Research Progression of the Genus Merremia: A Comprehensive Review on the Nutritional Value, Ethnomedicinal Uses, Phytochemistry, Pharmacology, and Toxicity
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Bibliometric Overview of the Genus—Merremia
3.2. Most Productive Countries
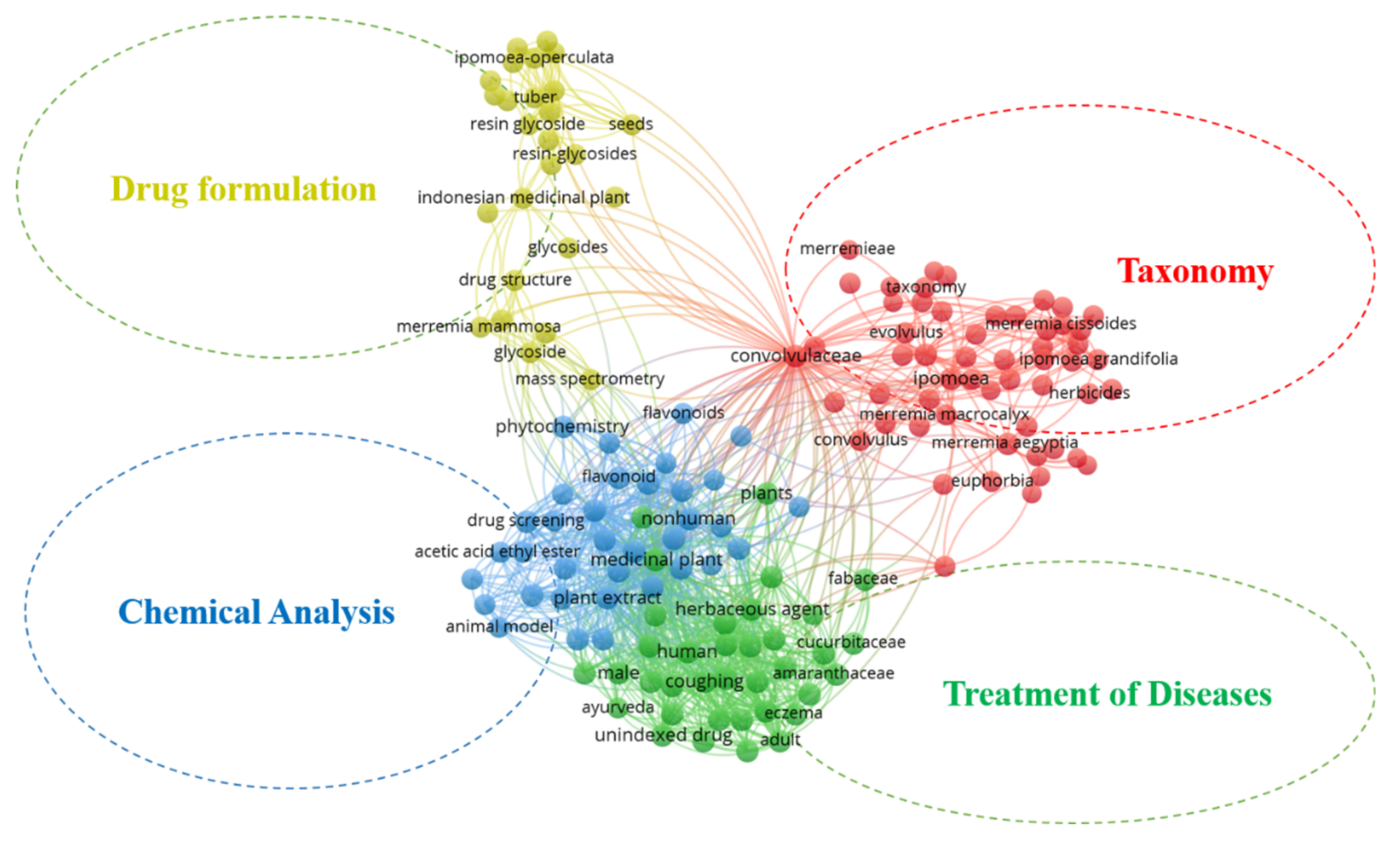
3.3. Keyword Analysis
3.4. Genus Merremia
3.4.1. Taxonomy, Botanical Description, and Distribution
3.4.2. Nutritional Value of Merremia Species
3.4.3. Ethnomedicinal Uses of Merremia Species
3.4.4. Phytochemistry of Species in the Genus Merremia
| Species | Isolated Compounds | Molecular Formula | Class of Isolated Compound | Part | Extraction Type | Bioactivity of the Tested Isolated Compound | Reference |
|---|---|---|---|---|---|---|---|
| M. emarginata | 8-prenylnaringenin (1) | C20H20O5 | Flavonoid | Leaf | Maceration | Antioxidant and antimycobacterial activities | [67] |
| Chlorogenic acid (41) | C16H18O9 | Phenolic compound | Leaf | Maceration | Diuretic activity | [79] | |
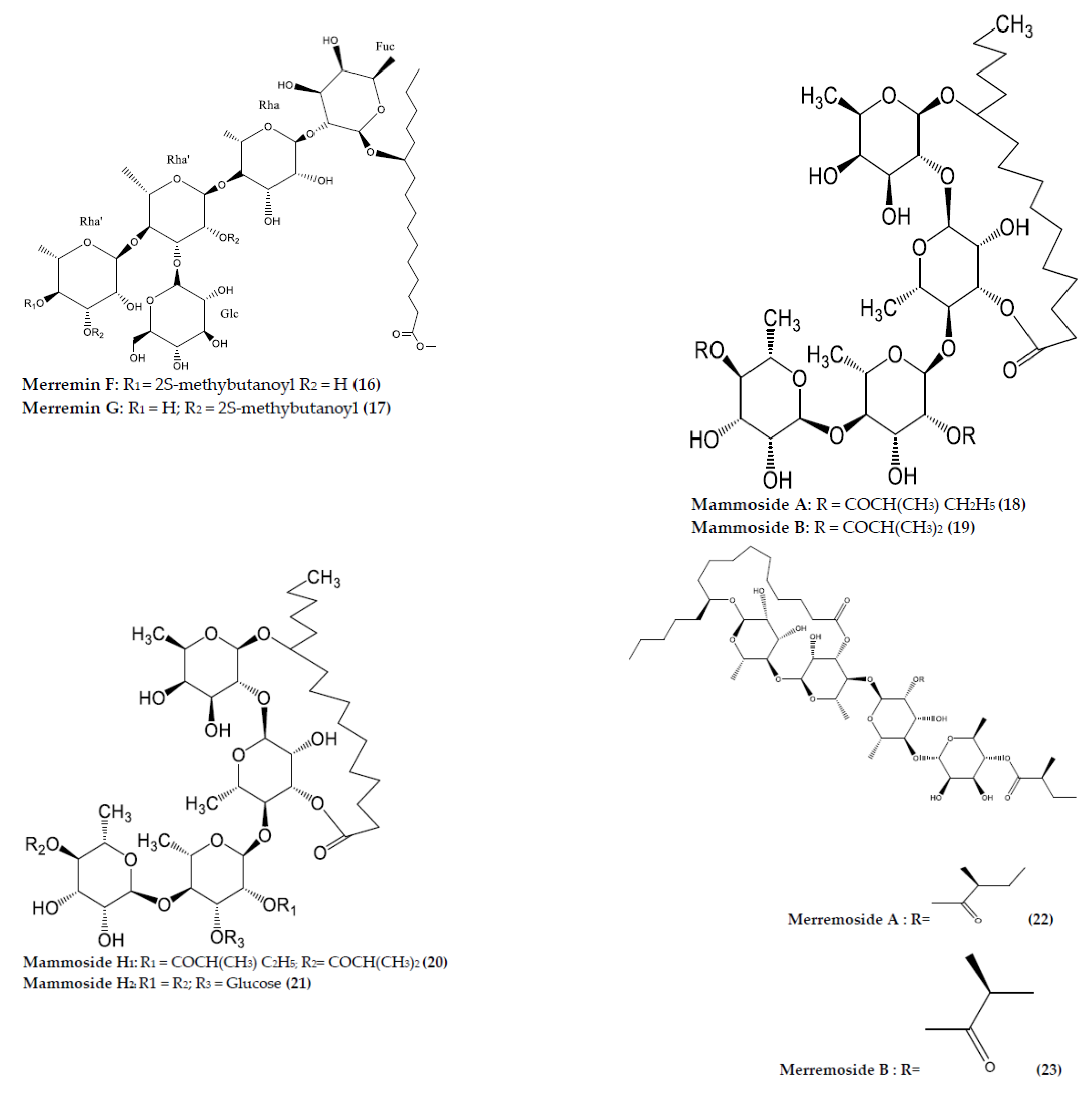
| M. hederacea | Merremin A (7) | C58H98O26 | Resin glycoside | Aerial | Infusion | Multidrug resistance reversal activity | [74] |
| Merremin B (8) | C61H104O26 | Resin glycoside | Aerial | Infusion | Multidrug resistance reversal activity | [74] | |
| Merremin C (9) | C59H98O26 | Resin glycoside | Aerial | Infusion | Multidrug resistance reversal activity | [74] | |
| Merremin D (10) | C63H108O26 | Resin glycoside | Aerial | Infusion | Multidrug resistance reversal activity | [74] | |
| Murucoidin IV (12) | C57H98O29 | Resin glycoside | Aerial | Infusion | Multidrug resistance reversal activity | [74] | |
| Stoloniferin IV (11) | C61H106O25 | Resin glycoside | Aerial | Infusion | Multidrug resistance reversal activity | [74] | |
| Merremin E (13) | C51H88O24 | Resin glycoside | Aerial | Infusion | Multidrug resistance reversal activity | [74] | |
| Murucoidin V (14) | C56H96O25 | Resin glycoside | Aerial | Infusion | Multidrug resistance reversal activity | [74] | |
| Murucoidin XVII (15) | C65H101O26 | Resin glycoside | Aerial | Infusion | Multidrug resistance reversal activity | [74] | |
| Merremin F (16) | C52H92O25 | Resin glycoside | Aerial | Infusion | Multidrug resistance reversal activity | [74] | |
| Merremin G (17) | C52H92O25 | Resin glycoside | Aerial | Infusion | Multidrug resistance reversal activity | [74] | |
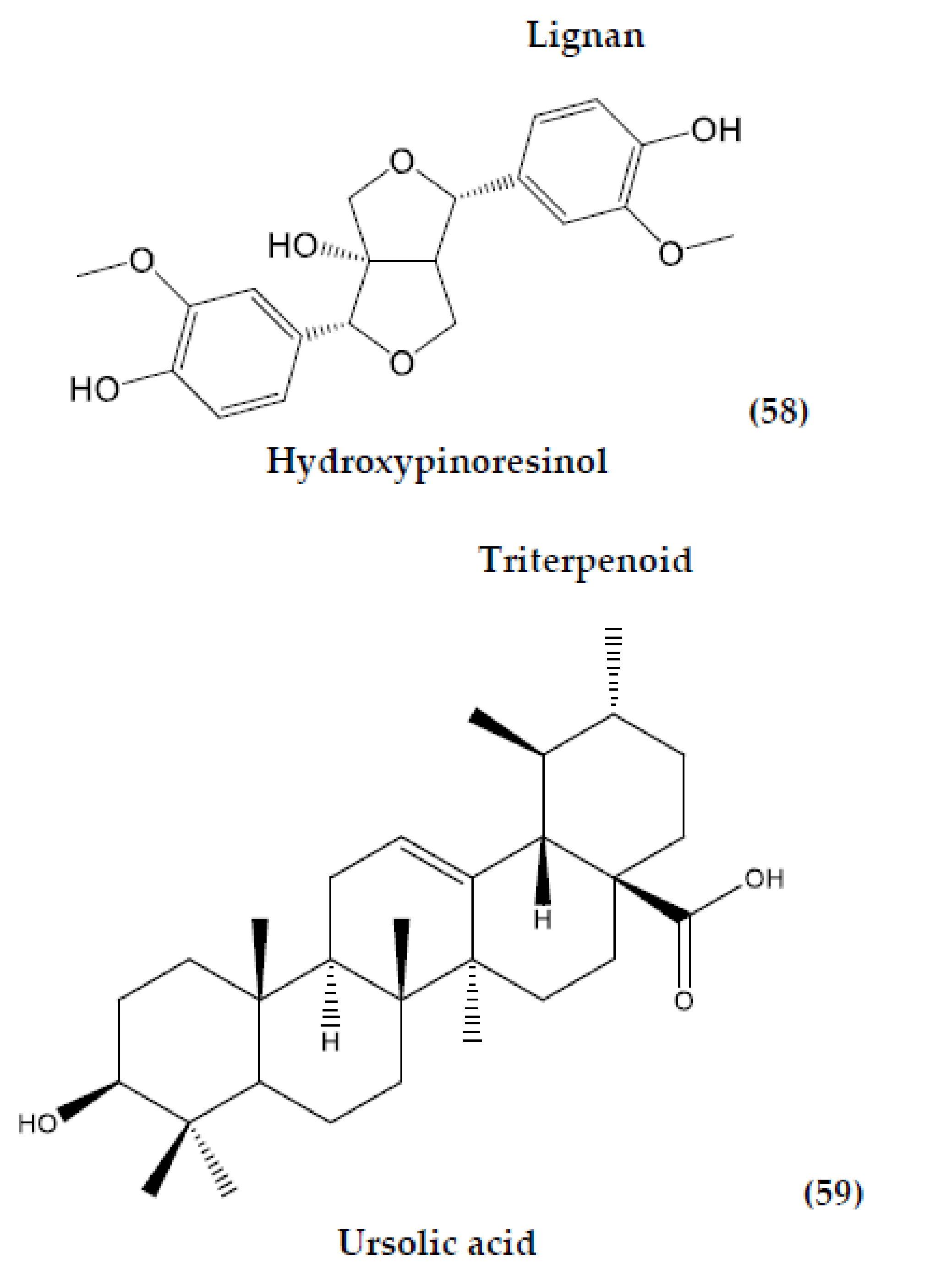
| M. tomentosa | Ursolic acid (59) | C30H48O3 | Triterpenoid | Leaf | Maceration | Insecticidal activity | [80] |
| cis-Tiliroside (2) | C30H26O13 | Flavonoid | Leaf | Maceration | Insecticidal activity | [80] | |
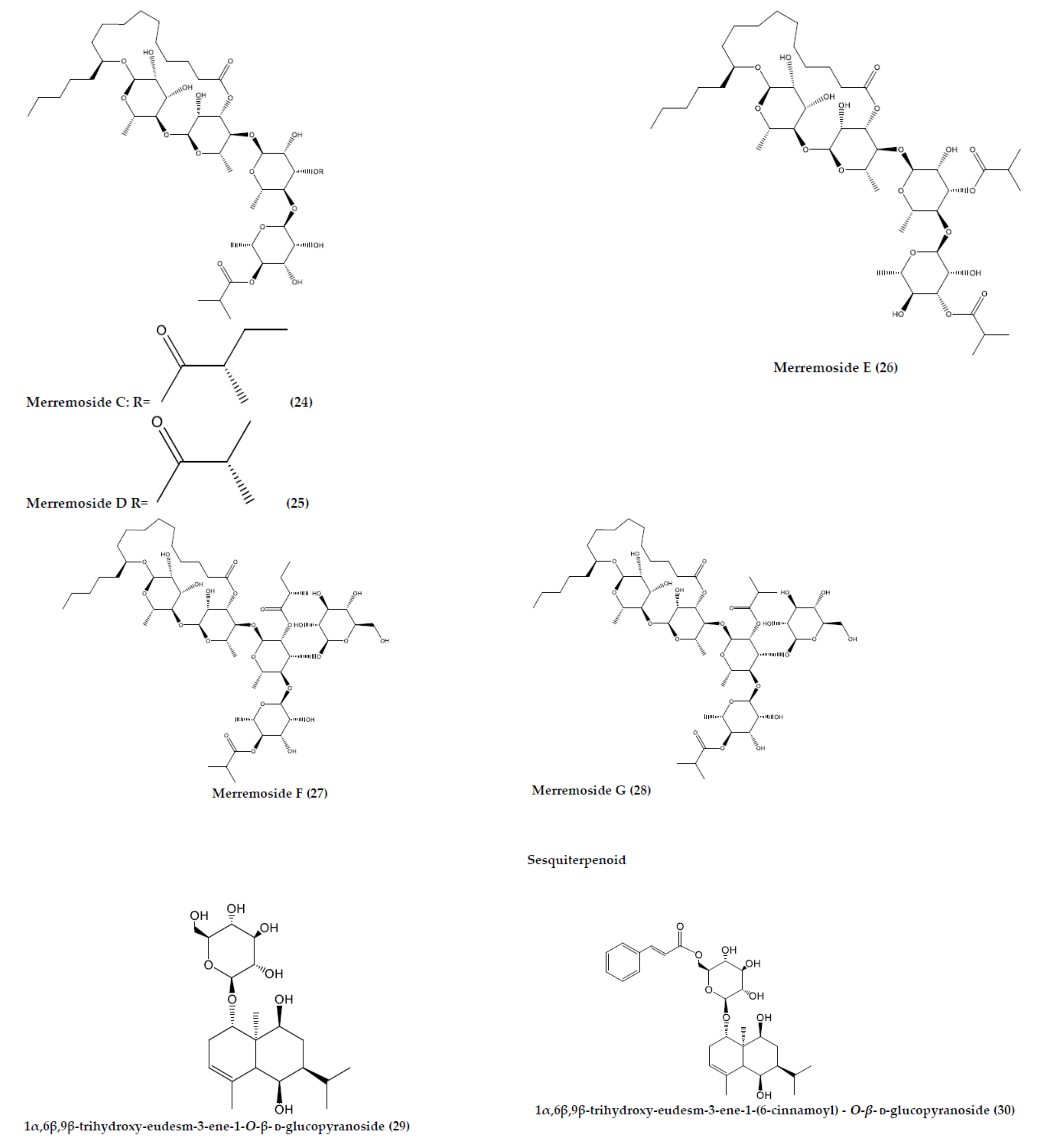
| M. yunnanensis | 1α,6β,9β-trihydroxy-eudesm-3-ene-1-O-β-d-glucopyranoside (29) | C21H36O8 | Sesquiterpenoid | Root | Decoction | N/A | [60] |
| 1α,6β,9β-trihydroxy-eudesm-3-ene-1-(6-cinnamoyl)-O-β-d-glucopyranoside (30) | C30H42O9 | Sesquiterpenoid | Root | Decoction | N/A | [60] | |
| Eeudesmane-1α,4β,8β,9β -tetrol-1-O-β- d -glucopyranoside (31) | C21H38O9 | Sesquiterpenoid | Root | Infusion | N/A | [61] | |
| Tyrosol (35) | C8H10O2 | Phenolic compound | Root | Infusion | N/A | [61] | |
| Hydroxypinoresinol (58) | C20H22O7 | Lignan | Leaf | Infusion | N/A | [61] | |
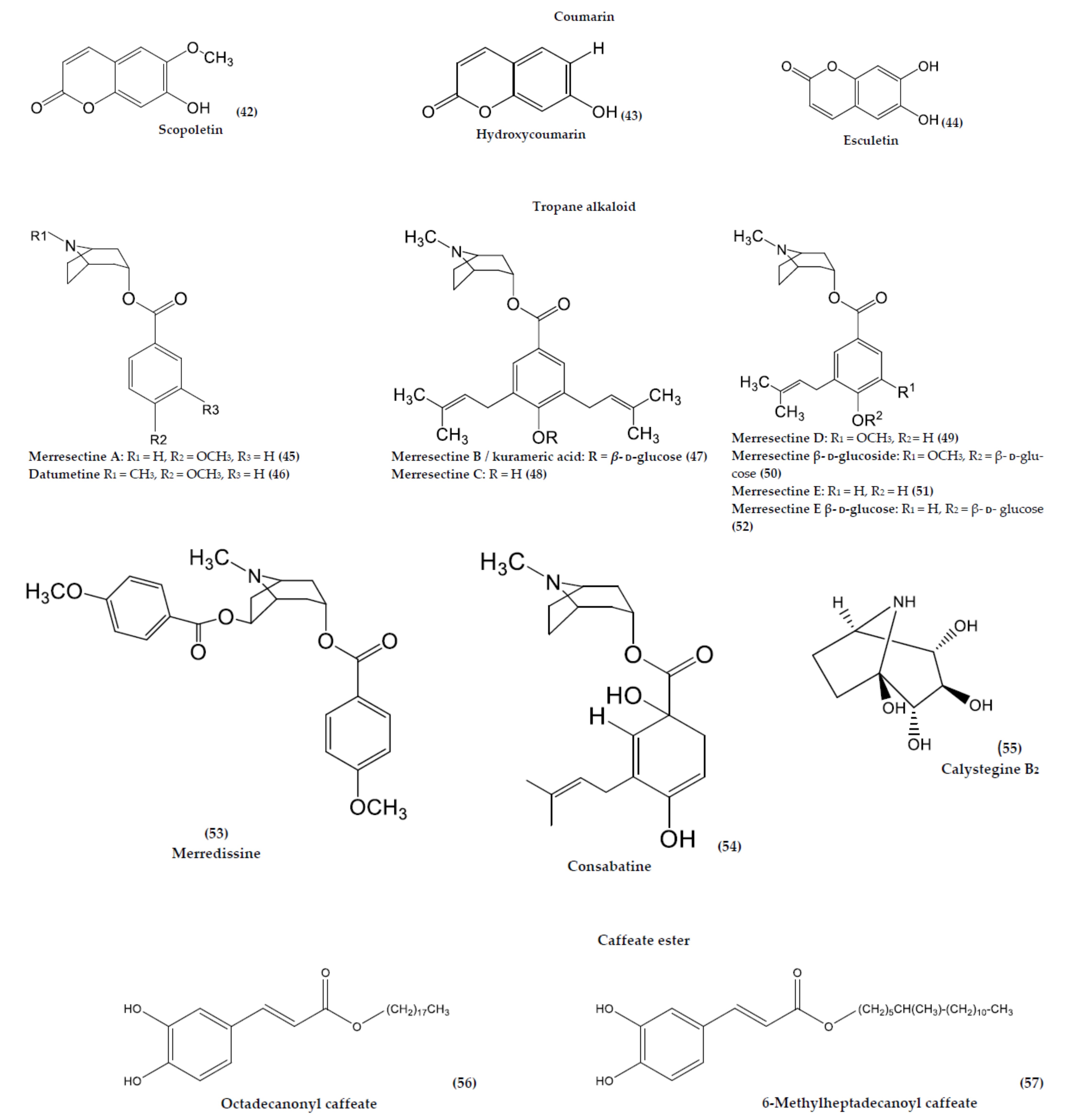
| Scopoletin (42) | C10H8O4 | Coumarin | Leaf | Infusion | N/A | [61] | |
| Hydroxycoumarin (43) | C9H6O3 | Coumarin | Leaf | Infusion | N/A | [61] | |
| Quercetin-7-O-glucoside (3) | C21H20O12 | Flavonoid | Root | Infusion | N/A | [61] | |
| 2-C-methylerythritol (6) | C5H12O4 | Polyol | Leaf | infusion | N/A | [61] | |
| M. umbellata | SA 2-O-β- d -(3′,6′-dicaffeoyl)-glucopyranoside (36) | C31H28O14 | Phenolic Compound | Whole plant | Maceration | Allelopathic effect on Arabidopsis seed germination | [75] |
| Rosmarinic acid (37) | C18H16O8 | Phenolic Compound | Whole plant | Maceration | Allelopathic effect on Arabidopsis seed germination | [64,75] | |
| Paprazine (38) | C17H17NO3 | Phenolic Compound | Whole plant | Maceration | Allelopathic effect on Arabidopsis seed germination | [75] | |
| N-p-cis-coumaroyltyramine (39) | C17H17NO | Phenolic Compound | Whole plant | Maceration | Allelopathic effect on Arabidopsis seed germination | [75] | |
| Caffeic acid (40) | C9H8O4 | Phenolic Compound | Whole plant | Maceration | Allelopathic effect on Arabidopsis seed germination | [64,75] | |
| Esculetin (44) | C9H6O4 | Coumarin | Whole plant | Maceration | Allelopathic effect on Arabidopsis seed germination | [75] | |
| Quercetin (4) | C15H10O7 | Flavonoid | Whole plant | Maceration | Allelopathic effect on Arabidopsis seed germination | [64,75] | |
| Luteolin (5) | C15H10O6 | Flavonoid | Whole plant | Maceration | Allelopathic effect on Arabidopsis seed germination | [75] | |
| M. kentrocaulos | Merrekentrones A and B (32) | C15H14O3 | Sesquiterpenoid | Root | Maceration | N/A | [81] |
| Merrekentrones C (33) | C15H16O4 | Sesquiterpenoid | Root | Maceration | N/A | [81] | |
| Merrekentrones D (34) | C15H18O3 | Sesquiterpenoid | Root | Maceration | N/A | [81] | |
| M. mammosa | Mammoside A (22) and B (23) | C48H82O20 | Resin glycosides | Root | Maceration | N/A | [82] |
| Mammoside H1 (20) | C54H92O25 | Resin glycosides | Root | Maceration | N/A | [82] | |
| Mammoside H2 (21) | C54H92O25 | Resin glycosides | Root | Maceration | N/A | [82] | |
| M. quinquefolia | Merresectine A (45) | C15H19NO3 | Tropane alkaloid | Root | Maceration | N/A | [15] |
| M. cissoides and M. quinquefolia | Merresectine B/kurameric acid (47) | C31H45NO8 | Tropane alkaloid | Root | Maceration | N/A | [15] |
| M. cissoides and M. quinquefolia | Merresectine C (48) | C25H35NO3 | Tropane alkaloid | Root | Maceration | N/A | [15] |
| M. cissoides and M. quinquefolia | Merredissine (53) | C24H27NO6 | Tropane alkaloid | Root | Maceration | N/A | [15] |
| M. dissecta | Datumetine (46) | C16H21NO3 | Tropane alkaloid | Root | Maceration | NA | [15] |
| M. guerichii | Merresectine D (49) | C27H39NO9 | Tropane alkaloid | Root | Maceration | N/A | [15] |
| Merresectine β-d–glucoside (50) | C27H39NO9 | Tropane alkaloid | Root | Maceration | N/A | [15] | |
| M. quinata | Consabatine (54) | C20H31NO4 | Tropane alkaloid | Root | Maceration | N/A | [15] |
| M. cissoides and M. quinquefolia | Merresectine E (51) and Merresectine E β-d-glucoside (52) | C20H27NO3 | Tropane alkaloid | Root | Maceration | N/A | [15] |
| M. tuberosa | Octadecanonyl caffeate (56) | C27H44O4 | Caffeate ester | Root | Maceration | NA | [83] |
| 6-Methylheptadecanoyl caffeate (57) | C27H42O5 | Caffeate ester | Root | Maceration | NA | [83] | |
| M. dissecta | Octadecanonyl caffeate (56) | C27H44O4 | Caffeate ester | Root | Maceration | NA | [83] |
| 6-Methylheptadecanoyl Caffeate (57) | C27H42O5 | Caffeate ester | Root | Maceration | NA | [83] | |
| Calystegine B2 (55) | C7H13NO4 | Tropane alkaloid | Leaf, Flower | N/A | Antidiabetic activity | [14,78] | |
| M. mammosa | Merremoside A (22) | C50H86O20 | Resin glycosides | Tuber | Maceration | NA | [84] |
| Merremoside B (23) | C48H82O20 | Resin glycosides | Tuber | Maceration | NA | [84] | |
| Merremoside C (24) | C49H84O20 | Resin glycosides | Tuber | Maceration | NA | [84] | |
| Merremoside D (25) | C48H82O20 | Resin glycosides | Tuber | Maceration | NA | [84] | |
| Merremoside E (26) | C48H82O20 | Resin glycosides | Tuber | Maceration | NA | [84] | |
| Merremoside F (27) | C55H94O25 | Resin glycosides | Tuber | Maceration | NA | [85] | |
| Merremoside G (28) | C54H92O25 | Resin glycosides | Tuber | Maceration | NA | [85] |
3.4.5. Biological and Pharmacological Activities
Cancer Cell Cytotoxicity
Anti-Diabetic Activity
Antimicrobial Activities
Anti-Influenza Activity
Anti-Inflammatory Activity
Anti-Nociceptive Activity
Thrombolytic Activity
Anti-Urolithiatic Activity
Nephroprotective Activity
Diuretic/Blood Pressure-Lowering Activity
Wound Healing Property
Antioxidant Activity
Insecticidal Activity
| Species | Biological Activity | Plant Part | Extract | Concentration or Dose | Model | Result | Reference |
|---|---|---|---|---|---|---|---|
| M. aegyptia | Antioxidant activity | Seeds | Methanol | 500 μg mL−1 | In vitro: DPPH assay; ascorbic acid was used as a standard | Extract had 90% (IC50 values of 84) free radical scavenging effect | [117] |
| Insecticidal activity | Leaves | Acetone and its hexane fraction | 250 µg mL−1 | In vivo: larvicidal activity against Aedes aegypti fourth instar larvae; Temephos served as a standard | Extracts exhibited over 100% activity (with LD50 values of 120.7 µg mL−1 and 144.3 µg mL−1, respectively) | [122] | |
| M. borneensis | Antifungal | Leaves | Methanol | 60–400 mg mL−1 | In vitro: antifungal activity against a mold strain (A. brasiliensis) and two yeast strains (Candida albicans and S. cerevisiae) using agar well diffusion method | Extract showed concentration-dependent activity (inhibition zones range: 5.5–15.5 mm) | [63] |
| Antioxidant activity | Leaves, stems | Aqueous ethanol | 100 µg mL−1 | In vitro: phosphomolybdenum method, β-carotene-linoleate model system and DPPH assays; ascorbic acid and BHA served as references | Phosphomolybdenum method: 31% antioxidant capacity, β-carotene bleaching: 84% inhibition, DPPH: 80% radical scavenging | [22] | |
| M. dissecta | Antibacterial | Leaves, flowers | Diethyl ether, methanol | 100 µg mL−1 | In vitro: antibacterial activity against S. aureus, P. aeruginosa by biofilm inhibition; quorum sensing inhibitor (azithromycin) served as a control | Extracts decreased the biosynthesis of N-acyl homoserine lactone by 72%, attenuated the expression of elastase activity (27%) and biofilm production (55%) in P. aeruginosa | [4] |
| Antioxidant activity | Leaves, seeds, stems | Methanol | 500 μg mL−1 | In vitro: DPPH assay; ascorbic acid was used as a standard | Leaves, seeds and stems extracts exhibited 75%, 90%, and 93%, free radical scavenging with IC50 of 82, 80, and 61 µg mL−1, respectively | [117] | |
| M. emarginata | Cancer cell cytotoxicity | Whole plant | Ethyl acetate | 50 μg mL−1 | In vitro: human cancer cell lines (prostate carcinoma (DU-145); Lung carcinoma (A549), mouth carcinoma (KB) and pancreas carcinoma (MIA-PaCa-2)) using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay | Ethyl acetate extract was the most effective, inhibiting the proliferation of cell lines A549, KB, MIA-PaCa-2, and DU-145 with the IC50 values of 28.5 μg mL−1, 37.2 μg mL−1, 51 μg mL−1, and 69.4 μg mL−1, respectively | [3] |
| Cancer cell cytotoxicity | Leaves | Hexane | 25 μg mL−1 | In vitro: human cancer cell lines (lung (A549), breast (MCF-7), stomach (AGS), colon (COLO 320 DM)) and monkey normal kidney epithelial cells (VERO) | Extract inhibited A549 and COLO 320 DM cell line proliferation (with IC50 values of 15.5 μg mL−1 and 18.4 μg mL−1, respectively) and had minimal toxicity (IC50 value of 65.2 μg mL−1) for VERO | [88] | |
| Anti-diabetic | Whole plant | Methanol | 100, 200, and 400 mg kg−1 | In vivo: streptozotocin-induced (intraperitoneally) diabetic male Wistar rats; assessment of blood glucose levels, plasma insulin and body weight, glycosylated hemoglobin, total hemoglobin, serum creatinine, serum urea, hexokinase, fructose-1, 6-bisphosphatase and glucose-6-phosphatase, total protein and pancreatic tissue histology | All extract doses lowered blood glucose levels to values comparable to standard drug (glibenclamide) and restored plasma insulin, body weight, glycosylated hemoglobin, total hemoglobin, serum creatinine, serum urea, hexokinase, fructose-1, 6-bisphosphatase, and glucose-6-phosphatase, and total protein to levels near normal control (sodium chloride). In the histological assessment, the extracts (100 and 200 mg kg−1, respectively) showed mild and moderate expansion of the diabetic rats’ pancreatic islets, while the 400 mg kg−1 extract showed prominent hyperplastic islet similar to that of the glibenclamide-treated group | [93] | |
| Antibacterial | Leaves | Aqueous, petroleum ether, and methanol | 10 µg mL−1, 25 µg mL−1, 50 µg mL−1, 100 µg mL−1 | In vitro: antibacterial activity against B. cereus, E. coli, P. aeruginosa, and S. aureus by disk diffusion assay; penicillin standard drug | All solvent extracts were effective against all the bacteria (zone of inhibition range: 0.7–13.7 mm) compared with penicillin (16.0–17.0 mm) | [95] | |
| Antibacterial | Leaves | Aqueous, acetone, chloroform, petroleum ether | 200 mg mL−1 | In vitro: antibacterial activity against seven bacteria by paper disk diffusion; Ciprofloxacin as a reference drug | All solvent extracts were effective against five bacteria, particularly L. rhamnosus (20% zone of inhibition each); the most effective (acetone extract) had maximum inhibitory (24%) against S. epidermidis | [96] | |
| Antibacterial | Leaves | Aqueous, thioglycolic acid-capped cadmium telluride quantum dots, their mixture (T-M complex) | 1 × 10−6 M, 100 µg | In vitro: antibacterial activity against E. coli using the Luria-Bertani agar disk diffusion assay | Extract alone and T-M complex showed 10-mm and 16-mm zones of inhibition, respectively | [21] | |
| Anti-inflammatory | Whole plant | Ethyl acetate | 50 μg mL−1 | In vitro: inhibition of pro-inflammatory cytokine, tumor necrosis factor alpha (TNF-α); recombinant human TNF-α served as standard | Extract inhibited TNF-α production with the IC50 of 5.9 μg mL−1. | [3] | |
| Anti-arthritic | Whole plant | Ethanol extract and its methanol and ethyl acetate fractions | 250 μg mL−1 | In vitro: anti-arthritic activity by protein denaturation inhibition method; diclofenac sodium served as a reference | Extracts had protein denaturation inhibition of 95.4%, 87.7%, and 72.6%, respectively, relative to the standard (98.4%) | [102] | |
| Antioxidant activity | Whole plant | Methanol | 300 μg mL−1 | In vitro: DPPH and superoxide radical scavenging assays; Vitamin C served as a standard drug | IC50 8.6 µg mL−1 relative to vitamin C (IC50 3.3 µg mL−1) | [116] | |
| Antioxidant activity | Leaves | Aqueous | 100 µg mL−1 or 1000 µg mL−1 | In vitro: reducing power, DPPH, ABTS, superoxide anion scavenging, and lipid peroxidation inhibition assays, fluorescence quenching using TGA-capped-CdTe QDs as fluorescent probes; butylated hydroxytoluene served as standards | Reducing power assay: optical density (0.79) comparable to butylated hydroxytoluene standard (1.32); DPPH assay: IC50 of 86.5 µg mL−1; ABTS assay: 71% inhibition with IC50 of 30.1 µg mL−1; superoxide anion scavenging activity: IC50 of 40.3 µg mL−1; TGA-capped-CdTe QD assay showed fluorescence emission quenching | [21] | |
| Antioxidant activity | Whole plant | Ethanol extract and its methanol fraction | In vitro: DPPH, hydrogen peroxide scavenging, ABTS scavenging, and hydroxy radical in the para-nitroso dimethylaniline assays; ascorbic acid, rutin, and BHA served as standards | DPPH assay: ethanol extract and its methanol fraction had IC50 of 26.5 µg mL−1 and 27.5 µg mL−1, respectively), ABTS assay: all extracts showed IC50 range of 15–38 µg mL−1 | [102] | ||
| Anti-urolithiatic activity | Whole plant | Methanol | 400 µg mL−1 | In vitro: anti-urolithiatic activity using a dissolution model; Cystone® served as a positive control | Extract had calcium phosphate and calcium oxalate mineralization inhibition of 81% and 84%, respectively, relative to the standard drug (91%) | [107] | |
| Blood pressure-lowering property | Aerial parts | Aqueous methanol | 30 mg Kg−1, 50 mg Kg−1; 0.1–3.0 mg Kg−1 | In vivo: angiotensin-converting-enzyme inhibitory (ACEI), diuretic and hypotensive effects in Sprague-Dawley rats; captopril and frusemide served as standard drugs, respectively | Extract exhibited 81% ACEI activity, increased the volume of urine and excretion of urinary Na+, drop in average arterial blood pressure range of 21.5–61.7% | [109] | |
| Insecticidal activity | Leaves | Leaf silver nanoparticles | 20 μg mL−1 | In vivo: larvicidal activity against late third instar larvae of A. stephensi, A. aegypti and C. quinquefasciatus; distilled water and silver nitrate served as a control | Larvicidal activity on A. stephensi, A. aegypti and C. quinquefasciatus larvae with LC50 values of 8.4 μg mL−1, 9.2 μg mL−1, and 10.0 μg mL−1, respectively | [123] | |
| Nephroprotective activity | Leaves | Ethanol | 250 mg kg−1 | In vivo: nephroprotective activity against gentamicin-induced (intraperitoneally) nephrotoxicity in adult albino Wistar rats using a histopathology examination | Extract caused regeneration of glomerular, tubular, and proximal tubular epithelial cells of damaged kidney | [64] | |
| M. hederacea | Multidrug resistance reversal activity | Aerial parts | Isolated compounds (merremins A−G and murucoidin IV, murucoidin V, stoloniferin IV, and murucoidin XVII) | 25 μM | In vivo: multidrug resistance reversal activity in KB/VCR cell lines using a sulforhodamine B assay; vinblastine served as a reference | Noncytotoxic inhibition ratios less than 50% (0.91%, −0.08%. 0.23%. 7.73%, respectively at 25 µM) for compounds A, E, F, and murucoidin V with IC50 values of 0.253, 0.036, 0.230, 0.004, and 0.570 | [74] |
| M. mammosa | Anti-diabetic | Whole plant | Hexane, ethyl acetate, methanol | 25 µg GAE mL−1, 100 µL | In vitro: α-amylase and α-glucosidase inhibition | Extracts exhibited α-amylase inhibition (48%, 43%, and 12%, respectively) and α-glucosidase inhibition (66%, 52%, and 13%, respectively) | [56] |
| Anti-influenza | Tuber | Ethyl acetate fraction of methanol extract | 1000 μg mL−1 | In vivo: anti-influenza A (subtype H1N1) activity using hemagglutinin assay; Zanamivir served as positive control | Extract had a strong anti-influenza effect similar to Zanamivir, reducing hemagglutinin virus titer by 94% and 100%, respectively, at 1000 μg mL−1 | [99] | |
| Antioxidant activity | Whole plant | Methanol | 2 mg GAE mL−1 | In vitro: DPPH, hydroxyl radical, and superoxide anion scavenging; methanol and vitamin C served as negative and positive controls, respectively | Methanol extract showed IC50 value of 0.7 GAE ml−1 relative to the positive control (0.9 GAE ml−1) in the DPPH assay, 92% inhibition in the hydroxyl radical scavenging assays, 25% inhibition in the superoxide anion assay | [56] | |
| Wound-healing property | Whole plant | Ethyl acetate, n-hexane, and water fractions of ethanol | 25 mg kg−1 | In vivo: wound-healing effect in streptozotocin-induced (intraperitoneally) diabetic male Winstar rats; wound was excised using the Morton method; aquadest and gentamicin served as negative and positive controls, respectively | Extracts showed wound diameter (72%, 62%, and 54%, respectively) compared with the negative control (114 mm), and percentage wound reduction (89%, 90%, and 93%, respectively) similar to the positive control (92%) | [112] | |
| Wound-healing property | Whole plant | Gel formulations (hydroxypropylmethylcellulose (HPMC), Carbopol or sodium carboxymethylcellulose (Na CMC) in 10% water fraction) of ethanol extract | 1.5% gelling agent | In vivo: vascular endothelial growth factor, hydroxyproline levels, and collagen density assessments in streptozotocin-induced (intraperitoneally) diabetic Winstar rats; wound was excised using the Morton method; distilled water (negative control), neomycin sulfate, and placenta extract gel (positive control) | Extracts had similar healing effect relative to the positive control in terms of optimum level vascular endothelial growth factor expression, hydroxyproline levels, and collagen density | [57] | |
| M. peltata | Anti-diabetic | Leaves and stem | Hexane, ethyl acetate, and methanol | 20-100 µg mL−1, | In vitro: alpha glucosidase enzyme inhibition | Stem methanolic extract had the best activity with IC50 value 47.44 μg/mL, almost two times better than acarbose as a positive control (IC50 = 98.38 μg/mL). Leaves methanolic extract, leaves ethyl acetate extract, and stem ethyl acetate extract also give better activity of alpha glucosidase inhibitors than acarbose with IC50 value 67.24 μg/mL, 69.38 μg/mL, and 72.85 μg/mL, respectively. | [90] |
| Antibacterial | Leaves | Ethanol | 5–20 µg mL−1 | In vitro: antibacterial activity against B. subtilis, S. aureus, P. aeruginosa, E. coli using the Kirby-Bauer disk diffusion method; streptomycin and chloramphenicol served as positive controls | Extract showed 5.7-mm average zone of inhibition against B. subtilis and S. aureus (at 10 µg mL−1 and 20 µg mL−1, respectively), 4.7-mm and 2.7-mm average zones of inhibition against P. aeruginosa and E. coli (at 15 µg mL−1 and 5 µg mL−1, respectively) | [19] | |
| Anti-diabetic | Leaves and stem | Hexane, ethyl acetate, and methanol | 20-100 µg mL−1, | In vitro: DPPH and FRAP | Stem methanolic extract had the highest antioxidant activity with IC50 value of 47.41 μg/mL in DPPH and total antioxidant power of 340.04 μmol/g in FRAP. | [90] | |
| M. tomentosa | Insecticidal activity | Leaves | Methanol and isolated compounds (ursolic acid and cis-tiliroside) | 8.9 mg mL−1 | In vivo: oviposition reduction in L. coffeella; chlorpyrifos serves as positive control | Extract and isolated compounds reduced L. coffeella oviposition to 6%, 0%, and 11%, respectively) relative to the control chlorpyrifos (0%) | [80] |
| M. tridentata | Anti-diabetic | Roots | Aqueous | 50–150 mg kg−1 | In vivo: normoglycemic, glucose-loaded hyperglycemic, and streptozotocin-induced (intraperitoneally) diabetic rats; blood glucose levels, serum insulin, triglycerides, total cholesterol, glycogen (in skeletal muscle and liver), and lipid peroxidation in pancreatic tissue estimations | All extract doses lowered blood glucose levels in normoglycemic, glucose-loaded hyperglycemic and streptozotocin-induced diabetic rats (range: 13.4–26.2%, 13.0–20.1%, and 28.6–49.7%), respectively) compared with their control groups; glibenclamide (reference drug) reduced blood glucose levels by 61.7%; in streptozotocin-induced diabetic rats, all extract doses increased serum insulin levels (maximum increase (14.9 U mL−1) was comparable to glibenclamide (18.3 U mL−1) at 150 mg kg−1; dose-dependent reduction (range: 13.9–25.1%) of serum triglycerides reduction; 100 mg kg−1 and 150 mg kg−1 extracts lowered serum cholesterol levels (ca. 60 mg dL−1) compared with both normal (66 mg dL−1) and diabetic (139 mg dL−1) controls, increased glycogen levels compared with streptozotocin-induced diabetic control and reduced lipid peroxidation levels (0.46 µM g−1, 0.47 µM g−1) to near glibenclamide (0.41 µM g−1) | [52] |
| Antibacterial | Leaves | Methanol | 0.01 mg mL−1, −1.00 mg mL−1;0.05 mg mL−1, −1 mg mL−1 | In vitro: antibacterial activity against Gram-positive (Bacillus subtilis and Staphylococcus aureus) and Gram-negative (Escherichia coli and Vibrio parahaemolyticus) bacteria using turbidity and zone formation methods | Gram-positive MIC range: 0.25–0.5 mg mL−1; zone formation range: 10‒14 mm; Gram-negative MIC range: 0.5–1 mg mL−1; zone formation range: 9–11 mm | [94] | |
| Antibacterial | Stem | Methanol | 100 mg mL−1 | In vitro: antibacterial activity against S. aureus and B. subtilis, Klebsiella pneumoniae, E. coli, and Pseudomonas aeruginosa; ampicillin and gentamicin standard drugs | Extract was effective against S. aureus and B. subtilis (inhibition zones: 12.0 mm and 11.3 mm; MIC: 3.13 mg mL−1 and 6.25 mg mL−1, respectively; minimum bactericidal concentration: 12.5 mg mL−1 and 100 mg mL−1, respectively) relative to both standard drugs (10 μg mL−1 | [66] | |
| Antifungal | Leaves | Methanol | 0.01–100 mg mL−1 | In vitro: antifungal activity against Aspergillus niger, Saccharomyces cerevisiae using turbidity and zone formation methods | Extracts were more effective against S. cerevisiae (MIC range: 0.25–0.5 mg mL−1; zone formation range: 10–15 mm). | [94] | |
| Anti-inflammatory | Whole plant | Ethanol | 100 mg kg−1, 200 mg kg−1 | In vivo: anti-inflammatory activity on male albino rats using the carrageenan-induced rat paw inflammation method; indomethacin served as standard drug | Relative to the standard drug (48.5%), extract was effective with 38.3% and 42.8% inflammation inhibition at 100 mg kg−1 and 200 mg kg−1 doses, respectively | [55] | |
| Anti-inflammatory | Roots | Solvent ether, ethyl acetate, butanol and butanone fractions of ethanol extract | 300 mg kg−1 | In vivo: anti-inflammatory activity in albino mice using the carrageenan-induced rat paw edema method; aspirin served as the standard drug | Extracts were effective (87%, 84%, 72%, and 68%, respectively) in inhibiting rat paw edema, relative to the standard (59.7%) | [53] | |
| Anti-arthritic | Whole plant | Ethanol | 100 mg kg−1 and 200 mg kg−1 | In vivo: anti-arthritic activity on male albino rats using the complete Freund’s adjuvant-induced arthritis model; indomethacin served as the standard drug | Extract had 49.0% and 51.7% anti-arthritic activity at 100 mg kg−1 and 200 mg kg−1, respectively, which were comparable to the standard drug (55.5%) | [55] | |
| Antioxidant activity | Roots, aerial parts | Acetone, chloroform, methanol, and hot water | 200 µg mL−1 | In vitro: DPPH and ABTS, OH•, FRAP, phosphomolybdenum reduction, Fe2+ chelation, β-carotene/linoleic acid peroxidation inhibition, and antihemolytic activity assays | Acetone root extract: IC50 26.6 µg mL−1 in DPPH assay; highest equivalent trolox, FRAP and phosphomolybdenum reduction values (26,270.8 µmol g−1 extract, 2656.7 mmol Fe (II) mg−1 extract and 56.7 g ascorbic acid/100 g extract, respectively); root and aerial parts extracts: OH• scavenging activity range of 29.6–59.3% and 34.8–52.9%; hot water extract showed activity of 8.0 mg EDTA g−1 extracts in the Fe2+ chelation assay; inhibition of β-carotene bleaching (20.7–36.1% by the root extract and 13.3–32.9% by the aerial parts extract); acetone extracts (aerial parts and roots) and methanol root extract exhibited activities (32.9%, 36.1%, and 31.8%, respectively) comparable with BHA standard (36.6%); higher peroxidation inhibition activity by all extracts compared with α-tocopherol standard; acetone root extract: 82.7% red blood cell hemolysis inhibition | [54] | |
| Wound-healing property | Roots | Solvent ether, petroleum ether, ethyl acetate, butanol, and butanone fractions of ethanol | 250 mg kg−1 or 300 mg kg−1 | In vivo: wound-healing effect in albino mice using the tensile strength of wound models (re-sutured incision and grass pith granuloma); Tween 80 solution served as the control | Extracts exhibited higher tensile strength (215.4 g, 213 g, 243.7 g, 236.1 g, and 232.9 g, respectively) than the control (144.3 g) in the re-sutured incision model; (221.7 g, 210 g, 264.1 g, 332.8 g, and 335.7 g, respectively) than the control (155.4 g) in the granuloma model | [53] | |
| M. umbellata | Antibacterial | Leaves | Ethanol | 1000 μg mL−1 | In vitro: antibacterial activity against Klebsiella pneumoniae, S. aureus, and Pseudomonas aeruginosa using the broth microdilution method; gentamicin was the positive control | Extract had 32%, 55%, and 67%, respectively, on the bacterial strains’ growth | [62] |
| M. vitifolia | Anti-arthritic | Leaves | Aqueous fraction from methanol extract | 500 μg mL−1 | In vitro: anti-arthritic activity by protein denaturation inhibition method; diclofenac sodium served as a reference | Extracts had protein denaturation inhibition of 64% relative the standard drug (87%) | [20] |
| Anti-nociceptive activity | Leaves | Aqueous fraction from methanol extract | 200 mg kg−1, 400 mg kg−1 | In vivo: anti-nociceptive activity in Swiss Albino mice using formalin-induced paw licking (at early and late stages) and acetic acid-induced writhing tests; diclofenac sodium (administered intraperitoneally) served as a standard drug | Extract showed anti-nociceptive activity (44% and 26%, 30% and 20% in both early and late test stages, respectively) relative to the control (20% and 15%, respectively) in the formalin-induced paw licking test; reduced (44% and 30%, respectively) abdominal contortions with increasing doses of APFME relative to the control (21%) | [20] | |
| Antioxidant activity | Stems | Ethanol | 120 μg mL−1 | In vitro: DPPH assay; gallic acid, L-ascorbic acid, and piperine were used as references | IC30 of 25 μg mL−1 | [118] | |
| Thrombolytic activity | Leaves | Aqueous fraction from methanol extract | 500 μg mL−1 | In vitro: thrombolytic activity in blood samples from male and female (1:1) adult human volunteers with no anticoagulant and oral contraceptive treatments history; streptokinase served as a positive control | Extract exhibited thrombolytic activity (42.5% clot lysis) relative to the positive control (72.2%) | [20] |
4. Toxicology
5. Discussion and Future Perspective
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A549 | Lung Carcinoma |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| ACE-2 | Angiotensin-Converting Enzyme 2 |
| ACEI | Angiotensin-Converting-Enzyme Inhibitory |
| AGS | Stomach Cancer Cells |
| APFME | Aqueous Portion of Fractionated Methanol Extract |
| BHA | Butylated Hydroxyanisole |
| BW | Body Weight |
| CAFDs | Caffeic Acid Derivatives |
| COLO 320 | Colon Cancer Cells |
| DM | Dry Matter |
| DMSO | Dimethyl sulfoxide |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| DU-145 | Prostate Carcinoma |
| EDTA | Ethylenediaminetetraacetic Acid |
| ESRD | End-Stage Renal Disease |
| EGFR | Epidermal Growth Factor Receptor |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FITC | Fluorescein Isothiocyanate-Conjugated |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalents |
| HPMC | Hydroxypropylmethylcellulose |
| IP | Intraperitoneally |
| KB | Mouth Carcinoma |
| LPS | Lipopolysaccharide |
| MCF-7 | Breast Cancer Cells |
| MIA-PaCa-2 | Pancreas Carcinoma |
| MIC | Minimum Inhibitory Concentration |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Na CMC | Sodium Carboxymethylcellulose |
| OH• | Hydroxyl Radicals |
| PI | Propidium Iodide |
| PO | Per os |
| RP-HPLC | Reverse Phase-High Performance Liquid Chromatography |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TGA-capped-CdTe QDs | Thioglycolic Acid-Capped Cadmium Telluride Quantum Dots |
| TNF-α | Tumour Necrosis Factor Alpha |
| UPLC-MS/MS | Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry |
| USDA | United State Department of Agriculture |
| VERO | Monkey Normal Kidney Epithelial Cells |
| WFO | World Flora Online |
| WHO | World Health Organization |
| WoS | Web of Science |
References
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept. ” J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Olukoya, B.M.; Sakpere, A.M.A.; Adetunji, A.E. Phytochemical, proximate and elemental analysis of Clerodendrum volubile (Lamiaceae) at pre-and post-flowering stages. Phytol. Balc. 2018, 24, 45–49. [Google Scholar]
- Vasu Babu, A.; Sujatha, K.; Srikanaka Valli, A.; Narayana, K.J.P.; Hari Babu, B.; Satyanarayana, P.V.V. Anticancer and anti-inflammatory activities of extracts of Merremia emerginata. Biosci. Biotechnol. Res. Asia 2009, 6, 835–838. [Google Scholar]
- Luciardi, M.C.; Pérez Hernández, M.V.; Muruaga, N.; Bardón, A.; Arena, M.E.; Cartagena, E. Volatiles from subtropical Convolvulaceae that interfere with bacterial cell-to-cell communication as potential antipathogenic drugs. Evid. Based Complement. Altern. Med. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, Y.J.; Wang, Z.J.; Hu, B.Y.; Xie, T.Z.; Xiao, X.; Zhou, Z.S.; Sang, X.Y.; Luo, X.D. A review of plant characteristics, phytochemistry and bioactivities of the genus Glechoma. J. Ethnopharmacol. 2021, 271, 113830. [Google Scholar] [CrossRef] [PubMed]
- Siebert, F.; Dreber, N. Forb ecology research in dry African savannas: Knowledge, gaps, and future perspectives. Ecol. Evol. 2019, 9, 7875–7891. [Google Scholar] [CrossRef] [PubMed]
- Bond, W.J.; Parr, C.L. Beyond the forest edge: Ecology, diversity and conservation of the grassy biomes. Biol. Conserv. 2010, 143, 2395–2404. [Google Scholar] [CrossRef]
- Adetunji, A.E. Physiological and Biochemical Investigations into the Reinvigoration of Deteriorated Brassica oleracea L. (Cabbage) and Lactuca sativa L. (Lettuce) Seeds with Antioxidants and Inorganic Salt Solutions; University of KwaZulu-Natal: Durban, South Africa, 2021. [Google Scholar]
- Staples, G. A Checklist of Merremia (Convolvulaceae) in Australasia and the Pacific. Gard. Bull. Singapore 2010, 61, 483–522. [Google Scholar]
- Demissew, S. A synopsis of the genus Merremia (Convolvulaceae) in the Flora of Ethiopia and Eritrea. Kew Bull. 2001, 56, 931–943. [Google Scholar] [CrossRef]
- Simoes, A.R.; Culham, A.; Carine, M. Resolving the unresolved tribe: A molecular phylogenetic framework for the Merremieae (Convolvulaceae). Bot. J. Linn. Soc. 2015, 179, 374–387. [Google Scholar] [CrossRef]
- Pereda-Miranda, R.; Rosas-Ramírez, D.; Castañeda-Gómez, J. Resin glycosides from the Morning Glory Family. In Fortschritte der Chemie Organischer Naturstoffe = Progress in the Chemistry of Organic Natural Products. Progres Dans la Chimie des Substances Organiques Naturelles; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Vienna, Austria, 2010; Volume 92, pp. 77–153. ISBN 9783211996614. [Google Scholar]
- Ono, M. Resin glycosides from Convolvulaceae plants. J. Nat. Med. 2017, 71, 591–604. [Google Scholar] [CrossRef]
- Schimming, T.; Jenett-Siems, K.; Mann, P.; Tofern-Reblin, B.; Milson, J.; Johnson, R.W.; Deroin, T.; Austin, D.F.; Eich, E. Calystegines as chemotaxonomic markers in the Convolvulaceae. Phytochemistry 2005, 66, 469–480. [Google Scholar] [CrossRef]
- Jenett-Siems, K.; Weigl, R.; Böhm, A.; Mann, P.; Tofern-Reblin, B.; Ott, S.C.; Ghomian, A.; Kaloga, M.; Siems, K.; Witte, L.; et al. Chemotaxonomy of the pantropical genus Merremia (Convolvulaceae) based on the distribution of tropane alkaloids. Phytochemistry 2005, 66, 1448–1464. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Chin, N.L. Antioxidant and anti-ulcer effects of ethyl acetate fraction of Merremia tridentata (L.) Hallier, F. Root. Agric. Agric. Sci. Procedia 2014, 2, 406–414. [Google Scholar] [CrossRef]
- Ediriweera, E.; Ratnasooriya, W. A review on herbs used in treatment of diabetes mellitus by Sri Lankan ayurvedic and traditional physicians. Ayurveda 2009, 30, 373–391. [Google Scholar]
- Yuvaraj, B.; Sathish, M. In Vitro and In Vivo antidiabetic activity on leaves of Merremia hederacea (Burm. f.) Hallier, F. Int. J. Green Pharm. 2019, 13, 384–391. [Google Scholar]
- Perez, K.J.; Jose, M.; Aranico, E.; Madamba, M.R. Phytochemical and antibacterial properties of the ethanolic leaf extract of Merremia peltata (L.) Merr. and Rubus SPP. Adv. Environ. Biol. 2015, 9, 50–56. [Google Scholar]
- Akter, S.; Jahan, I.; Riniara Khatun, M.; Forhad Khan, M.; Arshad, L.; Haque, M.A. Pharmacological insights into Merremia vitifolia (Burm.f.) Hallier f. leaf for its antioxidant, thrombolytic, anti-arthritic and anti-nociceptive potential. Biosci. Rep. 2021, 20203022. [Google Scholar] [CrossRef] [PubMed]
- Rameshkumar, A.; Sivasudha, T.; Jeyadevi, R.; Sangeetha, B.; Ananth, D.A.; Aseervatham, G.S.B.; Nagarajan, N.; Renganathan, R.; Kathiravan, A. In vitro antioxidant and antimicrobial activities of Merremia emarginata using thio glycolic acid-capped cadmium telluride quantum dots. Colloids Surf. B Biointerfaces 2013, 101, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Amzad Hossain, M.; Shah, M.D. A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arab. J. Chem. 2015, 8, 66–71. [Google Scholar] [CrossRef]
- Nahar, M.N.; Acharzo, A.K.; Rahaman, M.S.; Zabeen, I.A.; Haque, S.; Islam, M.A. Phytochemical screening and antioxidant, analgesic, and anthelmintic effect of ethanolic extract of Merremia umbellata stems. Clin. Phytoscience 2020, 6, 86. [Google Scholar] [CrossRef]
- Geaney, F.; Scutaru, C.; Kelly, C.; Glynn, R.W.; Perry, I.J. Type 2 Diabetes Research Yield, 1951-2012: Bibliometrics Analysis and Density-Equalizing Mapping. PLoS ONE 2015, 10, e0133009. [Google Scholar] [CrossRef][Green Version]
- Ellegaard, O.; Wallin, J.A. The bibliometric analysis of scholarly production: How great is the impact? Scientometrics 2015, 105, 1809–1831. [Google Scholar] [CrossRef]
- Ekundayo, T.C.; Okoh, A.I. A global bibliometric analysis of Plesiomonas-related research (1990–2017). PLoS ONE 2018, 13, e0207655. [Google Scholar] [CrossRef]
- Olisah, C.; Adams, J.B. Analysing 70 years of research output on South African estuaries using bibliometric indicators. Estuar. Coast. Shelf Sci. 2021, 252, 107285. [Google Scholar] [CrossRef]
- Olisah, C.; Adams, J.B. Systematic mapping of organophosphate contaminant (OPC) research trends between 1990 and 2018. Environ. Geochem. Health 2020, 42, 3481–3505. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.H.; Idris, O.A.; Maboeta, M.S. Global trends of green pesticide research from 1994 to 2019: A bibliometric analysis. J. Toxicol. 2021, 2021. [Google Scholar] [CrossRef]
- Stefanović, S.; Krueger, L.; Olmstead, R.G. Monophyly of the Convolvulaceae and circumscription of their major lineages based on DNA sequences of multiple chloroplast loci. Am. J. Bot. 2002, 89, 1510–1522. [Google Scholar] [CrossRef] [PubMed]
- Stefanović, S.; Austin, D.F.; Olmstead, R.G. Classification of Convolvulaceae: A phylogenetic approach. Syst. Bot. 2003, 28, 791–806. [Google Scholar] [CrossRef]
- The Plant List 2013. Available online: http://www.theplantlist.org/tpl1.1/search?q=Merremia (accessed on 3 March 2021).
- Sosef, M.S.M.; Gereau, R.E.; Janssens, S.B.; Kompanyi, M.; Simões, A.R. A curious new species of Xenostegia (Convolvulaceae) from Central Africa, with remarks on the phylogeny of the genus. Syst. Bot. 2019, 44, 405–414. [Google Scholar] [CrossRef]
- Fourie, R. Merremia palmate. Available online: https://www.inaturalist.org/observations/37152427 (accessed on 3 March 2021).
- Thierrycordenos. Merremia pterygocaulos. Available online: https://www.inaturalist.org/photos/101455002 (accessed on 3 March 2021).
- Hernández, A.L. Merremia platylphylla. Available online: https://www.inaturalist.org/photos/112408159 (accessed on 3 March 2021).
- Cock, M. Merremia peltata. Available online: https://www.cabi.org/isc/datasheet/33476#toPictures (accessed on 3 March 2021).
- Convolvulaceae Unlimited. Merremia emarginata. Available online: http://tropical.theferns.info/image.php?id=Merremia+emarginata (accessed on 3 March 2021).
- Wan-hsuan. Merremia gemella. Available online: https://www.inaturalist.org/observations/32514537 (accessed on 3 March 2021).
- Global Biodiversity Information Facility (GBIF). Merremia distribution map. Available online: https://www.gbif.org/species/2928639 (accessed on 3 March 2021).
- Thet, K.K.; Zar, K.; Lae, W.; Phyo, H.W.; Khin, A.N. Nutritional values and antioxidant activity analysis of Merremia emarginata (Burm.F). 3rd Myanmar Korea Conf. Res. J. 2020, 3, 1549–1555. [Google Scholar]
- Austin, D.F. Merremia dissecta (Convolvulaceae): Condiment, medicine, ornamental, and weed—A review. Econ. Bot. 2007, 61, 109–120. [Google Scholar] [CrossRef]
- Catarino, L.; Romeiras, M.M.; Bancessi, Q.; Duarte, D.; Faria, D.; Monteiro, F.; Moldão, M. Edible leafy vegetables from West Africa (Guinea-Bissau): Consumption, trade and food potential. Foods 2019, 8, 493. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.T.; Cabral, D.L.V.; Amorim, E.L.C.; Dos Santos, M.V.F.; Albuquerque, U.P. Plants used to feed ruminants in semi-arid Brazil: A study of nutritional composition guided by local ecological knowledge. J. Arid. Environ. 2016, 135, 96–103. [Google Scholar] [CrossRef]
- Aschfalk, A.; Steingass, H.; Müller, W.; Drochner, W. Merremia tridentata as a supplementary feed to the grass Panicum maximum for young West African Dwarf Sheep. Trop. Anim. Health Prod. 2002, 34, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Galat-Luong, A.; Nizinski, J.J.; Galat, G. Diet preferences of a Western giant’s (Lord Derby’s) eland group in a Sahelian dry habitat. Anim. Biol. 2011, 61, 485–492. [Google Scholar] [CrossRef]
- Rajendran, D.; Balakrishnan, V. Diet composition, biomass yield and mineral contents of vegetation in native tract of mecheri sheep. Anim. Nutr. Feed Technol. 2012, 12, 63–71. [Google Scholar]
- Herrero, M.; Thornton, P.K. Livestock and global change: Emerging issues for sustainable food systems. Proc. Natl. Acad. Sci. USA 2013, 110, 20878–20881. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Improving Animal Productivity by Supplementary Feeding of Multinutrient Blocks, Controlling Internal Parasites and Enhancing Utilization of Alternate Feed Resources; IAEA: Vienna, Austria, 2006; pp. 1–280, publication prepared under the framework of an RCA project with technical support of the Joint FAO/IAEA Programme of Nuclear Techniques in Food and Agriculture; Available online: https://www-pub.iaea.org/MTCD/publications/PDF/te_1495_web.pdfx. (accessed on 17 June 2021).
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Sachithanandam, P.; Parkavi, S.; Ganesh, P.; Swaminathan, C. Phytochemical analysis, antibacterial activity and antioxidant activity of leaf extracts of Merremia emarginata (Burm. F.). Int. J. Pharm. Sci. Res. 5214 IJPSR 2020, 11, 5214–5218. [Google Scholar] [CrossRef]
- Arunachalam, K.; Parimelazhagan, T. Antidiabetic activity of aqueous root extract of Merremia tridentata (L.) Hall. f. in streptozotocin-induced-diabetic rats. Asian Pac. J. Trop. Med. 2012, 5, 175–179. [Google Scholar] [CrossRef]
- Bidkar, A.A.; Sherje, A.P.; Gujar, K.N.; Bagul, U.S.; Miniyar, P.B.; Aphale, S.A. Phytochemical and pharmacological investigation of extracts of Merremia tridentata Linn. (Convolvulaceae). J. Nat. Remedies 2009, 9, 79–84. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Joseph, J.M.; Arunachalam, K.; Manian, S. Evaluation of Merremia tridentata (L.) Hallier f. for in vitro antioxidant activity. Food Sci. Biotechnol. 2010, 19, 663–669. [Google Scholar] [CrossRef]
- Kamalutheen, M.; Gopalakrishnan, S.; Syed Ismail, T. Anti-inflammatory and anti-arthritic activities of Merremia tridentata (L.) Hall. f. J. Chem. 2009, 6, 943–948. [Google Scholar] [CrossRef]
- Ratnadewi, A.A.I.; Wahyudi, L.D.; Rochman, J.; Susilowati; Nugraha, A.S.; Siswoyo, T.A. Revealing anti-diabetic potency of medicinal plants of Meru Betiri National Park, Jember-Indonesia. Arab. J. Chem. 2020, 13, 1831–1836. [Google Scholar] [CrossRef]
- Marchianti, A.C.N.; Sakinah, E.N.; Elfiah, U.; Putri, N.K.S.; Wahyuliswari, D.I.; Maulana, M.; Ulfa, E.U. Gel formulations of Merremia mammosa (Lour.) accelerated wound healing of the wound in diabetic rats. J. Tradit. Complement. Med. 2021, 11, 38–45. [Google Scholar] [CrossRef]
- Alen, Y.; Sari, P.; Aldi, Y.; Yulianis; Nakajima, S.; Baba, N.; Djamaan, A. Extraction, fractionation and cytotoxicity test of Merremia peltata (L.)Merr., (Fam. Convolvulaceae) Leaves. Der. Pharm. Lett. 2016, 8, 48–52. [Google Scholar]
- Hossain, M.A.; Shah, M.D.; Sang, S.V.; Sakari, M. Chemical composition and antibacterial properties of the essential oils and crude extracts of Merremia borneensis. J. King Saud Univ. Sci. 2012, 24, 243–249. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, M.; Li, J.; Wei, D.; Mei, S.; Cui, T.; Wang, J.; Zhu, Z.; Dong, X.; Wan, J. Structure elucidation and NMR assignment of two new eudesmane derivatives from Merremia yunnanensis. Magn. Reson. Chem. 2019, 57, 934–938. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, B.; Ding, Q.; Zhang, X.; Wei, D.; Mei, S.; Li, R.; Wan, J.; Li, Q. A new bicyclic sesquiterpenoid from Merremia yunnanensis. Biochem. Syst. Ecol. 2020, 89, 103964. [Google Scholar] [CrossRef]
- Rivera, D.E.; Ocampo, Y.C.; Castro, J.P.; Caro, D.; Franco, L.A. Antibacterial activity of Physalis angulata L., Merremia umbellata L., and Cryptostegia grandiflora Roxb. Ex R.Br.—medicinal plants of the Colombian Northern Coast. Orient. Pharm. Exp. Med. 2015, 15, 95–102. [Google Scholar] [CrossRef]
- Awang-Jamil, Z.; Aminuddin, M.F.; Zaidi, B.Q.; Basri, A.M.; Ahmad, N.; Taha, H. Phytochemicals and antimicrobial analysis of selected medicinal plants from Brunei Darussalam. Biodiversitas 2021, 22, 601–606. [Google Scholar] [CrossRef]
- Rameshkumar, A.; Sivasudha, T.; Jeyadevi, R.; Sangeetha, B.; Smilin Bell Aseervatham, G.; Maheshwari, M. Profiling of phenolic compounds using UPLC-Q-TOF-MS/MS and nephroprotective activity of Indian green leafy vegetable Merremia emarginata (Burm. f.). Food Res. Int. 2013, 50, 94–101. [Google Scholar] [CrossRef]
- Kim, H.R.; Chung, S.Y.; Jeong, Y.H.; Go, E.J.; Han, A.R.; Kim, N.H.; KyungSung, M.; Song, G.; Jang, J.O.; Nam, J.W.; et al. P-glycoprotein inhibitory activity of Indonesian medicinal plants in human breast cancer cells. Nat. Prod. Sci. 2004, 10, 268–271. [Google Scholar]
- Pavithra, P.S.; Janani, V.S.; Charumathi, K.H.; Indumathy, R.; Potala, S.; Verma, R.S. Antibacterial activity of plants used in Indian herbal medicine. Int. J. Green Pharm. 2010, 4, 22–28. [Google Scholar] [CrossRef]
- Ch, D.; Prasad, R.Y.; Babu, S.P.; Gananadhamu, S. Biological screening and phytochemical investigation of Merremia emarginata leaves. Int. J. Pharm. Sci. Res. 2021, 12, 507–514. [Google Scholar] [CrossRef]
- Anioł, M.; Świderska, A.; Stompor, M.; Żołnierczyk, A.K. Antiproliferative activity and synthesis of 8-prenylnaringenin derivatives by demethylation of 7-O-and 4’-O-substituted isoxanthohumols. Med. Chem. Res. 2012, 21, 4230–4238. [Google Scholar] [CrossRef][Green Version]
- Paulo, F.; Santos, L. Encapsulation of the Antioxidant tyrosol and characterization of loaded microparticles: An integrative approach on the study of the polymer-carriers and loading contents. Food Bioprocess Technol. 2020, 13, 764–785. [Google Scholar] [CrossRef]
- Adetunji, A.E.; Sershen, N.; Varghese, B.; Pammenter, N. Effects of exogenous application of five antioxidants on vigour, viability, oxidative metabolism and germination enzymes in aged cabbage and lettuce seeds. S. Afr. J. Bot. 2021, 137, 85–97. [Google Scholar] [CrossRef]
- Adetunji, A.E.; Sershen, N.; Varghese, B.; Pammenter, N.W. Effects of inorganic salt solutions on vigour, viability, oxidative metabolism and germination enzymes in aged cabbage and lettuce seeds. Plants 2020, 9, 1164. [Google Scholar] [CrossRef]
- Lee, J.; Han, S.I.; Yun, J.H.; Kim, J.H. Quercetin 3-O-glucoside suppresses epidermal growth factor–induced migration by inhibiting EGFR signaling in pancreatic cancer cells. Tumor Biol. 2015, 36, 9385–9393. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Tabrez, S.; Ali, R.; Alqahtani, A.S.; Ahmed, M.Z.; Rub, A. Molecular docking analysis of rutin reveals possible inhibition of SARS-CoV-2 vital proteins. J. Tradit. Complement. Med. 2021, 11, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Q.; Song, W.B.; Lan, X.J.; Huang, M.; Xuan, L.J. Merremins A-G, resin glycosides from Merremia hederacea with multidrug resistance reversal activity. J. Nat. Prod. 2014, 77, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bi, H.-H.; Liu, Y.-Z.; Zhang, M.; Zhou, Z.-Y.; Tan, J.-W. Phenolic compounds from Merremia umbellata subsp.orientalis and their allelopathic effects on Arabidopsis seed germination. Molecules 2010, 15, 8241–8250. [Google Scholar] [CrossRef]
- Adem, Ş.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A.F.; Ali, M.; Abdalla, M.; Ibrahim, I.M.; Elfiky, A.A. Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine 2020, 153310. [Google Scholar] [CrossRef]
- Gasparotto, A.J.; Tolouei, S.E.L.; Lívero, F.A.; Gasparotto, F.; Boeing, T.; De Souza, P. Natural agents modulating ACE-2: A review of compounds with potential against SARS-CoV-2 infections. Curr. Pharm. Des. 2021, 27. [Google Scholar] [CrossRef]
- Gaikwad, S.B.; Krishna Mohan, G.; Rani, M.S. Phytochemicals for diabetes management. Pharm. Crop. 2014, 5, 11–28. [Google Scholar] [CrossRef]
- Angappan, R.; Devanesan, A.A.; Thilagar, S. Diuretic effect of chlorogenic acid from traditional medicinal plant Merremia emarginata (Burm. F.) and its by product hippuric acid. Clin. Phytoscience 2018, 4, 29. [Google Scholar] [CrossRef]
- Santos Júnior, H.M.; Lopes, K.C.; Alves, D.S.; Carvalho, G.A.; Oliveira, D.F. Ursolic acid and cis-tiliroside produced by Merremia tomentosa affect oviposition of Leucoptera coffeella on coffee plants. Quim. Nova 2018, 41, 302–309. [Google Scholar] [CrossRef]
- Jenett-Siems, K.; Siems, K.; Witte, L.; Eich, E. Merrekentrones A-D, Ipomeamarone-like furanosesquiterpenes from Merremia kentrocaulos. J. Nat. Prod. 2001, 64, 1471–1473. [Google Scholar] [CrossRef]
- Kitagawa, I.; Ohashi, K.; Baek, N.I.; Sakagami, M.; Yoshikawa, M.; Shibuya, H. Indonesian Medicinal Plants. XIX. Chemical Structures of Four Additional Resin-Glycosides, Mammosides A, B, H1, and H2, from the Tuber of Merremia mammosa (Convolvulaceae). Chem. Pharm. Bull. 1997, 45, 786–794. [Google Scholar] [CrossRef][Green Version]
- García-Argáez, A.; Pérez-Amador, M.; Aguirre-Hernández, E.; Martínez-Vázquez, M. Two new caffeate esters from roots of Merremia tuberosa and M. dissecta. Planta Med. 1999, 65, 678–679. [Google Scholar] [CrossRef]
- Kitagawa, I.; Baek, N.I.; Kawashima, K.; Yokokawa, Y.; Yoshikawa, M.; Ohashi, K.; Shibuya, H. Indonesian medicinal plants. XV. Chemical structures of five new resin- glycosides, Merremosides A, B, C, D, and E, from the tuber of Merremia mammosa (Convolvulaceae). Chem. Pharm. Bull. 1996, 44, 1680–1692. [Google Scholar] [CrossRef]
- Kitagawa, I.; Baek, N.I.; Yokokawa, Y.; Yoshikawa, M.; Ohashi, K.; Shibuya, H. Indonesian Medicinal Plants. XVI. Chemical structures of four new resin-glycosides, Merremosides F, G, H1, and H2, from the tuber of Merremia mammosa (Convolvulaceae). Chem. Pharm. Bull. 1996, 44, 1693–1699. [Google Scholar] [CrossRef]
- Vilakazi, H.; Olasehinde, T.A.; Olaniran, A.O. Chemical characterization, antiproliferative and antioxidant activities of polyunsaturated fatty acid-rich extracts from Chlorella sp. S14. Molecules 2021, 26, 4109. [Google Scholar] [CrossRef]
- Chaudhary, S.K.; Sandasi, M.; Makolo, F.; Van Heerden, F.R.; Viljoen, A.M. Aspalathin: A rare dietary dihydrochalcone from Aspalathus linearis (rooibos tea). Phytochem. Rev. 2021, 1–32. [Google Scholar] [CrossRef]
- Baskar, A.A.; Al Numair, K.S.; Alsaif, M.A.; Ignacimuthu, S. In vitro antioxidant and antiproliferative potential of medicinal plants used in traditional Indian medicine to treat cancer. Redox Rep. 2012, 17, 145–156. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diabetes—WHO-Regional Office for Africa Diabetes—WHO-Regional Office for Africa. Available online: https://www.afro.who.int/health-topics/diabetes (accessed on 10 July 2021).
- Muthi’atul Af-Idah, B.; Hanafi, M.; Elya, B. Antioxidant and alpha glucosidase inhibitor screening of Merremia peltata L. as potential traditional treatment for diabetes mellitus. Orig. Artic. Pharm. J. 2021, 13, 902–908. [Google Scholar] [CrossRef]
- Corona, G.; Pizzocaro, A.; Vena, W.; Rastrelli, G.; Semeraro, F.; Isidori, A.M.; Pivonello, R.; Salonia, A.; Sforza, A.; Maggi, M. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: Systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2021, 22, 275–296. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.; Lim, M.A.; Pranata, R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—A systematic review, meta-analysis, and meta-regression: Diabetes and COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 395–403. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Sasikumar, P. Antidiabetic effect of Merremia emarginata Burm. F. in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 2, 281–286. [Google Scholar] [CrossRef]
- Kaladhar, D.S.V.G.K.; Harasreeramulu, S.; Vijaya Rachel, K.; Surekha, C.H. Evaluation of antimicrobial activity of methanolic leaf extracts of selected in vitro and in vivo grown Convolvulaceae members. J. Pure Appl. Microbiol. 2009, 3, 759–767. [Google Scholar]
- Elumalai, E.K.; Ramachandran, M.; Thirumalai, T.; Vinothkumar, P. Antibacterial activity of various leaf extracts of Merremia emarginata. Asian Pac. J. Trop. Biomed. 2011, 1, 406–408. [Google Scholar] [CrossRef]
- Diwan, P.D.; Gadhikar, Y.A. Assessment of phytochemical composition and antibacterial activity of different extracts of Barleria prionitis leaves against oral microflora to improve dental hygiene. Asian J. Pharm. Clin. Res. 2012, 5, 182–184. [Google Scholar]
- Yang, C.H.; Tan, D.H.; Hsu, W.L.; Jong, T.T.; Wen, C.L.; Hsu, S.L.; Chang, P.C. Anti-influenza virus activity of the ethanolic extract from Peperomia sui. J. Ethnopharmacol. 2014, 155, 320–325. [Google Scholar] [CrossRef] [PubMed]
- WHO Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 12 July 2021).
- Purwitasari, N.; Agil, M.; Studiawan, H. Activity of ethyl acetate fraction of Merremia mammosa Hall. as anti-influenza A (H1N1). Indian J. Forensic Med. Toxicol. 2020, 14, 2070–2073. [Google Scholar] [CrossRef]
- Khumalo, G.P.; Van Wyk, B.E.; Feng, Y.; Cock, I.E. A review of the traditional use of Southern African medicinal plants for the treatment of inflammation and inflammatory pain. J. Ethnopharmacol. 2021, 114436. [Google Scholar] [CrossRef]
- Shebaby, W.; Saliba, J.; Faour, W.H.; Ismail, J.; El Hage, M.; Daher, C.F.; Taleb, R.I.; Nehmeh, B.; Dagher, C.; Chrabieh, E.; et al. In Vivo and In Vitro anti-inflammatory activity evaluation of Lebanese Cannabis sativa L. ssp. indica (Lam.). J. Ethnopharmacol. 2021, 270, 113743. [Google Scholar] [CrossRef]
- Purushoth Prabhu, T.; Sudev, S.; Deepak Venkataraman, N.; Clement Atlee, W.; Shrikumar, S. In Vitro antioxidant and antiarthritis activity of extracts and fractions of Merremia emarginata. Der Pharm. Lett. 2014, 6, 227–231. [Google Scholar]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised international association for the study of pain, definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Ahmadimoghaddam, D.; Zarei, M.; Mohammadi, S.; Izadidastenaei, Z.; Salehi, I. Bupleurum falcatum L. alleviates nociceptive and neuropathic pain: Potential mechanisms of action. J. Ethnopharmacol. 2021, 273, 113990. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, D.M.G.; Ortega, Y.H.; Quero, P.C.; Martínez, R.S.; Pieters, L. Antiurolithiatic activity of Boldoa purpurascens aqueous extract: An in vitro and in vivo study. J. Ethnopharmacol. 2020, 253, 112691. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Arul, S.V. Anti-Urolithiatic activity of medicinal plants and Siddha formulatory medicine: A Review. J. Res. Biomed. Sci. 2020, 3, 7–12. [Google Scholar] [CrossRef]
- Neeraja Kamakshi, U.; Ganga Rao, B.; Venkateswara Rao, B. Comparison of in vitro antiurolithiatic activity of Aerva lanata, Sphaeranthus indicus, Merremia emarginata. Res. J. Pharm. Technol. 2017, 10, 1653–1656. [Google Scholar] [CrossRef]
- Sharma, V.C.; Kaushik, A.; Dey, Y.N.; Srivastava, B.; Wanjari, M.; Pawar, S.; Chougule, S. Nephroprotective potential of Anogeissus latifolia Roxb. (Dhava) against gentamicin-induced nephrotoxicity in rats. J. Ethnopharmacol. 2021, 273, 114001. [Google Scholar] [CrossRef]
- Jabeen, Q.; Aslam, N. Hypotensive, angiotensin converting enzyme (ACE) inhibitory and diuretic activities of the aqueous-methanol extract of Ipomoea reniformis. Iran. J. Pharm. Res. 2013, 12, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Rajoo, A.; Ramanathan, S.; Mansor, S.M.; Sasidharan, S. Formulation and evaluation of wound healing activity of Elaeis guineensis Jacq leaves in a Staphylococcus aureus infected Sprague Dawley rat model. J. Ethnopharmacol. 2021, 266, 113414. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Kostić, O.; Mataruga, Z.; Pavlović, D.; Pavlović, M.; Mitrović, M.; Pavlović, P. Traditional wound-healing plants used in the Balkan region (Southeast Europe). J. Ethnopharmacol. 2018, 211, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Sakinah, E.N.; Ulfa, E.U.; Marchianti, A.C.N. The effectiveness of Merremia mammosa (Lour.) extract fractions as diabetic wound healers on diabetic rat model. Hiroshima J. Med. Sci. 2018, 67, 70–77. [Google Scholar]
- Wei, X.; Zhao, Z.; Zhong, R.; Tan, X. A comprehensive review of herbacetin: From chemistry to pharmacological activities. J. Ethnopharmacol. 2021, 279, 114356. [Google Scholar] [CrossRef]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Phytochemical and antioxidant activities of Rumex crispus L. in treatment of gastrointestinal helminths in Eastern Cape Province, South Africa. Asian Pac. J. Trop. Biomed. 2017, 7, 1071–1078. [Google Scholar] [CrossRef]
- Gomathi, D.; Kalaiselvi, M.; Ravikumar, G.; Devaki, K.; Uma, C. GC-MS analysis of bioactive compounds from the whole plant ethanolic extract of Evolvulus alsinoides (L.) L. J. Food Sci. Technol. 2015, 52, 1212–1217. [Google Scholar] [CrossRef]
- Babu, A.V.; Rao, R.S.C.; Kumar, K.G.; Babu, B.H.; Satyanarayana, P.V.V. Biological activity of Merremia emarginata crude extracts in different solvents. Res. J. Med. Plant 2009, 3, 134–140. [Google Scholar] [CrossRef][Green Version]
- Joshi, R.; Mishra, P.; Patni, V. Untapped ornamental vines of Convolvulaceae-potential source of antioxidants. Int. J. Pharm. Sci. Rev. Res. 2016, 36, 120–123. [Google Scholar]
- Thamsermsang, O.; Akarasereenont, P.; Laohapand, T.; Panich, U. IL-1β-induced modulation of gene expression profile in human dermal fibroblasts: The effects of Thai herbal Sahatsatara formula, piperine and gallic acid possessing antioxidant properties. BMC Complement. Altern. Med. 2017, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Makajanma, M.M.; Taufik, I.; Faizal, A. Antioxidant and antibacterial activity of extract from two species of mosses: Leucobryum aduncum and Campylopus schmidii. Biodiversitas 2020, 21, 2751–2758. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Rakotosaona, R.; Nzekoue, F.K.; Canale, A.; Nicoletti, M.; Maggi, F. Insecticidal and mosquito repellent efficacy of the essential oils from stem bark and wood of Hazomalania voyronii. J. Ethnopharmacol. 2020, 248, 112333. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Warkentin, T.D. Botany, traditional uses, phytochemistry and biological activities of Cardamom [Elettaria cardamomum (L.) Maton]—A critical review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef]
- Oliveira, P.V.; Ferreira, J.C.; Moura, F.S.; Lima, G.S.; De Oliveira, F.M.; Oliveira, P.E.S.; Conserva, L.M.; Giulietti, A.M.; Lemos, R.P.L. Larvicidal activity of 94 extracts from ten plant species of Northeastern of Brazil against Aedes aegypti L. (Diptera: Culicidae). Parasitol. Res. 2010, 107, 403–407. [Google Scholar] [CrossRef]
- Azarudeen, R.M.S.T.; Govindarajan, M.; AlShebly, M.M.; AlQahtani, F.S.; Amsath, A.; Senthilmurugan, S.; Vijayan, P.; Benelli, G. Size-controlled biofabrication of silver nanoparticles using the Merremia emarginata leaf extract: Toxicity on Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) and non-target mosquito predators. J. Asia Pac. Entomol. 2017, 20, 359–366. [Google Scholar] [CrossRef]
- Kamble, S.; Kamble, V.S. Antiinflammatory activity of the methanolic root extract of Merremia tridentata (L.) Hall. F. J. Pharmacogn. Phytochem. 2017, 6, 470–471. [Google Scholar]
- Priya, P.; Arul An, C.N.; Gausunnisha, T.; Rajalakshmi, R.; Roselin, A.; Grandhi, U. Study of analgesic activity of Merremia emarginata (BURM. F) HALLIER F. Int. J. Pharm. Biol. Sci. 2012, 2, 299–302. [Google Scholar]
- Brito, L.B.; Silva Filho, G.B.; Chaves, H.A.S.; Nascimento, A.L.O.; Braga, T.C.; Pfister, J.; Correa, F.R.; Mendonça, F.S. Spontaneous and experimental poisoning by Merremia macrocalyx (Convolvulaceae) in cattle1. Pesqui. Vet. Bras. 2019, 39, 447–453. [Google Scholar] [CrossRef]
- Adebiyi, O.A.; Danladi, A.A.; Onyike, E.; James, D.B. Acute and chronic toxicity studies of ethanol Leaf extract of Merremia tridentata (Linn) Hallier, F. Eur. J. Adv. Chem. Res. 2021, 2, 14–20. [Google Scholar] [CrossRef]
- Miller, J.S. The Global Importance of Plants as Sources of Medicines and the Future Potential of Chinese Plants. In Drug Discovery and Traditional Chinese Medicine; Springer: Boston, MA, USA, 2001; pp. 33–42. [Google Scholar]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]





| Bibliographic Information | Numerical Outputs |
|---|---|
| Publications | 357 |
| Sources (Journals, Books, etc.) | 188 |
| Keywords Plus (ID) | 2727 |
| Author’s Keywords (DE) | 1005 |
| Period | 1990–2020 |
| Average citations per document | 10.39 |
| Authors | 948 |
| Author Appearances | 1537 |
| Authors of multi-authored documents | 937 |
| Single-authored documents | 18 |
| Average documents per author | 0.377 |
| Average authors per document | 2.66 |
| Average co-authors per document | 4.31 |
| Average collaboration index | 2.76 |
| Most Productive Countries | Total Number of Citations per Country | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Countries | Articles | % of Total | Freq | SCP | MCP | MCP/P Ratio | Rank | Country | Article Citations | Citation Average |
| 1 | Brazil | 116 | 32.5 | 0.34 | 112 | 4 | 0.03 | 1 | India | 769 | 15.1 |
| 2 | India | 51 | 14.3 | 0.15 | 49 | 2 | 0.04 | 2 | Brazil | 697 | 6.0 |
| 3 | Japan | 27 | 7.6 | 0.08 | 26 | 1 | 0.04 | 3 | Japan | 536 | 19.9 |
| 4 | USA | 26 | 7.3 | 0.08 | 19 | 7 | 0.27 | 4 | Germany | 399 | 30.7 |
| 5 | China | 18 | 5.0 | 0.05 | 16 | 2 | 0.11 | 5 | USA | 295 | 11.4 |
| 6 | Germany | 13 | 3.6 | 0.04 | 10 | 3 | 0.23 | 6 | Mexico | 173 | 17.3 |
| 7 | Indonesia | 13 | 3.6 | 0.04 | 13 | 0 | 0.00 | 7 | Malaysia | 117 | 29.3 |
| 8 | Mexico | 10 | 2.8 | 0.03 | 8 | 2 | 0.20 | 8 | China | 102 | 5.7 |
| 9 | Belgium | 6 | 1.7 | 0.02 | 3 | 3 | 0.50 | 9 | Panama | 89 | 22.3 |
| 10 | Venezuela | 6 | 1.7 | 0.02 | 5 | 1 | 0.17 | 10 | Spain | 77 | 25.7 |
| 11 | Australia | 5 | 1.4 | 0.01 | 4 | 1 | 0.20 | 11 | Colombia | 67 | 22.3 |
| 12 | South Africa | 5 | 1.4 | 0.01 | 5 | 0 | 0.00 | 12 | South Africa | 53 | 10.6 |
| 13 | Malaysia | 4 | 1.1 | 0.01 | 4 | 0 | 0.00 | 13 | Canada | 41 | 20.5 |
| 14 | Panama | 4 | 1.1 | 0.01 | 2 | 2 | 0.50 | 14 | Australia | 40 | 8.0 |
| 15 | Argentina | 3 | 0.8 | 0.01 | 2 | 1 | 0.33 | 15 | Argentina | 36 | 12.0 |
| 16 | Colombia | 3 | 0.8 | 0.01 | 1 | 2 | 0.67 | 16 | Belgium | 31 | 5.2 |
| 17 | Spain | 3 | 0.8 | 0.01 | 1 | 2 | 0.67 | 17 | Indonesia | 28 | 2.2 |
| 18 | Thailand | 3 | 0.8 | 0.01 | 3 | 0 | 0.00 | 18 | Venezuela | 26 | 4.3 |
| 19 | United Kingdom | 3 | 0.8 | 0.01 | 1 | 2 | 0.67 | 19 | Togo | 22 | 22.0 |
| 20 | Bangladesh | 2 | 0.6 | 0.01 | 2 | 0 | 0.00 | 20 | Thailand | 17 | 5.7 |
| Domain | Eukaryota |
| Kingdom | Plantae |
| Phylum | Spermatophyta |
| Subphylum | Angiospermae |
| Class | Dicotyledonae |
| Order | Solanales |
| Family | Convolvulaceae |
| Genus | Merremia |
| Species | Ethnomedicinal Uses | Part Used | Type of Extraction | Country | Location of Collection | Reference |
|---|---|---|---|---|---|---|
| M. borneensis | Relieve breast cancer | Leaf | Maceration | Malaysia | Unspecified | [22] |
| Hair treatment | Leaf | Maceration | Brunei Darussalam | Madang | [63] | |
| Relieve breast cancer | Aerial parts | Maceration | Malaysia | University of Malaysia, Sabah area | [59] | |
| M. emarginata | Rheumatism, neuralgia, cough, and headache | Leaf | Maceration | India | Dharmapuri, Tamil Nadu | [21] |
| Antimicrobial effect, anti-inflammatory activity | Leaf | Maceration | India | Dharmapuri, Tamil Nadu | [64] | |
| Fever, neuralgia, urinary infection, rheumatism, inflammation, liver and kidney diseases | Leaf | Maceration and decoction | India | Varakkalpattu village, Cuddalore District, Tamil Nadu | [51] | |
| M. mammosa | Breast cancer | Whole plant | Maceration | Indonesia | Surabaya | [65] |
| Diabetic therapy | Leaf | Infusion | Indonesia | Meru Betiri National Park, Jember | [56] | |
| Diabetic ulcers | Whole plant | Maceration | Indonesia | Klaten, Central Java Province | [57] | |
| M. peltata | Anti-inflammatory, analgesic, anti-cancer, anti-viral, anti-malarial, anti-bacterial, and anti-fungal | Whole plant | Maceration | Philippines | Rogongon, Iligan City | [19] |
| Anti-cancer, diarrhea, abdominal pain, cough, sore eyes, wound and inflammation | Leaf | Maceration and Fractionation | Indonesia | Padang City, West Sumatra | [58] | |
| M. tridentata | Rheumatism, hemiplegia, piles, swellings, and urinary disorders | Root | Maceration | India | Udupi, Manipal | [53] |
| Toothache | Whole plant | Maceration | India | Xavier’s College campus, Palayamkottai, Tirunelveli District, Tamil Nadu | [55] | |
| Astringent, calefacient, laxative, anodyne, hemiplegia, hemorrhoids, uropathy, mouth wash, piles, inflammation, fever, and leprosy | Aerial parts | Maceration | India | Tamil Nadu Medicinal Plant Farms and Herbal Medicine, Chennai | [66] | |
| Piles, swellings, rheumatism, stiffness of the joints, hemiplegia, and urinary infections | Whole plant | Maceration | India | Coimbatore, Tamil Nadu | [54] | |
| Treatment of diabetes | Root | Maceration | India | Coimbatore, Tamil Nadu | [52] | |
| M. umbellata | Antibacterial, antifungal, and anti-inflammatory | Leaf | Maceration | Colombia | Pueblo Nuevo, Bolívar, | [60] |
| M. yunnanensis | Typhoid and stroke treatment | Whole plant | Decoction | China | Heqing Country, Dali Prefecture, Yunnan province | [60] |
| Stroke hemiplegia, typhoid fever, and headache | Fruit/Leaf | Infusion | China | Heqing County of Yunnan Province | [61] | |
| M. vitifolia | Fever, headache, eye inflammation, rheumatism, dysentery, jaundice, and urinary diseases | Leaf | Maceration | Bangladesh | Chittagong | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olatunji, T.L.; Adetunji, A.E.; Olisah, C.; Idris, O.A.; Saliu, O.D.; Siebert, F. Research Progression of the Genus Merremia: A Comprehensive Review on the Nutritional Value, Ethnomedicinal Uses, Phytochemistry, Pharmacology, and Toxicity. Plants 2021, 10, 2070. https://doi.org/10.3390/plants10102070
Olatunji TL, Adetunji AE, Olisah C, Idris OA, Saliu OD, Siebert F. Research Progression of the Genus Merremia: A Comprehensive Review on the Nutritional Value, Ethnomedicinal Uses, Phytochemistry, Pharmacology, and Toxicity. Plants. 2021; 10(10):2070. https://doi.org/10.3390/plants10102070
Chicago/Turabian StyleOlatunji, Tomi Lois, Ademola Emmanuel Adetunji, Chijioke Olisah, Oladayo Amed Idris, Oluwaseyi Damilare Saliu, and Frances Siebert. 2021. "Research Progression of the Genus Merremia: A Comprehensive Review on the Nutritional Value, Ethnomedicinal Uses, Phytochemistry, Pharmacology, and Toxicity" Plants 10, no. 10: 2070. https://doi.org/10.3390/plants10102070
APA StyleOlatunji, T. L., Adetunji, A. E., Olisah, C., Idris, O. A., Saliu, O. D., & Siebert, F. (2021). Research Progression of the Genus Merremia: A Comprehensive Review on the Nutritional Value, Ethnomedicinal Uses, Phytochemistry, Pharmacology, and Toxicity. Plants, 10(10), 2070. https://doi.org/10.3390/plants10102070









