Metabolic Perturbation and Synthetic Biology Strategies for Plant Terpenoid Production—An Updated Overview
Abstract
:1. Introduction
2. Biosynthesis and Precursors of Terpenoids
2.1. Monoterpenoid Chemical Compounds
2.2. Sesquiterpenoid Chemical Compounds
3. Analytical Platforms for Terpenoids
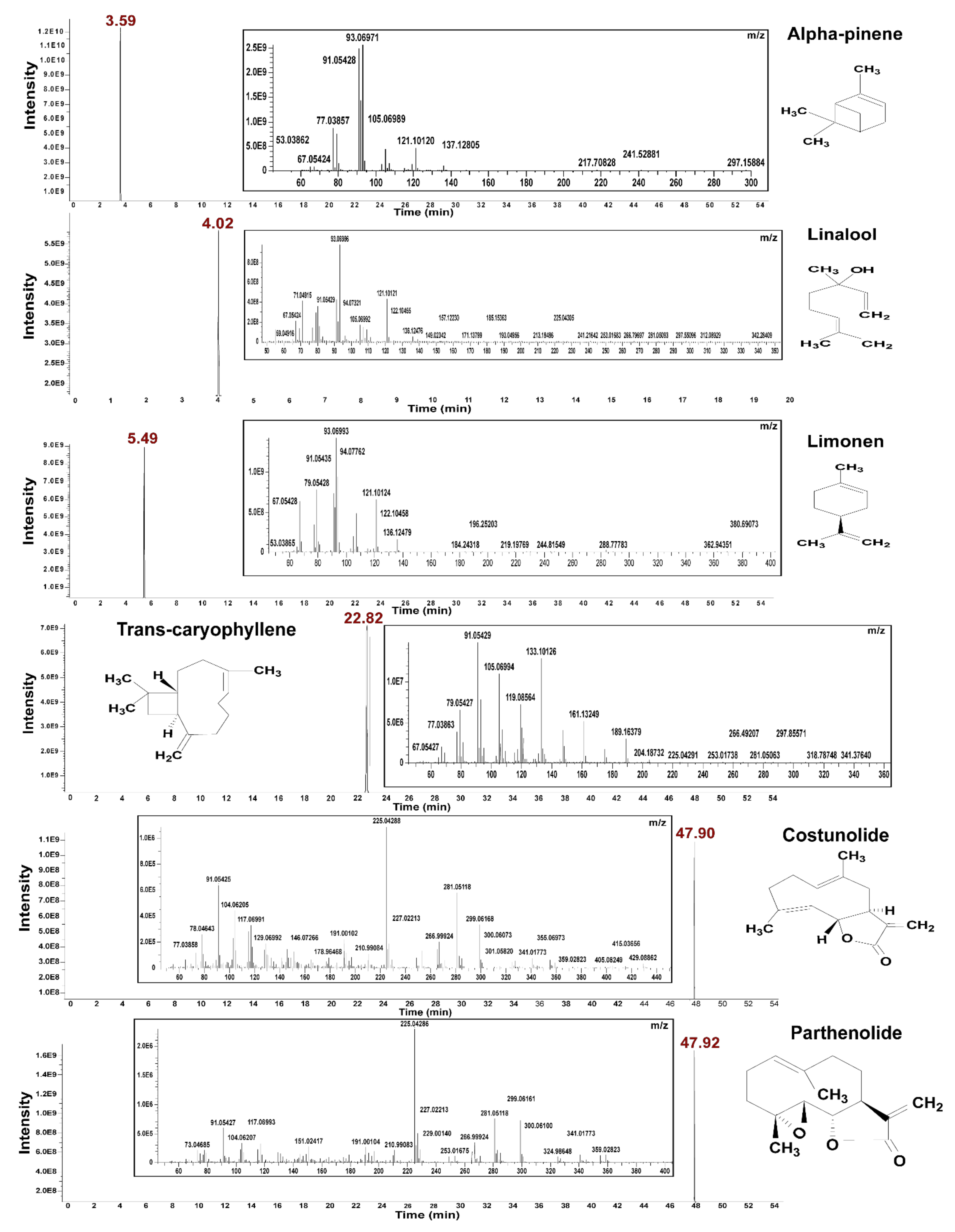
3.1. Chromatographic Techniques
3.2. Metabolic Profiling of Plant Terpenoids
4. Metabolic Engineering of Terpenoids in Plants
5. Synthetic Biology of Terpenoids
6. Terpenoids Pharmacological Activity
6.1. Monoterpenoids
6.1.1. Linalool
6.1.2. Limonene
6.1.3. Alpha-Pinene
6.1.4. Others
6.2. Sesquiterpenoids
6.2.1. Costunolide
6.2.2. Parthenolide
6.2.3. Trans-Caryophyllene
6.2.4. Others
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caputi, L.; Aprea, E. Use of terpenoids as natural flavouring compounds in food industry. Recent Patents Food. Nutr. Agric. 2012, 3, 9–16. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershenzon, J.; Croteau, R.B. Terpenoid biosynthesis: The basic pathway and formation of monoterpenes, sesquiterpenes and diterpenes. In Lipid Metabolism in Plants; Moore, T.S., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 340–388. [Google Scholar]
- Noriega, P. Terpenes in Essential Oils: Bioactivity and Applications; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Zhou, F.; Pichersky, E. More is better: The diversity of terpene metabolism in plants. Curr. Opin. Plant Biol. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Mahmoud, S.S.; Croteau, R.B. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 2002, 7, 366–373. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Boronat, A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef] [Green Version]
- Bick, J.A.; Lange, B.M. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: Unidirectional transport of intermediates across the chloroplast envelope membrane. Arch. Biochem. Biophys. 2003, 415, 146–154. [Google Scholar] [CrossRef]
- Lücker, J.; Bouwmeester, H.J.; Schwab, W.; Blaas, J.; Van Der Plas, L.H.W.; Verhoeven, H.A. Expression of clarkia S-linalool synthase in transgenic petunia plants results in the accumulation of S-linalyl-β-D-glucopyranoside. Plant J. 2001, 27, 315–324. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Schalechet, F.; Wilkinson, J.; Matsui, K.; Tadmor, Y.; Nam, K.; Amar, O.; Lastochkin, E.; Larkov, O.; Hiatt, W.; et al. Enhanced levels of the aroma and flavor compound S-Linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physol. 2001, 3, 1256–1265. [Google Scholar] [CrossRef]
- Lavy, M.; Zuker, A.; Lewinsohn, E.; Larkov, O.; Ravid, U.; Vainstein, A.; Weiss, D. Linalool and linalool oxide production in transgenic carnation flowers expressing the Clarkia breweri linalool synthase gene. Mol. Breed. 2002, 9, 103–111. [Google Scholar] [CrossRef]
- Aharoni, A.; Jongsma, M.A.; Kim, T.Y.; Ri, M.B.; Giri, A.P.; Verstappen, F.W.A.; Schwab, W.; Bouwmeester, H.J. Metabolic engineering of terpenoid biosynthesis in plants. Phytochem. Rev. 2006, 5, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Aharoni, A.; Giri, A.P.; Deuerlein, S.; Griepink, F.; De Kogel, W.J.; Verstappen, F.W.A.; Verhoeven, H.A.; Jongsma, M.A.; Schwab, W.; Bouwmeester, H.J. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 2003, 15, 2866–2884. [Google Scholar] [CrossRef] [Green Version]
- Krasnyanski, S.; May, R.A.; Loskutov, A.; Ball, T.M.; Sink, K.C. Transformation of the limonene synthase gene into peppermint (Mentha piperita L.) and preliminary studies on the essential oil profiles of single transgenic plants. Theor. Appl. Genet. 1999, 99, 676–682. [Google Scholar] [CrossRef]
- Aharoni, A.; Jongsma, M.A.; Bouwmeester, H.J. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 2005, 10, 594–602. [Google Scholar] [CrossRef]
- Wallaart, T.E.; Bouwmeester, H.J.; Hille, J.; Poppinga, L.; Maijers, N.C.A. Amorpha-4,11-diene synthase: Cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 2001, 212, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Hohn, T.M.; Ohlrogge, J.B. Expression of a fungal sesquiterpene cyclase gene in transgenic tobacco. Plant Physiol. 1991, 97, 460–462. [Google Scholar] [CrossRef] [Green Version]
- Petzold, C.J.; Chan, L.J.G.; Nhan, M.; Adams, P.D. Analytics for metabolic engineering. Front. Bioeng. Biotechnol. 2015, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Courdavault, V.; O’Connor, S.E.; Jensen, M.K.; Papon, N. Metabolic engineering for plant natural products biosynthesis: New procedures, concrete achievements and remaining limits. Nat. Prod. Rep. 2021. [Google Scholar] [CrossRef]
- Gutensohn, M.; Henry, L.K.; Gentry, S.A.; Lynch, J.H.; Nguyen, T.T.H.; Pichersky, E.; Dudareva, N. Overcoming bottlenecks for metabolic engineering of sesquiterpene production in tomato fruits. Front. Plant Sci. 2021, 12, 691754. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Seigler, D.S. Plant Secondary Metabolism; Springer Science Business Media: New York, NY, USA, 1995; ISBN 978-1-4613-7228-8. [Google Scholar] [CrossRef]
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant J. 2008, 54, 656–669. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Structure and dynamics of the isoprenoid pathway network. Mol. Plant 2012, 5, 318–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Ramawat, K.G.; Mérillon, J.M. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin, Germany, 2013; pp. 1–4242. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef] [Green Version]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and medicinal uses of terpenes. In Medicinal Plants: From Farm to Pharmacy; Springer Nature: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Mumm, R.; Posthumus, M.A.; Dicke, M. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ. 2008, 31, 575–585. [Google Scholar] [CrossRef]
- McCaskill, D.; Croteau, R. Some caveats for bioengineering terpenoid metabolism in plants. Trends Biotechnol. 1998, 16, 349–355. [Google Scholar] [CrossRef]
- Dudareva, N.; Andersson, S.; Orlova, I.; Gatto, N.; Reichelt, M.; Rhodes, D.; Boland, W.; Gershenzon, J. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc. Natl. Acad. Sci. USA 2005, 102, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Hemmerlin, A.; Harwood, J.L.; Bach, T.J. A raison d’être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lipid Res. 2012, 51, 95–148. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Huang, M.; Abel, C.; Sohrabi, R.; Petri, J.; Haupt, I.; Cosimano, J.; Gershenzon, J.; Tholl, D. Variation of herbivore-induced volatile terpenes among arabidopsis ecotypes depends on allelic differences and subcellular targeting of two terpene synthases, TPS02 and TPS03. Plant Physiol. 2010, 153, 1293–1310. [Google Scholar] [CrossRef] [Green Version]
- Lei, D.; Qiu, Z.; Qiao, J.; Zhao, G.R. Plasticity engineering of plant monoterpene synthases and application for microbial production of monoterpenoids. Biotechnol. Biofuels 2021, 14, 147. [Google Scholar] [CrossRef]
- Dewick, P.M. The Mevalonate and Methylerythritol Phosphate Pathways: Terpenoids and Steroids; John Wiley & Sons, Ltd.: Chichester, UK, 2009; ISBN 9780470741689. [Google Scholar]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef] [PubMed]
- Sülsen, V.P.; Martino, V.S. Sesquiterpene Lactones: Advances in Their Chemistry and Biological Aspects; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783319782744. [Google Scholar]
- Kozioł, A.; Stryjewska, A.; Librowski, T.; Sałat, K.; Gaweł, M.; Moniczewski, A.; Lochy´nski, S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural monoterpenes: Much more than only a scent. Chem. Biodivers. 2019, 16, e1900434. [Google Scholar] [CrossRef]
- Barreto, R.S.S.; Albuquerque-Júnior, R.L.C.; Araújo, A.A.S.; Almeida, J.R.G.S.; Santos, M.R.V.; Barreto, A.S.; DeSantana, J.M.; Siqueira-Lima, P.S.; Quintans, J.S.S.; Quintans-Júnior, L.J. A systematic review of the wound-healing effects of monoterpenes and iridoid derivatives. Molecules 2014, 19, 846–862. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and their derivatives—Recent development in biological and medical applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Singh, G. Chemistry of Terpenoids and Carotenoids; Discovery Publisher Pvt. Ltd.: New Delhi, India, 2007; pp. 1–286. [Google Scholar]
- Hohmann, M.S.N.; Longhi-Balbinot, D.T.; Guazelli, C.F.S.; Navarro, S.A.; Zarpelon, A.C.; Casagrande, R.; Arakawa, N.S.; Verri, W.A. Sesquiterpene Lactones: Structural diversity and perspectives as anti-inflammatory molecules. Stud. Nat. Prod. Chem. 2016, 49, 243–264. [Google Scholar] [CrossRef]
- Zwenger, S.; Basu, C. Plant terpenoids. Biotechnol. Mol. Biol. Rev. 2008, 3, 1–7. [Google Scholar]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Terpenoids; Academic Press: Boston, MA, USA, 2017; ISBN 9780128020999. [Google Scholar]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Krill, C.; Rochfort, S.; Spangenberg, G. A high-throughput method for the comprehensive analysis of terpenes and terpenoids in medicinal Cannabis biomass. Metabolites 2020, 10, 276. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Riordan-Short, S.; Dang, T.T.T.; O’Brien, R.; Noestheden, M. Quantitation of select terpenes/terpenoids and nicotine using gas chromatography-mass spectrometry with high-temperature headspace sampling. ACS Omega 2020, 5, 5565–5573. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Jiang, Q.; Sun, H.; Jiang, J.; Chen, S.; Guan, Z.; Fang, W.; Chen, F. GC-MS analysis of the volatile constituents in the leaves of 14 compositae plants. Molecules 2018, 23, 166. [Google Scholar] [CrossRef] [Green Version]
- Johnson, V.; Frost, J.M.; Clifford, B. Analysis of 21 Terpenes in 3 Cannabis Cultivars by HS-GCMS; Shimadzu Scientific Instruments: Columbia, MD, USA.
- Trass, M.; Anderson, T.; Krepich, S.; Ave, M. Analysis of 33 Primary and Secondary Terpenes Found in Cannabis by GC-FID; Phenomenex: Torrance, CA, USA, 2017. [Google Scholar]
- Fausett, A. Analysis of Terpene and Terpenoid Content in Cannabis Sativa Using Headspace with GC/MSD; Agilent Technologies: Santa Clara, CA, USA, 2020; pp. 1–7. [Google Scholar]
- Abualhasan, M.N.; Zaid, A.N.; Jaradat, N.; Mousa, A. GC Method validation for the analysis of menthol in suppository pharmaceutical dosage form. Int. J. Anal. Chem. 2017, 2017, 1728414. [Google Scholar] [CrossRef] [Green Version]
- James, A. Using Gas Chromatography for accurate terpene analysis in Cannabis. Cannabis Sci. Technol. 2019, 2. [Google Scholar]
- Henneman, L.; van Cruchten, A.G.; Denis, S.W.; Amolins, M.W.; Placzek, A.T.; Gibbs, R.A.; Kulik, W.; Waterham, H.R. Detection of nonsterol isoprenoids by HPLC-MS/MS. Anal. Biochem. 2008, 383, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Henneman, L.; Van Cruchten, A.G.; Kulik, W.; Waterham, H.R. Inhibition of the isoprenoid biosynthesis pathway; Detection of intermediates by UPLC-MS/MS. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2011, 1811, 227–233. [Google Scholar] [CrossRef]
- Kumar, R.; Bohra, A.; Pandey, A.K.; Pandey, M.K.; Kumar, A. Metabolomics for plant improvement: Status and prospects. Front. Plant Sci. 2017, 8, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Kempinski, C.; Chappell, J. Extraction and analysis of Terpenes/Terpenoids. Curr. Protoc. Plant Biol. 2016, 1, 345–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Zhou, Y.; Zeng, L.; Dong, F.; Mei, X.; Liao, Y.; Watanabe, N.; Yang, Z. Analytical method for metabolites involved in biosynthesis of plant volatile compounds. RSC Adv. 2017, 7, 19363–19372. [Google Scholar] [CrossRef] [Green Version]
- Myers, C.; Herrington, J.S.; Hamrah, P.; Anderson, K. Accelerated solvent extraction of terpenes in Cannabis coupled with various injection techniques for GC-MS analysis. Front. Chem. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Rocha, E.D.; Silva, V.E.A.; Pereira, F.C.S.; Jean, V.M.; Costa Souza, F.L.; Baratto, L.C.; Vieira, A.C.M.; Carvalho, V.M. Qualitative terpene profiling of Cannabis varieties cultivated for medical purposes. Rodriguesia 2020, 7, 48–51. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, B.; Zhang, X.; Wang, H.; Yan, A.; Zhang, G.; Wang, X.; Xu, H. The accumulation profiles of terpene metabolites in three Muscat table grape cultivars through HS-SPME-GCMS. Sci. Data 2020, 7, 1–6. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, C.; Xu, C.; Sun, J.; Grierson, D.; Zhang, B.; Chen, K. Integration of metabolite profiling and transcriptome analysis reveals genes related to volatile terpenoid metabolism in finger citron (C. Medica var. sarcodactylis). Molecules 2019, 24, 2564. [Google Scholar] [CrossRef] [Green Version]
- Kupska, M.; Wasilewski, T.; Jędrkiewicz, R.; Gromadzka, J.; Namieśnik, J. Determination of terpene profiles in potential superfruits. Int. J. Food Prop. 2016, 19, 2726–2738. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, Q.; Liang, Z.; Zhao, M.; Yu, X.; Yang, D.; Xu, X. The GC/MS analysis of volatile components extracted by different methods from Exocarpium citri grandis. J. Anal. Methods Chem. 2013, 2013, 918406. [Google Scholar] [CrossRef] [Green Version]
- Di Carro, M.; Ianni, C.; Magi, E. Determination of terpenoids in plant leaves by GC-MS: Development of the method and application to Ocimum basilicum and Nicotiana langsdorffii. Anal. Lett. 2013, 46, 630–639. [Google Scholar] [CrossRef]
- Ma, X.; Gang, D.R. Metabolic profiling of in vitro micropropagated and conventionally greenhouse grown ginger (Zingiber officinale). Phytochemistry 2006, 67, 2239–2255. [Google Scholar] [CrossRef]
- Li, C.; Sarangapani, S.; Wang, Q.; Nadimuthu, K.; Sarojam, R. Metabolic engineering of the native monoterpene pathway in spearmint for production of heterologous monoterpenes reveals complex metabolism and pathway interactions. Int. J. Mol. Sci. 2020, 21, 6164. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Gao, M.; Yin, H.; Wu, L.; Wang, Y. Overexpression of geranyl diphosphate synthase small subunit 1 (LcGPPS.SSU1) enhances the monoterpene content and biomass. Ind. Crops Prod. 2020, 143, 111926. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, M.; Guo, Y.; Shi, X.; Yang, Y.; Chen, J.; Zheng, T.; Han, Y.; Bao, F.; Ahmad, S. Overexpression of LiDXS and LiDXR from lily (Lilium ‘siberia’) enhances the terpenoid content in tobacco flowers. Front. Plant Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Yin, J.L.; Wong, W.S.; Jang, I.C.; Chua, N.H. Co-expression of peppermint geranyl diphosphate synthase small subunit enhances monoterpene production in transgenic tobacco plants. New Phytol. 2017, 213, 1133–1144. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Liu, C.; Zheng, R.; Cai, X.; Luo, J.; Zou, J.; Wang, C. Emission and accumulation of monoterpene and the key terpene synthase(TPS)associated with monoterpene biosynthesis in Osmanthus fragrans lour. Front. Plant Sci. 2016, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.M.; Park, D.J.; Lee, D.G.; Song, H.J.; Kang, S.M.; Min, J.Y.; Moon, B.C.; Lee, C.K.; Jeon, K.S.; Shivappakarigar, C.; et al. Over expression of IPP isomerase and limonene synthase enzymes in Mentha spicata and their influence on the terpenoid metabolism. Rom. Biotechnol. Lett. 2015, 20, 10358–10368. [Google Scholar]
- Amiri, P.; Shahpiri, A.; Asadollahi, M.A.; Momenbeik, F.; Partow, S. Metabolic engineering of Saccharomyces cerevisiae for linalool production. Biotechnol. Lett. 2016, 38, 503–508. [Google Scholar] [CrossRef]
- Gutensohn, M.; Orlova, I.; Nguyen, T.T.H.; Davidovich-Rikanati, R.; Ferruzzi, M.G.; Sitrit, Y.; Lewinsohn, E.; Pichersky, E.; Dudareva, N. Cytosolic monoterpene biosynthesis is supported by plastid-generated geranyl diphosphate substrate in transgenic tomato fruits. Plant J. 2013, 75, 351–363. [Google Scholar] [CrossRef]
- Lücker, J.; Schwab, W.; Van Hautum, B.; Blaas, J.; Van Der Plas, L.H.W.; Bouwmeester, H.J.; Verhoeven, H.A. Increased and altered fragrance of tobacco plants after metabolic engineering using three monoterpene synthases from Lemon. Plant Physiol. 2004, 134, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Ohara, K.; Ujihara, T.; Endo, T.; Sato, F.; Yazaki, K. Limonene production in tobacco with Perilla limonene synthase cDNA. J. Exp. Bot. 2003, 54, 2635–2642. [Google Scholar] [CrossRef]
- Muthusamy, S.; Vetukuri, R.R.; Lundgren, A.; Ganji, S.; Zhu, L.H.; Brodelius, P.E.; Kanagarajan, S. Transient expression and purification of β-caryophyllene synthase in Nicotiana benthamiana to produce β-caryophyllene in vitro. PeerJ 2020, 2020, 1–21. [Google Scholar] [CrossRef]
- Mirzaee, H.; Sharafi, A.; Hashemi Sohi, H. In vitro regeneration and transient expression of recombinant sesquiterpene cyclase (SQC) in Artemisia annua L. South African J. Bot. 2016, 104, 225–231. [Google Scholar] [CrossRef]
- Liu, Q.; Manzano, D.; Tanić, N.; Pesic, M.; Bankovic, J.; Pateraki, I.; Ricard, L.; Ferrer, A.; de Vos, R.; van de Krol, S.; et al. Elucidation and in planta reconstitution of the parthenolide biosynthetic pathway. Metab. Eng. 2014, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kanagarajan, S.; Muthusamy, S.; Gliszczyńska, A.; Lundgren, A.; Brodelius, P.E. Functional expression and characterization of sesquiterpene synthases from Artemisia annua L. using transient expression system in Nicotiana benthamiana. Plant Cell Rep. 2012, 31, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Majdi, M.; Cankar, K.; Goedbloed, M.; Charnikhova, T.; Verstappen, F.W.A.; de Vos, R.C.H.; Beekwilder, J.; van der Krol, S.; Bouwmeester, H.J. Reconstitution of the costunolide biosynthetic pathway in yeast and Nicotiana benthamiana. PLoS ONE 2011, 6, e23255. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Kerbler, S.M.; Fernie, A.R.; Zhang, Y. Plant cell cultures as heterologous bio-factories for secondary metabolite production. Plant Commun. 2021, 2, 100235. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, X.; Liu, T.; Wang, Y.; Ahmed, N.; Li, Z.; Jiang, H. Synthetic biology of plant natural products: From pathway elucidation to engineered biosynthesis in plant cells. Plant Commun. 2021, 2, 100229. [Google Scholar] [CrossRef]
- Farhi, M.; Marhevka, E.; Ben-Ari, J.; Algamas-Dimantov, A.; Liang, Z.; Zeevi, V.; Edelbaum, O.; Spitzer-Rimon, B.; Abeliovich, H.; Schwartz, B.; et al. Generation of the potent anti-malarial drug artemisinin in tobacco. Nat. Biotechnol. 2011, 29, 1072–1074. [Google Scholar] [CrossRef]
- Zhang, Y.; Nowak, G.; Reed, D.W.; Covello, P.S. The production of artemisinin precursors in tobacco. Plant Biotechnol. J. 2011, 9, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, K.; Subramaniyan, M.; Rawat, K.; Kalamuddin, M.; Qureshi, M.I.; Malhotra, P.; Mohmmed, A.; Cornish, K.; Daniell, H.; Kumar, S. Compartmentalized metabolic engineering for Artemisinin biosynthesis and effective malaria treatment by oral delivery of plant cells. Mol. Plant. 2016, 9, 1464–1477. [Google Scholar] [CrossRef] [Green Version]
- Zebec, Z.; Wilkes, J.; Jervis, A.J.; Scrutton, N.S.; Takano, E.; Breitling, R. Towards synthesis of monoterpenes and derivatives using synthetic biology. Curr. Opin. Chem. Biol. 2016, 34, 37–43. [Google Scholar] [CrossRef]
- Mai, J.; Li, W.; Ledesma-Amaro, R.; Ji, X.J. Engineering Plant Sesquiterpene Synthesis into Yeasts: A Review. J. Agric. Food Chem. 2021, 69, 9498–9510. [Google Scholar] [CrossRef]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Gutierrez, J.; Chan, R.; Batth, T.S.; Adams, P.D.; Keasling, J.D.; Petzold, C.J.; Lee, T.S. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 2013, 19, 33–41. [Google Scholar] [CrossRef]
- Du, F.L.; Yu, H.L.; Xu, J.H.; Li, C.X. Enhanced limonene production by optimizing the expression of limonene biosynthesis and MEP pathway genes in E. Coli. Bioresour. Bioprocess. 2014, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Sun, J.; Li, D.; Lu, W. Heterologous biosynthesis of (+)-nootkatone in unconventional yeast Yarrowia lipolytica. Biochem. Eng. J. 2018, 137, 125–131. [Google Scholar] [CrossRef]
- Hartley, J.L.; Temple, G.F.; Brasch, M.A. DNA cloning using in vitro site-specific recombination. Genome Res. 2000, 10, 1788–1795. [Google Scholar] [CrossRef] [Green Version]
- Gibson, D.G. Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 2011, 498, 349–361. [Google Scholar] [CrossRef]
- Quan, J.; Tian, J. Circular polymerase extension cloning for high-throughput cloning of complex and combinatorial DNA libraries. Nat. Protoc. 2011, 6, 242–251. [Google Scholar] [CrossRef]
- Irfan, M.; Chavez, B.; Rizzo, P.; D’Auria, J.C.; Moghe, G.D. Evolution-aided engineering of plant specialized metabolism. aBIOTECH 2021, 2, 240–263. [Google Scholar] [CrossRef]
- Engler, C.; Marillonnet, S. Generation of families of construct variants using golden gate shuffling. Methods Mol. Biol. 2011, 729, 167–181. [Google Scholar] [CrossRef]
- Emami, S.; Yee, M.C.; Dinneny, J.R. A robust family of Golden Gate Agrobacterium vectors for plant synthetic biology. Front. Plant Sci. 2013, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Engler, C.; Youles, M.; Gruetzner, R.; Ehnert, T.M.; Werner, S.; Jones, J.D.G.; Patron, N.J.; Marillonnet, S. A Golden Gate modular cloning toolbox for plants. ACS Synth. Biol. 2014, 3, 839–843. [Google Scholar] [CrossRef]
- Ilc, T.; Parage, C.; Boachon, B.; Navrot, N.; Werck-Reichhart, D. Monoterpenol oxidative metabolism: Role in plant adaptation and potential applications. Front. Plant Sci. 2016, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Van Zyl, R.L.; Seatlholo, S.T.; Van Vuuren, S.F.; Viljoen, A.M. The biological activities of 20 nature identical essential oil constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M. Linalool—A Review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008, 3, 1934578X0800300727. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.H.; Yeh, S.Y.; Lin, M.Y.; Shih, M.C.; Yang, K.T.; Hwang, S.Y. Major chemotypes and antioxidative activity of the leaf essential oils of Cinnamomum osmophloeum Kaneh. from a clonal orchard. Food Chem. 2007, 105, 133–139. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef]
- Moretti, M.D.L.; Peana, A.T.; Satta, M. A study on anti-inflammatory and peripheral analgesic action of salvia sclareaoil and its main components. J. Essent. Oil Res. 1997, 9, 199–204. [Google Scholar] [CrossRef]
- Peana, A.T.; Moretti, M.D.L. Pharmacological activities and applications of Salvia sclarea and Salvia desoleana essential oils. Stud. Nat. Prod. Chem. 2002, 26, 391–423. [Google Scholar] [CrossRef]
- Erasto, P.; Viljoen, A.M. Limonene—A review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008, 3, 1934578X0800300728. [Google Scholar] [CrossRef] [Green Version]
- Dabbah, R.; Edwards, V.M.; Moats, W.A. Antimicrobial action of some citrus fruit oils on selected food-borne bacteria. Appl. Microbiol. 1970, 19, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Keinan, E.; Alt, A.; Amir, G.; Bentur, L.; Bibi, H.; Shoseyov, D. Natural ozone scavenger prevents asthma in sensitized rats. Bioorganic Med. Chem. 2005, 13, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Winnacker, M. Pinenes: Abundant and renewable building blocks for a variety of sustainable polymers. Angew. Chemie-Int. Ed. 2018, 57, 14362–14371. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.R.; Lopes, P.M.; De Azevedo, M.M.B.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [Green Version]
- Sybilska, D.; Kowalczyk, J.; Asztemborska, M.; Ochocka, R.J.; Lamparczyk, H. Chromatographic studies of the enantiomeric composition of some therapeutic compositions applied in the treatment of liver and kidney diseases. J. Chromatogr. A 1994, 665, 67–73. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Di Tang, F.; Mao, G.G.; Bian, R.L. Effect of α-pinene on nuclear translocation of NF-κB in THP-1 cells. Acta Pharmacol. Sin. 2004, 25, 480–484. [Google Scholar]
- Thomsett, M.R.; Moore, J.C.; Buchard, A.; Stockman, R.A.; Howdle, S.M. New renewably-sourced polyesters from limonene-derived monomers. Green Chem. 2019, 21, 149–156. [Google Scholar] [CrossRef]
- Farco, J.A.; Grundmann, O. Menthol—Pharmacology of an important naturally medicinal “Cool”. Mini Rev. Med. Chem. 2012, 13, 124–131. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [Green Version]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef]
- Kim, D.Y.; Choi, B.Y. Costunolide—A bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential. Int. J. Mol. Sci. 2019, 20, 2926. [Google Scholar] [CrossRef] [Green Version]
- Rasul, A.; Parveen, S.; Ma, T. Costunolide: A novel anti-cancer sesquiterpene lactone. Bangladesh J. Pharmacol. 2012, 7, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.I.; Kim, J.H.; Lee, K.T.; Choi, J.H. Costunolide induces apoptosis in platinum-resistant human ovarian cancer cells by generating reactive oxygen species. Gynecol. Oncol. 2011, 123, 588–596. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, K.T. Costunolide-induced apoptosis in human leukemia cells: Involvement of c-Jun N-terminal kinase activation. Biol. Pharm. Bull. 2009, 32, 1803–1808. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.L.; Pan, S.L.; Ho, Y.F.; Hwang, T.L.; Kung, F.L.; Guh, J.H. Costunolide induces apoptosis through nuclear calcium2+ overload and DNA damage response in human prostate cancer. J. Urol. 2011, 185, 1967–1974. [Google Scholar] [CrossRef]
- Jeong, S.J.; Itokawa, T.; Shibuya, M.; Kuwano, M.; Ono, M.; Higuchi, R.; Miyamoto, T. Costunolide, a sesquiterpene lactone from Saussurea lappa, inhibits the VEGFR KDR/Flk-1 signaling pathway. Cancer Lett. 2002, 187, 129–133. [Google Scholar] [CrossRef]
- Bocca, C.; Gabriel, L.; Bozzo, F.; Miglietta, A. A sesquiterpene lactone, costunolide, interacts with microtubule protein and inhibits the growth of MCF-7 cells. Chem. Biol. Interact. 2004, 147, 79–86. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W. The Current and Potential Therapeutic Uses of Parthenolide, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 58, ISBN 9780444640567. [Google Scholar]
- Brown, A.M.G.; Lowe, K.C.; Davey, M.R.; Brian Power, J.; Knight, D.W.; Heptinstall, S. Comparison of extraction procedures for parthenolide in Tanacetum parthenium. Phytochem. Anal. 1996, 7, 86–91. [Google Scholar] [CrossRef]
- Schinella, G.R.; Giner, R.M.; Del Carmen Recio, M.; De Buschiazzo, P.M.; Ríos, J.L.; Máñez, S. Anti-inflammatory effects of South American Tanacetum vulgare. J. Pharm. Pharmacol. 1998, 50, 1069–1074. [Google Scholar] [CrossRef]
- Schnitzler, P.; Astani, A.; Reichling, J. Screening for antiviral activities of isolated compounds from essential oils. Evid.based Complement. Altern. Med. 2011, 2011, 253643. [Google Scholar] [CrossRef] [Green Version]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef]
- Medeiros, R.; Passos, G.F.; Vitor, C.E.; Koepp, J.; Mazzuco, T.L.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br. J. Pharmacol. 2007, 151, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Pinho-Da-Silva, L.; Mendes-Maia, P.V.; Do Nascimento Garcia Teófilo, T.M.; Barbosa, R.; Ceccatto, V.M.; Coelho-De-Souza, A.N.; Cruz, J.S.; Leal-Cardoso, J.H. Trans-caryophyllene, a natural sesquiterpene, causes tracheal smooth muscle relaxation through blockade of voltage-dependent Ca2+ channels. Molecules 2012, 17, 11965–11977. [Google Scholar] [CrossRef]
- Kayani, W.K.; Kiani, B.H.; Dilshad, E.; Mirza, B. Biotechnological approaches for artemisinin production in Artemisia. World J. Microbiol. Biotechnol. 2018, 34, 54. [Google Scholar] [CrossRef] [Green Version]
- Bryant, L.; Flatley, B.; Patole, C.; Brown, G.D.; Cramer, R. Proteomic analysis of Artemisia annua—Towards elucidating the biosynthetic pathways of the antimalarial pro-drug artemisinin. BMC Plant Biol. 2015, 15, 175. [Google Scholar] [CrossRef] [Green Version]

| Instrument | Column Name | Dimension (Length, Inner Diameter & Thickness) | Oven Program | References |
|---|---|---|---|---|
| GC-MS | DB-5MS-DG, DB-17, VF-35 | 30 m × 0.25 µm ID × 0.25 µm, 30 m × 0.25 µm ID × 1.0 µm | 60 °C (2 min), Ramp: 5 °C/min to 200 °C | [52] |
| GC-MS | TG-624 SilMS | 30 m × 0.25 mm ID × 1.4 µm | 60 °C (30 s), Ramp 1: 15 °C/min to 130 (3 min); Ramp 2 5 °C/min to 140 °C (1 min); Ramp 3: 22° C/min 280 °C (3 min) | [53] |
| GC-MS | HP-5 | 30 m × 0.25 µm ID × 0.25 µm | 50 °C (2 min), Ramp 1: 5 °C min to 180 °C, Ramp 2: 20 °C/min to 270 °C | [54] |
| GC-MS | Rxi-624 Sil MS | 30 m × 0.25 mm ID × 1.4 µm | 80 °C (1 min), Ramp 1: 12 °C/min to 150 (1 min); Ramp 2 9 °C/min to 250 (1 min) | [55] |
| GC-FID | ZB-5 PLUSTM | 20 m × 0.18 mm ID × 0.36 µm | Ramp 1: 35 °C to 105 °C 10 °C/min to 205 °C Ramp 2: 15 °C/min to 360 °C Ramp 3: 35 °C/min for 1.9 min | [56] |
| GC-MSD | DB-HeavyWax | 30 m × 250 µm ID × 1.4 µm | 50 °C (0.75 min), Ramp 1: 80 °C (0 min); Ramp 2: 240 °C (5 min) | [57] |
| GC-FID | VF-624 ms | 60 m × 0.32 mm ID × 1.8 µm | 90° C (1 min), Ramp 1: 15 °C min to 181° C (3 min) | [58] |
| GC-MS | Elite-5 | 30 m × 0.25 µm ID × 0.25 µm | 100 °C (5 min), Ramp 1: 20 C/min (200° C), Ramp 2: 10 °C/min (270 °C) | [59] |
| Source | Instrument * | Method | Identified Terpenes | References |
|---|---|---|---|---|
| Cannabis | GC-MS | ASE | 23 | [65] |
| Cannabis | GC-MS | Headspace, SPME and Liquid injection | 49 | [52] |
| Cannabis varieties | GC-MS | HS-SPME | 30 | [66] |
| Cannabis | GC-MS | Headsapce | 30 | [53] |
| Muscat grape | GC-MS | HS-SPME | 28 | [67] |
| Finger Cirton | GC-MS | SPME | 62 | [68] |
| Fourteen Compositae plants | GC-MS | n-hexane | 213 | [54] |
| Goose berry, crabapple, cherry silver berry, scarlet hawthornq | GC × GC-TOF-MS | SPME | 79 | [69] |
| Exocarpium citri Grandis | GC-MS | SP, HS-SPME & solvent extraction | 81 | [70] |
| Basil & Tobacco | GC-MS | SP, Ultrasound-Assisted | 18 | [71] |
| Zinger | GC-MS/LC-ESI-MS | MeOH | 102 | [72] |
| Source | Species | Targeted Genes | Up/Downregulated | References |
|---|---|---|---|---|
| Monoterpenoids (C15) | ||||
| Mentha | Mentha spicata | Limonene synthase | Incresead in sesquiterpenoid | [73] |
| Lour | Litsea cubeba | Geranyl diphosphate synthase small subunit 1 | Increase in monoterpene content | [74] |
| Lilium | Lilium “Siberia” | 1-deoxy-d-xylulose-5-phosphate synthase, 1-deoxy-d-xylulose-5-phosphate reductoisomerase | Linalool (mono), Caryophyllene (sesqui) | [75] |
| Mentha X piperita | Nicotiania benthamiana & Nicotiania tabacum | Geranyl diphosphate synthase small subunit | (−) Limonene, (−)-Linalool, (−)-β-pinene, (−)-α-pinene, Myrcene | [76] |
| Sweet osmanthus | Osmanthus fragrans | Terpene synthase | β-linalool, trans-β-ocimene, α-farnesene | [77] |
| Mentha | Mentha spicata | IPP isomerase & limonen synthase | 1,8-cineole, linalool, camphor, terpinene, lomonene, borneol, safranal, geraniol, thymol, 1-α-terpineol, methyl eugenol, menthone, menthol-isomer, thymol, piperitone | [78] |
| English lavender | Lavandula angustifolia | Linalool synthase, HMG-CoA reductase | Linalool | [79] |
| Snapdragon | Antirrhinum majus | Geranyl diphosphate synthase small sub unit | Increase in monoterpene and sesquiterpene content | [80] |
| Tobacco | Nicotiania tabacum | ϒ-Terpinene synthase | ϒ-Terpinene, limonene, β-pinene and side products | [81] |
| Arabidopsis | Arabidopsis thalina | Linalool/nerolidol synthase | Linalool, hydroxylated and glycosylated linalool | [14] |
| Tobacco | Nicotiania tabacum | Limonene synthase | Limonene | [82] |
| Petunia | Petunia hybrida | Linalool synthase | Linalool glycoside | [10] |
| Tomato | Lycopersicon esculentum | Linalool synthase | Fruit-Linalool & hydroxylated linalool | [11] |
| Sesquiterpenoids (C15) | ||||
| Sweet wormwood | Nicotiania benthamiana | β-caryophyllene synthase | β-caryophyllene | [83] |
| Sweet wormwood | Artemisia annua | Sesquiterpene cyclase | Atremisinin | [84] |
| Feverfew | Tanacetum parthenium | Parthenolide synthase | Parthenolide | [85] |
| Sweet wormwood | Nicotiania benthamiana | Sesquiterpene synthase | Amorpha-4,11-diene & epi-cedrol | [86] |
| Lettuce | Lactuca sativa | Costunolide synthase | Costunolide | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, V.; Park, S.; Kim, J.A.; Lee, S.I.; Lee, K. Metabolic Perturbation and Synthetic Biology Strategies for Plant Terpenoid Production—An Updated Overview. Plants 2021, 10, 2179. https://doi.org/10.3390/plants10102179
Mani V, Park S, Kim JA, Lee SI, Lee K. Metabolic Perturbation and Synthetic Biology Strategies for Plant Terpenoid Production—An Updated Overview. Plants. 2021; 10(10):2179. https://doi.org/10.3390/plants10102179
Chicago/Turabian StyleMani, Vimalraj, Soyoung Park, Jin A Kim, Soo In Lee, and Kijong Lee. 2021. "Metabolic Perturbation and Synthetic Biology Strategies for Plant Terpenoid Production—An Updated Overview" Plants 10, no. 10: 2179. https://doi.org/10.3390/plants10102179
APA StyleMani, V., Park, S., Kim, J. A., Lee, S. I., & Lee, K. (2021). Metabolic Perturbation and Synthetic Biology Strategies for Plant Terpenoid Production—An Updated Overview. Plants, 10(10), 2179. https://doi.org/10.3390/plants10102179







