Vescalagin from Pink Wax Apple (Syzygium samarangense (Blume) Merrill and Perry) Protects Pancreatic β-Cells against Methylglyoxal-Induced Inflammation in Rats
Abstract
:1. Introduction
2. Results
2.1. Diet Intake and Body Weight in Rats Orally Administered with MG
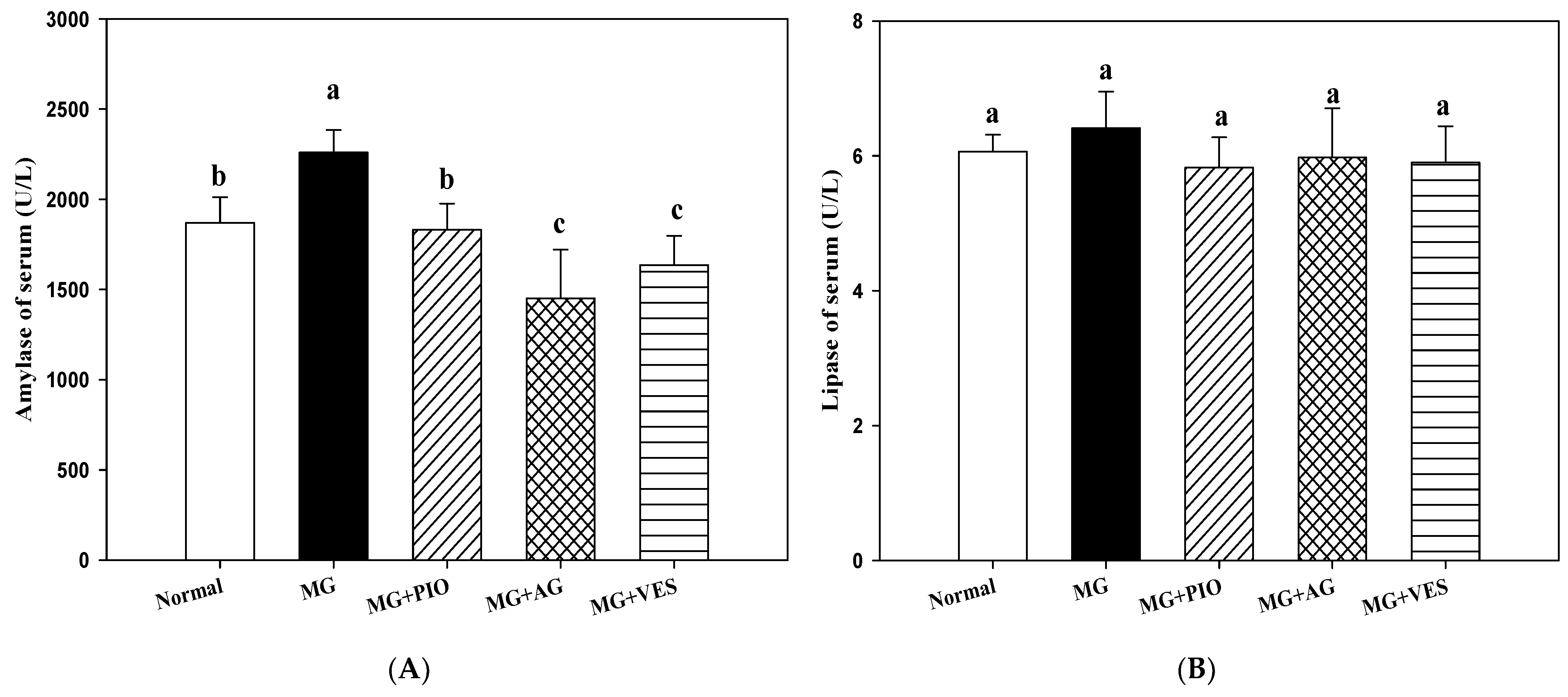
2.2. Amylase and Lipase Activities in the Rats
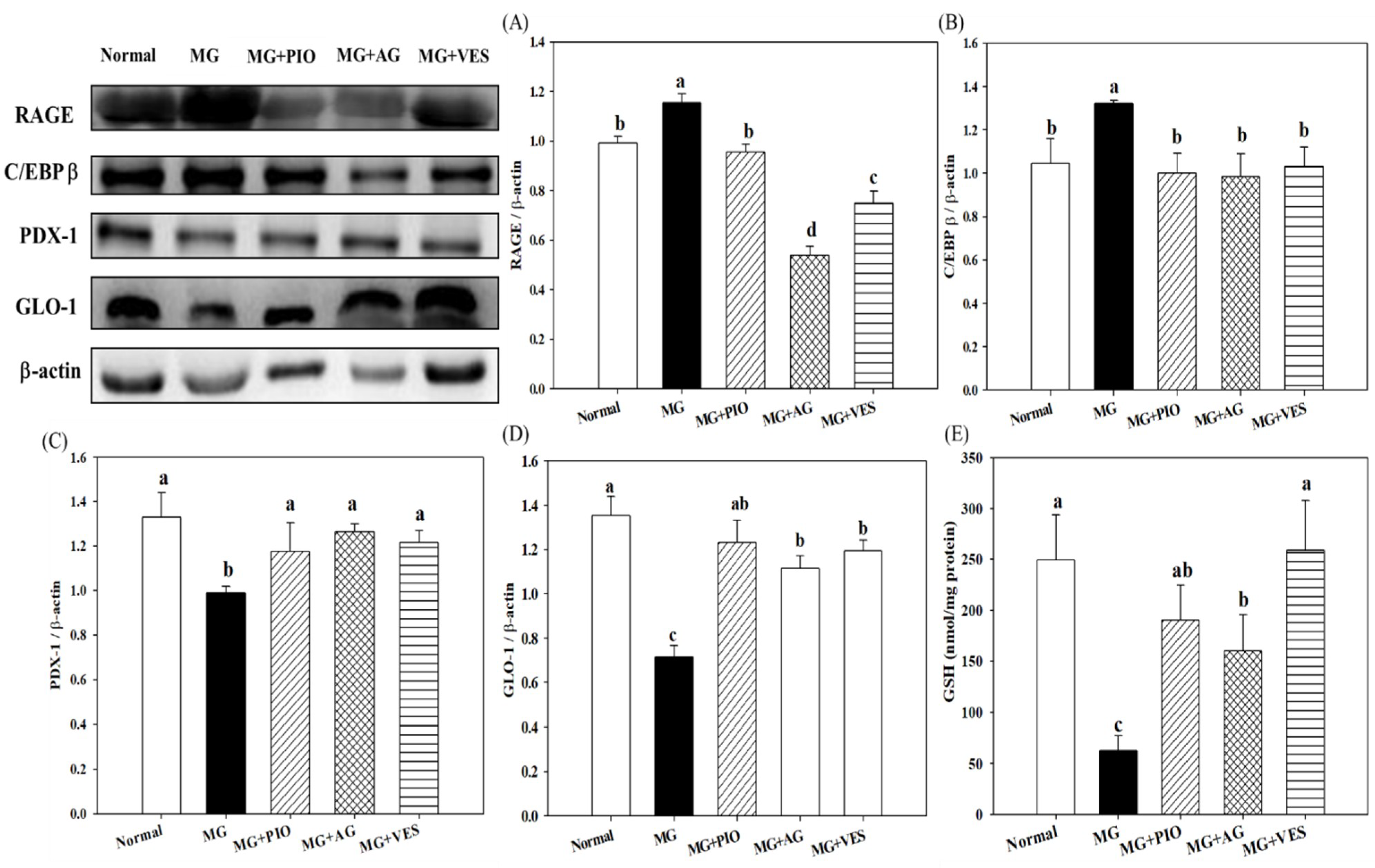
2.3. RAGE and Insulin Secretion-Related Protein Expression Levels in Pancreatic β-Cells of the Rats
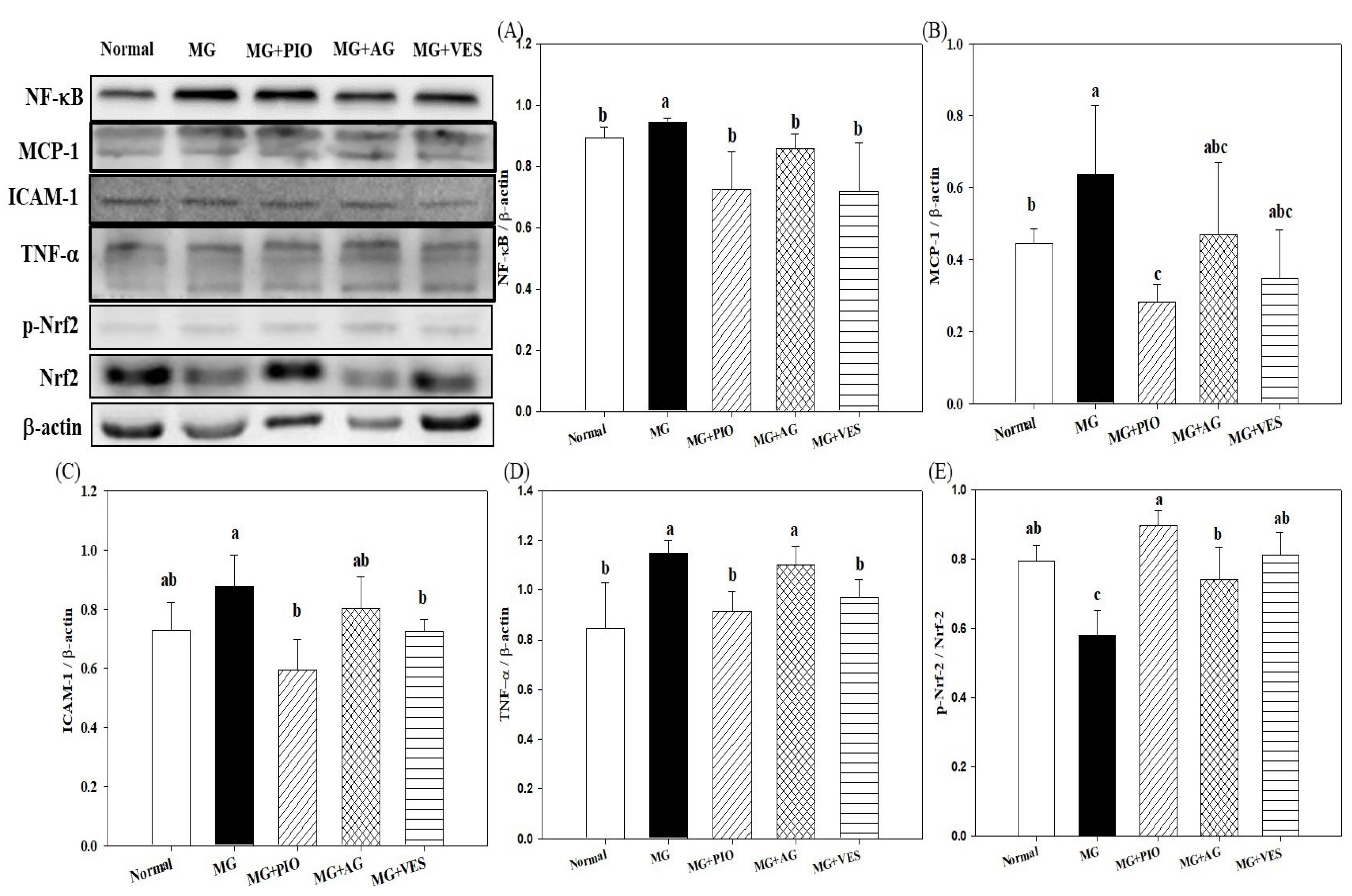
2.4. Expression Levels of Inflammation Proteins in Pancreatic β-Cells of the Rats
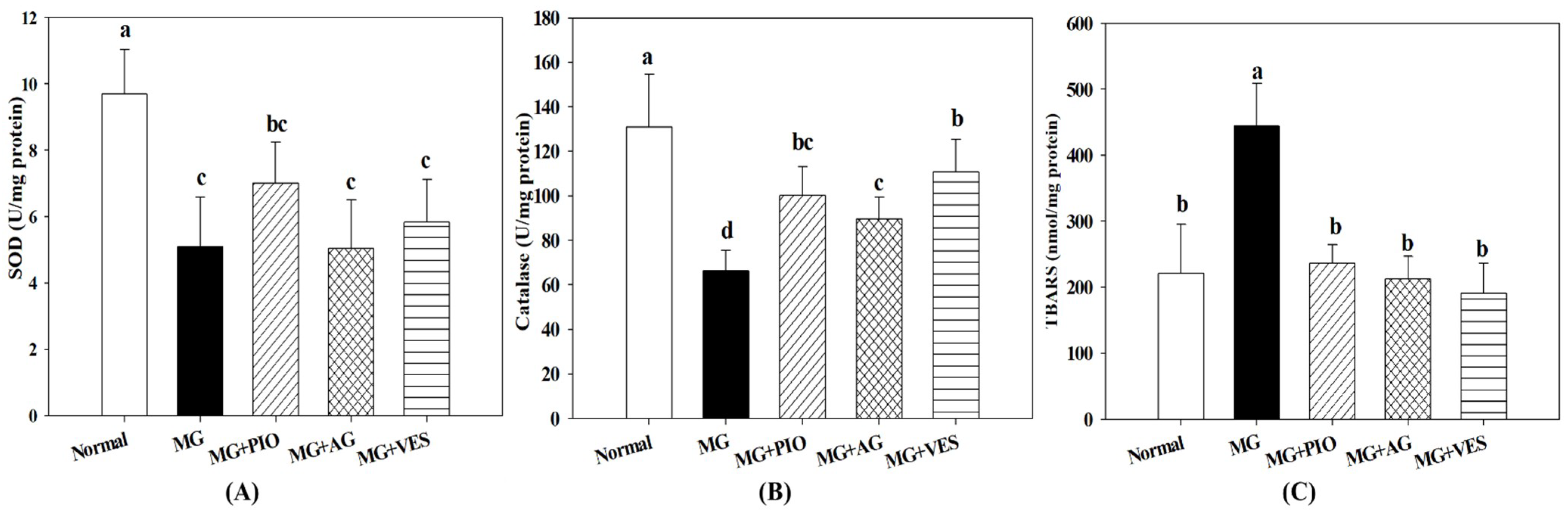
2.5. Activities of Antioxidant Enzymes in Pancreatic β-Cells of the Rats
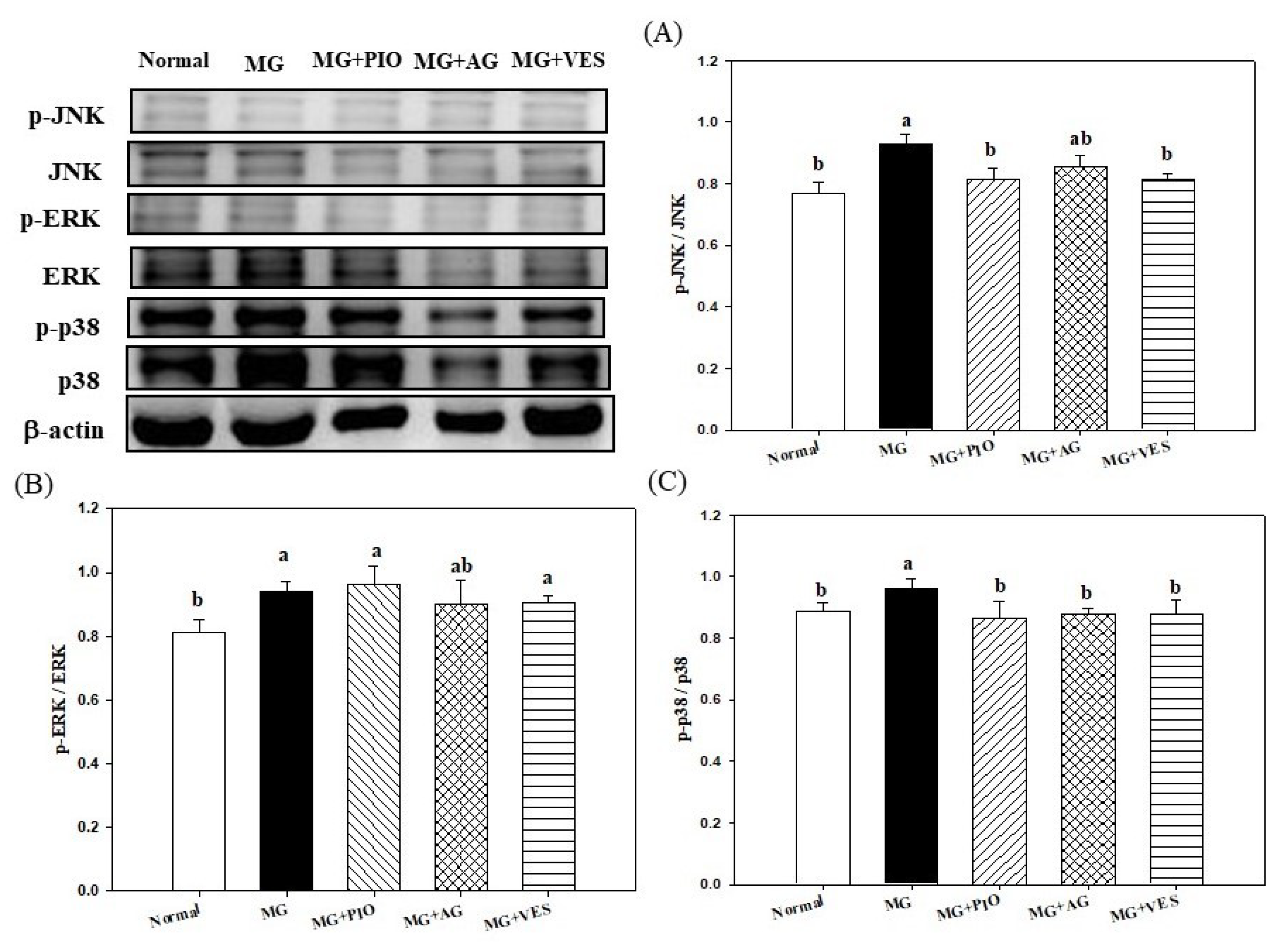
2.6. Mitogen-Activated Protein Kinases (MAPKs) and Inflammation in Pancreatic β-Cells of the Rats
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals and Diets
4.3. Blood and Pancrease Tissue Sample Collection
4.4. Biochemical Analyses
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, D.W.; Shen, S.C.; Wu, J.S.B. Effects of caffeic acid and cinnamic acid on glucose uptake in insulin-resistant mouse hepatocytes. J. Agric. Food Chem. 2009, 57, 7687–7692. [Google Scholar] [CrossRef]
- Nemet, I.; Varga-Defterdarovic, L.; Turk, Z. Methylglyoxal in food and living organisms. Mol. Nutr. Food Res. 2006, 50, 1105–1117. [Google Scholar] [CrossRef]
- Lin, S.; Yang, Z.; Liu, H.; Tang, L.; Cai, Z. Beyond glucose: Metabolic shifts in responses to the effects of oral glucose tolerance test and the high-fructose diet in rats. Mol. BioSyst. 2011, 7, 1537–1548. [Google Scholar] [CrossRef]
- Matafome, P.; Sena, C.; Seiça, R. Methylglyoxal, obesity, and diabetes. Endocrine 2013, 43, 472–484. [Google Scholar] [CrossRef]
- Dhar, A.; Dhar, I.; Jiang, B.; Desai, K.M.; Wu, L. Chronic methylglyoxal infusion by minipump causes pancreatic β-cell dysfunction and induces type 2 diabetes in sprague-dawley rats. Diabetes 2011, 60, 899–908. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Hsu, W.H.; Chang, Y.Y.; Kuo, H.F.; Hsu, Y.W.; Pan, T.M. Ankaflavin: A natural novel PPARγ agonist upregulates Nrf2 to attenuate methylglyoxal-induced diabetes in vivo. Free Radic. Biol. Med. 2012, 53, 2008–2016. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.; Schmidt, A. Receptor for AGE (RAGE): Signaling mechanisms in the pathogenesis of diabetes and its complications. Ann. N. Y. Acad. Sci. 2011, 1243, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Novotny, G.W.; Lundh, M.; Backe, M.B.; Christensen, D.P.; Hansen, J.B.; Dahllof, M.S.; Pallesen, E.M.H.; Mandrup-Poulsen, T. Transcriptional and translational regulation of cytokine signaling in inflammatory β-cell dysfunction and apoptosis. Arch. Biochem. Biophys. 2012, 528, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Fiory, F.; Lombardi, A.; Miele, C.; Giudicelli, J.; Beguinot, F.; Van Obberghen, E. Methylglyoxal impairs insulin signalling and insulin action on glucose-induced insulin secretion in the pancreatic beta cell line INS-1E. Diabetologia 2011, 54, 2941–2952. [Google Scholar] [CrossRef] [Green Version]
- Dhar, A.; Desai, K.M.; Wu, L. Alagebrium attenuates acute methylglyoxal-induced glucose intolerance in sprague-dawley rats. Brit. J. Pharmacol. 2010, 159, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Revel, G.; Bertrand, A. A method for the detection of carbonyl compounds in wine: Glyoxal and methylglyoxal. J. Sci. Food Agric. 1993, 61, 267–272. [Google Scholar] [CrossRef]
- Barros, A.; Rodrigues, J.A.; Almeida, P.J.; Oliva-Teles, M.T. Determination of glyoxal, methylglyoxal and diacetyl in selected beer and wine by HPLC with UV spectrophotometric detection after derivatization with o-phenylendiamine. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 2061–2069. [Google Scholar] [CrossRef]
- Desai, K.; Wu, L. Methylglyoxal and advanced glycation endproducts: New therapeutic horizons? Recent Pat. Cardiovasc. Drug Discov. 2007, 2, 89–99. [Google Scholar] [CrossRef]
- Vander Jagt, D.L.; Hunsaker, L.A. Methylglyoxal metabolism and diabetic complications: Roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem. Biol. Interact. 2003, 143, 341–351. [Google Scholar] [CrossRef]
- Lee, B.H.; Hsu, W.H.; Hsu, Y.W.; Pan, T.M. Dimerumic acid protects pancreas damage and elevates insulin production in methylglyoxal-treated pancreatic RINm5F cells. J. Funct. Foods 2013, 5, 642–650. [Google Scholar] [CrossRef]
- Heber, D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008, 269, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Fridrich, D.; Glabasnia, A.; Fritz, J.; Esselen, M.; Pahlke, G.; Hofmann, T.; Marko, D. Oak ellagitannins suppress the phosphorylation of the epidermal growth factor receptor in human colon carcinoma cells. J. Agric. Food Chem. 2008, 56, 3010–3015. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.C.; Chang, W.C. Hypotriglyceridemic and hypoglycemic effects of vescalagin from Pink wax apple [Syzygium samarangense (Blume) Merrill and Perry cv. Pink] in high-fructose diet-induced diabetic rats. Food Chem. 2013, 136, 858–863. [Google Scholar] [CrossRef]
- Chang, W.C.; Shen, S.C.; Wu, J.S.B. Protective effects of vescalagin from pink wax apple [Syzygium samarangense (Blume) Merrill and Perry] fruit against methylglyoxal-induced inflammation and carbohydrate metabolic disorder in rats. J. Agric. Food Chem. 2013, 61, 7102–7109. [Google Scholar] [CrossRef]
- Koyu, A.; Gokalp, O.; Gumral, N.; Oktem, F.; Karahan, N.; Yilmaz, N.; Saygin, M. Impact of caffeic acid phenethyl ester treatment on vancomycin-induced pancreatic damage in rats. Toxicol. Ind. Health 2013, 32, 306–312. [Google Scholar] [CrossRef]
- Malloy, J.; Gurney, K.; Shan, K.; Yan, P.; Chen, S. Increased variability and abnormalities in pancreatic enzyme concentrations in otherwise asymptomatic subjects with type 2 diabetes. Diabetes Metab. Syndr. Obes. 2012, 5, 419–424. [Google Scholar]
- Liu, S.; Li, D.; Huang, B.; Chen, Y.; Lu, X.; Wang, Y. Inhibition of pancreatic lipase, α-glucosidase, α-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J. Ethnopharmacol. 2013, 149, 263–269. [Google Scholar] [CrossRef]
- Mnafgui, K.; Kchaou, M.; Hamden, K.; Derbali, F.; Slama, S.; Nasri, M.; Salah, H.B.; Allouche, N.; Elfeki, A. Inhibition of carbohydrate and lipid digestive enzymes activities by Zygophyllum album extracts: Effect on blood and pancreas inflammatory biomarkers in alloxan-induced diabetic rats. J. Physiol. Biochem. 2014, 70, 93–106. [Google Scholar] [CrossRef]
- McDougall, G.J.; Stewart, D. The inhibitory effects of berry polyphenols on digestive enzymes. BioFactors 2005, 23, 189–195. [Google Scholar] [CrossRef]
- Irwin, N.; McKinney, J.M.; Bailey, C.J.; McClenaghan, N.H.; Flatt, P.R. Acute and long-term effects of peroxisome proliferator-activated receptor-γ activation on the function and insulin secretory responsiveness of clonal beta-cells. Horm. Metab. Res. 2011, 43, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.H.; Zuo, D.M. Aminoguanidine inhibits semicarbazide- sensitive amine oxidase activity; implication for advanced glycation and angiopathy in diabetes. Diabetologia 1997, 363, 1243–1250. [Google Scholar] [CrossRef]
- Yildiz, G.; Demiryürek, A.T.; Sahin-Erdemli, I.; Kanzik, I. Comparison of antioxidant activities of aminoguanidine, methylguanidine and guanidine by luminol-enhanced chemiluminescence. Birt. J. Pharmacol. 1998, 124, 905–910. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, glyoxalase 1 and dicarbonyl proteome. Amino Acids 2012, 42, 1133–1142. [Google Scholar] [CrossRef]
- More, S.S.; Vartak, A.P.; Vince, R. Restoration of glyoxalase enzyme activity precludes cognitive dysfunction in a mouse model of Alzheimer’s disease. ACS Chem. Neurosci. 2013, 4, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack, M.; Ryals, J.; Wright, D. Protection from diabetes-induced peripheral sensory neuropathy-a role for elevated glyoxalase I? Exp. Neurol. 2012, 234, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chartoumpekis, D.V.; Ziors, P.G.; Psyrogiannis, A.I.; Papavassiliou, A.G.; Kyriazopoulou, V.E.; Sykiotis, G.P.; Habeos, I.G. Nrf2 represses FGF21 during long-term high-fat diet-induced obesity in mice. Diabetes 2011, 60, 2465–2473. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.W.; Li, D.; Ling, W.H.; Jin, T.R. Role of nuclear factor (erythroid-derived 2)-like 2 in metabolic homeostasis and insulin action: A novel opportunity for diabetes treatment? World J. Diabetes 2012, 3, 19–28. [Google Scholar] [CrossRef]
- Pi, J.; Zhang, Q.; Fu, J.; Woods, C.G.; Hou, Y.; Corkey, B.E.; Collins, S.; Andersen, M.E. ROS signaling oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol. Appl. Pharm. 2010, 244, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gu, C.; He, W.; Ye, X.; Chen, H.; Zhang, X.; Hai, C. Glucose oxidase induces insulin resistance via influencing multiple targets in vitro and in vivo: The central role of oxidative stress. Biochimie 2012, 94, 1705–1717. [Google Scholar] [CrossRef]
- Nain, P.; Saini, V.; Sharma, S.; Nain, J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J. Ethnopharmacol. 2012, 142, 65–71. [Google Scholar] [CrossRef]
- Duh, P.D.; Lin, S.L.; Wu, S.C. Hepatoprotection of Graptopetalum paraguayense E. Walther on CCl4-induced liver damage and inflammation. J. Ethnopharmacol. 2011, 134, 379–385. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Chang, W.C.; Shen, S.C. Effect of water extracts from edible Myrtaceae plants on uptake of 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) in tumor necrosis factor-α-treated FL83B mouse hepatocytes. Phytother. Res. 2013, 27, 236–243. [Google Scholar] [CrossRef]
| Items/Groups | Normal | MG | MG+PIO | MG+AG | MG+VES |
|---|---|---|---|---|---|
| Diet intake (g/rat/day) | 28.93 ± 5.66 a | 29.60 ± 5.42 a | 30.48 ± 5.41 a | 27.60 ± 5.76 a | 28.99 ± 4.84 a |
| Drink intake (mL/rat/day) | 42.40 ± 3.29 a | 44.23 ± 3.67 a | 49.67 ± 5.09 a | 43.42 ± 4.76 a | 46.63 ± 4.78 a |
| Body weight (g) | 416.11 ± 9.58 a | 444.50 ± 19.0 a | 420.64 ± 17.6 a | 411.01 ± 36.3 a | 424.37 ± 21.6 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-C.; Wu, J.S.-B.; Shen, S.-C. Vescalagin from Pink Wax Apple (Syzygium samarangense (Blume) Merrill and Perry) Protects Pancreatic β-Cells against Methylglyoxal-Induced Inflammation in Rats. Plants 2021, 10, 1448. https://doi.org/10.3390/plants10071448
Chang W-C, Wu JS-B, Shen S-C. Vescalagin from Pink Wax Apple (Syzygium samarangense (Blume) Merrill and Perry) Protects Pancreatic β-Cells against Methylglyoxal-Induced Inflammation in Rats. Plants. 2021; 10(7):1448. https://doi.org/10.3390/plants10071448
Chicago/Turabian StyleChang, Wen-Chang, James Swi-Bea Wu, and Szu-Chuan Shen. 2021. "Vescalagin from Pink Wax Apple (Syzygium samarangense (Blume) Merrill and Perry) Protects Pancreatic β-Cells against Methylglyoxal-Induced Inflammation in Rats" Plants 10, no. 7: 1448. https://doi.org/10.3390/plants10071448
APA StyleChang, W.-C., Wu, J. S.-B., & Shen, S.-C. (2021). Vescalagin from Pink Wax Apple (Syzygium samarangense (Blume) Merrill and Perry) Protects Pancreatic β-Cells against Methylglyoxal-Induced Inflammation in Rats. Plants, 10(7), 1448. https://doi.org/10.3390/plants10071448






