Niche Differentiation at Multiple Spatial Scales on Large and Small Mediterranean Islands for the Endemic Silene velutina Pourr. ex Loisel. (Caryophyllaceae)
Abstract
1. Introduction
2. Results
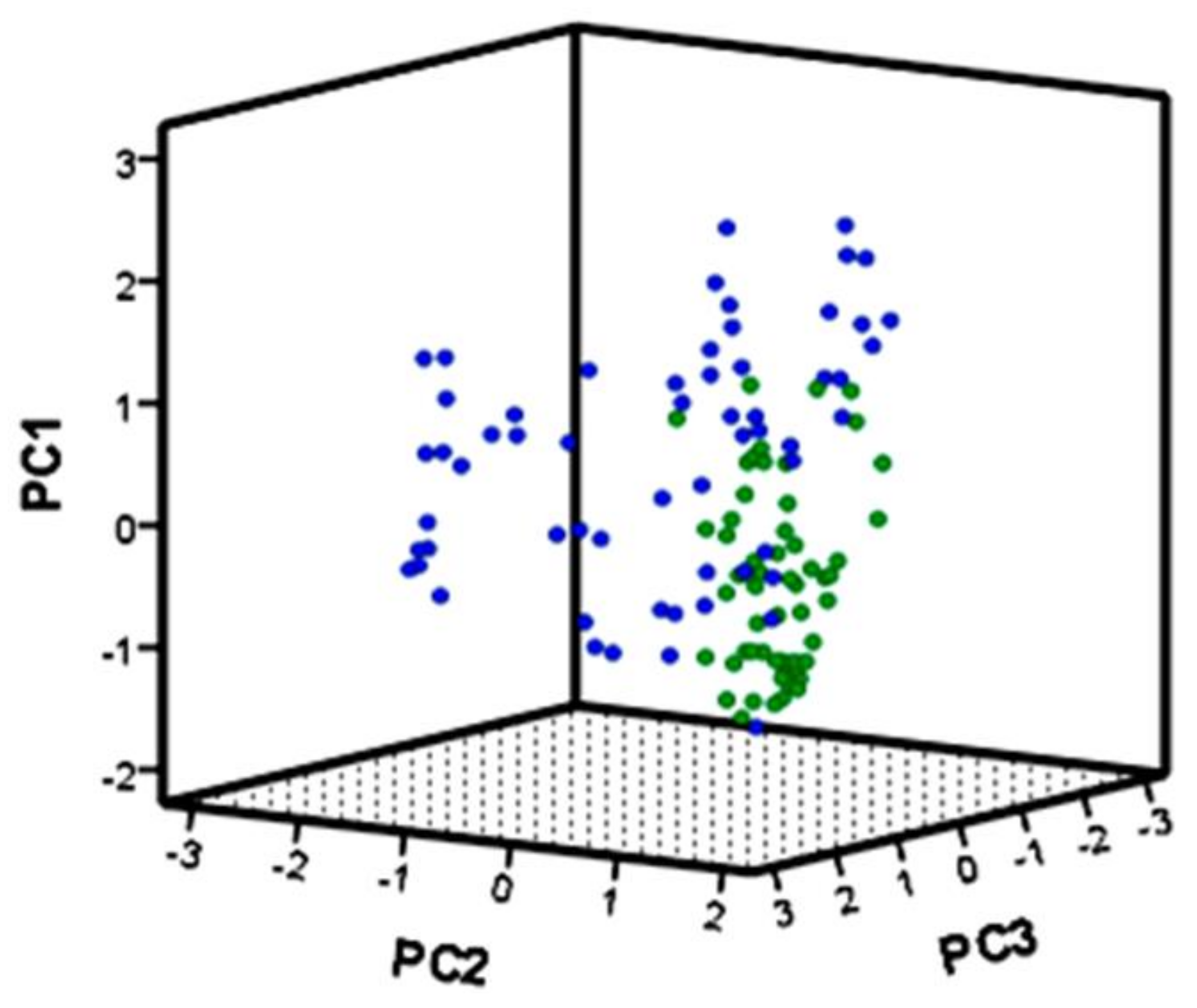
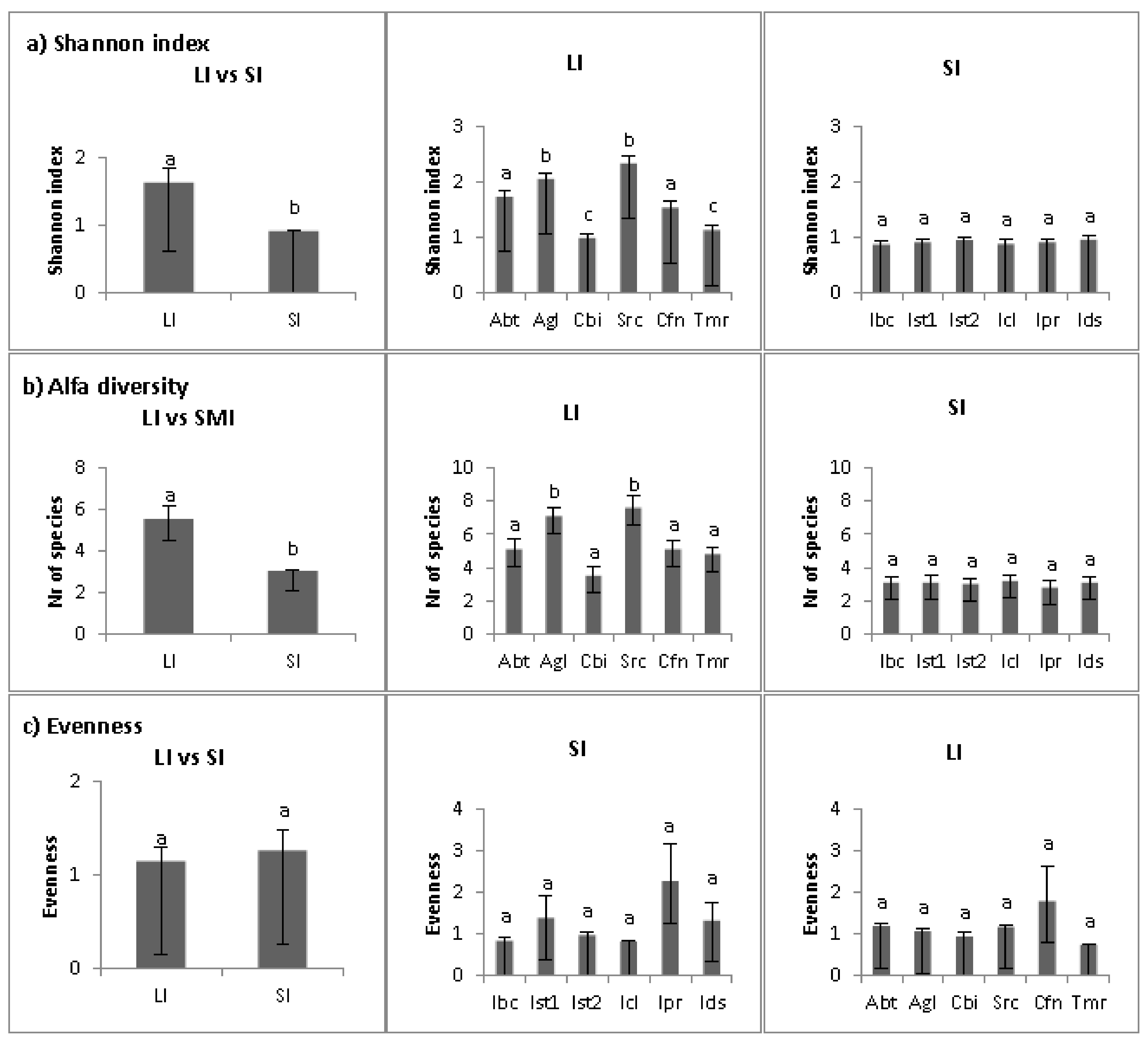
2.1. Multivariate Analyses
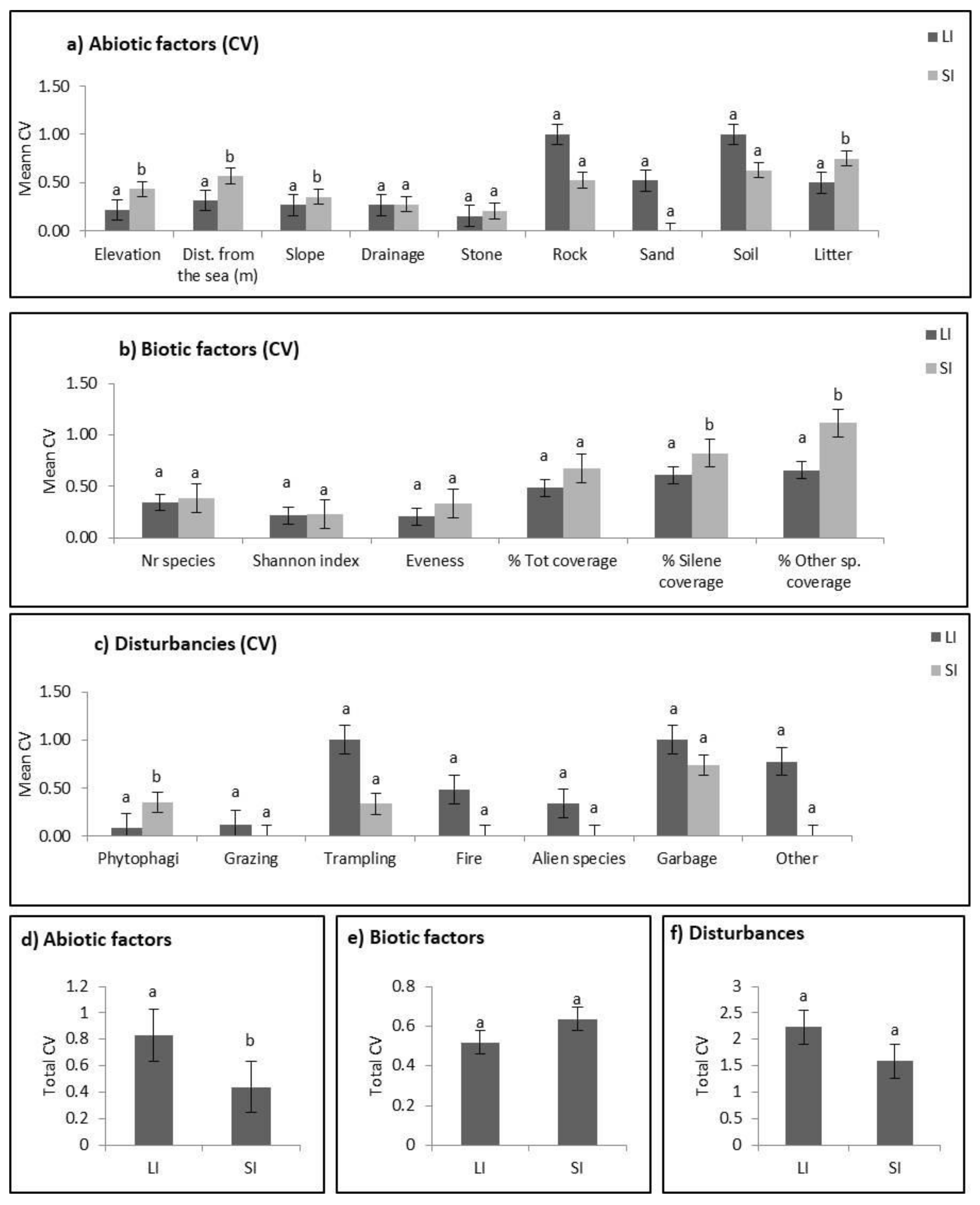
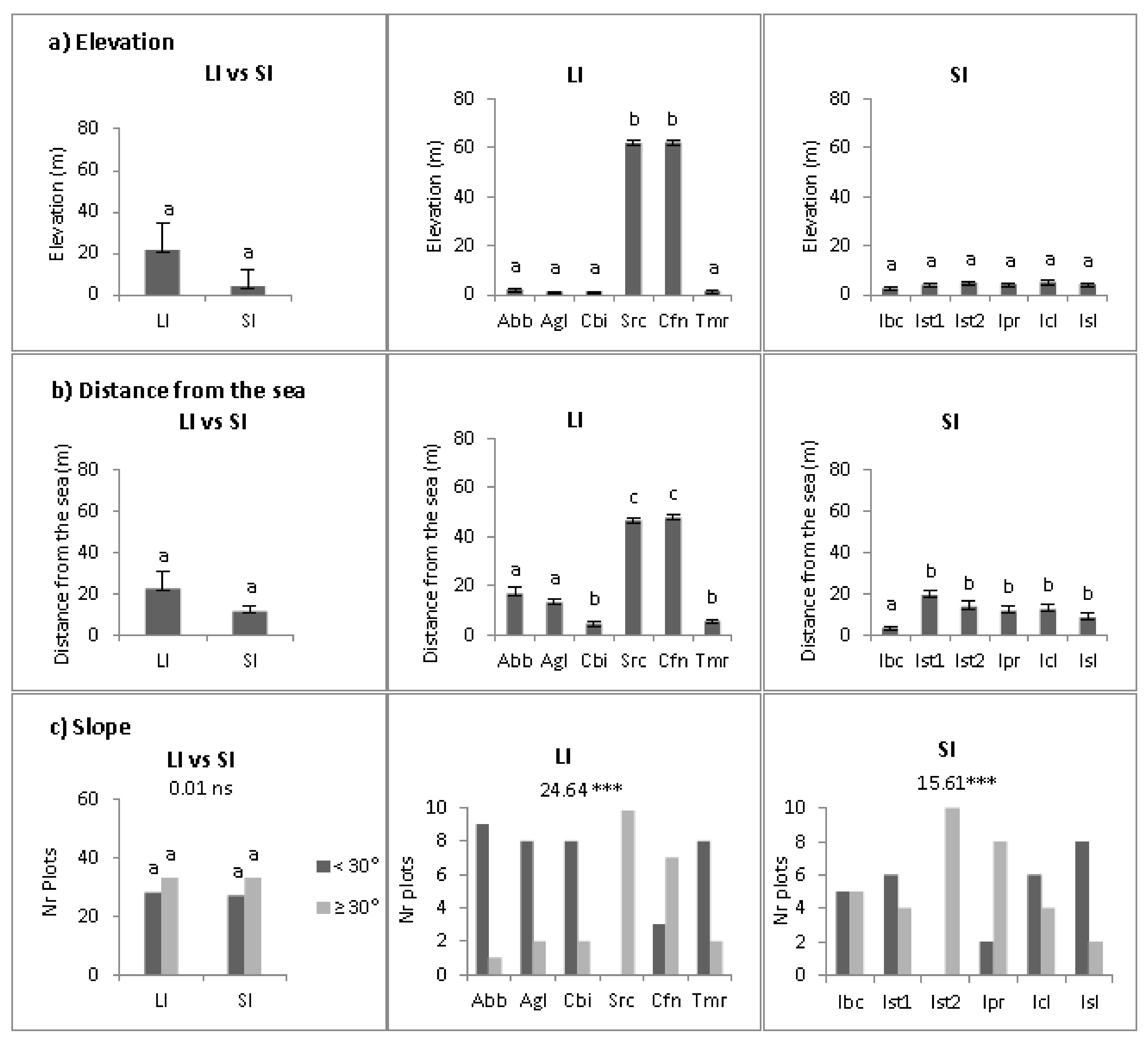
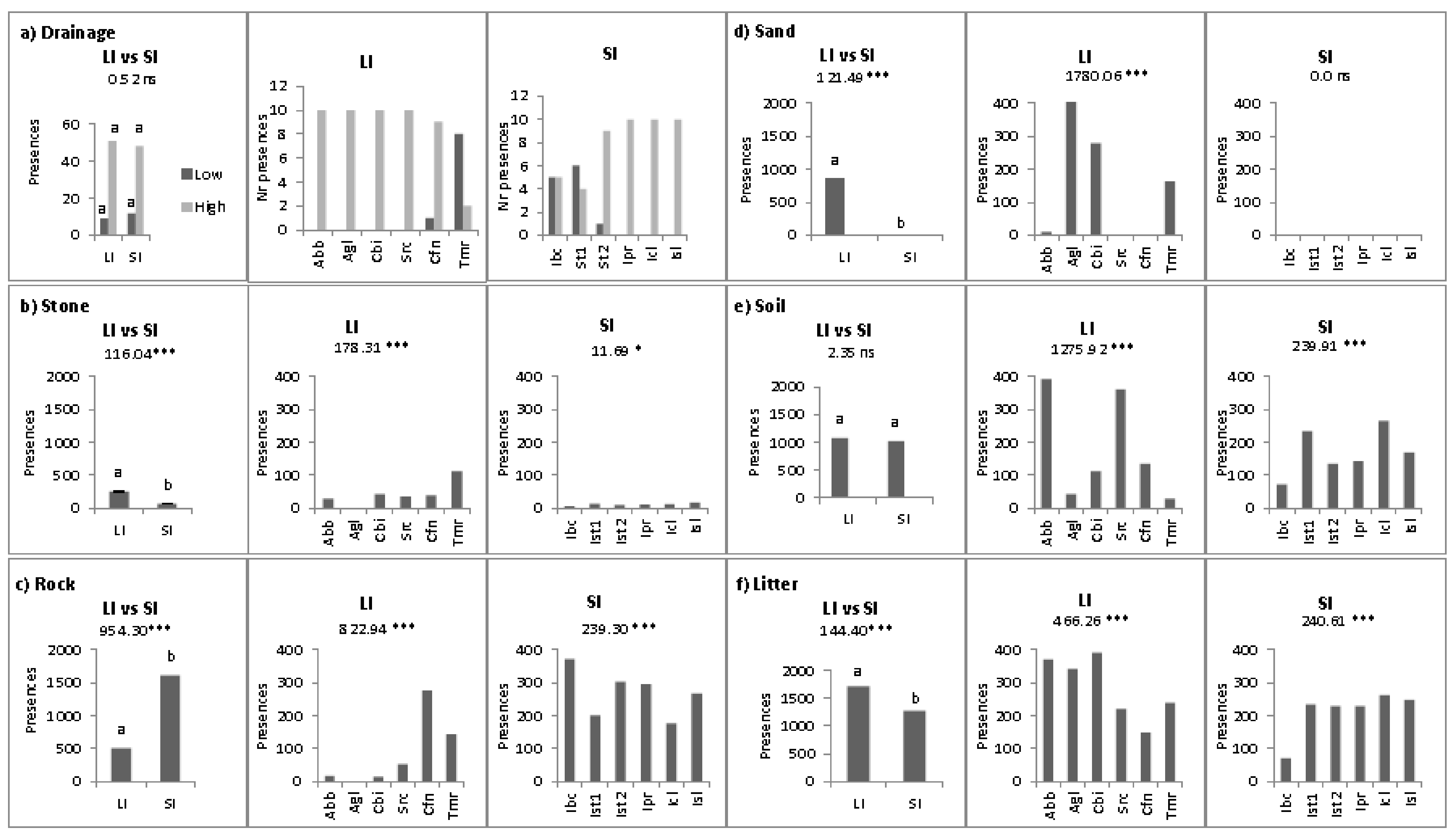
2.2. Abiotic Niche Components
2.3. Biotic Niche Components
2.4. Disturbance
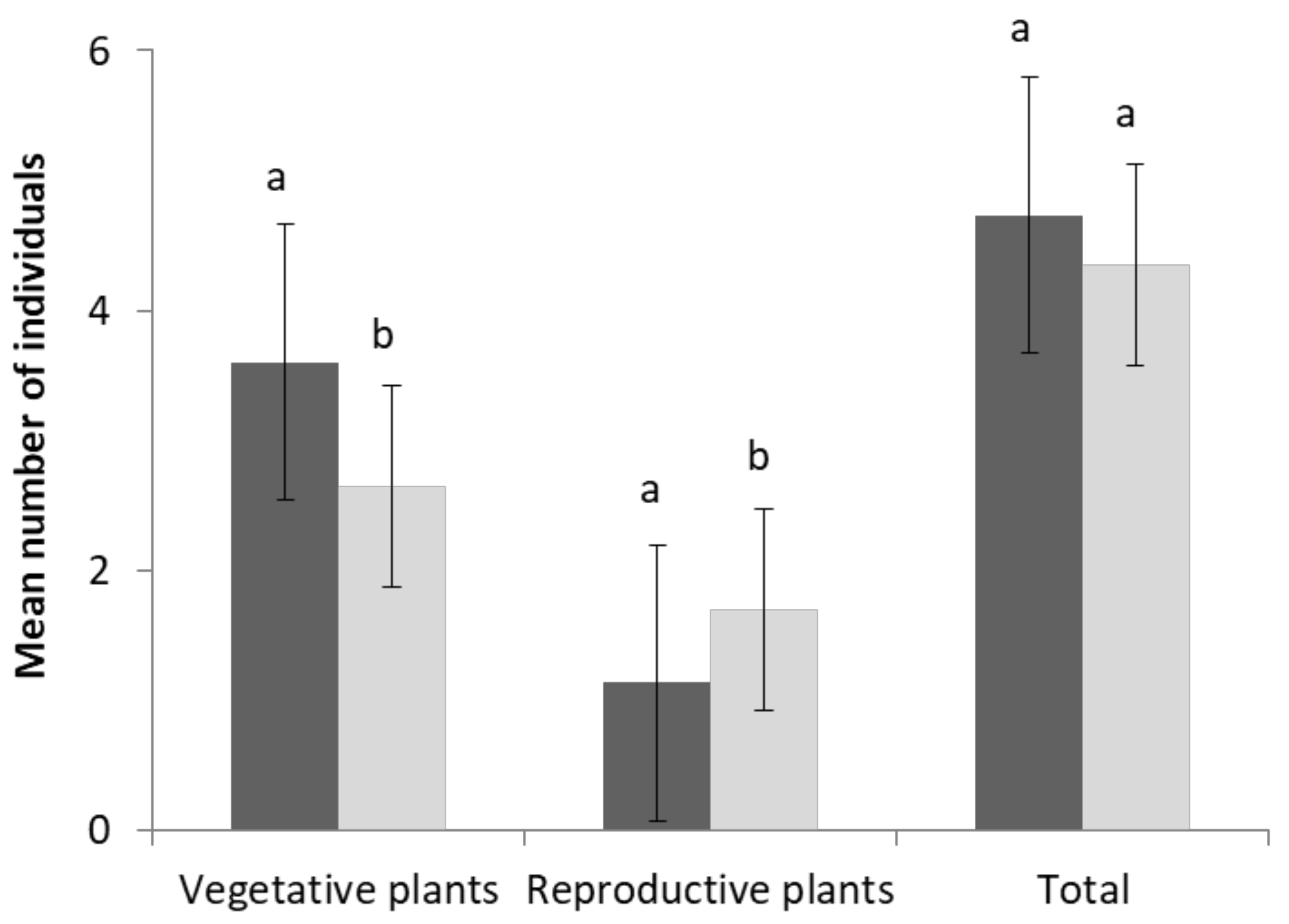
2.5. Population Parameters
3. Discussion
4. Materials and Methods
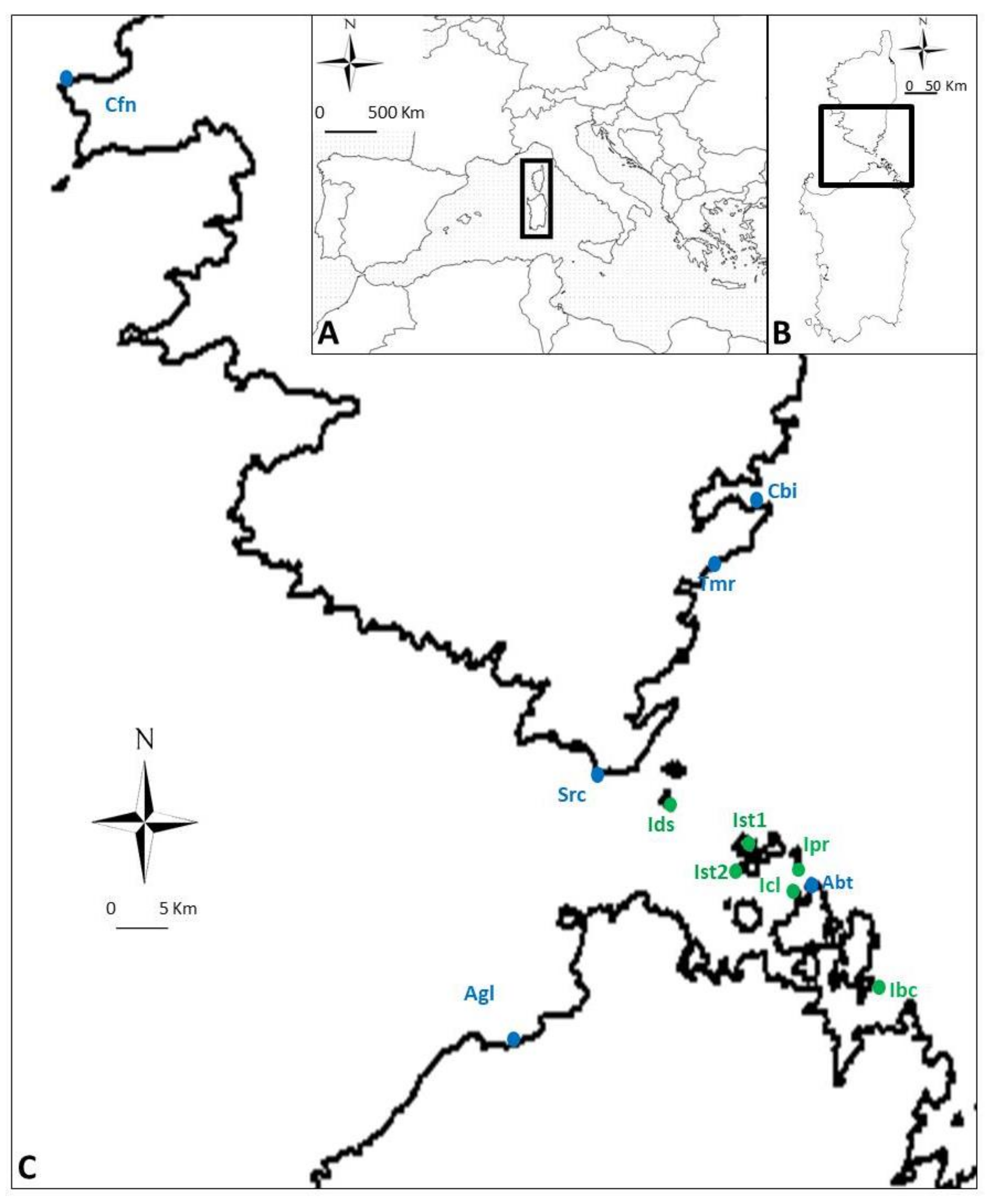
4.1. Study Species and Sites
4.2. Sampling Design and Response Variables
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darwin, C. On the Origin of the Species by Natural Selection; D. Appleton and Company: New York, NY, USA, 1859; p. 466. [Google Scholar]
- Wallace, A.R. Island Life: Or the Phenomena and Causes of Insular Faunas and Floras, Including a Revision and Attempted Solution of the Problem of Geological Climates; Macmillan and Co.: London, UK, 1880; p. 526. [Google Scholar]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967; p. 224. [Google Scholar]
- Wardle, D.A.; Zackrisson, O.; Hornberg, G.; Gallet, C. The influence of island area on ecosystem properties. Science 1997, 277, 1296–1299. [Google Scholar] [CrossRef]
- Cody, M.L. Plants on Islands: Diversity and Dynamics on a Continental Archipelago; University of California Press: Berkeley, CA, USA, 2006; p. 269. [Google Scholar]
- Yu, M.; Hu, G.; Feeley, K.J.; Wu, J.; Ding, P. Richness and composition of plants and birds on land-bridge islands: Effects of island attributes and differential responses of species groups. J. Biogeogr. 2012, 39, 1124–1133. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001; p. 392. [Google Scholar]
- Williams, C.B. Patterns in the Balance of Nature and Related Problems in Quantitative Ecology; Academic Press: London, UK; New York, NY, USA, 1964; p. 324. [Google Scholar]
- Kohn, D.D.; Walsh, D.M. Plant species richness—The effect of island size and habitat diversity. J. Ecol. 1994, 82, 367–377. [Google Scholar] [CrossRef]
- Triantis, K.A.; Mylonas, M.; Lika, K.; Vardinoyannis, K. A model for the species–area–habitat relationship. J. Biogeogr. 2003, 30, 19–27. [Google Scholar] [CrossRef]
- Panitsa, M.; Tzanoudakis, D.; Sfenthourakis, S. Turnover of plants on small islets of the eastern Aegean Sea within two decades. J. Biogeogr. 2008, 35, 1049–1061. [Google Scholar] [CrossRef]
- Panitsa, M.; Trigas, P.; Iatrou, G.; Sfenthourakis, S. Factors affecting plant species richness and endemism on land-bridge islands: An example from the East Aegean archipelago. Acta Oecol. 2010, 36, 431–437. [Google Scholar] [CrossRef]
- Hortal, J.; Triantis, K.A.; Meiri, S.; Thébault, E.; Sfenthourakis, S. Island species richness increases with habitat diversity. Am. Nat. 2009, 174, 205–217. [Google Scholar] [CrossRef]
- Whitehead, D.R.; Jones, C.E. Small islands and the equilibrium theory of insular biogeography. Evolution 1969, 23, 171–179. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Weiser, M.D. Towards a more general species–area relationship: Diversity on all islands, great and small. J. Biogeogr. 2001, 28, 431–445. [Google Scholar] [CrossRef]
- Triantis, K.A.; Vardinoyannis, K.; Tsolaki, E.P.; Botsaris, I.; Lika, K.; Mylonas, M. Re-approaching the small island effect. J. Biogeogr. 2006, 33, 914–923. [Google Scholar] [CrossRef]
- Chen, C.W.; Yang, X.R.; Tan, X.W.; Wang, Y.P. The role of habitat diversity in generating the small island effect. Ecography 2020, 43, 1241–1249. [Google Scholar] [CrossRef]
- Farris, E.; Pisanu, S.; Ceccherelli, G.; Filigheddu, R. Effects of the management regime on the performance of the endangered Mediterranean Centaurea horrida Badarò (Asteraceae). J. Nat. Conserv. 2009, 17, 15–24. [Google Scholar] [CrossRef]
- Sheil, D. Disturbance and distributions: Avoiding exclusion in a warming world. Ecol. Soc. 2016, 21, 10. [Google Scholar] [CrossRef]
- Fois, M.; Fenu, G.; Bacchetta, G. Global analyses underrate part of the story: Finding applicable results for the conservation planning of small Sardinian islets′ flora. Biodivers. Conserv. 2016, 25, 1091–1106. [Google Scholar] [CrossRef]
- Thompson, J.D. Plant Evolution in the Mediterranean. Insignts for Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2020; p. 464. [Google Scholar]
- Whittaker, R.J.; Fernandez-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2007; p. 416. [Google Scholar]
- Médail, F.; Vidal, E. Organisation de la richesse et de la composition floristiques d’iles de la Méditerranée occidentale (sud-est de la France). Can. J. Bot. 1998, 76, 321–331. [Google Scholar]
- Kallimanis, A.S.; Mazaris, A.D.; Tzanopoulos, J.; Halley, J.M.; Pantis, J.D.; Sgardelis, S.P. How does habitat diversity affect the species–area relationship? Glob. Ecol. Biogeogr. 2008, 17, 532–538. [Google Scholar] [CrossRef]
- Fois, M.; Podda, L.; Médail, F.; Bacchetta, G. Endemic and alien vascular plant diversity in the small Mediterranean islands of Sardinia: Drivers and implications for their conservation. Biol. Conserv. 2020, 244, 108519. [Google Scholar] [CrossRef]
- Médail, F. Plant Biogeography and Vegetation Patterns of the Mediterranean Islands. Bot. Rev. 2021, 1–67. [Google Scholar] [CrossRef]
- García, D.; Zamora, R. Persistence, multiple demographic strategies and conservation in long-lived Mediterranean plants. J. Veg. Sci. 2003, 14, 921–926. [Google Scholar] [CrossRef]
- Baumel, A.; Affre, L.; Véla, E.; Auda, P.; Torre, F.; Youssef, S.; Tatoni, T. Ecological magnitude and fine scale dynamics of the Mediterranean narrow endemic therophyte, Arenaria provincialis (Caryophyllaceae). Acta Bot. Gall. 2009, 156, 259–272. [Google Scholar] [CrossRef][Green Version]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Sparrow, A.D. Resprouting as a life history strategy in woody plant communities. Oikos 2000, 89, 409–416. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Grove, A.T.; Rackham, O. The Nature of Mediterranean Europe: An Ecological History; Yale University Press: New Haven, CT, USA, 2001; p. 384. [Google Scholar]
- Quézel, P.; Médail, F. Ecologie et Biogéographie des Forêts du Bassin Méditerranéen; Elsevier: Paris, France, 2003; p. 576. [Google Scholar]
- Lavergne, S.; Thompson, J.D.; Garnier, E.; Debussche, M. The biology and ecology of narrow endemic and widespread plants: A comparative study of trait variation in 20 congeneric pairs. Oikos 2004, 107, 505–518. [Google Scholar] [CrossRef]
- Lavergne, S.; Thuiller, W.; Molina, J.; Debussche, M. Environmental and human factors influencing rare plant local occurrence, extinction and persistence: A 115-year study in the Mediterranean region. J. Biogeogr. 2005, 32, 799–811. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M.; Lawton, J.H. Interspecific abundance range size relationships: An appraisal of mechanisms. J. Anim. Ecol. 1997, 66, 579–601. [Google Scholar] [CrossRef]
- Hughes, J.B. The scale of resource specialization and the distribution and abundance of lycaenid butterflies. Oecologia 2000, 123, 375–383. [Google Scholar] [CrossRef]
- Devictor, V.; Clavel, J.; Julliard, R.; Lavergne, S.; Mouillot, D.; Thuiller, W.; Venail, P.; Villeger, S.; Mouquet, N. Defining and measuring ecological specialization. J. Appl. Ecol. 2010, 47, 15–25. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Ingram, T.; Stutz, W.E.; Snowberg, L.K.; Lau, O.L.; Paull, J.S. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc. R. Soc. Lond. B Biol. Sci. 2010, 277, 1789–1797. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R.M. Positive interactions in communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- Grubb, P.J. The maintenance of species richness in plant communities: The importance of the regeneration niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; John Wiley & Sons: Chichester, UK, 2001; p. 456. [Google Scholar]
- Büchi, L.; Vuilleumier, S. Ecological strategies in stable and disturbed environments depend on species specialisation. Oikos 2016, 125, 1408–1420. [Google Scholar] [CrossRef]
- Debussche, M.; Thompson, J.D. Habitat differentiation between two closely related Mediterranean plant species, the endemic Cyclamen balearicum and the widespread C. Repandum. Acta Oecol. 2003, 24, 35–45. [Google Scholar] [CrossRef]
- Larson, D.W.; Matthes, U.; Gerrath, J.A.; Gerrath, J.M.; Nekola, J.C.; Walker, G.L.; Porembski, S.; Charlton, A.; Larson, N.W.K. Ancient stunted trees on cliffs. Nature 1999, 398, 382–383. [Google Scholar] [CrossRef]
- Larson, D.W.; Matthes, U.; Kelly, P.E. Cliff Ecology. Pattern and Process in Cliff Ecosystems; Cambridge University Press: Cambridge, UK, 2000; p. 360. [Google Scholar]
- Van Valen, L. Morphological variation and width of ecological niche. Am. Nat. 1965, 99, 377–390. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptive radiation and molecular systematics: Issues and approaches. In Molecular Evolution and Adaptive Radiation; Givnish, T.J., Sytsma, K.J., Eds.; Oxford University Press: London, UK, 1997; pp. 1–54. [Google Scholar]
- Gillespie, R.G.; Clague, D.A. Encyclopedia of Islands; University of California Press: Berkeley, CA, USA, 2009; p. 1111. [Google Scholar]
- Futuyma, D.J.; Moreno, G. The evolution of ecological specialisation. Ann. Rev. Ecol. Syst. 1988, 19, 207–233. [Google Scholar] [CrossRef]
- Devictor, V.; Julliard, R.; Jiguet, F. Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 2008, 117, 507–514. [Google Scholar] [CrossRef]
- Smith, T.M.; Smith, R.L. Elements of Ecology, 6th ed.; Pearson Benjamin Cummings: San Francisco, CA, USA, 2006; p. 655. [Google Scholar]
- Shimizu, Y.; Tabata, H. Forest structure, composition, and distribution on a Pacific island, with reference to ecological release and speciation. Pac. Sci. 1991, 45, 28–49. [Google Scholar]
- Mesquita, D.O.; Colli, G.R.; Vitt, L.J. Ecological release in lizard assemblages of neotropical savannas. Oecologia 2007, 153, 185–195. [Google Scholar] [CrossRef]
- Pannek, A.; Manthey, M.; Diekmann, M. Comparing resource-based and co-occurrence-based methods for estimating species niche breadth. J. Veg. Sci. 2016, 27, 596–605. [Google Scholar] [CrossRef]
- Slatyer, R.A.; Hirst, M.; Sexton, J.P. Niche breadth predicts geographical range size: A general ecological pattern. Ecol. Lett. 2013, 16, 1104–1114. [Google Scholar] [CrossRef]
- Vetaas, O.R. Realized and potential climate niches: A comparison of four Rhododendron tree species. J. Biogeogr. 2002, 29, 545–554. [Google Scholar] [CrossRef]
- Chase, J.M.; Leibold, M.A. Ecological Niches: Linking Classical and Contemporary Approaches; University of Chicago Press: Chicago, IL, USA, 2003; p. 221. [Google Scholar]
- Jeanmonod, D. Révision de la section Siphonomorpha Otth du genre Silene, L. en Méditerranée occidentale. II: Le Group Du Silene mollissima. Candollea 1984, 39, 195–259. [Google Scholar]
- Murru, V.; Santo, A.; Piazza, C.; Hugot, L.; Bacchetta, G. Seed germination, salt-stress tolerance, and the effect of nitrate on three Tyrrhenian coastal species of the Silene mollissima aggregate (Caryophyllaceae). Botany 2015, 93, 881–892. [Google Scholar] [CrossRef]
- Olivier, L.; Galland, J.P.; Maurin, H.; Roux, J.P. Livre Rouge de la Flore Menacée de France. Tome I: Espèces Prioritaires. Muséum National d'Histoire Naturelle; Ministère de l’Environnement, Service Patrimoine Naturel, Conservatoire Botanique National de Porquerolles: Paris, France, 1995; p. 135.
- Conti, F.; Manzi, A.; Pedrotti, F. Liste Rosse Regionali delle Piante d’Italia; WWF Italia, MATTM, Società Botanica Italiana, Poligrafica Editrice: Camerino, Italy, 1997; p. 637. [Google Scholar]
- Pisanu, S.; Caria, M.C.; Sotgiu, S.; Bagella, S. Silene velutina Loisel. Inf. Bot. Ital. 2014, 46, 148–150. [Google Scholar]
- Buord, S.; Gargano, D.; Gigot, G.; Montagnani, C. Silene Velutina. The IUCN Red List of Threatened Species. 2011. Version 3.1, e.T161830A5501371. Available online: http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T161830A5501371.en (accessed on 23 August 2021).
- Papuga, G.; Gauthier, P.; Pons, V.; Farris, E.; Thompson, J.D. Ecological niche differentiation in peripheral populations: A comparative analysis of eleven Mediterranean plant species. Ecography 2018, 41, 1–15. [Google Scholar] [CrossRef]
- Arrigoni, P.V. La Flora dell'Isola di Sardegna; Carlo Delfino Editore: Sassari, Italy, 2006; Volume 1–6. [Google Scholar]
- Jeanmonod, D.; Gamisans, J. Flora Corsica, 2nd ed.; Société Botanique du Centre Ouest: Jarnac, France, 2013. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d'Italia, 2nd ed.; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Euro+Med. The Euro+Med PlantBase, the Information Resource for Euro-Mediterranean Plant Diversity. 2006. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 23 August 2021).
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.N.M.G.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; p. 144. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed.; Freeman and Company: New York, NY, USA, 1995; p. 887. [Google Scholar]
- Underwood, A.J. Experiments in Ecology: Their Logic Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997; p. 504. [Google Scholar]
- Duda, R.; Hart, P.; Stork, D. Pattern Classification, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2000; p. 680. [Google Scholar]
- Holden, J.E.; Finch, W.H.; Kelley, K. A comparison of two-group classification methods. Educ. Psychol. Meas. 2011, 20, 1–32. [Google Scholar] [CrossRef]
- Kuhn, M.; Johnson, K. Applied Predictive Modeling; Springer: New York, NY, USA, 2013; p. 600. [Google Scholar]
- Rencher, A.C.; Christensen, W.F. Methods of Multivariate Analysis; John Wiley: New York, NY, USA, 2012; p. 800. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murru, V.; Farris, E.; Santo, A.; Grillo, O.; Piazza, C.; Gaio, A.; Bacchetta, G.; Thompson, J.D. Niche Differentiation at Multiple Spatial Scales on Large and Small Mediterranean Islands for the Endemic Silene velutina Pourr. ex Loisel. (Caryophyllaceae). Plants 2021, 10, 2298. https://doi.org/10.3390/plants10112298
Murru V, Farris E, Santo A, Grillo O, Piazza C, Gaio A, Bacchetta G, Thompson JD. Niche Differentiation at Multiple Spatial Scales on Large and Small Mediterranean Islands for the Endemic Silene velutina Pourr. ex Loisel. (Caryophyllaceae). Plants. 2021; 10(11):2298. https://doi.org/10.3390/plants10112298
Chicago/Turabian StyleMurru, Valentina, Emmanuele Farris, Andrea Santo, Oscar Grillo, Carole Piazza, Antonella Gaio, Gianluigi Bacchetta, and John D. Thompson. 2021. "Niche Differentiation at Multiple Spatial Scales on Large and Small Mediterranean Islands for the Endemic Silene velutina Pourr. ex Loisel. (Caryophyllaceae)" Plants 10, no. 11: 2298. https://doi.org/10.3390/plants10112298
APA StyleMurru, V., Farris, E., Santo, A., Grillo, O., Piazza, C., Gaio, A., Bacchetta, G., & Thompson, J. D. (2021). Niche Differentiation at Multiple Spatial Scales on Large and Small Mediterranean Islands for the Endemic Silene velutina Pourr. ex Loisel. (Caryophyllaceae). Plants, 10(11), 2298. https://doi.org/10.3390/plants10112298






