Developing Novel Rice Genotypes Harboring Specific QTL Alleles Associated with High Grain Yield under Water Shortage Stress
Abstract
:1. Introduction
2. Results
2.1. The Response of the Newly Developed Lines to Water Shortage Stress
2.2. High Heritability in the Broad Sense under Water-Shortage Stress
2.3. Marker Selection Based on Parents’ Genotyping
2.4. Marker-Trait Association for GY under Water Stress and Normal Conditions
2.5. Consistent Allele Effect across the Different Water Regimes
3. Discussion
3.1. Phenotypic Response and Lines Performance under Well-Watered and Stress Conditions
3.2. GY Heritability under Water Shortage Stress Condition
3.3. Genotypic Evaluation of the Parents with the Markers Linked to GY QTLs
3.4. Marker GY Association Analysis and Common Parent Allele Effect
3.5. Consistent QTL Effect under Both Stress and Well-Watered Conditions
3.6. The Newly Developed Lines Harboring High Effect QTLs
4. Materials and Methods
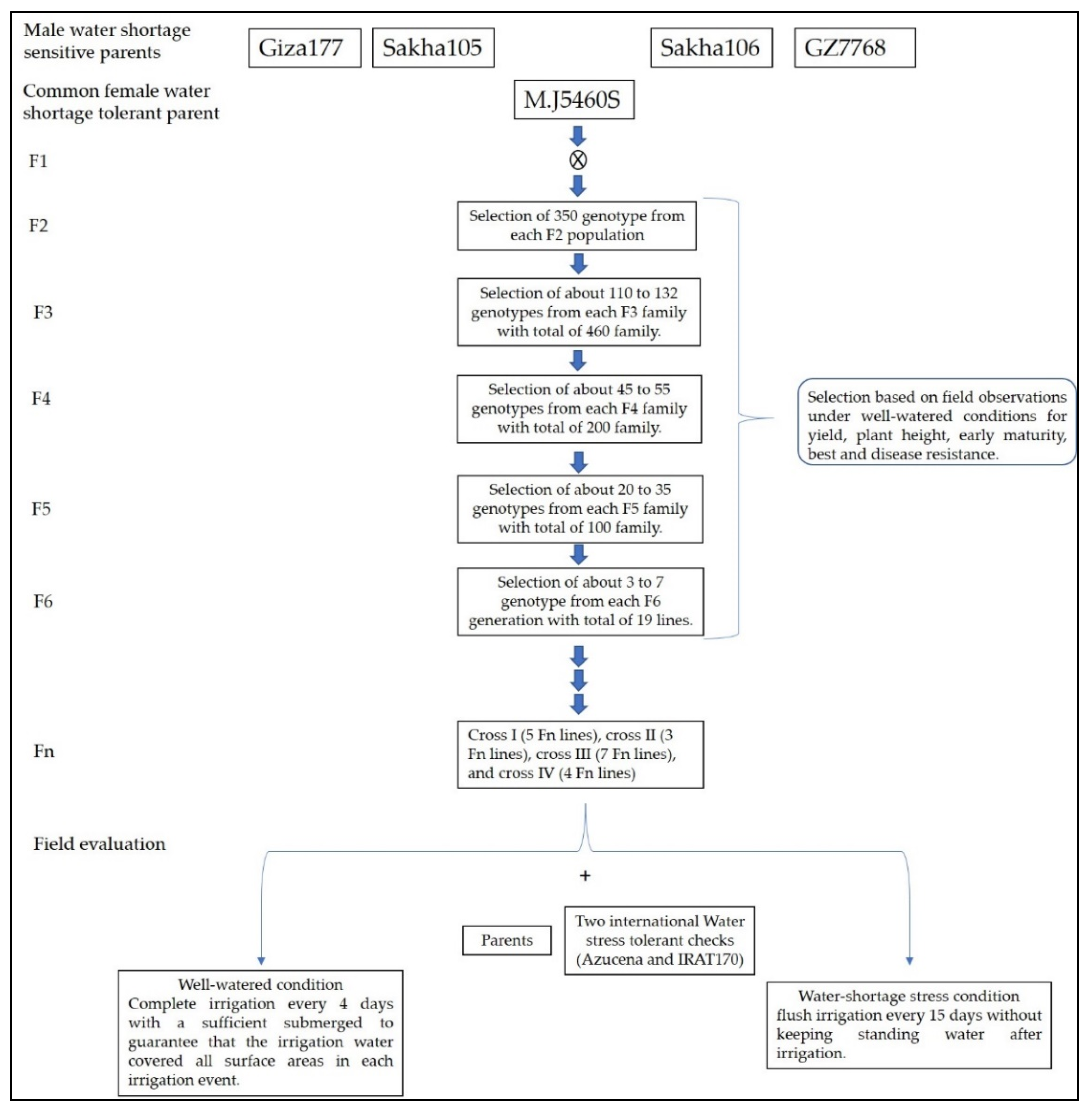
4.1. Plant Material Development
4.2. Climate and Soil Properties
4.3. Field Evaluation Procedures
4.4. Phenotypic Data Statistical Analysis
4.5. Molecular Analysis
4.6. Genetic Analysis and Single Marker Analysis (SMA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, S.P.; Ort, D.R. More than taking the heat: Crops and global change. Curr. Opin. Plant Biol. 2010, 13, 240–247. [Google Scholar] [CrossRef]
- Samal, R.; Roy, P.S.; Sahoo, A.; Kar, M.K.; Patra, B.C.; Marndi, B.C.; Gundimeda, J.N.R. Morphological and molecular dissection of wild rices from eastern India suggests distinct speciation between O. rufipogon and O. nivara populations. Sci. Rep. 2018, 8, 2773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Sayed, M.A.; Kheir, A.M.; Hussein, F.A.; Ali, E.F.; Selim, M.E.; Majrashi, A.; El Shamey, E.A. Developing new lines of Japonica rice for higher quality and yield under arid conditions. PeerJ 2021, 9, e11592. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Mishra, S.S.; Behera, P.K. Drought Tolerance in Rice: Focus on Recent Mechanisms and Approaches. Rice Sci. 2021, 28, 119–132. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Chen, L.; Li, P.; Cao, C. The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci. Rep. 2019, 9, 3742. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, S. Water quality worldwide. In Handbook of Water Purity and Quality; Elsevier: Amsterdam, The Netherlands, 2021; pp. 19–33. [Google Scholar]
- World Water Assessment Programme. World Water Assessment Programme: The United Nations World Water Development Report 4: Managing Water under Uncertainty and Risk; World Water Assessment Programme: Washington, DC, USA, 2012. [Google Scholar]
- Abdelaal, H.S.A.; Thilmany, D. Grains production prospects and long run food security in Egypt. Sustainability 2019, 11, 4457. [Google Scholar] [CrossRef] [Green Version]
- Mehana, M.; Abdelrahman, M.; Emadeldin, Y.; Rohila, J.S.; Karthikeyan, R. Impact of Genetic Improvements of Rice on Its Water Use and Effects of Climate Variability in Egypt. Agriculture 2021, 11, 865. [Google Scholar] [CrossRef]
- ElShamey, E.A.; Ali, E.F.; Selim, M.E.; ElSayed, M.A.; Ahmed, M.; Alotaibi, F.A.; Kamara, M.M.; Kheir, A.M. Water deficit induced physiological and amino acid responses in some rice varieties using NMR-metabolic analysis. Agron. J. 2021. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, S.; Bi, J.; Sun, H.; Wang, C.; Zhang, J. Drought-resistance rice variety with water-saving management reduces greenhouse gas emissions from paddies while maintaining rice yields. Agric. Ecosyst. Environ. 2021, 320, 107592. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Aravinthasamy, P.; Subramani, T.; Kumar, D.; Setia, R. Investigation of health risks related with multipath entry of groundwater nitrate using Sobol sensitivity indicators in an urban-industrial sector of south India. Environ. Res. 2021, 200, 111726. [Google Scholar] [CrossRef]
- Sharma, L.; Dalal, M.; Verma, R.K.; Kumar, S.V.; Yadav, S.K.; Pushkar, S.; Kushwaha, S.R.; Bhowmik, A.; Chinnusamy, V. Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ. Exp. Bot. 2018, 150, 9–24. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, S.; Ram, T.; Yadaw, R.; Mishra, K.; Mandal, N. Breeding high-yielding drought-tolerant rice: Genetic variations and conventional and molecular approaches. J. Exp. Bot. 2014, 65, 6265–6278. [Google Scholar] [CrossRef] [Green Version]
- Venuprasad, R.; Dalid, C.; Del Valle, M.; Zhao, D.; Espiritu, M.; Cruz, M.S.; Amante, M.; Kumar, A.; Atlin, G. Identification and characterization of large-effect quantitative trait loci for grain yield under lowland drought stress in rice using bulk-segregant analysis. Theor. Appl. Genet. 2009, 120, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Vikram, P.; Sta Cruz, T.; Espiritu, M.; Valle, M.; Singh, A.; Kumar, A. Major effect QTLs for grain yield under lowland drought stress. In Proceedings of the International Rice Genetics Symposium, Manila, Philippines, 16–19 November 2009; pp. 16–19. [Google Scholar]
- Dixit, S.; Singh, A.; Kumar, A. Rice breeding for high grain yield under drought: A strategic solution to a complex problem. Int. J. Agron. 2014, 2014, 863683. [Google Scholar] [CrossRef]
- Swamy, B.M.; Shamsudin, N.A.A.; Abd Rahman, S.N.; Mauleon, R.; Ratnam, W.; Cruz, M.T.S.; Kumar, A. Association mapping of yield and yield-related traits under reproductive stage drought stress in rice (Oryza sativa L.). Rice 2017, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Dwivedi, S.; Singh, S.; Jha, S.; Lekshmy, S.; Elanchezhian, R.; Singh, O.; Bhatt, B. Identification of drought tolerant rice genotypes by analysing drought tolerance indices and morpho-physiological traits. SABRAO J. Breed. Genet. 2014, 46. [Google Scholar]
- Garin, V.; Malosetti, M.; van Eeuwijk, F. Multi-parent multi-environment QTL analysis: An illustration with the EU-NAM Flint population. Theor. Appl. Genet. 2020, 133, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.; Tardieu, F.; Tuberosa, R. QTL approaches for improving crop performance under abiotic stress conditions: Where do we stand. Plant Physiol. 2008, 147, 469–486. [Google Scholar] [CrossRef] [Green Version]
- Gaballah, M.M.; Metwally, A.M.; Skalicky, M.; Hassan, M.M.; Brestic, M.; El Sabagh, A.; Fayed, A.M. Genetic diversity of selected rice genotypes under water stress conditions. Plants 2021, 10, 27. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, G.; Wu, Z.; Wan, J.; Yang, J.; Wang, F.; Wang, F.; Yu, D.; Xu, P. Identification and fine mapping of rtms1-D, a gene responsible for reverse thermosensitive genic male sterility from Diannong S-1X. Plant Divers. 2021. [Google Scholar] [CrossRef]
- Swamy BP, M.; Ahmed, H.U.; Henry, A.; Mauleon, R.; Dixit, S.; Vikram, P.; Tilatto, R.; Verulkar, S.B.; Perraju, P.; Mandal, N.P. Genetic, physiological, and gene expression analyses reveal that multiple QTL enhance yield of rice mega-variety IR64 under drought. PLoS ONE 2013, 8, e62795. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.A.A.; Swamy, B.M.; Ratnam, W.; Cruz, M.T.S.; Raman, A.; Kumar, A. Marker assisted pyramiding of drought yield QTLs into a popular Malaysian rice cultivar, MR219. BMC Genet. 2016, 17, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamsudin, N.A.A.; Swamy, B.M.; Ratnam, W.; Cruz, M.T.S.; Sandhu, N.; Raman, A.K.; Kumar, A. Pyramiding of drought yield QTLs into a high quality Malaysian rice cultivar MRQ74 improves yield under reproductive stage drought. Rice 2016, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kamarudin, Z.S.; Muhammad, I.I.; Kolapo, K. Drought resistance in rice from conventional to molecular breeding: A review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, M.; Huang, M.; Qiu, H.; Chun, Y.; Li, L.; Kumar, A.; Fang, J.; Zhao, J.; He, H.; Li, X. Genome-Wide Association Study of the Genetic Basis of Effective Tiller Number in Rice. Rice 2021, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Vikram, P.; Swamy, B.M.; Dixit, S.; Ahmed, H.U.; Cruz, M.T.S.; Singh, A.K.; Kumar, A. qDTY 1.1, a major QTL for rice grain yield under reproductive-stage drought stress with a consistent effect in multiple elite genetic backgrounds. BMC Genet. 2011, 12, 89. [Google Scholar] [CrossRef] [Green Version]
- Atlin, G.; Lafitte, H.; Tao, D.; Laza, M.; Amante, M.; Courtois, B. Developing rice cultivars for high-fertility upland systems in the Asian tropics. Field Crop. Res. 2006, 97, 43–52. [Google Scholar] [CrossRef]
- Kumar, A.; Bernier, J.; Verulkar, S.; Lafitte, H.; Atlin, G. Breeding for drought tolerance: Direct selection for yield, response to selection and use of drought-tolerant donors in upland and lowland-adapted populations. Field Crop. Res. 2008, 107, 221–231. [Google Scholar] [CrossRef]
- Sakran, R.; El Shamey, E.; Anis, G. Diallel Analysis of Different Rice Genotypes under Water Deficiency Conditions and Assessing Genetic Diversity Using SSR Markers. J. Plant Prod. 2020, 11, 1319–1332. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.; Ali, O.A.; Hafez, E.M.; ElShamey, E.A.; Zhou, Z.; Wang, B.; Ge, Y.; Fahmy, A.E.; Seleiman, M.F. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar] [CrossRef]
- Gogarten, S.M.; Sofer, T.; Chen, H.; Yu, C.; Brody, J.A.; Thornton, T.A.; Rice, K.M.; Conomos, M.P. Genetic association testing using the GENESIS R/Bioconductor package. Bioinformatics 2019, 35, 5346–5348. [Google Scholar] [CrossRef] [PubMed]
- Kamoshita, A.; Babu, R.C.; Boopathi, N.M.; Fukai, S. Phenotypic and genotypic analysis of drought-resistance traits for development of rice cultivars adapted to rainfed environments. Field Crop. Res. 2008, 109, 1–23. [Google Scholar] [CrossRef]
- Khahani, B.; Tavakol, E.; Shariati, V.; Rossini, L. Meta-QTL and ortho-MQTL analyses identified genomic regions controlling rice yield, yield-related traits and root architecture under water deficit conditions. Sci. Rep. 2021, 11, 6942. [Google Scholar] [CrossRef]
- Lanceras, J.C.; Pantuwan, G.; Jongdee, B.; Toojinda, T. Quantitative trait loci associated with drought tolerance at reproductive stage in rice. Plant Physiol. 2004, 135, 384–399. [Google Scholar] [CrossRef] [Green Version]
- Bernier, J.; Kumar, A.; Ramaiah, V.; Spaner, D.; Atlin, G. A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop. Sci. 2007, 47, 507–516. [Google Scholar] [CrossRef]
- Beena, R.; Kirubakaran, S.; Nithya, N.; Sah, R.; Abida, P.; Sreekumar, J.; Jaslam, M.; Rejeth, R.; Jayalekshmy, V.; Roy, S. Association Mapping of Drought Avoidance and Agronomic Traits in Rice (Oryza Sativa L.) Landraces with SSR Markers and Genotyping-By-Sequencing Approach. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Snoek, B.L.; Volkers, R.J.; Nijveen, H.; Petersen, C.; Dirksen, P.; Sterken, M.G.; Nakad, R.; Riksen, J.A.; Rosenstiel, P.; Stastna, J.J. A multi-parent recombinant inbred line population of C. elegans allows identification of novel QTLs for complex life history traits. BMC Biol. 2019, 17, 24. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.; El-Denary, M.; Ammar, M.; Abdelkhalik, A.; Draz, A.; Dora, S. QTL validation for grain yield and nitrogen use efficiency under different nitrogen levels in rice. Egypt. J. Genet. Cytol. 2015, 44, 235–251. [Google Scholar] [CrossRef] [Green Version]
- Achar, D.; Awati, M.G.; Udayakumar, M.; Prasad, T. Identification of putative molecular markers associated with root traits in Coffea canephora Pierre ex Froehner. Mol. Biol. Int. 2015, 2015, 532386. [Google Scholar] [CrossRef] [Green Version]
- Sallam, A.; Amro, A.; Elakhdar, A.; Dawood, M.F.; Moursi, Y.S.; Baenziger, P.S. Marker–trait association for grain weight of spring barley in well-watered and drought environments. Mol. Biol. Rep. 2019, 46, 2907–2918. [Google Scholar] [CrossRef]
- Yue, B.; Xiong, L.; Xue, W.; Xing, Y.; Luo, L.; Xu, C. Genetic analysis for drought resistance of rice at reproductive stage in field with different types of soil. Theor. Appl. Genet. 2005, 111, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Akkareddy, S.; Vemireddy, L.; Hariprasad, A.; Jayaprada, M.; Sridhar, S.; Ramanarao, P.; Anuradha, G.; Siddiq, E. Identification and mapping of landrace derived QTL associated with yield and its components in rice under different nitrogen levels and environments. Int. J. Plant Breed. Genet. 2010, 4, 210–227. [Google Scholar]
- Negarestani, M.; Tohidi-Nejad, E.; Khajoei-Nejad, G.; Nakhoda, B.; Mohammadi-Nejad, G. Comparison of Different Multivariate Statistical Methods for Screening the Drought Tolerant Genotypes of Pearl Millet (Pennisetum americanum L.) and Sorghum (Sorghum bicolor L.). Agronomy 2019, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Murray, M.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabi, M.; Mokrani, L.; Shoaib, A.; Jawhar, M. Identification of AFLP markers associated with spot blotch resistance through single marker analysis in barley (Hordeum vulgare L.). Cereal Res. Commun. 2021, 49, 285–290. [Google Scholar] [CrossRef]

| Characters | Days to Heading | Plant Height (cm) | Panicle Weight (g) | Panicle Length (cm) | Yield (kg/m2) | Seed Set (%) | 1000-Grain Weight (g) | Drought Susceptibility Index | Reduction % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes (Fn) | N | S | N | S | N | S | N | S | N | S | N | S | N | S | ||
| M.J5460S/GIZA177-3 | 88.73 | 95.26 | 90.36 | 75.33 | 5.83 | 3.39 | 20.85 | 18.06 | 1.38 | 0.59 | 94.51 | 69.28 | 30.31 | 24.13 | 0.572 | 57.195 |
| M.J5460S/GIZA177-12 | 92.06 | 96.26 | 101.67 | 80.33 | 5.43 | 2.73 | 21.30 | 20.42 | 1.20 | 0.54 | 95.65 | 67.18 | 29.42 | 23.40 | 0.548 | 54.849 |
| M.J5460S/GIZA177-18 | 91.22 | 96.38 | 99.70 | 95.33 | 4.07 | 2.76 | 23.55 | 17.22 | 1.08 | 0.46 | 94.58 | 63.14 | 28.59 | 23.10 | 0.570 | 56.957 |
| M.J5460S/GIZA177-22 | 93.40 | 100.93 | 120.16 | 76.33 | 5.21 | 1.19 | 23.22 | 16.62 | 1.05 | 0.17 | 92.76 | 29.60 | 30.13 | 22.17 | 0.842 | 84.244 |
| M.J5460S/GIZA177-28 | 92.09 | 93.93 | 113.38 | 96.67 | 5.22 | 1.34 | 26.57 | 22.13 | 1.13 | 0.20 | 91.76 | 38.07 | 27.39 | 23.13 | 0.828 | 82.764 |
| M.J5460S/SAKHA105-6 | 90.53 | 97.93 | 97.13 | 85.33 | 5.72 | 2.38 | 25.51 | 21.71 | 1.19 | 0.38 | 95.29 | 56.77 | 31.42 | 23.40 | 0.679 | 67.896 |
| M.J5460S/SAKHA105-15 | 92.22 | 103.60 | 99.09 | 75.33 | 5.48 | 2.32 | 20.84 | 15.44 | 1.12 | 0.43 | 95.38 | 55.49 | 32.45 | 21.47 | 0.614 | 61.358 |
| M.J5460S/SAKHA105-20 | 95.15 | 99.93 | 98.11 | 79.67 | 5.64 | 1.21 | 19.00 | 16.18 | 1.06 | 0.20 | 92.68 | 27.63 | 34.22 | 21.03 | 0.808 | 80.784 |
| M.J5460S/SAKHA106-1 | 90.07 | 95.93 | 98.42 | 79.67 | 5.63 | 1.22 | 21.83 | 18.16 | 1.22 | 0.19 | 93.41 | 24.15 | 32.48 | 22.33 | 0.842 | 84.242 |
| M.J5460S/SAKHA106-5 | 95.46 | 103.60 | 112.74 | 90.11 | 5.54 | 1.40 | 22.90 | 21.00 | 1.34 | 0.24 | 93.55 | 32.39 | 28.22 | 23.25 | 0.819 | 81.882 |
| M.J5460S/SAKHA106-6 | 88.58 | 102.60 | 86.58 | 75.33 | 5.79 | 1.36 | 23.09 | 16.59 | 1.28 | 0.18 | 94.41 | 21.67 | 30.13 | 21.42 | 0.857 | 85.725 |
| M.J5460S/SAKHA106-12 | 98.87 | 105.15 | 112.50 | 73.56 | 5.59 | 1.72 | 22.96 | 17.12 | 1.28 | 0.24 | 91.61 | 35.01 | 29.38 | 21.77 | 0.816 | 81.612 |
| M.J5460S/SAKHA106-15 | 90.41 | 110.04 | 114.76 | 60.44 | 5.27 | 2.30 | 23.93 | 16.64 | 1.24 | 0.38 | 91.65 | 54.08 | 30.51 | 23.28 | 0.692 | 69.208 |
| M.J5460S/SAKHA106-18 | 91.73 | 98.93 | 87.71 | 80.11 | 3.53 | 1.51 | 20.30 | 17.22 | 1.00 | 0.20 | 92.12 | 18.39 | 28.36 | 22.33 | 0.798 | 79.821 |
| M.J5460S/SAKHA106-25 | 95.47 | 111.93 | 90.64 | 75.78 | 4.65 | 2.92 | 18.27 | 15.40 | 1.07 | 0.40 | 94.49 | 50.68 | 26.29 | 21.20 | 0.625 | 62.496 |
| M.J5460S/GZ.7768-4 | 94.57 | 102.93 | 85.73 | 75.44 | 5.58 | 2.28 | 22.31 | 19.06 | 1.18 | 0.36 | 92.34 | 56.60 | 31.56 | 22.20 | 0.693 | 69.318 |
| M.J5460S/GZ.7768-7 | 97.07 | 114.93 | 92.39 | 75.67 | 5.64 | 1.34 | 24.30 | 20.06 | 1.23 | 0.19 | 93.73 | 28.70 | 26.79 | 23.00 | 0.843 | 84.328 |
| M.J5460S/GZ.7768-10 | 95.47 | 100.93 | 106.20 | 80.00 | 5.53 | 3.08 | 20.44 | 16.36 | 1.28 | 0.47 | 92.07 | 67.09 | 28.44 | 23.20 | 0.634 | 63.396 |
| M.J5460S/GZ.7768-30 | 92.80 | 102.93 | 90.84 | 77.33 | 5.59 | 2.69 | 22.22 | 19.66 | 1.38 | 0.38 | 93.41 | 56.12 | 30.07 | 24.20 | 0.724 | 72.368 |
| M.J5460S | 84.00 | 89.93 | 95.67 | 82.33 | 5.75 | 3.48 | 21.86 | 18.31 | 1.20 | 0.60 | 93.14 | 72.25 | 29.05 | 24.17 | 0.498 | 49.847 |
| Giza177 | 85.71 | 95.93 | 102.59 | 65.33 | 3.45 | 1.10 | 23.54 | 16.07 | 0.96 | 0.33 | 95.11 | 61.17 | 28.71 | 23.10 | 0.659 | 65.892 |
| Sakha105 | 87.51 | 94.93 | 99.94 | 60.44 | 3.56 | 1.24 | 23.97 | 16.60 | 0.98 | 0.20 | 93.88 | 33.07 | 29.44 | 23.15 | 0.795 | 79.477 |
| Sakha106 | 88.94 | 94.93 | 104.41 | 73.11 | 3.48 | 1.68 | 24.13 | 17.11 | 1.10 | 0.20 | 93.25 | 45.31 | 26.76 | 23.87 | 0.816 | 81.620 |
| GZ7768 | 87.86 | 116.93 | 105.64 | 70.44 | 3.64 | 1.09 | 21.07 | 17.47 | 1.06 | 0.19 | 95.77 | 31.38 | 26.98 | 22.17 | 0.817 | 81.681 |
| Azucena | 101.64 | 108.93 | 158.53 | 122.56 | 4.05 | 3.02 | 26.06 | 23.27 | 0.91 | 0.62 | 90.90 | 74.19 | 31.86 | 24.50 | 0.318 | 31.783 |
| IRAT 170 | 86.83 | 94.93 | 128.00 | 110.56 | 4.89 | 3.13 | 27.84 | 22.48 | 0.92 | 0.65 | 90.49 | 72.76 | 31.85 | 24.27 | 0.294 | 29.433 |
| Mean | 91.86 | 101.18 | 103.57 | 80.48 | 4.99 | 2.07 | 22.76 | 18.32 | 1.15 | 0.35 | 93.38 | 47.78 | 29.65 | 22.89 | 0.69 | 69.24 |
| LSD | 1.98 | 1.44 | 1.99 | 1.67 | 0.65 | 0.53 | 1.09 | 0.83 | 0.06 | 0.01 | 0.82 | 0.55 | 0.73 | 0.44 | - | - |
| Characters | Replications (df = 2) | Genotypes (df = 25) | Coefficient of Variation | |||
|---|---|---|---|---|---|---|
| N | S | N | S | N | S | |
| Days to heading | 1.637 ns | 0.073 ns | 51.49 ** | 141.35 ** | 0.844 | 0.406 |
| Plant height (cm) | 1.187 ns | 0.639 ns | 718.86 ** | 563.00 ** | 0.756 | 0.684 |
| Panicle weight (g) | 0.123 ns | 0.002 ns | 2.22 ** | 1.976 ** | 1.677 | 2.704 |
| Panicle length (cm) | 0.263 ns | 0.184 ns | 15.33 ** | 15.939 ** | 1.033 | 0.739 |
| Yield M-2 (kg/m2) | 0.001 ns | 0.001 ns | 0.055 ** | 0.076 ** | 0.067 | 0.004 |
| Seed set (%) | 0.057 ns | 0.012 ns | 6.66 ** | 968.72 ** | 0.142 | 0.125 |
| 1000-grain weight (g) | 0.101 ns | 0.041 ns | 12.33 ** | 3.044 ** | 0.359 | 0.165 |
| Characters | Mean | Range | GCV (%) | ECV (%) | PCV (%) | H2 (%) | |
|---|---|---|---|---|---|---|---|
| Min | Max | ||||||
| Well-watered condition | |||||||
| Days to heading | 91.861 | 84.00 | 101.64 | 4.48 | 1.69 | 4.78 | 87.510 |
| Plant height (cm) | 103.573 | 85.73 | 158.53 | 14.94 | 1.35 | 15.00 | 99.184 |
| Panicle weight (g) | 4.991 | 3.45 | 5.83 | 16.93 | 9.10 | 19.22 | 77.564 |
| Panicle length (cm) | 22.765 | 18.27 | 27.84 | 9.85 | 3.10 | 10.33 | 90.989 |
| Yield M-2 (kg/m2) | 1.149 | 0.91 | 1.38 | 11.73 | 2.89 | 12.09 | 94.269 |
| Seed set (%) | 93.383 | 90.49 | 95.77 | 1.58 | 0.47 | 1.65 | 92.015 |
| 1000-grain weight (g) | 29.648 | 26.29 | 34.22 | 6.81 | 1.54 | 6.98 | 95.147 |
| Water-stress condition | |||||||
| Days to heading | 101.178 | 89.93 | 116.93 | 6.77 | 0.69 | 6.81 | 98.980 |
| Plant height (cm) | 80.483 | 60.44 | 122.56 | 17.01 | 1.36 | 17.07 | 99.370 |
| Panicle weight (g) | 2.072 | 1.09 | 3.48 | 38.62 | 11.61 | 40.33 | 91.709 |
| Panicle length (cm) | 18.321 | 15.40 | 23.27 | 12.53 | 3.08 | 12.90 | 94.287 |
| Yield M-2 (kg/m2) | 0.347 | 0.17 | 0.65 | 45.81 | 2.53 | 45.88 | 99.695 |
| Seed set (%) | 47.776 | 18.39 | 74.19 | 37.61 | 0.56 | 37.62 | 99.978 |
| 1000-grain weight (g) | 22.891 | 21.03 | 24.50 | 4.37 | 1.22 | 4.54 | 92.729 |
| Marker Allele | QTL | Water Stress Condition | Well Irrigation Condition | ||
|---|---|---|---|---|---|
| R2 | Allele Effect (kg/m2) | R2 | Allele Effect (kg/m2) | ||
| RM555_240 | 2.2 | 17.8 * | 0.12 | 1.1 ns | 0.03 |
| RM525_144 | 2.2 | 6.2 ns | 0.08 | 0.1 ns | 0.01 |
| RM14551_620 | 3.1 | 25.3 ** | 0.17 | 34.7 ** | 0.17 |
| RM3199_186 | 3.2 | 15.9 * | 0.11 | 0.0 ns | 0.00 |
| RM410_195 | 9.1 | 27.8 ** | 0.17 | 22.5 ** | 0.13 |
| RM257_166 | 9.1 | 31.6 ** | 0.16 | 11.8 ns | 0.08 |
| RM242_208 | 9.1 | 60.3 ** | 0.21 | 2.60 ns | 0.04 |
| No | Entries | Parents | Origin | Water-Stress Response |
|---|---|---|---|---|
| 1 | M.J5460S | rT60-6 MS | China | Tolerant |
| 2 | Giza177 | [Giza171] Ymji Ni.1//PiNo.4 | Egypt | Sensitive |
| 3 | Sakha105 | GZ5581/GZ4316 | Egypt | Sensitive |
| 4 | Sakha106 | Giza177/Hexi30 | Egypt | Sensitive |
| 5 | GZ7768 | GZ5320/Taninung70 | Egypt | Sensitive |
| 6 | Azucena | Landrace | Philippines | Tolerant |
| 7 | IRAT170 | IRAT13/Palawan | Ivory Cost | Tolerant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, M.; Selim, M.E.; ElSayed, M.A.; Ammar, M.H.; Hussein, F.A.; ElKholy, N.K.; ElShamey, E.A.; Khan, N.; Attia, K.A. Developing Novel Rice Genotypes Harboring Specific QTL Alleles Associated with High Grain Yield under Water Shortage Stress. Plants 2021, 10, 2219. https://doi.org/10.3390/plants10102219
Abdelrahman M, Selim ME, ElSayed MA, Ammar MH, Hussein FA, ElKholy NK, ElShamey EA, Khan N, Attia KA. Developing Novel Rice Genotypes Harboring Specific QTL Alleles Associated with High Grain Yield under Water Shortage Stress. Plants. 2021; 10(10):2219. https://doi.org/10.3390/plants10102219
Chicago/Turabian StyleAbdelrahman, Mohamed, Mahmoud E. Selim, Mahmoud A. ElSayed, Megahed H. Ammar, Fatma A. Hussein, Neama K. ElKholy, Essam A. ElShamey, Naeem Khan, and Kotb A. Attia. 2021. "Developing Novel Rice Genotypes Harboring Specific QTL Alleles Associated with High Grain Yield under Water Shortage Stress" Plants 10, no. 10: 2219. https://doi.org/10.3390/plants10102219
APA StyleAbdelrahman, M., Selim, M. E., ElSayed, M. A., Ammar, M. H., Hussein, F. A., ElKholy, N. K., ElShamey, E. A., Khan, N., & Attia, K. A. (2021). Developing Novel Rice Genotypes Harboring Specific QTL Alleles Associated with High Grain Yield under Water Shortage Stress. Plants, 10(10), 2219. https://doi.org/10.3390/plants10102219








