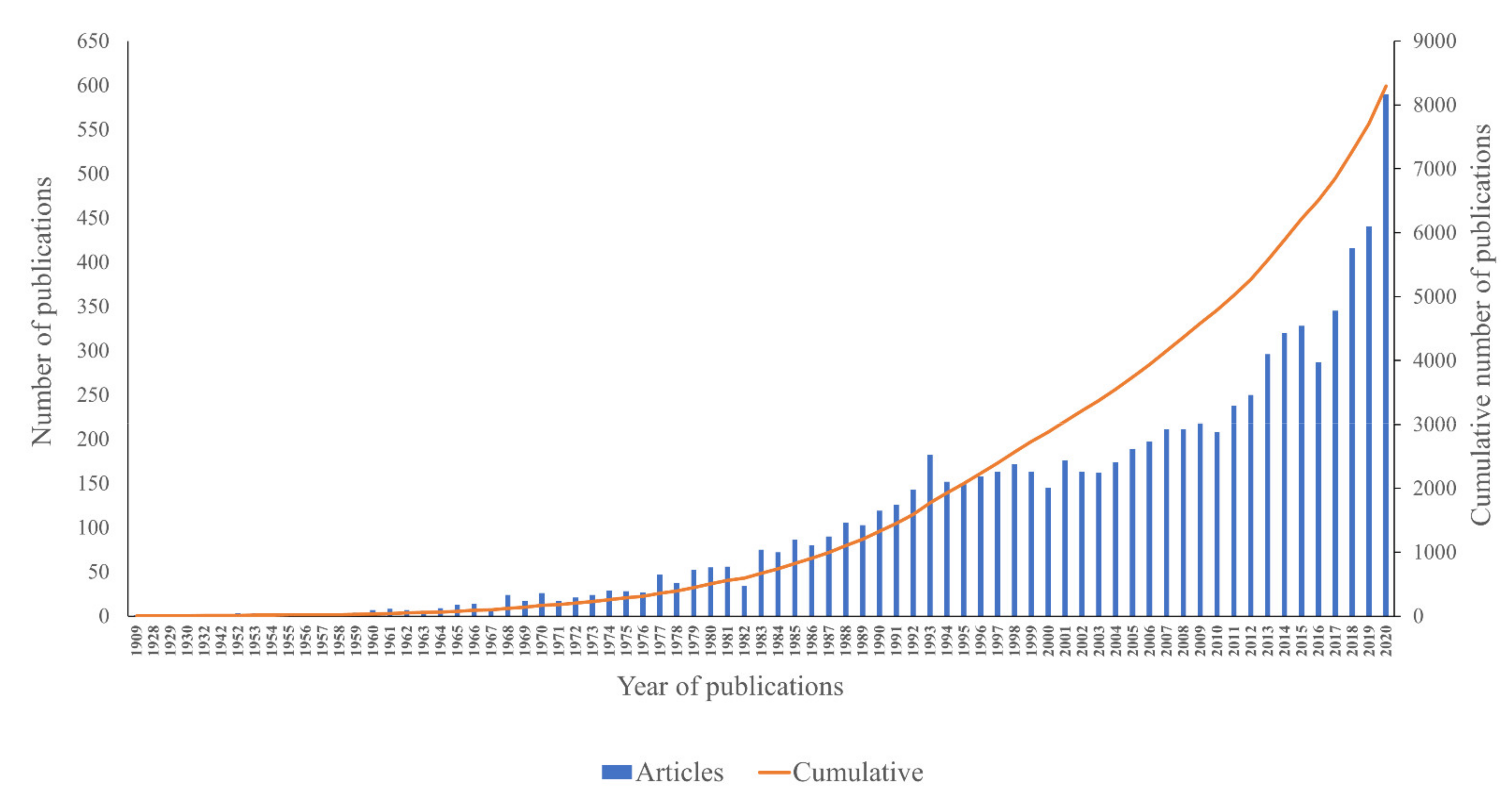
2.3.1. Annual Publication Trend
2.3.2. Analysis of Growth Trend
2.3.3. Highly Cited Publications
2.3.4. Performance of Publications by Countries
2.3.5. Performance of Publications by Institutions
2.3.6. Performance of Publications by Authors
2.4. Main Research Hotspot and Trends
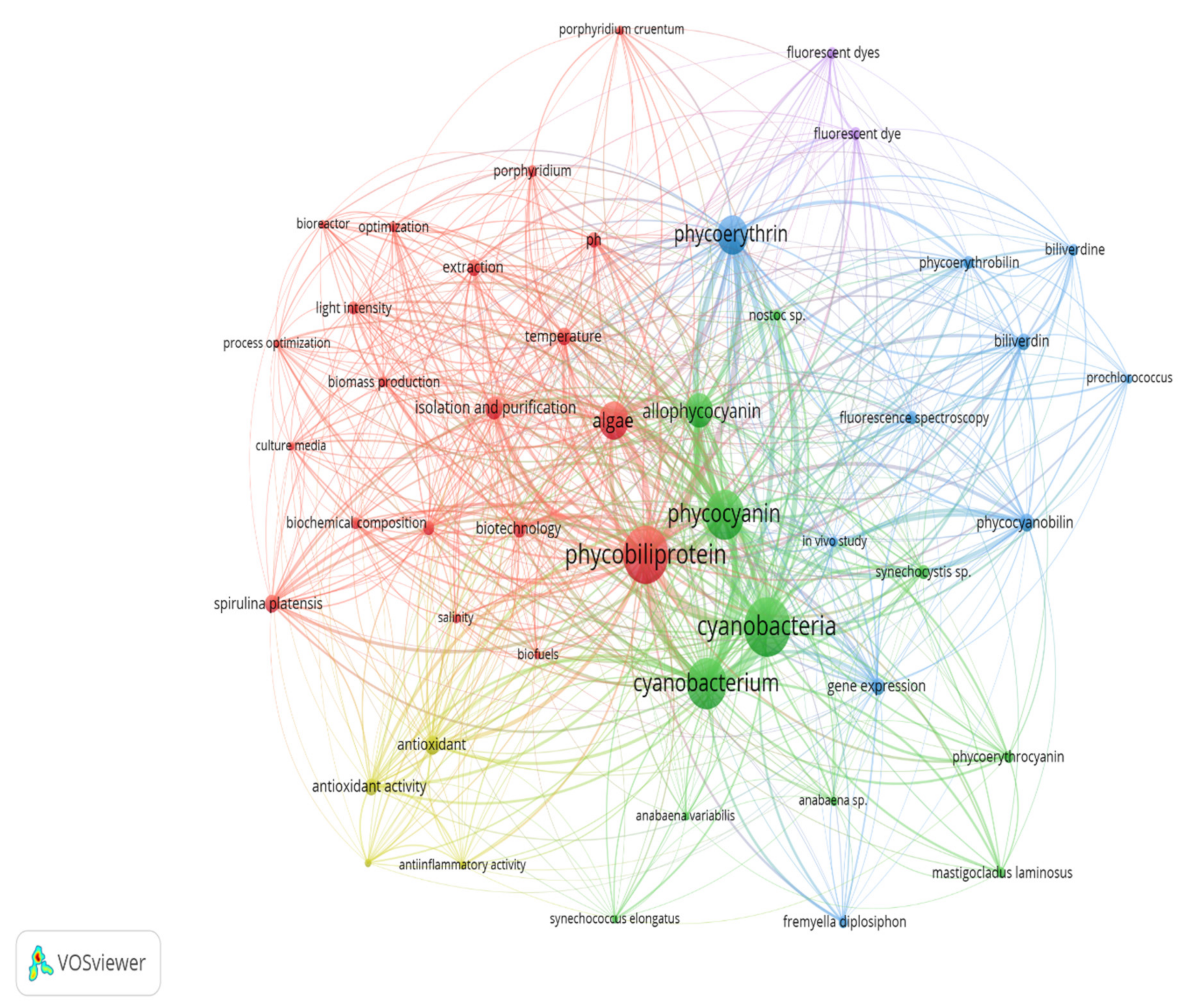
2.4.1. Keywords Analysis
2.4.2. Analysis of Research Trend–Burst Detection Analysis
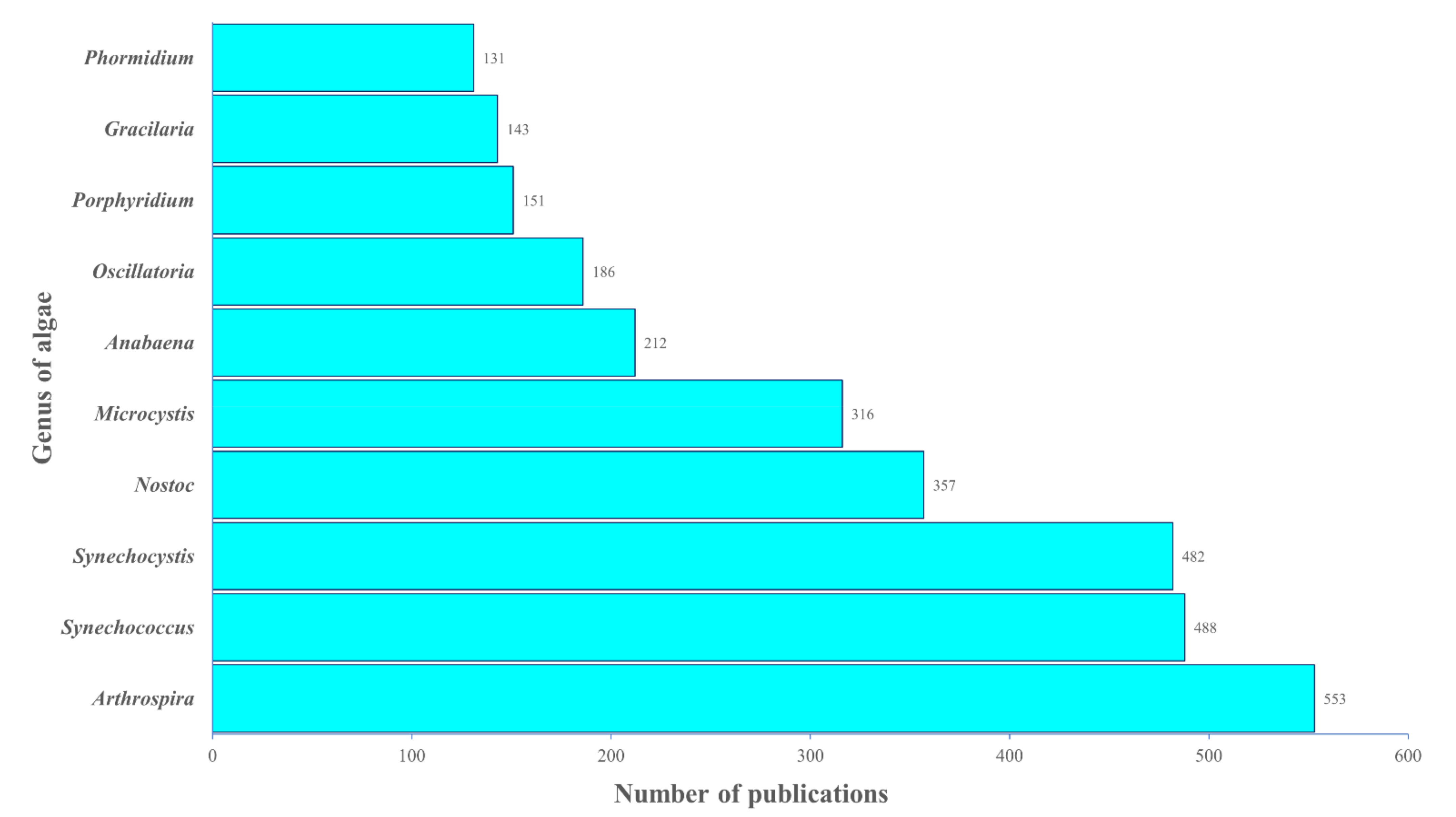
2.4.3. Analysis of Research Trend: Algae Genera
3. Overview of Previous Phycobiliprotein Research
3.1. General Characteristics of Research Publications in Phycobiliproteins Study
3.2. Annual Publication Trend in Phycobiliproteins Research
3.3. Country Involved in Phycobiliproteins Research
3.4. International Collaboration in Phycobiliproteins Field
3.5. Research Trend in Phycobiliproteins Research
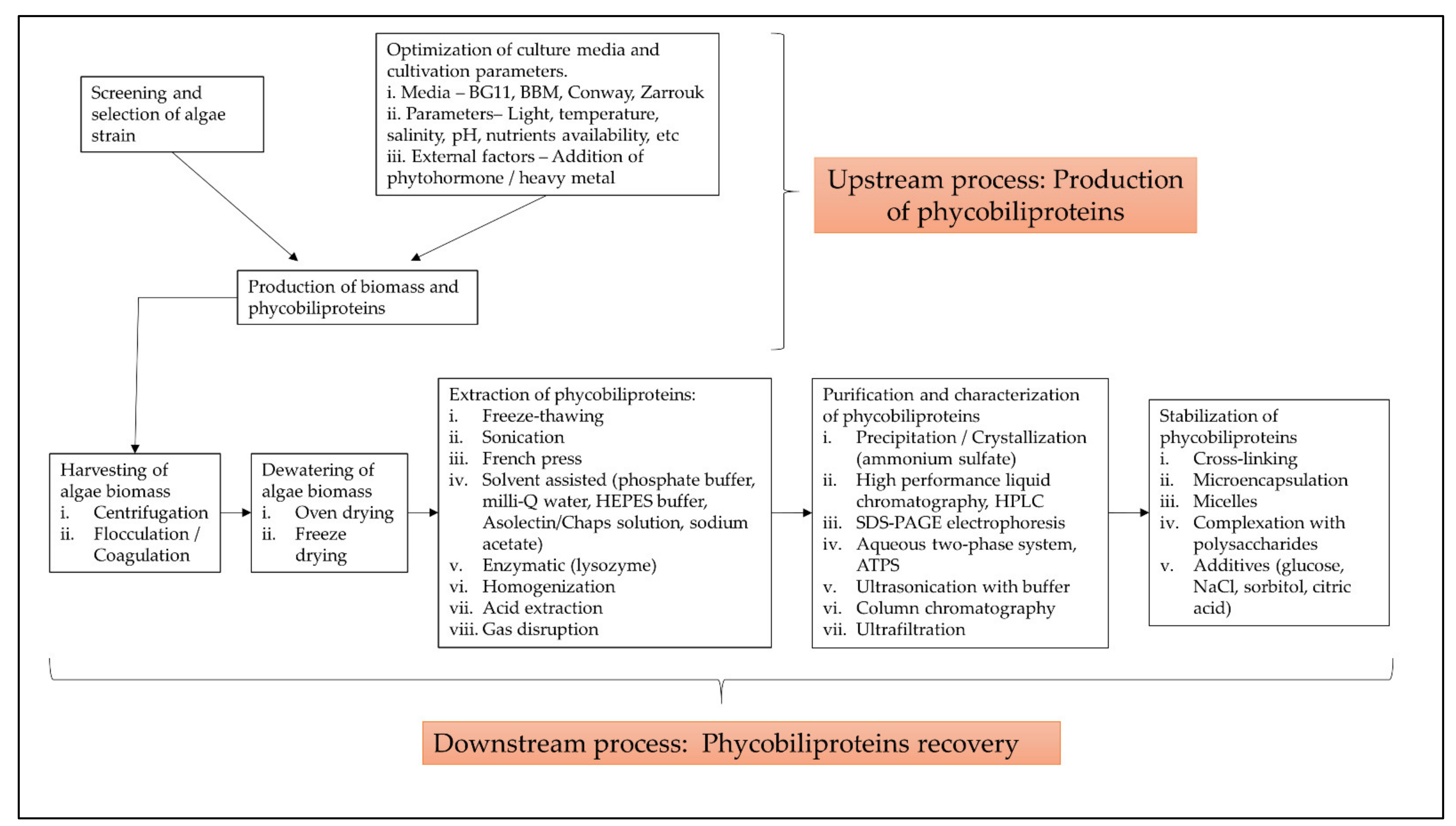
3.5.1. Optimization of Cyanobacteria Cultivation and Phycobiliproteins Harvesting Process
3.5.2. Classification with Structure
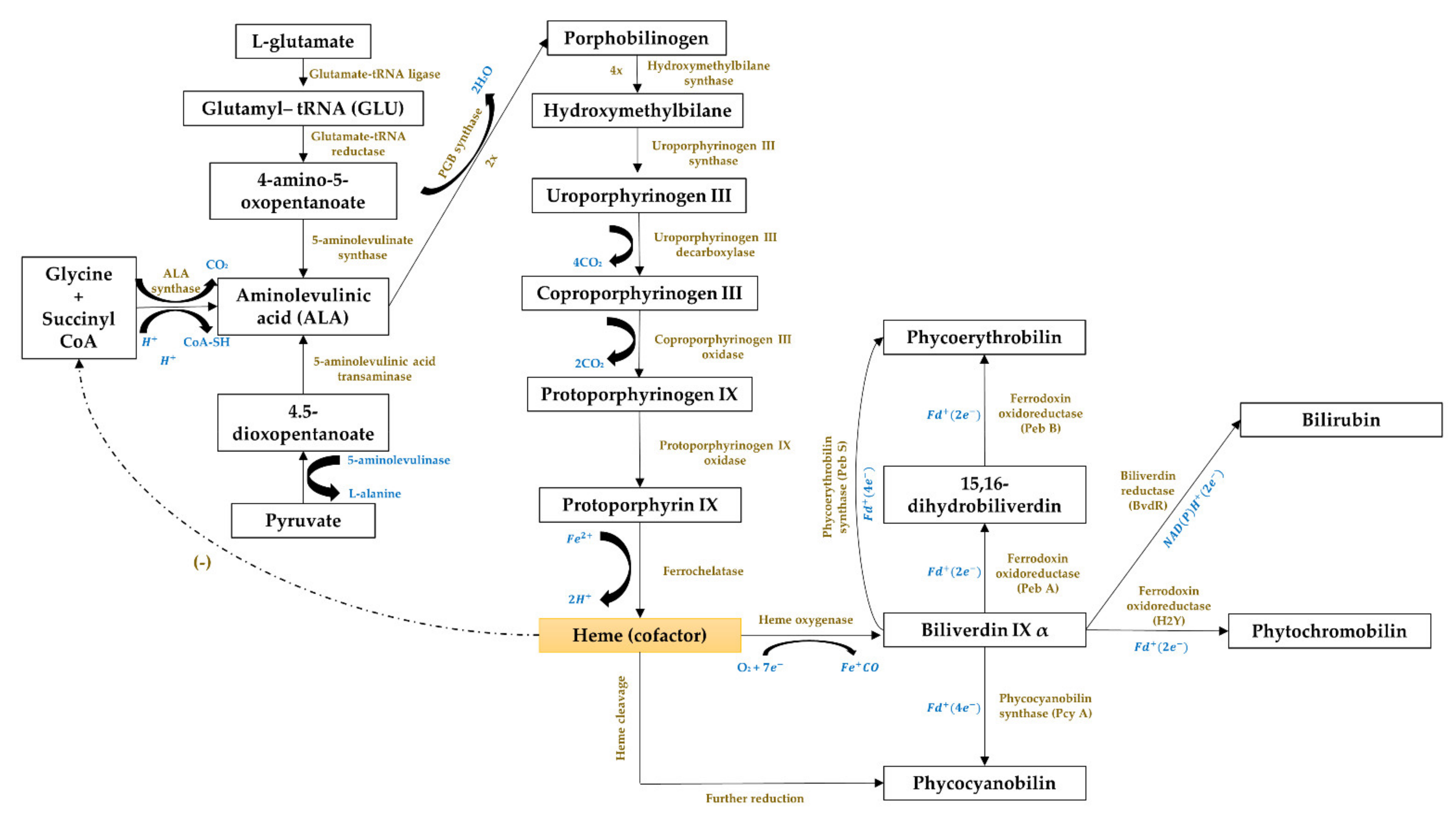
3.5.3. Gene Expression of the Phycobiliproteins Biosynthesis Pathway
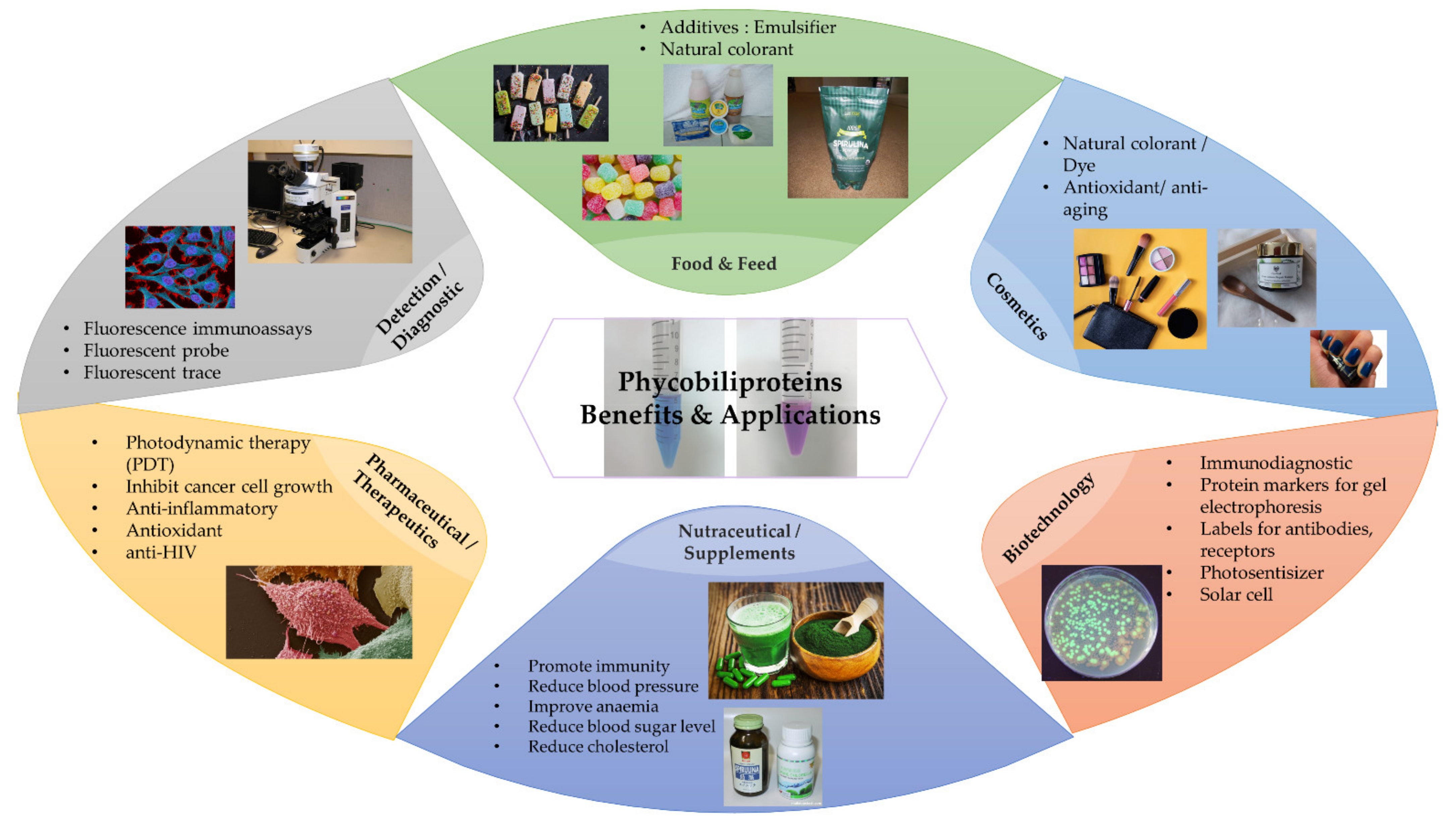
3.5.4. Bioactivities and Applications of Phycobiliproteins
3.6. Future Research Prospects in Phycobiliproteins Study
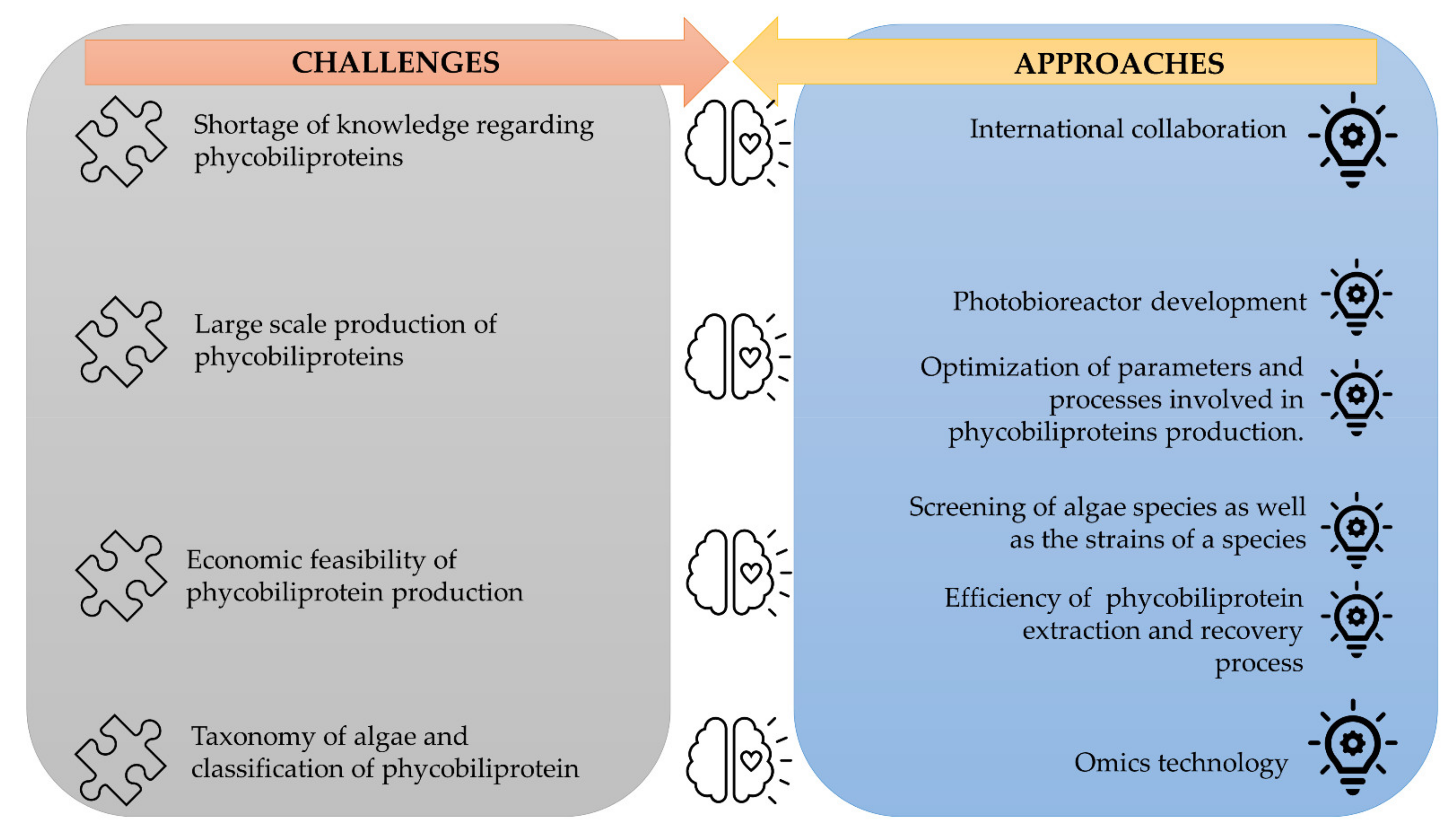
3.7. Challenges and Approaches in the Phycobiliprotein Field
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from Cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Viskari, P.J.; Colyer, C.L. Rapid extraction of phycobiliproteins from cultured Cyanobacteria samples. Anal. Biochem. 2003, 319, 263–271. [Google Scholar] [CrossRef]
- Stadnichuk, I.N.; Tropin, I.V. Phycobiliproteins: Structure, functions and biotechnological applications. Appl. Biochem. Microbiol. 2017, 53, 1–10. [Google Scholar] [CrossRef]
- Bryant, D.A.; Guglielmi, G.; de Marsac, N.T.; Castets, A.M.; Cohen-Bazire, G. The structure of Cyanobacterial Phycobilisomes: A model. Arch. Microbiol. 1979, 123, 113–127. [Google Scholar] [CrossRef]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential microalgae derived pharmaceutical and biological reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Wildman, R.B.; Bowen, C.C. Phycobilisomes in blue green algae. J. Bacteriol. 1974, 117, 866–881. [Google Scholar] [CrossRef]
- Fleurence, J. R-phycoerythrin from red macroalgae: Strategies for extraction and potential application in biotechnological area. Appl. Biotechnol. Food Sci. Policy 2003, 1, 3–9. [Google Scholar]
- Van Der Weij-De Wit, C.D.; Doust, A.B.; Van Stokkum, I.H.M.; Dekker, J.P.; Wilk, K.E.; Curmi, P.M.G.; Van Grondelle, R. Phycocyanin sensitizes both photosystem I and photosystem II in Cryptophyte Chroomonas CCMP270 Cells. Biophys. J. 2008, 94, 2423–2433. [Google Scholar] [CrossRef]
- Silva, S.C.; Ferreira, I.C.; Dias, M.M.; Barreiro, M.F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- Santiago-Santos, M.C.; Ponce-Noyola, T.; Olvera-Ramírez, R.; Ortega-López, J.; Cañizares-Villanueva, R.O. Extraction and purification of phycocyanin from Calothrix sp. Process. Biochem. 2004, 39, 2047–2052. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Wang, G.H.; Xiang, W.Z.; Li, T.; He, H. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef]
- Pradeep, H.N.; Nayak, C.A. Enhanced stability of C-phycocyanin colorant by extrusion encapsulation. J. Food Sci. Technol. 2019, 56, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, F.M.; Banerjee, S.; Nagao, N.; Imaizumi, Y.; Shariff, M.; Toda, T. Use of microalgae pigments in aquaculture. In Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M.I., Zepka, L.Q., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 471–513. [Google Scholar] [CrossRef]
- Pritchard, A. Statistical bibliography or bibliometrics? J. Doc. 1962, 25, 348–349. [Google Scholar]
- Rumin, J.; Nicolau, E.; de Oliveira, R.G.; Fuentes-Grünewald, C.; Flynn, K.J.; Picot, L. A bibliometric analysis of microalgae research in the world, Europe, and the European Atlantic area. Mar. Drugs 2020, 18, 79. [Google Scholar] [CrossRef]
- Tang, Y.; Xin, H.; Yang, F.; Long, X. A historical review and bibliometric analysis of nanoparticles toxicity on algae. J. Nanopart. Res. 2018, 20, 92. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, X.; Hu, Z.; Song, C.; Cui, Y. Bibliometric analysis of algal-bacterial symbiosis in wastewater treatment. Int. J. Environ. Res. Public Health 2019, 16, 1077. [Google Scholar] [CrossRef]
- Lim, Z.S.; Wong, R.R.; Wong, C.Y.; Zulkharnain, A.; Shaharuddin, N.A.; Ahmad, S.A. Bibliometric analysis of research on diesel pollution in Antarctica and a review on remediation techniques. Appl. Sci. 2021, 11, 1123. [Google Scholar] [CrossRef]
- Li, Z.; Ho, Y.S. Use of citation per publication as an indicator to evaluate contingent valuation research. Scientometrics 2008, 75, 97–110. [Google Scholar] [CrossRef]
- Gan, C.; Wang, W. Research Characteristics and Status on Social Media in China: A Bibliometric and Co-Word Analysis. Scientometrics 2015, 105, 1167–1182. [Google Scholar] [CrossRef]
- Macías-Chapula, C.A.; Mijangos-Nolasco, A. Bibliometric analysis of AIDS literature in Central Africa. Scientometrics 2002, 54, 309–317. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Müller, M.N.; Santos, E.P.; Dantas, D.M.M.; Gálvez, A.O. A scientometric overview of global dinoflagellate research. Publications 2020, 8, 52. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae research worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Rowley, J.; Slack, F. Conducting a literature review. Manag. Res. News 2004, 27, 31–39. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Assoc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Croce, R.; Van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylort, W.R.; Thornton, J.M. A new approach to protein fold recognition. Nature 1992, 358, 86–89. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Braud, V.M.; Allan, D.S.J.; O’Callaghan, C.A.; Soderstrom, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef]
- Lyons, A.B.; Parish, C.R. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 1994, 171, 131–137. [Google Scholar] [CrossRef]
- Hardy, R.R.; Carmack, C.E.; Shinton, S.A.; Kemp, J.D.; Hayakawa, K. Resolution and characterization of pro-B and pre-pro-B cell Stages in normal mouse bone marrow. J. Exp. Med. 1991, 173, 1213–1225. [Google Scholar] [CrossRef]
- Chee, M.; Yang, R.; Hubbell, E.; Berno, A.; Huang, X.C.; Stern, D.; Winkler, J.; Lockhart, D.J.; Morris, M.S.; Fodor, S.P.A. Accessing genetic information with high-density DNA arrays. Science 1996, 274, 610–614. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Guo, Y.-M.; Huang, Z.L.; Guo, J.; Li, H.; Guo, X.-R.; Nkeli, M.J. Bibliometric analysis on smart cities research. Sustainability 2019, 11, 3606. [Google Scholar] [CrossRef]
- Eck, N.J.V.; Waltman, L. VOSviewer Manual; University Leiden: Leiden, The Netherlands, 2018; Volume 1, pp. 1–51. [Google Scholar]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical application of Spirulina Platensis derived C-phycocyanin. Evid. Based Complement. Altern. Med. 2016, 2016, 7803846. [Google Scholar] [CrossRef]
- Li, W.; Su, H.N.; Pu, Y.; Chen, J.; Liu, L.N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Kuddus, M.; Singh, P.; Thomas, G.; Al-Hazimi, A. Recent developments in production and biotechnological applications of C-phycocyanin. Biomed. Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Sui, S.-F. Structure of phycobilisomes. Annu. Rev. Biophys. 2021, 50, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Fernandes e Silva, E.; Figueira, F.d.S.; Lettnin, A.P.; Carrett-Dias, M.; Filgueira, D.d.M.V.B.; Kalil, S.; Trindade, G.S.; Votto, A.P.d.S. C-phycocyanin: Cellular targets, mechanisms of action and multi drug resistance in cancer. Pharmacol. Rep. 2018, 70, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Nyakuma, B.B.; Nordin, A.H.; Lee, C.T.; Ngadi, N.; Wong, K.Y.; Oladokun, O. Uncovering the dynamics in global carbon dioxide utilization research: A bibliometric analysis (1995–2019). Environ. Sci. Pollut. Res. 2021, 28, 13842–13860. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, N.T. Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef]
- Manirafasha, E.; Guo, L.; Jing, K. Nutraceutical and Pharmaceutical Applications of Phycobiliproteins; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Ma, J.; Chen, H.; Qin, S.; Lin, H. Applications of natural and artificial phycobiliproteins in solar cells. Curr. Biotechnol. 2015, 4, 275–281. [Google Scholar] [CrossRef]
- Wang, L.; Tian, L.; Deng, X.; Zhang, M.; Sun, S.; Zhang, W.; Zhao, L. Photosensitizers from Spirulina for solar cell. J. Chem. 2014, 2014, 430806. [Google Scholar] [CrossRef]
- Bogorad, L. Studies of phycobiliproteins. Rec. Chem. Prog. 1965, 26, 1–12. [Google Scholar] [PubMed]
- Grossman, K.E.A.; Grosman, A.R. Characterization and transcript analysis of the majar phycobiliprotein subunit genes from Aglaothamnion Neglectum (Rhodophyta). Plant. Mol. Biol. 1993, 21, 27–38. [Google Scholar]
- Shen, G.; Schluchter, W.M.; Bryant, D.A. Biogenesis of phycobiliproteins: I. CpcS-I and CpcU mutants of the Cyanobacterium Synechococcus Sp. PCC 7002 define a heterodimeric phyococyanobilin lyase specific for β-phycocyanin and allophycocyanin subunits. J. Biol. Chem. 2008, 283, 7503–7512. [Google Scholar] [CrossRef] [PubMed]
- Sonani, R.R. Recent advances in production, purification and applications of phycobiliproteins. World J. Biol. Chem. 2016, 7, 100. [Google Scholar] [CrossRef]
- Maurya, S.S.; Maurya, J.N.; Pandey, V.D. Factors regulating phycobiliprotein production in cyanobacteria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 764–771. [Google Scholar]
- Kronick, M.N.; Grossman, P.D. Immunoassay techniques with fluorescent phycobiliprotein conjugates. Clin. Chem. 1983, 29, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Díaz Domínguez, G.; Marsán Suárez, V.; del Valle Pérez, L.O. Main immunomodulatory and anti-inflamatory properties of phycobiliproteins C-phycocyanin. Rev. Cuba. Hematol. Inmunol. Hemoter. 2016, 32, 447–454. [Google Scholar]
- Oi, V.T.; Glazer, A.N.; Stryer, L. Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. J. Cell Biol. 1982, 93, 981–986. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, S.; Ke, F.; Gao, H. Bibliometric analysis of research on microcystins in China and worldwide from 1991 to 2011. Desalin. Water Treat. 2015, 53, 272–283. [Google Scholar] [CrossRef]
- Md Khudzari, J.; Kurian, J.; Tartakovsky, B.; Raghavan, G.S.V. Bibliometric analysis of global research trends on microbial fuel cells using scopus database. Biochem. Eng. J. 2018, 136, 51–60. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Chen, L.; Gong, X. Promising fluorescent probes from phycobiliproteins. IEEE J. Sel. Top. Quantum Electron. 2003, 9, 177–188. [Google Scholar] [CrossRef]
- Mindeli, L.E.; Markusova, V.A. Bibliometric studies of scientific collaboration: International trends. Autom. Doc. Math. Linguist. 2015, 49, 59–64. [Google Scholar] [CrossRef]
- Jones, K.S.; Jackson, D.M. The use of automatically-obtained keyword classifications for information retrieval. Inf. Storage Retr. 1970, 5, 175–201. [Google Scholar] [CrossRef]
- Wang, C.C.; Ho, Y.S. Research trend of metal–Organic frameworks: A bibliometric analysis. Scientometrics 2016, 109, 481–513. [Google Scholar] [CrossRef]
- Mutanda, T.; Naidoo, D.; Bwapwa, J.K.; Anandraj, A. Biotechnological applications of microalgal oleaginous compounds: Current trends on microalgal bioprocessing of products. Front. Energy Res. 2020, 8, 598803. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.T.; Khong, N.M.H.; Khaw, Y.S.; Ahmad, S.A.; Yusoff, F.M. Optimization of the freezing-thawing method for extracting phycobiliproteins from Arthrospira sp. Molecules 2020, 25, 3894. [Google Scholar] [CrossRef]
- Chamorro-Cevallos, G. Methods for extraction, isolation and purification of C-phycocyanin: 50 years of research in review. Int. J. Food Nutr. Sci. 2016, 3, 1–10. [Google Scholar] [CrossRef][Green Version]
- Begum, H.; Khatoon, H.; Yusoff, F.M.; Shariff, M. Production and purity of phycobiliproteins from selected marine and freshwater cyanobacteria subjected to different drying methods. Asian Fish. Sci. 2020, 33, 258–265. [Google Scholar] [CrossRef]
- Yu, P.; Wu, Y.; Wang, G.; Jia, T.; Zhang, Y. Purification and bioactivities of phycocyanin. Crit. Rev. Food Sci. Nutr. 2017, 57, 3840–3849. [Google Scholar] [CrossRef]
- Hashimoto, E.; Yabuta, Y.; Watanabe, F.; Morimoto, M.; Yamaguchi, Y.; Takenaka, H. Purification and characterization of phycobiliproteins from edible Cyanobacterium Nostochopsis sp. Food Sci. Technol. Res. 2012, 18, 485–490. [Google Scholar] [CrossRef]
- Apt, K.E.; Collier, J.L.; Grossman, A.R. Evolution of the phycobiliproteins. J. Mol. Biol. 1995, 248, 79–96. [Google Scholar] [CrossRef]
- Scheer, H.; Zhao, K.H. Biliprotein maturation: The chromophore attachment. Mol. Microbiol. 2008, 68, 263–276. [Google Scholar] [CrossRef]
- Montgomery, B.L.; Casey, E.S.; Grossman, A.R.; Kehoe, D.M. AplA, a member of a new class of phycobiliproteins lacking a traditional role in photosynthetic light harvesting. Society 2004, 186, 7420–7428. [Google Scholar] [CrossRef]
- Wedemayer, G.J.; Wemmer, D.E.; Glazer, A.N. Phycobilins of Cryptophycean algae: Structures of novel bilins with acryloyl substituents from phycoerythrin 566. J. Biol. Chem. 1991, 266, 4731–4741. [Google Scholar] [CrossRef]
- Wedemayer, G.J.; Kidd, D.G.; Wemmer, D.E.; Glazer, A.N. Phycobilins of Cryptophycean algae. Occurrence of dihydrobiliverdin and mesobiliverdin in cryptomonad biliproteins. J. Biol. Chem. 1992, 267, 7315–7331. [Google Scholar] [CrossRef]
- Schluchter, W.M.; Shen, G.; Alvey, R.M.; Biswas, A.; Saunée, N.A.; Williams, S.R.; Mille, C.A.; Bryant, D.A. Phycobiliprotein Biosynthesis in Cyanobacteria: Structure and Function of Enzymes Involved in Post-Translational Modification; Springer: New York, NY, USA, 2010; Volume 675. [Google Scholar] [CrossRef]
- Belford, H.S.; Offier, G.D.; Troxler, R.F. Phycobiliprotein synthesis in the unicellular rhodophyte, Cyanidiurn cazdurium. J. Biol. Chem. 1983, 258, 4503–4510. [Google Scholar] [CrossRef]
- McErlean, M.; Liu, X.; Cui, Z.; Gust, B.; Van Lanen, S.G. Identification and characterization of enzymes involved in the biosynthesis of pyrimidine nucleoside antibiotics. Nat. Prod. Rep. 2021, 7. [Google Scholar] [CrossRef]
- Brown, S.B.; Holroyd, J.A.; Vernon, D.I. Biosynthesis of phycobiliproteins. Biochem. J. 1984, 219, 905–909. [Google Scholar] [CrossRef]
- Kumar, V.; Maurya, P.K.; Mondal, S.; Sinha, R.P.; Singh, S.P. Photomorphogenesis in the Cyanobacterium Fremyella Diplosiphon Improves Photosynthetic Efficiency; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Singh, N.K.; Sonani, R.R.; Prasad Rastogi, R.; Madamwar, D. The phycobilisomes: An early requisite for efficient photosynthesis in Cyanobacteria. EXCLI J. 2015, 14, 268–289. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Baky, H.H.; El-Baroty, G.S. Characterization and bioactivity of phycocyanin isolated from Spirulina Maxima grown under salt stress. Food Funct. 2012, 3, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Amiri, R.Z.; Esmaeilzadeh Kenari, R. Antioxidant and antibacterial activities of C-Phycocyanin from common name Spirulina platensis. Iran. J. Fish. Sci. 2020, 19, 1911–1927. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Chen, C. Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. USA 2004, 101, 5303–5310. [Google Scholar] [CrossRef]
- Jiang, M.; Qi, Y.; Liu, H.; Chen, Y. The role of nanomaterials and nanotechnologies in wastewater treatment: A bibliometric analysis. Nanoscale Res. Lett. 2018, 13, 233. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace: A Practical Guide for Mapping Scientific Literature; Nova Science Publishers: Hauppauge, NY, USA, 2016. [Google Scholar]
- Sundström, V.; van Grondelle, R. Dynamics of excitation energy transfer in photosynthetic bacteria. Chlorophylls 1991, 2137, 627–704. [Google Scholar]
- Jung, T.M.; Dailey, M.O. A novel and inexpensive source of allophycocyanin for multicolor flow cytometry. J. Immunol. Methods 1989, 121, 9–18. [Google Scholar] [CrossRef]
- Glazer, A.N. Phycobiliproteins—A family of valuable, widely used fluorophores. J. Appl. Phycol. 1994, 6, 105–112. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K.S.M.S. Simple and efficient method for extraction of C-phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Jáuregui, M.; Medina, E.; Jaime, C.; Cerezal, P. Rapid green extractions of C-phycocyanin from Arthrospira Maxima for functional applications. Appl. Sci. 2019, 9, 1987. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Liu, D.; Qin, S.; Sun, S.; Zhao, J.; Sui, S.F. Structure of phycobilisome from the red alga Griffithsia pacifica. Nature 2017, 551, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; You, X.; Sun, S.; Wang, X.; Qin, S.; Sui, S.F. Structural basis of energy transfer in Porphyridium Purpureum phycobilisome. Nature 2020, 579, 146–151. [Google Scholar] [CrossRef]
- Farrar, W.V. Tecuitlatl: A Glimpse of Aztec food technology. Nature 1966, 211, 341–342. [Google Scholar] [CrossRef]
- Hamed, I. The evolution and versatility of microalgal biotechnology: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef]
- Abdulqader, G.; Barsanti, L.; Tredici, M.R. Harvest of Arthrospira Platensis from Lake Kossorom (Chad) and its household usage among the Kanembu. J. Appl. Phycol. 2000, 12, 493–498. [Google Scholar] [CrossRef]
- Ludwig, M.; Bryant, D.A. Synechococcus Sp. Strain PCC 7002 transcriptome: Acclimation to temperature, salinity, oxidative stress, and mixotrophic growth conditions. Front. Microbiol. 2012, 3, 354. [Google Scholar] [CrossRef]
- Coutinho, F.; Tschoeke, D.A.; Thompson, F.; Thompson, C. Comparative genomics of Synechococcus and proposal of the new GENUS Parasynechococcus. PeerJ 2016, 2016, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Singh, D.; Tyagi, M.B.; Kumar, A. Cyanobacteria: Applications in Biotechnology; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 7421. [Google Scholar] [CrossRef]
- Wang, K.; Wommack, K.E.; Chen, F. Abundance and distribution of Synechococcus spp. and cyanophages in the Chesapeake Bay. Appl. Environ. Microbiol. 2011, 77, 7459–7468. [Google Scholar] [CrossRef] [PubMed]
- Cordara, A.; Re, A.; Pagliano, C.; Van Alphen, P.; Pirone, R.; Saracco, G.; dos Santos, F.B.; Hellingwerf, K.; Vasile, N. Analysis of the light intensity dependence of the growth of Synechocystis and of the light distribution in a photobioreactor energized by 635 Nm light. PeerJ 2018, 2018, e5256. [Google Scholar] [CrossRef]
- Li, Z.; Guo, M. Healthy efficacy of Nostoc Commune vaucher. Oncotarget 2018, 9, 14669–14679. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Liang, S.; Lee, J. Toxin-producing Cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. J. Microbiol. 2013, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Atia, A. Review on freshwater blue-green algae (Cyanobacteria): Occurrence, classification and toxicology. Biosci. Biotechnol. Res. Asia 2014, 11, 1319–1325. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.M.; Wosu, C.I.; Ben-Amotz, A.; Harvey, P.J. Potential of new isolates of Dunaliella Salina for natural β-carotene production. Biology 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef]
- Yusoff, F.M.; Nagao, N.; Imaizumi, Y.; Toda, T. Bioreactor for microalgal cultivation systems: Strategy and development. In Prospects of Renewable Bioprocessing in Future Energy Systems, Biofuel and Biorefinery Technologies; Springer: Berlin/Heidelberg, Germany, 2019; pp. 117–159. [Google Scholar] [CrossRef]
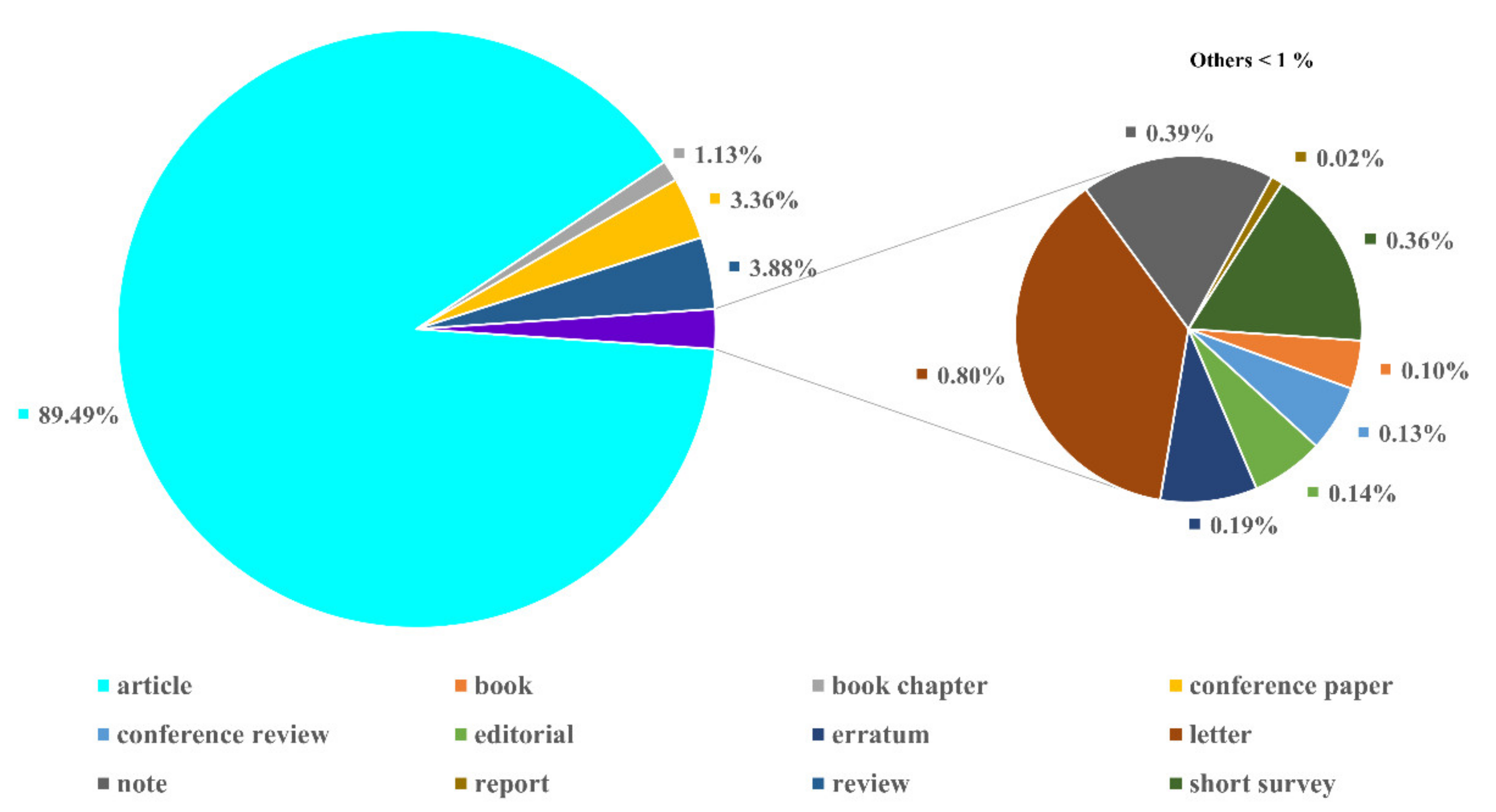
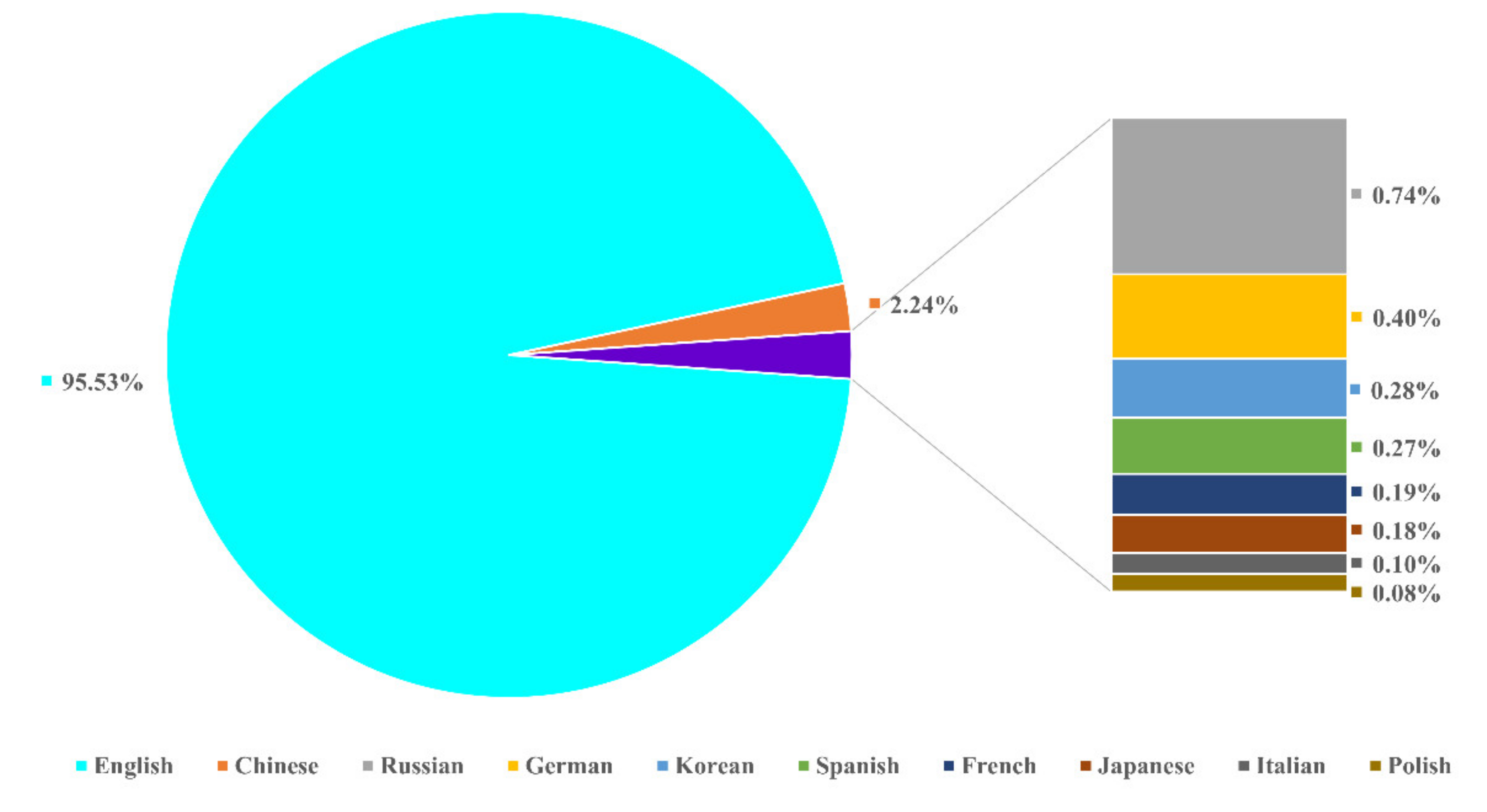


| Description | Results |
|---|---|
| Period | 1909–2020 |
| Sources (journals, books, etc.) | 2214 |
| Documents | 8296 |
| Average years from publication | 16.7 |
| Average citations per documents | 30.88 |
| Average citations per year per doc | 1.85 |
| Annual growth rate of scientific production | 9% |
| Authors | 22,871 |
| Authors of single-authored documents | 331 |
| Authors of multi-authored documents | 22,540 |
| Subject Area | Percentage (%) | Rank |
|---|---|---|
| Biochemistry, Genetics, and Molecular Biology | 26.00 | 1 |
| Agricultural and Biological Sciences | 14.60 | 2 |
| Medicine | 12.60 | 3 |
| Immunology and Microbiology | 9.10 | 4 |
| Chemistry | 7.00 | 5 |
| Environmental Science | 5.90 | 6 |
| Chemical Engineering | 4.00 | 7 |
| Engineering | 3.32 | 8 |
| Pharmacology, Toxicology, and Pharmaceutics | 3.30 | 9 |
| Physics and Astronomy | 2.80 | 10 |
| Sources | TP | TP (%) | Cumulative (%) | TC | IF 2020 | Rank by JIF | h- Index | Publisher |
|---|---|---|---|---|---|---|---|---|
| Journal of Applied Phycology | 184 | 2.22 | 2.22 | 4122 | 3.215 | Q1 | 93 | Springer |
| Journal of Biological Chemistry | 137 | 1.65 | 3.87 | 6672 | 5.157 | Q1 | 477 | American Society for Biochemistry and Molecular Biology |
| Journal of Phycology | 127 | 1.53 | 5.40 | 5584 | 2.923 | Q1 | 114 | Wiley |
| Cytometry | 121 | 1.46 | 6.86 | 5396 | 2.698 | n/a | 61 | Wiley |
| Photochemistry and Photobiology | 96 | 1.16 | 8.02 | 1979 | 3.421 | Q3 | 120 | Wiley |
| Photosynthesis Research | 95 | 1.15 | 9.17 | 2173 | 3.573 | Q1 | 99 | Springer |
| Proceedings of the National Academy of Sciences of the United States of America | 83 | 1.00 | 10.17 | 5923 | 11.205 | Q1 | 699 | United States National Academy of Sciences (U.S.A.) |
| Biochimica et Biophysica Acta—Bioenergetics | 82 | 0.99 | 11.16 | 2556 | 3.991 | Q2 | 154 | Elsevier |
| Archives of Microbiology | 81 | 0.98 | 12.14 | 3654 | 2.552 | Q3 | 94 | Springer |
| Biochemistry | 77 | 0.93 | 13.07 | 3144 | 3.162 | Q3 | 253 | American Chemical Society |
| PY | TP | TA | TA/TP | TR | TR/TP | TC | TC/TP |
|---|---|---|---|---|---|---|---|
| ≤1960 | 33 | 46 | 1.39 | 11 | 0.03 | 841 | 25.48 |
| 1961–1970 | 133 | 161 | 1.21 | 413 | 3.11 | 3736 | 28.09 |
| 1971–1980 | 337 | 422 | 1.25 | 5691 | 16.89 | 11,462 | 34.01 |
| 1981–1990 | 821 | 1547 | 1.88 | 15,380 | 18.73 | 30,248 | 36.84 |
| 1991–2000 | 1553 | 4175 | 2.69 | 35,553 | 22.89 | 70,619 | 45.47 |
| 2001–2010 | 1909 | 6410 | 3.36 | 58,387 | 30.59 | 81,576 | 42.73 |
| 2011–2020 | 3510 | 14,000 | 3.99 | 158,176 | 45.06 | 57,738 | 16.45 |
| Title of Publications | Journal | TC | PD | References |
|---|---|---|---|---|
| The Chemistry behind Antioxidant Capacity Assays | Journal of Agricultural and Food Chemistry | 4010 | Feb 2005 | [30] |
| Commercial Applications of Microalgae | Journal of Bioscience and Bioengineering | 2574 | Oct 2006 | [31] |
| Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships | Free Radical Biology and Medicine | 2038 | June 1997 | [32] |
| Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe | Journal of Agricultural and Food Chemistry | 2024 | Jan 2001 | [35] |
| HLA-E Binds to Natural Killer Cell Receptors CD94/NKG2A, B and C | Nature | 1592 | Feb 1998 | [36] |
| Determination of Lymphocyte Division by Flow Cytometry | Journal of Immunological Method | 1439 | Feb 1994 | [37] |
| Resolution and Characterization of Pro-B and Pre-Pro-B Cell Stages in Normal Mouse Bone Marrow | Journal of Experimental Medicine | 1317 | May 1991 | [38] |
| Accessing Genetic Information with High-Density DNA Arrays | Science | 1309 | Oct 1996 | [39] |
| Oxygen-Radical Absorbance Capacity Assay for Antioxidants | Free Radical Biology and Medicine | 1256 | Jan 1993 | [40] |
| A New Approach to Protein Fold Recognition | Nature | 1035 | July 1992 | [34] |
| Country | TP (%) | TC | TC/TP | h | Number of Publications | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤1960 | 1961–1970 | 1971–1980 | 1981–1990 | 1991–2000 | 2001–2010 | 2011–2020 | |||||
| United States of America (U.S.A.) | 2240 (27.00) | 103,660 | 46.28 | 132 | 11 | 65 | 138 | 353 | 538 | 470 | 665 |
| China | 1177 (14.19) | 17,961 | 15.26 | 60 | 0 | 0 | 0 | 8 | 89 | 289 | 791 |
| Germany | 775 (9.34) | 24,613 | 31.76 | 71 | 0 | 9 | 46 | 112 | 179 | 201 | 228 |
| India | 637 (7.67) | 13,752 | 21.59 | 56 | 0 | 1 | 7 | 21 | 79 | 148 | 381 |
| Japan | 534 (6.44) | 13,814 | 25.87 | 59 | 6 | 7 | 23 | 61 | 116 | 151 | 170 |
| France | 441 (5.32) | 20,542 | 46.58 | 71 | 0 | 3 | 12 | 41 | 111 | 130 | 144 |
| United Kingdom | 382 (4.60) | 17,008 | 44.52 | 64 | 3 | 10 | 7 | 46 | 100 | 91 | 125 |
| Spain | 299 (3.60) | 9303 | 31.11 | 52 | 0 | 0 | 0 | 10 | 61 | 86 | 142 |
| Italy | 264 (3.18) | 8229 | 31.17 | 47 | 0 | 1 | 4 | 8 | 57 | 59 | 135 |
| Canada | 246 (2.97) | 9106 | 37.02 | 54 | 0 | 1 | 4 | 25 | 62 | 59 | 95 |
| Institution | Country | TP (%) | TC | TC/TP | h | Number of Publications | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤1960 | 1961–1970 | 1971–1980 | 1981–1990 | 1991–2000 | 2001–2010 | 2011–2020 | ||||||
| Chinese Academy of Sciences | China | 352 (4.24) | 6599 | 18.75 | 44 | 0 | 0 | 0 | 6 | 63 | 96 | 187 |
| CNRS (Centre National de la Recherche Scientifique) | France | 150 (1.80) | 6658 | 44.39 | 45 | 0 | 3 | 5 | 14 | 34 | 36 | 58 |
| Ludwig-Maximilians-Universität München | Germany | 132 (1.59) | 3703 | 28.05 | 35 | 0 | 1 | 20 | 36 | 30 | 24 | 21 |
| University of California, Berkeley | U.S.A. | 130 (1.57) | 7574 | 58.26 | 49 | 1 | 2 | 13 | 52 | 39 | 6 | 17 |
| Russian Academy of Sciences | Russia | 105 (1.27) | 2080 | 19.81 | 22 | 0 | 3 | 10 | 6 | 22 | 21 | 43 |
| Ministry of Education China | China | 94 (1.13) | 1089 | 11.59 | 18 | 0 | 0 | 0 | 0 | 0 | 10 | 84 |
| The University of Tokyo | Japan | 91 (1.10) | 2981 | 32.76 | 29 | 2 | 5 | 11 | 10 | 14 | 25 | 24 |
| University of Chinese Academy of Sciences | China | 91 (1.10) | 1571 | 17.26 | 24 | 0 | 0 | 0 | 0 | 0 | 39 | 52 |
| New York State Department of Health | U.S.A. | 81 (0.98) | 2141 | 26.43 | 22 | 0 | 10 | 25 | 22 | 20 | 3 | 1 |
| Lomonosov Moscow State University | Russia | 73 (0.88) | 1078 | 14.77 | 19 | 0 | 0 | 2 | 4 | 17 | 16 | 34 |
| Author | Country | Institutions | TP (%) | h | Number of Publications | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤1960 | 1961–1970 | 1971–1980 | 1981–1990 | 1991–2000 | 2001–2010 | 2011–2020 | ||||||
| Glazer, Alexander N. | U.S.A. | University of California | 118 (1.42) | 65 | glazer@berkeley.edu | 0 | 0 | 28 | 57 | 28 | 5 | 0 |
| Scheer, Hugo | Germany | Ludwig-Maximilians-Universität München | 89 (1.07) | 49 | hugo.scheer@lmu.de | 0 | 0 | 6 | 23 | 26 | 16 | 18 |
| Bryant, Donald A. | U.S.A. | Pennsylvania State University | 64 (0.77) | 72 | dab14@psu.edu | 0 | 0 | 5 | 16 | 14 | 12 | 17 |
| Qin, Song | China | Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, | 62 (0.75) | 35 | sqin@yic.ac.cn | 0 | 0 | 0 | 0 | 1 | 25 | 36 |
| Berns, Donald S. | Israel | Israel Ministry of Health | 59 (0.71) | 24 | d.berns@netvision.net.il | 0 | 14 | 28 | 13 | 4 | 0 | 0 |
| Zhao, Kaihong | China | Huazhong Agricultural University | 55 (0.66) | 26 | kaihongzhao@mail.hzau.edu.cn | 0 | 0 | 0 | 0 | 5 | 20 | 30 |
| MacColl, Robert | U.S.A. | Wadsworth Center for Laboratories and Research | 52 (0.63) | 25 | maccoll@wadsworth.ph.albany.edu | 0 | 0 | 14 | 15 | 19 | 3 | 1 |
| Madamwar, Datta B. | India | Sardar Patel University | 45 (0.54) | 49 | datta_madamwar@yahoo.com | 0 | 0 | 0 | 0 | 1 | 8 | 36 |
| Bekasova, Olga D. | Russia | Bach Institute of Biochemistry, | 40 (0.48) | 7 | bekasova@inbi.ras.ru | 0 | 3 | 14 | 8 | 1 | 6 | 6 |
| Gantt, Elisabeth | U.S.A. | University of Maryland | 40 (0.48) | 43 | egantt@umd.edu | 0 | 2 | 19 | 15 | 3 | 0 | 1 |
| Clusters | Co-Words | Themes |
|---|---|---|
| Cluster I (21 items) | Algae, Arthrospira platensis, biomass production, bioreactor, biotechnology, culture media, extraction, isolation and purification, light intensity, light quality, optimization, oscillatoria, pH, photobioreactor, phycobiliprotein, Porphyridium, Porphyridium cruentum, process optimization, salinity, spirulina platensis and temperature | Optimization of cyanobacteria cultivation and phycobiliprotein harvesting process |
| Cluster II (12 items) | Allophycocyanin, Anabaena sp., Anabaena variabilis, cyanobacteria, cyanobacterium, Fremyella diplosiphon, Mastigocladus laminosu, nostoc sp., phycocyanin, phycoerythrocyanin, Synechococcus elongatus and Synechocystis sp. | Classification of phycobiliproteins from different cyanobacteria |
| Cluster III (11 items) | Biliverdin, biliverdine, biosynthesis, ferredoxin, fremyella diplosiphon, gene expression, phycobilins, phycocyanobilin, phycoerythrin, phycoerythrobilin and prochlorococcus | Gene expression of the phycobiliprotein biosynthesis pathway |
| Cluster IV (4 items) | Anti-inflammatory activity, antineoplastic agent, antioxidant, antioxidant activity | Bioactivities of phycobiliprotein |
| Cluster V (4 items) | Biofuels, fluorescent dye, fluorescent dyes, fluorescent spectroscopy | Applications of phycobiliprotein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.T.; Yusoff, F.M.; Khaw, Y.S.; Ahmad, S.A.; Shaharuddin, N.A. Uncovering Research Trends of Phycobiliproteins Using Bibliometric Approach. Plants 2021, 10, 2358. https://doi.org/10.3390/plants10112358
Tan HT, Yusoff FM, Khaw YS, Ahmad SA, Shaharuddin NA. Uncovering Research Trends of Phycobiliproteins Using Bibliometric Approach. Plants. 2021; 10(11):2358. https://doi.org/10.3390/plants10112358
Chicago/Turabian StyleTan, Hui Teng, Fatimah Md. Yusoff, Yam Sim Khaw, Siti Aqlima Ahmad, and Noor Azmi Shaharuddin. 2021. "Uncovering Research Trends of Phycobiliproteins Using Bibliometric Approach" Plants 10, no. 11: 2358. https://doi.org/10.3390/plants10112358
APA StyleTan, H. T., Yusoff, F. M., Khaw, Y. S., Ahmad, S. A., & Shaharuddin, N. A. (2021). Uncovering Research Trends of Phycobiliproteins Using Bibliometric Approach. Plants, 10(11), 2358. https://doi.org/10.3390/plants10112358