Growth Quality and Development of Olive Plants Cultured In-Vitro under Different Illumination Regimes
Abstract
1. Introduction
2. Results
2.1. Effect of Light Quality and Intensity on Stem Growth and Development
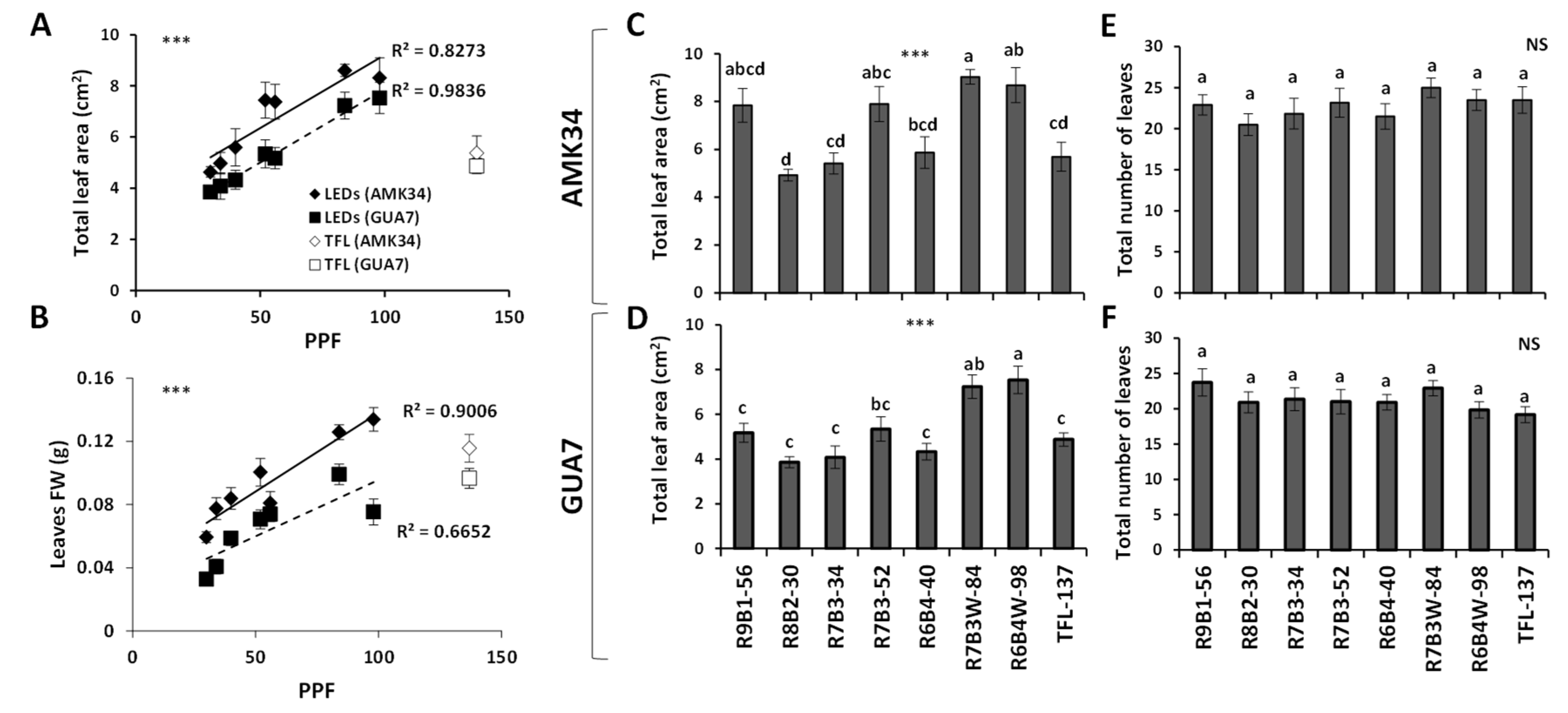
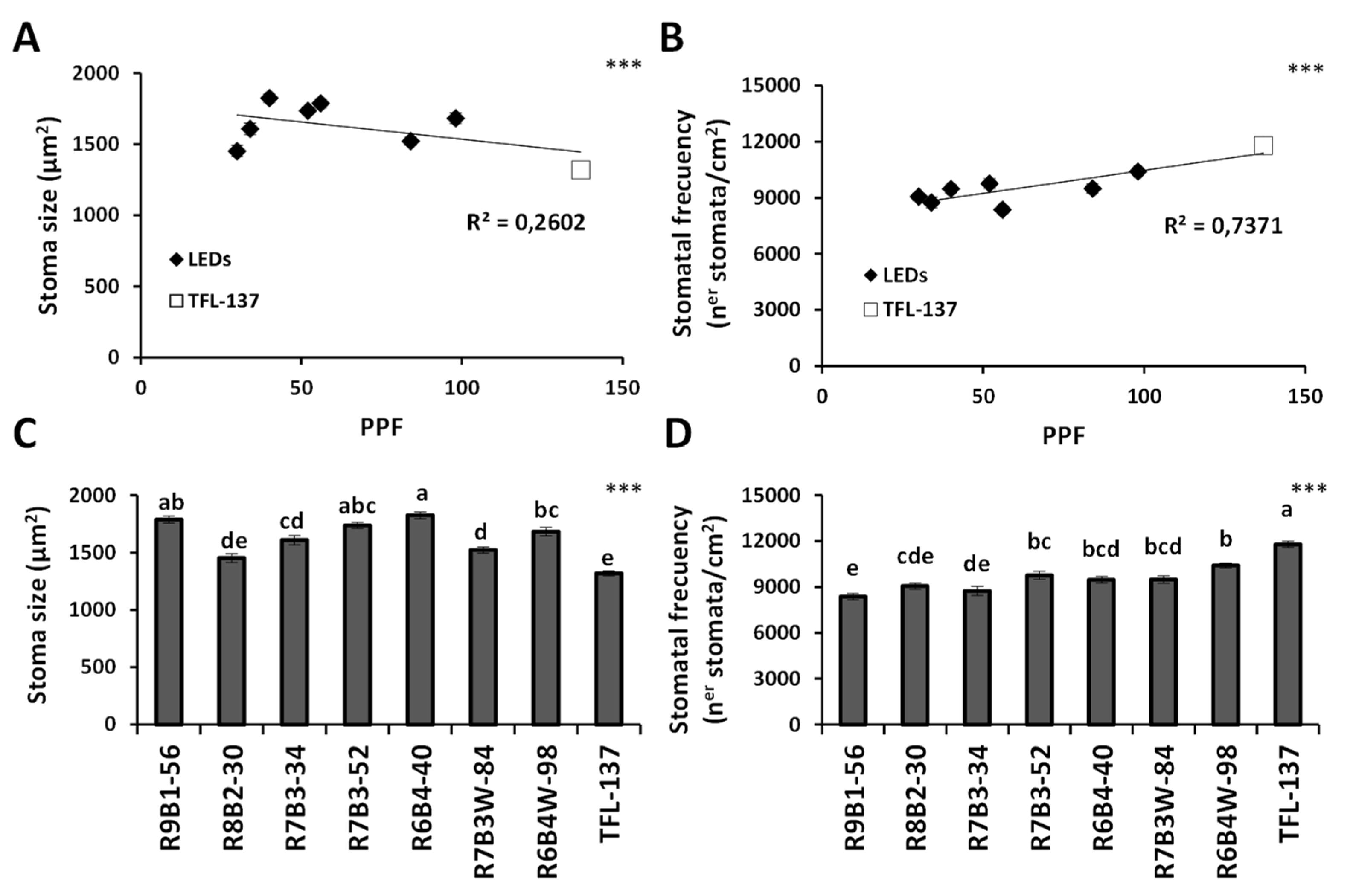
2.2. Effect of Light Quality on Leaf Growth, Development and Pigment Composition
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Illumination Treatments
4.3. Determination of Growth and Developmental Parameters
4.4. Determination of Photosynthetic Pigments
4.5. Number and Size of Leaf Stomata and Trichomes
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin, 4th ed.; Oxford University Press: Oxford, UK, 2012; ISBN 978-0-19-954906-1. [Google Scholar]
- Barranco, D.; Fernández-Escobar, R.; Rallo, L. El Cultivo del Olivo; Junta de Andalucía: Sevilla, Spain, 1998; ISBN 978-84-89802-19-3. [Google Scholar]
- Hoenecke, M.; Bula, R.; Tibbitts, T. Importance of ‘Blue’ Photon Levels for Lettuce Seedlings Grown under Red-light-emitting Diodes. HortScience 1992, 27, 427–430. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Saebo, A.; Krekling, T.; Appelgren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. PCTOC 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Vieira, L.D.N.; Fraga, H.; Dos Anjos, K.G.; Puttkammer, C.C.; Scherer, R.F.; Da Silva, D.A.; Guerra, M.P. Light-emitting diodes (LED) increase the stomata formation and chlorophyll content in Musa acuminata (AAA) ‘Nanicão Corupá’ in vitro plantlets. Theor. Exp. Plant Physiol. 2015, 27, 91–98. [Google Scholar] [CrossRef]
- Beattie, G.A.; Hatfield, B.M.; Dong, H.; McGrane, R.S. Seeing the Light: The Roles of Red- and Blue-Light Sensing in Plant Microbes. Annu. Rev. Phytopathol. 2018, 56, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2021, 1–39. [Google Scholar] [CrossRef]
- Quail, P.H. Phytochromes. Curr. Biol. 2010, 20, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.A.; Burns, C. Photobiology in protected horticulture. Food Energy Secur. 2016, 5, 223–238. [Google Scholar] [CrossRef]
- Shinomura, T.; Nagatani, A.; Hanzawa, H.; Kubota, M.; Watanabe, M.; Furuya, M. Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 8129–8133. [Google Scholar] [CrossRef]
- Marks, T.; Simpson, S. Effect of irradiance on shoot development in vitro. Plant Growth Regul. 1999, 28, 133–142. [Google Scholar] [CrossRef]
- Lazzarin, M.; Meisenburg, M.; Meijer, D.; van Ieperen, W.; Marcelis, L.; Kappers, I.; van der Krol, A.; van Loon, J.; Dicke, M. LEDs Make It Resilient: Effects on Plant Growth and Defense. Trends Plant Sci. 2021, 26, 496–508. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; He, H.; Song, W. Application of Light-emitting Diodes and the Effect of Light Quality on Horticultural Crops: A Review. HortScience 2019, 54, 1656–1661. [Google Scholar] [CrossRef]
- Wang, Y.; Maruhnich, S.A.; Mageroy, M.H.; Justice, J.R.; Folta, K.M. Phototropin 1 and cryptochrome action in response to green light in combination with other wavelengths. Planta 2013, 237, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and Photomorphogenesis of Pepper Plants under Red Light-emitting Diodes with Supplemental Blue or Far-red Lighting. J. Am. Soc. Hortic. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Bula, R.; Morrow, R.; Tibbitts, T.; Barta, D.; Ignatius, R.; Martin, T. Light-emitting Diodes as a Radiation Source for Plants. HortScience 1991, 26, 203–205. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Tang, C. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tissue Organ Cult. PCTOC 2010, 103, 155–163. [Google Scholar] [CrossRef]
- Benedict, C.R.; McCree, K.J.; Kohel, R.J. High Photosynthetic Rate of a Chlorophyll Mutant of Cotton. Plant Physiol. 1972, 49, 968–971. [Google Scholar] [CrossRef]
- Moon, H.K.; Park, S.-Y.; Kim, Y.W.; Kim, C.S. Growth of Tsuru-rindo (Tripterospermum japonicum) cultured in vitro under various sources of light-emitting diode (LED) irradiation. J. Plant Biol. 2006, 49, 174–179. [Google Scholar] [CrossRef]
- Gupta, S.D.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Chaves, M.C.; Freitas, J.C.E.; Nery, F.C.; Paiva, R.; Prudente, D.D.O.; Costa, B.G.P.; Daubermann, A.G.; Bernardes, M.M.; Grazul, R.M. Influence of colorful light-emitting diodes on growth, biochemistry, and production of volatile organic compounds in vitro of Lippia filifolia (Verbenaceae). J. Photochem. Photobiol. B Biol. 2020, 212, 112040. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, C.; Xu, Z. The effects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Miranda, N.A.; Xavier, A.; Otoni, W.C.; Gallo, R.; Gatti, K.C.; De Moura, L.C.; Souza, D.M.S.C.; Maggioni, J.H.; de Santos, S.S.O. Quality and Intensity of Light in the In Vitro Development of Microstumps of Eucalyptus urophylla in a Photoautotrophic System. For. Sci. 2020, 66, 754–760. [Google Scholar] [CrossRef]
- Oliveira, T.D.R.D.; Aragão, V.P.M.; Moharana, K.C.; Fedosejevs, E.; Amaral, F.P.D.; de Sousa, K.R.; Thelen, J.J.; Venâncio, T.M.; Silveira, V.; Santa-Catarina, C. Light spectra affect the in vitro shoot development of Cedrela fissilis Vell. (Meliaceae) by changing the protein profile and polyamine contents. Biochim. Biophys. Acta BBA Proteins Proteom. 2020, 1868, 140529. [Google Scholar] [CrossRef]
- Smirnakou, S.; Ouzounis, T.; Radoglou, K. Effects of continuous spectrum LEDs used in indoor cultivation of two coniferous species Pinus sylvestris L. and Abies borisii-regis Mattf. Scand. J. For. Res. 2017, 32, 115–122. [Google Scholar] [CrossRef]
- Ausin, I.; Alonso-Blanco, C.; Martinez-Zapater, J.-M. Environmental regulation of flowering. Int. J. Dev. Biol. 2005, 49, 689–705. [Google Scholar] [CrossRef]
- Chang, H.S.; Charkabarty, D.; Hahn, E.J.; Paek, K.Y. Micropropagation of calla lily (Zantedeschia albomaculata) via in vitro shoot tip proliferation. In Vitro Cell. Dev. Biol. Anim. 2003, 39, 129–134. [Google Scholar] [CrossRef]
- Chory, J. Light modulation of vegetative development. Plant Cell 1997, 9, 1225–1234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chory, J.; Chatterjee, M.; Cook, R.K.; Elich, T.; Fankhauser, C.; Li, J.; Nagpal, P.; Neff, M.; Pepper, A.; Poole, D.; et al. From seed germination to flowering, light controls plant development via the pigment phytochrome. Proc. Natl. Acad. Sci. USA 1996, 93, 12066–12071. [Google Scholar] [CrossRef]
- Folta, K.M.; Maruhnich, S.A. Green light: A signal to slow down or stop. J. Exp. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef]
- Kim, S.-J.; Hahn, E.-J.; Heo, J.-W.; Paek, K.-Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Pharis, R.P. Light signaling and the phytohormonal regulation of shoot growth. Plant Sci. 2014, 229, 280–289. [Google Scholar] [CrossRef]
- Luan, V.Q.; Huy, N.P.; Nam, N.B.; Huong, T.T.; Hien, V.T.; Hien, N.T.T.; Hai, N.T.; Thinh, D.K.; Nhut, D.T. Ex vitro and in vitro Paphiopedilum delenatii Guillaumin stem elongation under light-emitting diodes and shoot regeneration via stem node culture. Acta Physiol. Plant. 2015, 37, 136. [Google Scholar] [CrossRef]
- McNellis, T.W.; Deng, X.W. Light control of seedling morphogenetic pattern. Plant Cell 1995, 7, 1749–1761. [Google Scholar] [CrossRef]
- Leva, A.; Muleo, R.; Petruccelli, R. Long-Term Somatic Embryogenesis from Inmature Olive Cotyledons. J. Hortic. Sci. 1995, 70, 417–421. [Google Scholar] [CrossRef]
- Trabelsi, E.B.; Naija, S.; Elloumi, N.; Belfeleh, Z.; Msellem, M.; Ghezel, R.; Bouzid, S. Somatic embryogenesis in cell suspension cultures of olive Olea europaea (L.) ‘Chetoui’. Acta Physiol. Plant. 2011, 33, 319–324. [Google Scholar] [CrossRef]
- Mazri, M.A.; Naciri, R.; Belkoura, I. Maturation and Conversion of Somatic Embryos Derived from Seeds of Olive (Olea europaea L.) cv. Dahbia: Occurrence of Secondary Embryogenesis and Adventitious Bud Formation. Plants 2020, 9, 1489. [Google Scholar] [CrossRef]
- García, J.; Troncoso, J.; Sarmiento, R. Influence of carbon source and concentration on the in vitro development of olive (Olea europaea L.) zygotic embryos and explants raised from them. Plant Cell Tissue Organ Cult. PCTOC 2002, 69, 95–100. [Google Scholar] [CrossRef]
- Gentile, L.; Uccella, N.A. Selected bioactives from callus cultures of olives (Olea europaea L. Var. Coratina) by LC-MS. Food Res. Int. 2014, 55, 128–136. [Google Scholar] [CrossRef]
- Mohammad, S.; Khan, M.A.; Ali, A.; Khan, L.; Khan, M.S.; Mashwani, Z.-U. Feasible production of biomass and natural antioxidants through callus cultures in response to varying light intensities in olive (Olea europaea. L) cult. Arbosana. J. Photochem. Photobiol. B Biol. 2019, 193, 140–147. [Google Scholar] [CrossRef]
- Zacchini, M.; De Agazio, M. Micropropagation of a Local Olive Cultivar for Germplasm Preservation. Biol. Plant. 2004, 48, 589–592. [Google Scholar] [CrossRef]
- Leva, A. Innovative protocol for “ex vitro rooting” on olive micropropagation. Open Life Sci. 2011, 6, 352–358. [Google Scholar] [CrossRef]
- Mirzaei, L.; Yadollahi, A.; Kermani, M.J.; Naderpour, M.; Zeinanloo, A.A.; Farsi, M.; Davoodi, D. Evaluation of genetic stability in olive callus-induced and meristem-induced shoots using flow cytometry and amplified fragment length polymorphism techniques. Plant Methods 2021, 17, 31. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Eleftheriou, E.; Vasilakakis, M. Hidden hyperhydricity may be responsible for abnormal development and acclimatization problems of micropropagated olive plantlets: An anatomical leaf study. Acta Hortic. 2007, 103–106. [Google Scholar] [CrossRef]
- Akoyunoglou, G.; Anni, H. Blue light effect on chloroplast development in higher plants. In Blue Light Effects in Biological Systems; Senger, H., Ed.; Proceedings in Life Sciences; Springer: Berlin, Germany, 1984; pp. 397–406. ISBN 978-3-642-69769-2. [Google Scholar]
- Saito, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. The effect of light quality on growth of lettuce. IFAC Proc. Vol. 2010, 43, 294–298. [Google Scholar] [CrossRef]
- Silva, S.T.; Bertolucci, S.K.V.; Da Cunha, S.H.B.; Lazzarini, L.E.S.; Tavares, M.C.; Pinto, J.E.B.P. Effect of light and natural ventilation systems on the growth parameters and carvacrol content in the in vitro cultures of Plectranthus amboinicus (Lour.) Spreng. Plant Cell Tissue Organ Cult. PCTOC 2017, 129, 501–510. [Google Scholar] [CrossRef]
- de Hsie, B.S.; Bueno, A.I.S.; Bertolucci, S.K.V.; de Carvalho, A.A.; da Cunha, S.H.B.; Martins, E.R.; Pinto, J.E.B.P. Study of the influence of wavelengths and intensities of LEDs on the growth, photosynthetic pigment, and volatile compounds production of Lippia rotundifolia Cham in vitro. J. Photochem. Photobiol. B Biol. 2019, 198, 111577. [Google Scholar] [CrossRef]
- Pierik, R.L.M. Rejuvenation and micropropagation. In Progress in Plant Cellular and Molecular Biology; Nijkamp, H.J.J., Van Der Plas, L.H.W., Van Aartrijk, J., Eds.; Current Plant Science and Biotechnology in Agriculture; Springer: Dordrecht, The Netherlands, 1990; Volume 9, pp. 91–101. ISBN 978-94-010-7445-2. [Google Scholar]
- Serret, M.; Trillas, M.; Araus, J. The Effect of In Vitro Culture Conditions on the Pattern of Photoinhibition during Acclimation of Gardenia Plantlets to Ex Vitro Conditions. Photosynthetica 2001, 39, 67–73. [Google Scholar] [CrossRef]
- da Silva, M.M.; Debergh, P. The effect of light quality on the morphogenesis of in vitro cultures of Azorina vidalii (Wats.) Feer. Plant Cell Tissue Organ Cult. PCTOC 1997, 51, 187–193. [Google Scholar] [CrossRef]
- Mortensen, L.; Strømme, E. Effects of light quality on some greenhouse crops. Sci. Hortic. 1987, 33, 27–36. [Google Scholar] [CrossRef]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of red- and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tissue Organ Cult. PCTOC 2008, 92, 147–153. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Brown, C.S.; Stryjewski, E.C. Anatomical Features of Pepper Plants (Capsicum annuum L.) Grown under Red Light-emitting Diodes Supplemented with Blue or Far-red Light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Simlat, M.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]
- Islam, M.A.; Kuwar, G.; Clarke, J.L.; Blystad, D.-R.; Gislerød, H.R.; Olsen, J.E.; Torre, S. Artificial light from light emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Senger, H. The effect of blue light on plants and microorganisms. Photochem. Photobiol. 1982, 35, 911–920. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.-M. Blue and Red LED Illumination Improves Growth and Bioactive Compounds Contents in Acyanic and Cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [PubMed]
- Abidi, F.; Girault, T.; Douillet, O.; Guillemain, G.; Sintes, G.; Laffaire, M.; Ben Ahmed, H.; Smiti, S.; Huché-Thélier, L.; Leduc, N. Blue light effects on rose photosynthesis and photomorphogenesis: Blue Light and Rose Development. Plant Biol. 2013, 15, 67–74. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Hogewoning, S.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Batista, D.; Felipe, S.H.S.; Silva, T.D.; De Castro, K.M.; Mamedes-Rodrigues, T.C.; Miranda, N.; Ríos, A.M.R.; Faria, D.; Fortini, E.A.; Chagas, K.; et al. Light quality in plant tissue culture: Does it matter? In Vitro Cell. Dev. Biol. Anim. 2018, 54, 195–215. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Yang, Q.; Li, T. Red/blue light ratio strongly affects steady-state photosynthesis, but hardly affects photosynthetic induction in tomato (Solanum lycopersicum). Physiol. Plant. 2019, 167, 144–158. [Google Scholar] [CrossRef]
- Morini, S.; D’Onofrio, C.; Bellocchi, G.; Fisichella, M. Effect of 2,4-D and light quality on callus production and differentiation from in vitro cultured quince leaves. Plant Cell Tissue Organ Cult. PCTOC 2000, 63, 47–55. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Morini, S. Effect of light quality on in vitro production of callus in explants of three poinsettia cultivars. Acta Hortic. 2001, 449–452. [Google Scholar] [CrossRef]
- Paek, K.-Y.; Hahn, E.-J. Cytokinins, auxins and activated charcoal affect organogenesis and anatomical characteristics of shoot-tip cultures of lisianthus [Eustoma grandiflorum (RAF.) Shinn]. In Vitro Cell. Dev. Biol. Anim. 2000, 36, 128–132. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Torii, K.U. Mechanisms of Stomatal Development. Annu. Rev. Plant Biol. 2012, 63, 591–614. [Google Scholar] [CrossRef]
- Wetzstein, H.; Sommer, H. Scanning Electron Microscopy of in Vitro-Cultured Liquidambar Styraciflua Plantlets during Acclimatization. J. Am. Soc. Hortic. Sci. 1983, 108, 475–480. [Google Scholar]
- Franco-Navarro, J.D.; Rosales, M.A.; Cubero-Font, P.; Calvo, P.; Alvarez, R.; Diaz-Espejo, A.; Colmenero-Flores, J.M. Chloride as a macronutrient increases water-use efficiency by anatomically driven reduced stomatal conductance and increased mesophyll diffusion to CO2. Plant J. 2019, 99, 815–831. [Google Scholar] [CrossRef]
- Díaz-Rueda, P.; Franco-Navarro, J.D.; Messora, R.; Espartero, J.; Rivero-Núñez, C.M.; Aleza, P.; Capote, N.; Cantos, M.; García-Fernández, J.L.; De Cires, A.; et al. SILVOLIVE, a Germplasm Collection of Wild Subspecies with High Genetic Variability as a Source of Rootstocks and Resistance Genes for Olive Breeding. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Rugini, E. In vitro propagation of some olive (Olea europaea sativa L.) cultivars with different root-ability, and medium development using analytical data from developing shoots and embryos. Sci. Hortic. 1984, 24, 123–134. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Gitz, D.C.; Baker, J.T. Methods for Creating Stomatal Impressions Directly onto Archivable Slides. Agron. J. 2009, 101, 232–236. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Brumos, J.; Rosales, M.A.; Cubero-Font, P.; Talón, M.; Colmenero-Flores, J.M. Chloride regulates leaf cell size and water relations in tobacco plants. J. Exp. Bot. 2016, 67, 873–891. [Google Scholar] [CrossRef] [PubMed]




| Red/Blue Ratio (%) | Photon Flux (µmol m−2 s−1) | Nomenclature |
|---|---|---|
| LED 90/10 | 56 | R9B1-56 |
| LED 80/20 | 30 | R8B2-30 |
| LED 70/30 | 34 | R7B3-34 |
| LED 70/30 | 52 | R7B3-52 |
| LED 60/40 | 40 | R6B4-40 |
| LED 70/30 + white | 84 | R7B3W-84 |
| LED 60/40 + white | 98 | R6B4W-98 |
| Fluorescent | 137 | TFL-137 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Rueda, P.; Cantos-Barragán, M.; Colmenero-Flores, J.M. Growth Quality and Development of Olive Plants Cultured In-Vitro under Different Illumination Regimes. Plants 2021, 10, 2214. https://doi.org/10.3390/plants10102214
Díaz-Rueda P, Cantos-Barragán M, Colmenero-Flores JM. Growth Quality and Development of Olive Plants Cultured In-Vitro under Different Illumination Regimes. Plants. 2021; 10(10):2214. https://doi.org/10.3390/plants10102214
Chicago/Turabian StyleDíaz-Rueda, Pablo, Manuel Cantos-Barragán, and José Manuel Colmenero-Flores. 2021. "Growth Quality and Development of Olive Plants Cultured In-Vitro under Different Illumination Regimes" Plants 10, no. 10: 2214. https://doi.org/10.3390/plants10102214
APA StyleDíaz-Rueda, P., Cantos-Barragán, M., & Colmenero-Flores, J. M. (2021). Growth Quality and Development of Olive Plants Cultured In-Vitro under Different Illumination Regimes. Plants, 10(10), 2214. https://doi.org/10.3390/plants10102214







