The Effect of Low Irradiance on Leaf Nitrogen Allocation and Mesophyll Conductance to CO2 in Seedlings of Four Tree Species in Subtropical China
Abstract
:1. Introduction
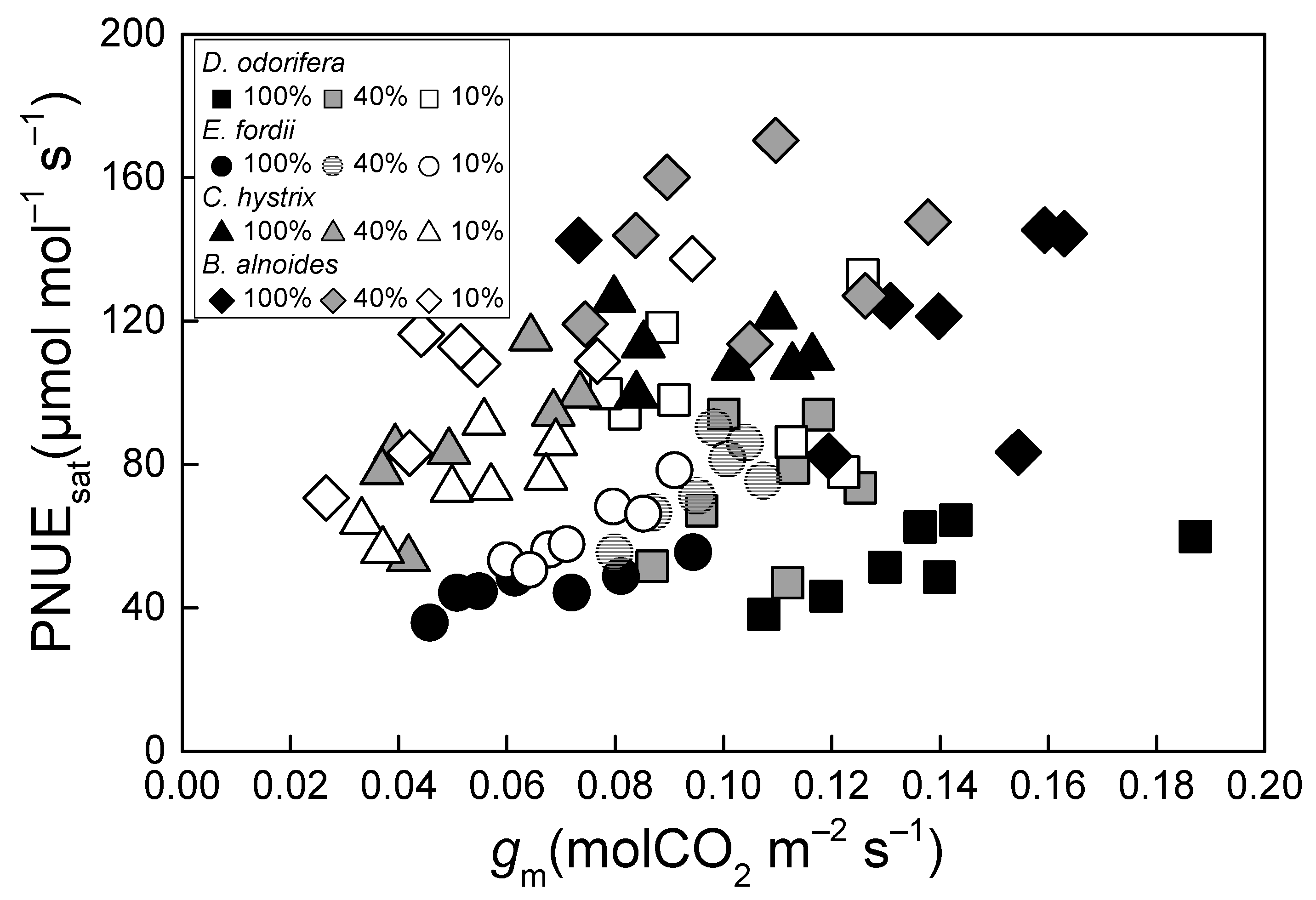
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Area and Plant Material
4.2. Determination of Gas Exchange and Fluorescence Parameters
4.3. Determination of Mesophyll Conductance, Vcmax, and Jmax
4.4. Determination of Additional Leaf Traits
4.5. Calculation of N Allocation in the Photosynthetic Apparatus
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, X.; Liu, G.; Jiang, J.; Lei, C.; Zhang, Y.; Wang, L.; Liu, X. Effects of growth irradiance on photosynthesis and photorespiration of Phoebe bournei leaves. Funct. Plant Biol. 2020, 47, 1053. [Google Scholar] [CrossRef]
- Warren, C.R.; Adams, M. Distribution of N, Rubisco and photosynthesis in Pinus pinaster and acclimation to light. Plant Cell Environ. 2001, 24, 597–609. [Google Scholar] [CrossRef]
- Coble, A.P.; Cavaleri, M.A. Vertical leaf mass per area gradient of mature sugar maple reflects both height-driven increases in vascular tissue and light-driven increases in palisade layer thickness. Tree Physiol. 2017, 37, 1337–1351. [Google Scholar] [CrossRef]
- Coble, A.P.; Fogel, M.L.; Parker, G.G. Canopy gradients in leaf functional traits for species that differ in growth strategies and shade tolerance. Tree Physiol. 2017, 37, 1415–1425. [Google Scholar] [CrossRef]
- Bachofen, C.; D’Odorico, P.; Buchmann, N. Light and VPD gradients drive foliar nitrogen partitioning and photosynthesis in the canopy of European beech and silver fir. Oecologia 2020, 192, 323–339. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.; Harbinson, J.; Van Ieperen, W. Photosynthetic acclimation in relation to nitrogen allocation in cucumber leaves in response to changes in irradiance. Physiol. Plant. 2011, 142, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-W.; Kuang, S.-B.; Long, G.-Q.; Meng, Z.-G.; Li, L.-G.; Chen, Z.-J.; Zhang, G.-H.; Yang, S.-C. Steady-state and dynamic photosynthetic performance and nitrogen partitioning in the shade-demanding plant Panax notoginseng under different levels of growth irradiance. Acta Physiol. Plant. 2014, 36, 2409–2420. [Google Scholar] [CrossRef]
- Osada, N.; Onoda, Y.; Hikosaka, K. Effects of atmospheric CO2 concentration, irradiance, and soil nitrogen availability on leaf photosynthetic traits of Polygonum sachalinense around natural CO2 springs in northern Japan. Oecologia 2010, 164, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Delagrange, S. Light- and seasonal-induced plasticity in leaf morphology, N partitioning and photosynthetic capacity of two temperate deciduous species. Environ. Exp. Bot. 2011, 70, 1–10. [Google Scholar] [CrossRef]
- Ruan, J.; Haerdter, R.; Gerendás, J. Impact of nitrogen supply on carbon/nitrogen allocation: A case study on amino acids and catechins in green tea [Camellia sinensis (L.) O. Kuntze] plants. Plant Biol. 2010, 12, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Glenwinkel, L.A.; Sack, L. Differential Allocation to Photosynthetic and Non-Photosynthetic Nitrogen Fractions among Native and Invasive Species. PLoS ONE 2013, 8, e64502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stitt, M.; Schulze, D. Does Rubisco control the rate of photosynthesis and plant growth? An exercise in molecular ecophysiology. Plant Cell Environ. 1994, 17, 465–487. [Google Scholar] [CrossRef]
- Annighöfer, P.; Petritan, A.M.; Petritan, I.C.; Ammer, C. Disentangling juvenile growth strategies of three shade-tolerant temperate forest tree species responding to a light gradient. For. Ecol. Manag. 2017, 391, 115–126. [Google Scholar] [CrossRef]
- Oguchi, R.; Hikosaka, K.; Hirose, T. Leaf anatomy as a constraint for photosynthetic acclimation: Differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ. 2005, 28, 916–927. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tazoe, Y.; Vyas, P.; Yano, S. Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 2005, 57, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009, 60, 2235–2248. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü.; Díaz-Espejo, A.; Flexas, J.; Galmés, J.; Warren, C.R. Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J. Exp. Bot. 2009, 60, 2249–2270. [Google Scholar] [CrossRef] [Green Version]
- Fini, A.; Loreto, F.; Tattini, M.; Giordano, C.; Ferrini, F.; Brunetti, C.; Centritto, M. Mesophyll conductance plays a central role in leaf functioning of Oleaceae species exposed to contrasting sunlight irradiance. Physiol. Plant. 2016, 157, 54–68. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H. Rice (Oryza sativa L.) with reduced chlorophyll content exhibit higher photosynthetic rate and efficiency, improved canopy light distribution, and greater yields than normally pigmented plants. Field Crop. Res. 2017, 200, 58–70. [Google Scholar] [CrossRef]
- Rocha, J.S.; Calzavara, A.K.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R.; Oliveira, H.C. Nitrogen supplementation improves the high-light acclimation of Guazuma ulmifolia Lam. seedlings. Trees 2018, 33, 421–431. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Hu, H.; Huang, W. The Light Dependence of Mesophyll Conductance and Relative Limitations on Photosynthesis in Evergreen Sclerophyllous Rhododendron Species. Plants 2020, 9, 1536. [Google Scholar] [CrossRef]
- Hikosaka, K. Mechanisms underlying interspecific variation in photosynthetic capacity across wild plant species. Plant Biotechnol. 2010, 27, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Hou, W.; Tränkner, M.; Lu, J.; Yan, J.; Huang, S.; Ren, T.; Cong, R.; Li, X. Interactive effects of nitrogen and potassium on photosynthesis and photosynthetic nitrogen allocation of rice leaves. BMC Plant Biol. 2019, 19, 302. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Yin, L.; Xu, H.; Dong, S.; Chu, J.; Dai, X.; He, M. Optimised nitrogen allocation favours improvement in canopy photosynthetic nitrogen-use efficiency: Evidence from late-sown winter wheat. Environ. Exp. Bot. 2019, 159, 75–86. [Google Scholar] [CrossRef]
- Wang, S.; Cai, Y.F.; Li, Z.L.; Li, S.F. Effect of different light intensities on photosynthetic characteristics of rhododendron ‘Furnivall’s Daughter’. Acta Bot. Boreali-Occident. Sin. 2012, 32, 2095–2101. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Feng, Y.-L. The relationships between photosynthetic capacity and lamina mass per unit area, nitrogen content and partitioning in seedlings of two ficus species grown under different irradiance. J. Plant Physiol. Mol. Boil. 2004, 30, 269–276. [Google Scholar]
- Wyka, T.P.; Oleksyn, J.; Żytkowiak, R.; Karolewski, P.; Jagodzinski, A.; Reich, P. Responses of leaf structure and photosynthetic properties to intra-canopy light gradients: A common garden test with four broadleaf deciduous angiosperm and seven evergreen conifer tree species. Oecologia 2012, 170, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Catoni, R.; Gratani, L.; Sartori, F.; Varone, L.; Granata, M.U. Carbon gain optimization in five broadleaf deciduous trees in response to light variation within the crown: Correlations among morphological, anatomical and physiological leaf traits. Acta Bot. Croat. 2015, 74, 71–94. [Google Scholar] [CrossRef] [Green Version]
- Townsend, A.J.; Retkute, R.; Chinnathambi, K.; Randall, J.W.P.; Foulkes, J.; Carmo-Silva, E.; Murchie, E.H. Suboptimal Acclimation of Photosynthesis to Light in Wheat Canopies. Plant Physiol. 2018, 176, 1233–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niinemets, Ü.; Flexas, J.; Penuelas, J. Evergreens favored by higher responsiveness to increased CO2. Trends Ecol. Evol. 2011, 26, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Bahar, N.H.A.; Ishida, F.Y.; Weerasinghe, L.K.; Guerrieri, R.; O’Sullivan, O.S.; Bloomfield, K.J.; Asner, G.P.; Martin, R.E.; Lloyd, J.; Malhi, Y.; et al. Leaf-level photosynthetic capacity in lowland Amazonian and high-elevation Andean tropical moist forests of Peru. New Phytol. 2017, 214, 1002–1018. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sisó, S.; Flexas, J.; Galmés, J.; Niinemets, Ü.; Sancho-Knapik, D.; Gil-Pelegrín, E. Coordinated modifications in mesophyll conductance, photosynthetic potentials and leaf nitrogen contribute to explain the large variation in foliage net assimilation rates across Quercus ilex provenances. Tree Physiol. 2017, 37, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Cousins, A.B.; Mullendore, D.L.; Sonawane, B.V. Recent developments in mesophyll conductance in C3, C4, and crassulacean acid metabolism plants. Plant J. 2020, 101, 816–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, C.R. Stand aside stomata, another actor deserves centre stage: The forgotten role of the internal conductance to CO2 transfer. J. Exp. Bot. 2007, 59, 1475–1487. [Google Scholar] [CrossRef] [Green Version]
- Scafaro, A.P.; VON Caemmerer, S.; Evans, J.R.; Atwell, B.J. Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant Cell Environ. 2011, 34, 1999–2008. [Google Scholar] [CrossRef]
- Niinemets, U.; Cescatti, A.; Rodeghiero, M.; Tosens, T. Leaf internal diffusion conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaved species. Plant Cell Environ. 2005, 28, 1552–1566. [Google Scholar] [CrossRef]
- Qing, H.; Cai, Y.; Xiao, Y.; Yao, Y.; An, S. Leaf nitrogen partition between photosynthesis and structural defense in invasive and native tall form Spartina alterniflora populations: Effects of nitrogen treatments. Biol. Invasions 2012, 14, 2039–2048. [Google Scholar] [CrossRef]
- Chen, L.; Dong, T.; Duan, B. Sex-specific carbon and nitrogen partitioning under N deposition in Populus cathayana. Trees 2014, 28, 793–806. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Diaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193–194, 70–84. [Google Scholar] [CrossRef]
- Buckley, T.N.; Warren, C.R. The role of mesophyll conductance in the economics of nitrogen and water use in photosynthesis. Photosynth. Res. 2014, 119, 77–88. [Google Scholar] [CrossRef]
- Olsson, T.; Leverenz, J.W. Non-uniform stomatal closure and the apparent convexity of the photosynthetic photon flux density response curve. Plant Cell Environ. 1994, 17, 701–710. [Google Scholar] [CrossRef]
- Tang, J.; Sun, B.; Cheng, R.; Shi, Z.; Luo, D.; Liu, S.; Centritto, M. Seedling leaves allocate lower fractions of nitrogen to photosynthetic apparatus in nitrogen fixing trees than in non-nitrogen fixing trees in subtropical China. PLoS ONE 2019, 14, e0208971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Sun, B.; Cheng, R.; Shi, Z.; Luo, D.; Liu, S.; Centritto, M. Effects of soil nitrogen (N) deficiency on photosynthetic N-use efficiency in N-fixing and non-N-fixing tree seedlings in subtropical China. Sci. Rep. 2019, 9, 4604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fotis, A.T.; Curtis, P.S. Effects of structural complexity on within-canopy light environments and leaf traits in a northern mixed deciduous forest. Tree Physiol. 2017, 37, 1426–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikosaka, K.; Hanba, Y.T.; Hirose, T.; Terashima, I. Photosynthetic nitrogen-use efficiency in leaves of woody and herbaceous species. Funct. Ecol. 1998, 12, 896–905. [Google Scholar] [CrossRef]
- Wang, W.-X.; Shi, Z.-M.; Luo, D.; Liu, S.-R.; Lu, L.-H. Characteristics of soil microbial biomass and community composition in three types of plantations in southern subtropical area of China. J. Appl. Ecol. 2013, 24, 1784–1792. [Google Scholar]
- Tang, J.C.; Shi, Z.M.; Luo, D.; Liu, S.R. Photosynthetic nitrogen-use efficiency of Manglietia glauca seedling leaves under different shading levels. Acta Ecol. Sin. 2017, 37, 7493–7502. [Google Scholar] [CrossRef] [Green Version]
- Harley, P.C.; Loreto, F.; Di Marco, G.; Sharkey, T. Theoretical Considerations when Estimating the Mesophyll Conductance to CO2 Flux by Analysis of the Response of Photosynthesis to CO2. Plant Physiol. 1992, 98, 1429–1436. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Pallardy, S.G.; Tu, K.; Law, B.E.; Wullschleger, S.D. Reliable estimation of biochemical parameters from C3 leaf photosynthesis-intercellular carbon dioxide response curves. Plant Cell Environ. 2010, 33, 1852–1874. [Google Scholar] [CrossRef] [PubMed]
- Ethier, G.J.; Livingston, N.J. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ. 2004, 27, 137–153. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Onoda, Y.; Hikosaka, K.; Hirose, T. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct. Ecol. 2004, 18, 419–425. [Google Scholar] [CrossRef]
- Niinemets, U.; Tenhunen, J.D. A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ. 1997, 20, 845–866. [Google Scholar] [CrossRef]

| Tree Species | Irradiance Treatment | Asat (μmol·m−2·s−1) | Narea (g·m−2) | Nmass (mg·g−1) | LMA (g·m−2) | PNUEsat (μmol·mol−1·s−1) |
|---|---|---|---|---|---|---|
| Dalbergia odorifera | 100% | 8.04 ± 0.46 aA | 2.19 ± 0.13 aA | 31.7 ± 0.76 aA | 69.0 ± 3.90 aB | 52.6 ± 3.78 bB |
| 40% | 8.30 ± 0.76 aA | 1.62 ± 0.04 bA | 31.2± 0.65 aA | 51.8 ± 0.65 bB | 72.3 ± 7.03 bB | |
| 10% | 6.88 ± 0.30 aA | 0.97 ± 0.04 cB | 33.0 ± 1.11 aA | 29.3 ± 0.67 cC | 101.0 ± 7.12 aA | |
| F | 1.967 | 54.700 *** | 1.196 | 73.752 *** | 15.533 *** | |
| Erythrophleum fordii | 100% | 6.60 ± 0.50 bB | 2.01 ± 0.12 aA | 28.1 ± 1.49 bB | 71.4 ± 0.89 aB | 45.9 ± 2.24 cB |
| 40% | 9.34 ± 0.49 aA | 1.75 ± 0.03 bA | 33.0 ± 0.46 bA | 53.1 ± 0.99 bB | 75.0 ± 4.56 aB | |
| 10% | 6.87 ± 0.50 bA | 1.56 ± 0.04 bA | 35.3 ± 0.88 aA | 44.3 ± 1.47 cB | 61.6 ± 3.72 bB | |
| F | 9.042 ** | 9.223 ** | 12.658 *** | 145.227 *** | 15.877 *** | |
| Castanopsis hystrix | 100% | 8.16 ± 0.18 aA | 1.02 ± 0.06 aB | 10.2 ± 1.80 bD | 100.1 ± 2.60 aA | 112.0 ± 4.62 aA |
| 40% | 4.57 ± 0.23 bB | 0.75 ± 0.05 bB | 9.6 ± 0.50 bC | 78.8 ± 1.11 bA | 87.0± 7.26 bB | |
| 10% | 4.18 ± 0.25 bB | 0.79 ± 0.03 bC | 13.7 ± 0.49 aC | 57.9 ± 1.29 cA | 74.4 ± 4.59 bB | |
| F | 95.630 *** | 20.060 *** | 28.220 *** | 138.877 *** | 12.868 *** | |
| Betula alnoides | 100% | 8.55 ± 0.60 aA | 1.03 ± 0.09 aB | 15.4 ± 1.04 bC | 67.6 ± 5.45 aB | 120.5 ± 5.18 abA |
| 40% | 7.42 ± 0.30 aA | 0.75 ± 0.04 bB | 15.4 ± 0.45 bB | 49.1 ± 3.36 bB | 140.3 ± 8.02 aA | |
| 10% | 4.26 ± 0.52 bB | 0.56 ± 0.04 bD | 19.0 ± 0.62 aB | 29.6 ± 2.14 cC | 105.3 ± 8.33 bA | |
| F | 20.458 *** | 13.371 *** | 7.790 ** | 23.833 *** | 3.815 * |
| Tree Species | Irradiance Treatment | gs (molCO2·m−2·s−1) | gm (molCO2·m−2·s−1) | Ci (μmol·mol−1) | Cc (μmol·mol−1) | Ci-Cc (μmol·mol−1) |
|---|---|---|---|---|---|---|
| Dalbergia odorifera | 100% | 0.067 ± 0.004 bBC | 0.137 ± 0.010 aA | 251.5 ± 6.44 bBC | 190.8 ± 6.92 bB | 60.8 ± 2.21 aC |
| 40% | 0.091 ± 0.009 aA | 0.107 ± 0.005 bA | 288.5 ± 3.93 aA | 210.0 ± 8.82 bA | 78.6 ± 7.50 aAB | |
| 10% | 0.089 ± 0.003 aA | 0.100 ± 0.007 bA | 302.6 ± 1.94 aA | 231.3 ± 6.20 aA | 71.2 ± 5.27 aB | |
| F | 6.562 ** | 6.823 ** | 34.333 *** | 7.536 ** | 2.698 | |
| Erythrophleum fordii | 100% | 0.046 ± 0.002 bC | 0.066 ± 0.007 bC | 235.6 ± 6.19 bC | 132.6 ± 6.90 bD | 103.0 ± 4.83 aA |
| 40% | 0.075 ± 0.005 aA | 0.096 ± 0.004 aA | 254.1 ± 3.81 abB | 156.8 ± 4.09 aB | 97.3 ± 2.37 aA | |
| 10% | 0.060 ± 0.005 abB | 0.074 ± 0.004 bB | 264.4 ± 4.18 aB | 171.7 ± 4.45 aB | 92.7 ± 1.56 aA | |
| F | 11.744 ** | 9.789 ** | 7.982 ** | 13.855 *** | 2.553 | |
| Castanopsis hystrix | 100% | 0.074 ± 0.004 aB | 0.099 ± 0.006 aB | 256.8 ± 5.24 bB | 168.0 ± 6.04 bC | 88.8 ± 6.26 aB |
| 40% | 0.039 ± 0.004 bB | 0.053 ± 0.006 bB | 280.1 ± 3.48 aA | 196.4 ± 5.84 aA | 83.7 ± 4.14 aAB | |
| 10% | 0.036 ± 0.001 bC | 0.053 ± 0.005 bB | 268.0 ± 5.65 abB | 186.5 ± 6.27 abB | 81.5 ± 5.03 aAB | |
| F | 38.06 *** | 22.353 *** | 5.711 * | 5.691 * | 0.511 | |
| Betula alnoides | 100% | 0.100 ± 0.013 aA | 0.134 ± 0.012 aA | 292.9 ± 5.94 aA | 226.4 ± 9.57 aA | 66.5 ± 4.64 aC |
| 40% | 0.095 ± 0.011 aA | 0.104 ± 0.009 aA | 297.1 ± 7.04 aA | 222.9 ± 12.19 aA | 74.2 ± 5.69 aB | |
| 10% | 0.063 ± 0.005 bB | 0.056 ± 0.009 bB | 312.3 ± 4.73 aA | 232.1 ± 9.07 aA | 80.3 ± 6.14 aAB | |
| F | 4.195 * | 16.261 *** | 2.93 | 0.198 | 1.568 |
| Tree Species | Irradiance Treatment | Vcmax (μmol·m−2·s−1) | Jmax (μmol·m−2·s−1) |
|---|---|---|---|
| Dalbergia odorifera | 100% | 78.1 ± 4.59 bB | 100.7 ± 5.80 aBC |
| 40% | 95.2 ± 8.01 aB | 118.5 ± 7.39 aB | |
| 10% | 68.6 ± 3.96 cB | 79.1 ± 2.76 bB | |
| F | 5.405 * | 12.154 *** | |
| Erythrophleum fordii | 100% | 99.8 ± 9.37 bA | 128.8 ± 11.20 bAB |
| 40% | 141.4 ± 5.24 aA | 168.9 ± 3.36 aA | |
| 10% | 80.1 ± 4.07 cA | 99.8 ± 3.83 cA | |
| F | 22.233 *** | 23.930 *** | |
| Castanopsis hystrix | 100% | 82.8 ± 4.47 aB | 109.3 ± 3.40 aABC |
| 40% | 46.5 ± 2.51 bC | 57.6 ± 4.49 bC | |
| 10% | 47.7 ± 2.92 bC | 66.5 ± 3.80 bC | |
| F | 75.031 *** | 49.677 *** | |
| Betula alnoides | 100% | 73.0 ± 3.51 aB | 98.4 ± 5.37 aBC |
| 40% | 82.6 ± 5.46 aB | 97.8 ± 5.39 aB | |
| 10% | 41.6 ± 4.80 bC | 56.0 ± 4.59 bC | |
| F | 25.05 *** | 22.465 *** |
| Tree Species | Irradiance Treatment | PR (g·g−1) | PB (g·g−1) | PL (g·g−1) | PP (g·g−1) | PCW (g·g−1) | POther (g·g−1) |
|---|---|---|---|---|---|---|---|
| Dalbergia odorifera | 100% | 0.135 ± 0.009 bB | 0.030 ± 0.002 bB | 0.105 ± 0.008 cA | 0.269 ± 0.016 cB | 0.068 ± 0.004 aC | 0.663 ± 0.015 aA |
| 40% | 0.201 ± 0.018 aA | 0.047 ± 0.003 aC | 0.132 ± 0.002 bA | 0.381 ± 0.021 bB | 0.067 ± 0.006 aC | 0.552 ± 0.017 bA | |
| 10% | 0.242 ± 0.016 aAB | 0.054 ± 0.003 aA | 0.183 ± 0.005 aA | 0.479 ± 0.021 aA | 0.061 ± 0.004 aC | 0.461 ± 0.023 cB | |
| F | 14.001 *** | 27.585 *** | 54.347 *** | 29.423 *** | 0.632 | 29.390 *** | |
| Erythrophleum fordii | 100% | 0.164 ± 0.010 cB | 0.043 ± 0.003 bB | 0.060 ± 0.009 cB | 0.266 ± 0.018 cB | 0.052 ± 0.002 aC | 0.683 ± 0.019 aA |
| 40% | 0.268 ± 0.011 aB | 0.065 ± 0.003 aB | 0.129 ± 0.004 bA | 0.462 ± 0.007 aB | 0.038 ± 0.001 bC | 0.500 ± 0.007 cA | |
| 10% | 0.203 ± 0.011 bB | 0.041 ± 0.002 bB | 0.150 ± 0.008 aB | 0.394 ± 0.016 bB | 0.039 ± 0.002 bC | 0.568 ± 0.015 bA | |
| F | 24.021 *** | 25.215 *** | 38.638 *** | 47.577 *** | 24.303 *** | 40.909 *** | |
| Castanopsis hystrix | 100% | 0.302 ± 0.012 aA | 0.068 ± 0.003 aA | 0.072 ± 0.008 bB | 0.441 ± 0.018 aA | 0.267 ± 0.010 bA | 0.292 ± 0.019 aB |
| 40% | 0.231 ± 0.018 bB | 0.049 ± 0.005 bC | 0.130 ± 0.014 aA | 0.411 ± 0.032 aB | 0.443 ± 0.022 aA | 0.146 ± 0.023 cB | |
| 10% | 0.247 ± 0.010 bAB | 0.054 ± 0.003 bA | 0.164 ± 0.013 aAB | 0.466 ± 0.015 aA | 0.342 ± 0.028 bA | 0.192 ± 0.031 bD | |
| F | 7.010 ** | 6.229 ** | 15.540 *** | 1.475 | 17.205 *** | 4.023 * | |
| Betula alnoides | 100% | 0.256 ± 0.028 bA | 0.066 ± 0.007 bA | 0.116 ± 0.011 bA | 0.439 ± 0.042 cA | 0.221 ± 0.011 aB | 0.340 ± 0.042 aB |
| 40% | 0.369 ± 0.026 aB | 0.089 ± 0.006 aA | 0.119 ± 0.005 bA | 0.577 ± 0.033 aA | 0.197 ± 0.011 aB | 0.227 ± 0.031 aB | |
| 10% | 0.281 ± 0.017 bA | 0.063 ± 0.003 bA | 0.176 ± 0.005 aAB | 0.521 ± 0.016 bA | 0.150 ± 0.010 bB | 0.329 ± 0.021 aC | |
| F | 5.979 ** | 6.189 ** | 20.386 *** | 4.641 * | 10.826 ** | 3.774 * |
| Tree Species | Irradiance Treatment | AQY (mol·mol−1) | Rn (μmol·m−2·s−1) | LCP (μmol·m−2·s−1) | LSP (μmol·m−2·s−1) |
|---|---|---|---|---|---|
| Dalbergia odorifera | 100% | 0.052 ± 0.004 aA | 0.909 ± 0.050 aBC | 22.1 ± 1.68 aA | 822.9 ± 27.5 aA |
| 40% | 0.059 ± 0.002 aA | 0.845 ± 0.050 aA | 14.4 ± 0.73 bB | 724.3 ± 37.0 aBA | |
| 10% | 0.058 ± 0.002 aA | 0.760 ± 0.038 aB | 7.4 ± 0.43 cB | 684.3 ± 23.1 bA | |
| F | 2.300 | 2.587 | 46.127 *** | 5.378 * | |
| Erythrophleum fordii | 100% | 0.047 ± 0.003 bA | 1.129 ± 0.051 aA | 13.9 ± 0.81 aB | 637.1 ± 29.6 aB |
| 40% | 0.062 ± 0.002 aA | 0.873 ± 0.050 bA | 13.1 ± 1.10 aB | 633.6 ± 17.1 aA | |
| 10% | 0.059 ± 0.001 aA | 0.936 ± 0.030 bAB | 7.5 ± 0.79 bB | 522.1 ± 17.2 bB | |
| F | 15.924 *** | 8.811 ** | 14.464 *** | 6.569 ** | |
| Castanopsis hystrix | 100% | 0.047 ± 0.003 aA | 1.005 ± 0.067 aAB | 14.0 ± 1.21 aB | 632.9 ± 23.4 aB |
| 40% | 0.054 ± 0.002 aA | 0.988 ± 0.040 aA | 7.3 ± 1.00 bC | 307.1 ± 26.8 bC | |
| 10% | 0.054 ± 0.003 aA | 1.048 ± 0.088 aA | 7.4 ± 1.21 bB | 262.1 ± 27.7 bC | |
| F | 2.737 | 0.206 | 11.226 ** | 60.359 *** | |
| Betula alnoides | 100% | 0.049 ± 0.001 aA | 0.889 ± 0.039 aBC | 20.8 ± 0.93 aA | 886.4 ± 43.5 aA |
| 40% | 0.055 ± 0.003 aA | 0.844 ± 0.055 aA | 18.6 ± 2.49 aA | 519.3 ± 27.9 bB | |
| 10% | 0.051 ± 0.003 aA | 0.834 ± 0.048 aAB | 10.5 ± 0.94 bA | 268.6 ± 30.5 cC | |
| F | 1.415 | 0.384 | 10.989 ** | 80.343 *** |
| Tree Species | Irradiance Treatment | A100 (μmol·m−2·s−1) | A400 (μmol·m−2·s−1) | PNUE100 (μmol·mol−1·s−1) | PNUE400 (μmol·mol−1·s−1) |
|---|---|---|---|---|---|
| Dalbergia odorifera | 100% | 2.78 ± 0.41 bA | 7.38 ± 0.45 abAB | 20.7 ± 1.36 cB | 48.2 ± 3.65 cB |
| 40% | 4.06 ± 0.08 aA | 8.02 ± 0.30 aA | 35.2 ± 0.66 bB | 69.8 ± 3.35 bB | |
| 10% | 4.03 ± 0.17 aA | 6.65 ± 0.36 bA | 59.2 ± 4.12 aAB | 97.4 ± 7.32 aA | |
| F | 7.114 * | 6.206 * | 58.81 *** | 23.261 *** | |
| Erythrophleum fordii | 100% | 3.42 ± 0.16 bA | 6.07 ± 0.24 bB | 24.2 ± 1.38 bB | 42.7 ± 1.48 cB |
| 40% | 4.04 ± 0.08 aA | 8.53 ± 0.22 aA | 32.3 ± 0.72 aB | 68.4 ± 2.42 aB | |
| 10% | 3.98 ± 0.18 aA | 6.31 ± 0.43 bA | 35.8 ± 1.29 aC | 56.6 ± 3.11 bB | |
| F | 5.426 * | 16.016 *** | 26.501 *** | 22.155 *** | |
| Castanopsis hystrix | 100% | 3.57 ± 0.12 aA | 7.67 ± 0.42 aAB | 49.1 ± 1.83 aA | 105.4 ± 6.18 aA |
| 40% | 3.00 ± 0.15 bB | 3.98 ± 0.29 bB | 57.3 ± 4.57 aA | 76.2 ± 8.00 bB | |
| 10% | 2.92 ± 0.19 bB | 3.68 ± 0.31 bB | 51.8 ± 3.22 aBC | 65.3 ± 4.99 bB | |
| F | 5.107 * | 41.202 *** | 1.506 | 10.123 ** | |
| Betula alnoides | 100% | 3.27 ± 0.15 aA | 7.95 ± 0.52 aA | 46.4 ± 4.09 bA | 113.7 ± 12.66 aA |
| 40% | 3.52 ± 0.28 aAB | 7.16 ± 0.68 aA | 67.3 ± 7.62 abA | 135.9 ± 14.91 aA | |
| 10% | 2.92 ± 0.32 aB | 3.91 ± 0.66 bB | 72.9 ± 6.33 aA | 95.6 ± 11.85 aA | |
| F | 1.358 | 12.425 *** | 5.074 * | 2.852 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Sun, B.; Cheng, R.; Shi, Z.; Luo, D.; Liu, S.; Centritto, M. The Effect of Low Irradiance on Leaf Nitrogen Allocation and Mesophyll Conductance to CO2 in Seedlings of Four Tree Species in Subtropical China. Plants 2021, 10, 2213. https://doi.org/10.3390/plants10102213
Tang J, Sun B, Cheng R, Shi Z, Luo D, Liu S, Centritto M. The Effect of Low Irradiance on Leaf Nitrogen Allocation and Mesophyll Conductance to CO2 in Seedlings of Four Tree Species in Subtropical China. Plants. 2021; 10(10):2213. https://doi.org/10.3390/plants10102213
Chicago/Turabian StyleTang, Jingchao, Baodi Sun, Ruimei Cheng, Zuomin Shi, Da Luo, Shirong Liu, and Mauro Centritto. 2021. "The Effect of Low Irradiance on Leaf Nitrogen Allocation and Mesophyll Conductance to CO2 in Seedlings of Four Tree Species in Subtropical China" Plants 10, no. 10: 2213. https://doi.org/10.3390/plants10102213








