Hypoglycemic Effect of Two Mexican Medicinal Plants
Abstract
:1. Introduction
2. Results
2.1. Ethnobotany
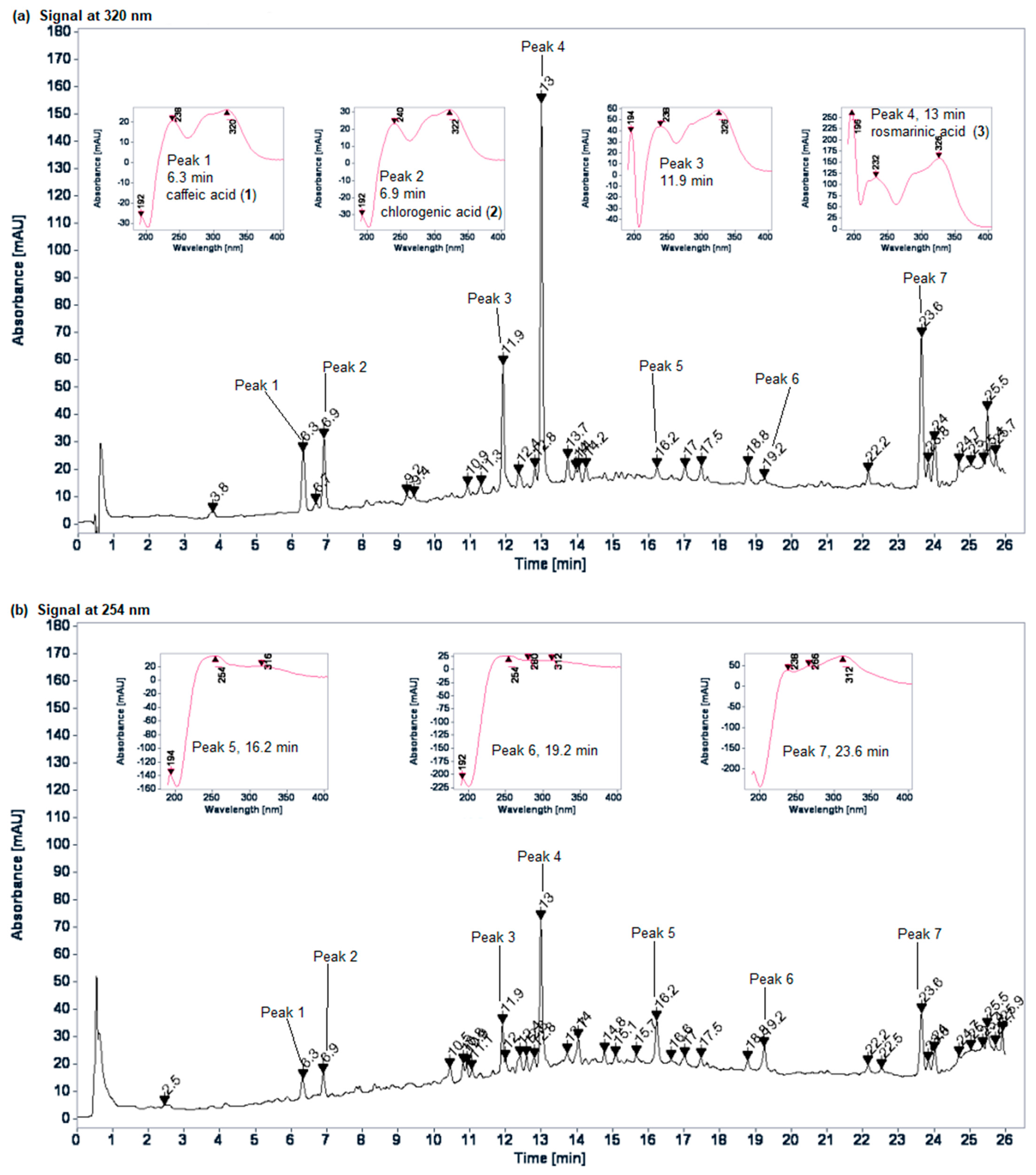
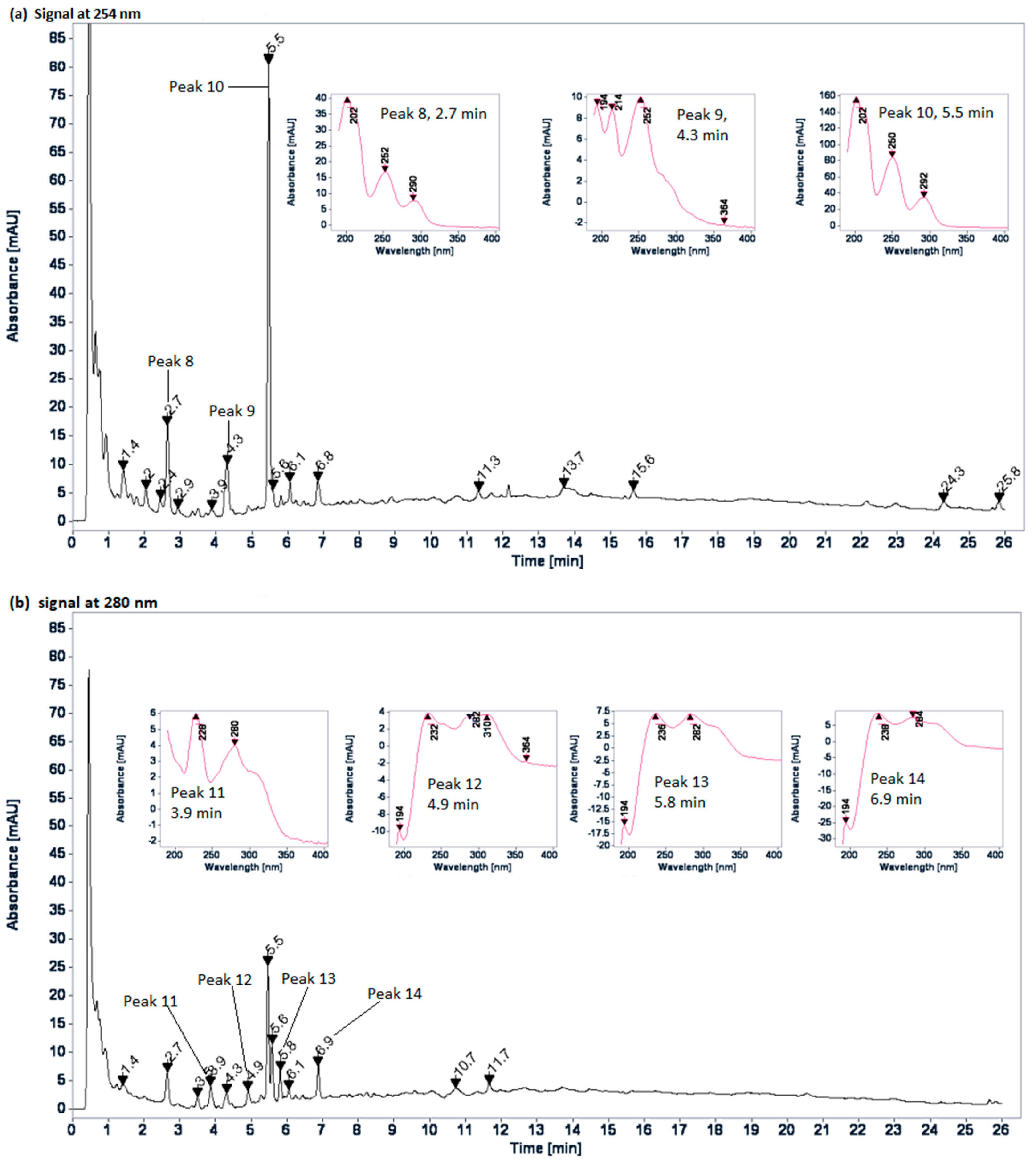
2.2. Chromatographic Profiles
2.3. Acute Oral Toxicity Tests
2.4. Hypoglycemic Effect of Plant Extracts
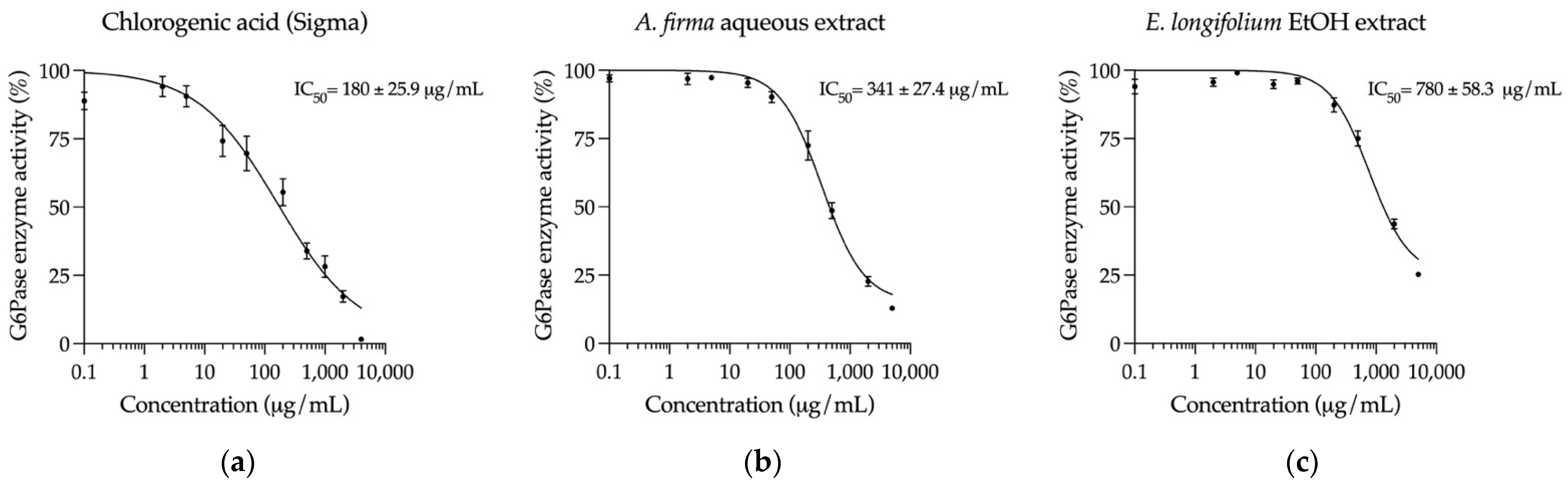
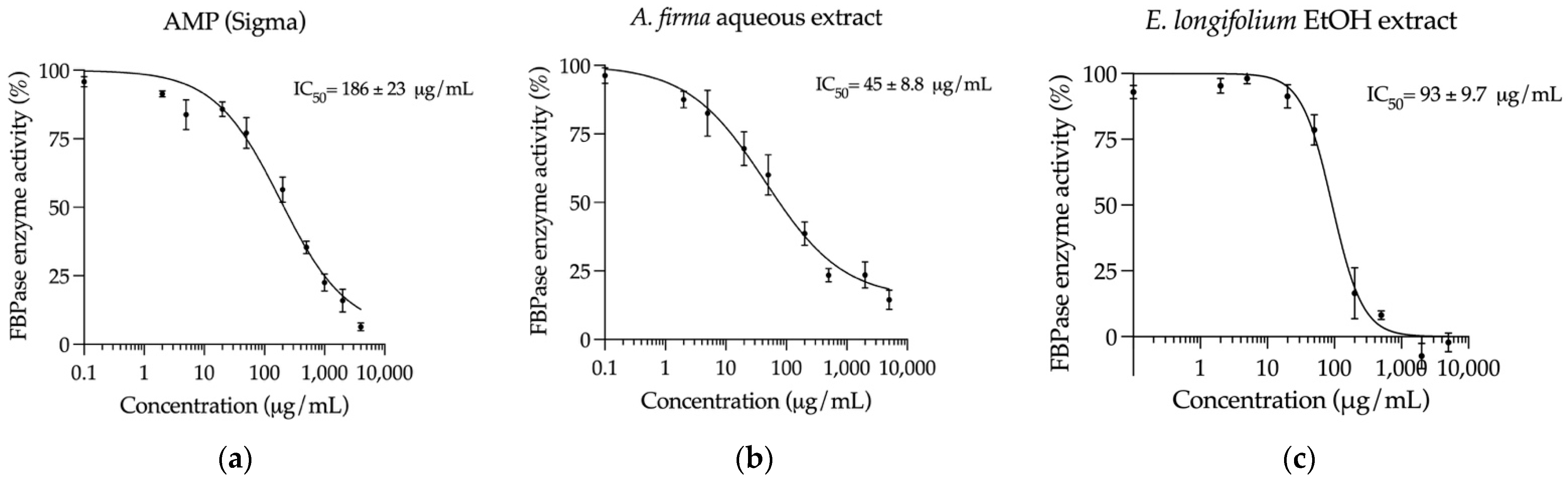
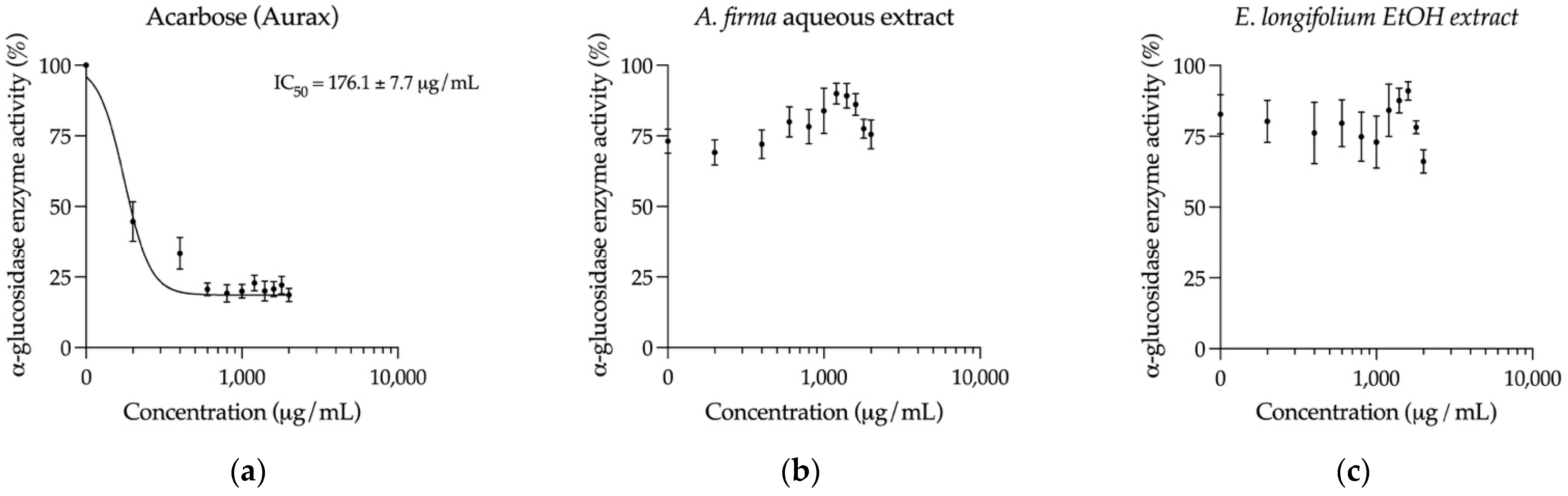
2.5. Inhibition of Key Hyperglycemia-Related Enzymes
3. Discussion
4. Materials and Methods
4.1. Ethnobotany
4.2. Elaboration of Traditional Extracts and Dose Calculation
4.3. Chemicals and Reagents
4.4. HPLC Analysis
4.5. Experimental Animals
4.6. Acute Oral Toxicity Tests
4.7. Induction of Hyperglycemia
4.8. Assessment of Hypoglycemic Effect
4.9. Glucose 6-phosphatase Inhibition Assay
4.10. Fructose 1,6-bisphosphatase Inhibition Assay
4.11. α-glucosidase Inhibition Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). WHO Traditional Medicine Strategy 2014–2023; World Health Organization (WHO): Geneva, Switzerland, 2013; pp. 1–76. [Google Scholar]
- Andrade-Cetto, A.; Becerra-Jiménez, J.; Martínez-Zurita, E.; Ortega-Larrocea, P.; Heinrich, M. Disease-Consensus Index as a tool of selecting potential hypoglycemic plants in Chikindzonot, Yucatán, México. J. Ethnopharmacol. 2006, 107, 199–204. [Google Scholar] [CrossRef]
- Andrade-Cetto, A. Ethnobotanical study of the medicinal plants from Tlanchinol, Hidalgo, México. J. Ethnopharmacol. 2009, 122, 163–171. [Google Scholar] [CrossRef]
- American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- International Diabetes Federation (IDF). IDF Diabetes Atlas; International Diabetes Federation (IDF): Brussels, Belgium, 2019; pp. 1–168. [Google Scholar]
- Rizza, R.A. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: Implications for therapy. Diabetes 2010, 59, 2697–2707. [Google Scholar] [CrossRef] [Green Version]
- Ghani, U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur. J. Med. Chem. 2015, 103, 133–162. [Google Scholar] [CrossRef]
- CONABIO. Capital Natural de México; Sarukhán, J., Koleff, P., Carabias, J., Soberón, J., Dirzo, R., Llorente-Bousquets, J., Halffter, G., González, R., March, I., Mohar, A., et al., Eds.; Comisión Nacional Para El Conocimiento Y Uso de la Biodiversidad: Mexico City, Mexico, 2017; ISBN 9786077607021. [Google Scholar]
- INEGI Lengua Indígena. Available online: https://www.inegi.org.mx/temas/lengua/ (accessed on 26 August 2021).
- CONEVAL Pobreza en México. Available online: https://www.coneval.org.mx/socialdeprivationorincomeMedicion/Paginas/PobrezaInicio.aspx (accessed on 29 August 2021).
- CONABIO Enciclo Vida. Available online: http://enciclovida.mx/especies/185075 (accessed on 3 September 2021).
- Palá, J. Contribución Al Conocimiento de Los Aceites Esenciales Del Género “Eryngium” L, En La Península Ibérica; Universidad Complutense De Madrid: Madrid, Spain, 2002. [Google Scholar]
- Uphof, J. Dictionary of Economic Plants; Weinheim, H. R. Engelmann (J. Cramer): New York, NY, USA, 1959. [Google Scholar]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970; pp. 165–167. ISBN 978-3-642-88460-3. [Google Scholar]
- Cádiz-Gurrea, M.d.l.L.; Fernández-Arroyo, S.; Joven, J.; Segura-Carretero, A. Comprehensive characterization by UHPLC-ESI-Q-TOF-MS from an Eryngium bourgatii extract and their antioxidant and anti-inflammatory activities. Food Res. Int. 2013, 50, 197–204. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Ownby, S.; Wang, P.; Yuan, W.; Zhang, W.; Scott Beasley, R. Phenolic compounds and rare polyhydroxylated triterpenoid saponins from Eryngium yuccifolium. Phytochemistry 2008, 69, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- OECD/OCDE (Testing Guidelines 425). OECD Guidelines for the Testing of Chemicals: Acute Oral Toxicity—Up and Down Procedure (UDP); OECD Publishing: Paris, France, 2008; Volume 27. [Google Scholar]
- Rojas Martínez, R.; Aguilar Salinas, C. Epidemiología de la diabetes mellitus en México. Rev. Fac. Med. UNAM 1994, 37, 15–28. [Google Scholar]
- Cruz, E.C.; Andrade-Cetto, A. Ethnopharmacological field study of the plants used to treat type 2 diabetes among the Cakchiquels in Guatemala. J. Ethnopharmacol. 2015, 159, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Serrano, V.; McLaren, B.; Carrasco, J.C.; Alday, J.G.; Fiallos, L.; Amigo, J.; Onaindia, M. Traditional ecological knowledge and medicinal plant diversity in Ecuadorian Amazon home gardens. Glob. Ecol. Conserv. 2019, 17, e00524. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Vasconcelos, T.H.C.; Alencar, A.A.; Batista, L.M.; Oliveira, R.A.G.; Guedes, D.N.; Falcão, H.d.S.; Moura, M.D.; Diniz, M.F.F.M.; Modesto-Filho, J. Plants and their active constituents from South, Central, and North America with hypoglycemic activity. Rev. Bras. Farmacogn. 2005, 15, 392–413. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Escandón-Rivera, S.M.; Mata, R.; Andrade-Cetto, A. Molecules isolated from Mexican hypoglycemic plants: A review. Molecules 2020, 25, 4145. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.D.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-Dependent and Insulin-Independent Mechanisms: An Integrative Approach. Nutrients 2021, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.P.; Tan, E.N.Y.; Lim, Q.Y.; Nafiah, M.A. Phyllanthus acidus (L.) Skeels: A review of its traditional uses, phytochemistry, and pharmacological properties. J. Ethnopharmacol. 2020, 253, 112610. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhai, Y.Y.; Xu, J.; Yao, W.F.; Cao, Y.D.; Cheng, F.F.; Bao, B.H.; Zhang, L. A review on traditional uses, phytochemistry and pharmacology of Eclipta prostrata (L.). J. Ethnopharmacol. 2019, 245, 112109. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Cruz, E.C.; Cabello-Hernández, C.A.; Cárdenas-Vázquez, R. Hypoglycemic Activity of Medicinal Plants Used among the Cakchiquels in Guatemala for the Treatment of Type 2 Diabetes. Evid.-Based Complement. Altern. Med. 2019, 2019, 2168603. [Google Scholar] [CrossRef]
- Silva-Rivas, R.; Bailon-Moscoso, N.; Cartuche, L.; Romero-Benavides, J.C. The antioxidant and hypoglycemic properties and phytochemical profile of Clusia latipes extracts. Pharmacogn. J. 2020, 12, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Gomes, J.H.d.S.; Mbiakop, U.C.; Oliveira, R.L.; Stehmann, J.R.; de Pádua, R.M.; Cortes, S.F.; Braga, F.C. Polyphenol-rich extract and fractions of Terminalia phaeocarpa Eichler possess hypoglycemic effect, reduce the release of cytokines, and inhibit lipase, α-glucosidase, and α-amilase enzymes. J. Ethnopharmacol. 2021, 271, 113847. [Google Scholar] [CrossRef]
- Szkudelski, T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp. Biol. Med. 2012, 237, 481–490. [Google Scholar] [CrossRef]
- Wang, P. Phytochemical Constituents and Pharmacological Activities of Eryngium L. (Apiaceae). Pharm. Crop. 2012, 3, 99–120. [Google Scholar] [CrossRef]
- Ayuso, M.; Pinela, J.; Dias, M.I.; Barros, L.; Ivanov, M.; Calhelha, R.C.; Soković, M.; Ramil-Rego, P.; Barreal, M.E.; Gallego, P.P.; et al. Phenolic composition and biological activities of the in vitro cultured endangered Eryngium viviparum J. Gay. Ind. Crop. Prod. 2020, 148, 112325. [Google Scholar] [CrossRef]
- Espinoza-Hernández, F.; Andrade-Cetto, A.; Escandón-Rivera, S.; Mata-Torres, G.; Mata, R. Contribution of fasting and postprandial glucose-lowering mechanisms to the acute hypoglycemic effect of traditionally used Eryngium cymosum F. Delaroche. J. Ethnopharmacol. 2021, 114339. [Google Scholar] [CrossRef]
- Saadeldeen, F.S.A.; Niu, Y.; Wang, H.; Zhou, L.; Meng, L.; Chen, S.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Liu, Z.; Kang, W. Natural products: Regulating glucose metabolism and improving insulin resistance. Food Sci. Hum. Wellness 2020, 9, 214–228. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Jayanthy, G.; Subramanian, S. Rosmarinic acid, a polyphenol, ameliorates hyperglycemia by regulating the key enzymes of carbohydrate metabolism in high fat diet—STZ induced experimental diabetes mellitus. Biomed. Prev. Nutr. 2014, 4, 431–437. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- National Research Council (US); Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Masiello, P.; Broca, C.; Gross, R.; Roye, M.; Manteghetti, M.; Hillarire-Buys, D.; Novelli, M.; Ribes, G.; Hillaire-buys, D.; Novelli, M.; et al. Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998, 47, 224–229. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Medina-Hernández, A.E. Hypoglycemic effect of Bromelia plumieri (E. Morren) L.B. Sm., leaves in STZ-NA-induced diabetic rats. Front. Pharmacol. 2013, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Arion, W.J. [7] Measurement of intactness of rat liver endoplasmic reticulum. In Methods in Enzymology; Fleischer, S., Fleischer, B., Abelson, J., Simon, M., Eds.; Academic Press: Cambridge, MA, USA, 1989; Volume 174, pp. 58–67. [Google Scholar]
- Andrade-Cetto, A.; Cárdenas-Vázquez, R. Gluconeogenesis inhibition and phytochemical composition of two Cecropia species. J. Ethnopharmacol. 2010, 130, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Tashima, Y.; Yoshimura, N. Control of rabbit liver fructose-1, 6-diphosphatase activity by magnesium ions. J. Biochem. 1975, 78, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Escandón-Rivera, S.; González-Andrade, M.; Bye, R.; Linares, E.; Navarrete, A.; Mata, R. α-Glucosidase inhibitors from Brickellia cavanillesii. J. Nat. Prod. 2012, 75, 968–974. [Google Scholar] [CrossRef] [PubMed]


| Group | Dose | 0 min | 60 min | 120 min | 180 min | |
|---|---|---|---|---|---|---|
| Normoglycemic Control | n/a | 120 ± 3 a | 118 ± 2 a | 116 ± 2 a | 119 ± 3 a | |
| Hyperglycemic Control | n/a | 192 ± 4 | 190 ± 5 b | 188 ± 6 b | 193 ± 5 b | |
| Hyperglycemic + Glibenclamide Control | 5 mg/kg | 187 ± 5 | 152 ± 6 a,* | 128 ± 3 a,* | 124 ± 3 a,* | |
| Hyperglycemic + A. firma Rhizome | Aqueous Extract | 16 mg/kg | 195 ± 4 | 157 ± 5 a,* | 136 ± 4 a,* | 132 ± 3 a,* |
| 160 mg/kg | 190 ± 3 | 159 ± 5 * | 150 ± 3 a,b,* | 140 ± 2 a,* | ||
| EtOH Extract | 37 mg/kg | 199 ± 5 | 166 ± 4 * | 149 ± 4 a,b,* | 147 ± 5 a,b,* | |
| 374 mg/kg | 205 ± 4 | 184 ± 5 b | 158 ± a,b,* | 153 ± 4 a,b,* | ||
| Hyperglycemic + E. longifolium Aerial Part | Aqueous Extract | 30 mg/kg | 197 ± 6 | 169 ± 7 * | 148 ± 4 a,b,* | 149 ± 4 a,,b* |
| 310 mg/kg | 204 ± 7 | 164 ± 7 a,* | 135 ± 4 a,* | 136 ± 5 a,* | ||
| EtOH Extract | 32 mg/kg | 186 ± 6 | 156 ± 5 a,* | 141 ± 3 a,* | 121 ± 3 a,* | |
| 318 mg/kg | 193 ± 5 | 152 ± 4 a,* | 141 ± 3 a,* | 132 ± 3 a,* | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade-Cetto, A.; Espinoza-Hernández, F.; Mata-Torres, G.; Escandón-Rivera, S. Hypoglycemic Effect of Two Mexican Medicinal Plants. Plants 2021, 10, 2060. https://doi.org/10.3390/plants10102060
Andrade-Cetto A, Espinoza-Hernández F, Mata-Torres G, Escandón-Rivera S. Hypoglycemic Effect of Two Mexican Medicinal Plants. Plants. 2021; 10(10):2060. https://doi.org/10.3390/plants10102060
Chicago/Turabian StyleAndrade-Cetto, Adolfo, Fernanda Espinoza-Hernández, Gerardo Mata-Torres, and Sonia Escandón-Rivera. 2021. "Hypoglycemic Effect of Two Mexican Medicinal Plants" Plants 10, no. 10: 2060. https://doi.org/10.3390/plants10102060








