The Arabidopsis Rho of Plants GTPase ROP1 Is a Potential Calcium-Dependent Protein Kinase (CDPK) Substrate
Abstract
1. Introduction
2. Results
2.1. The Arabidopsis ROP1 GTPase Does Not Serve as In Vitro Substrate of the AGC 1.7 Kinase
2.2. The Arabidopsis Calcium-Dependent Protein Kinases CPK17 and CPK34 Can In Vitro Phosphorylate the ROP1 GTPase Dependent on Its Conformation but Not at the S74 Residue
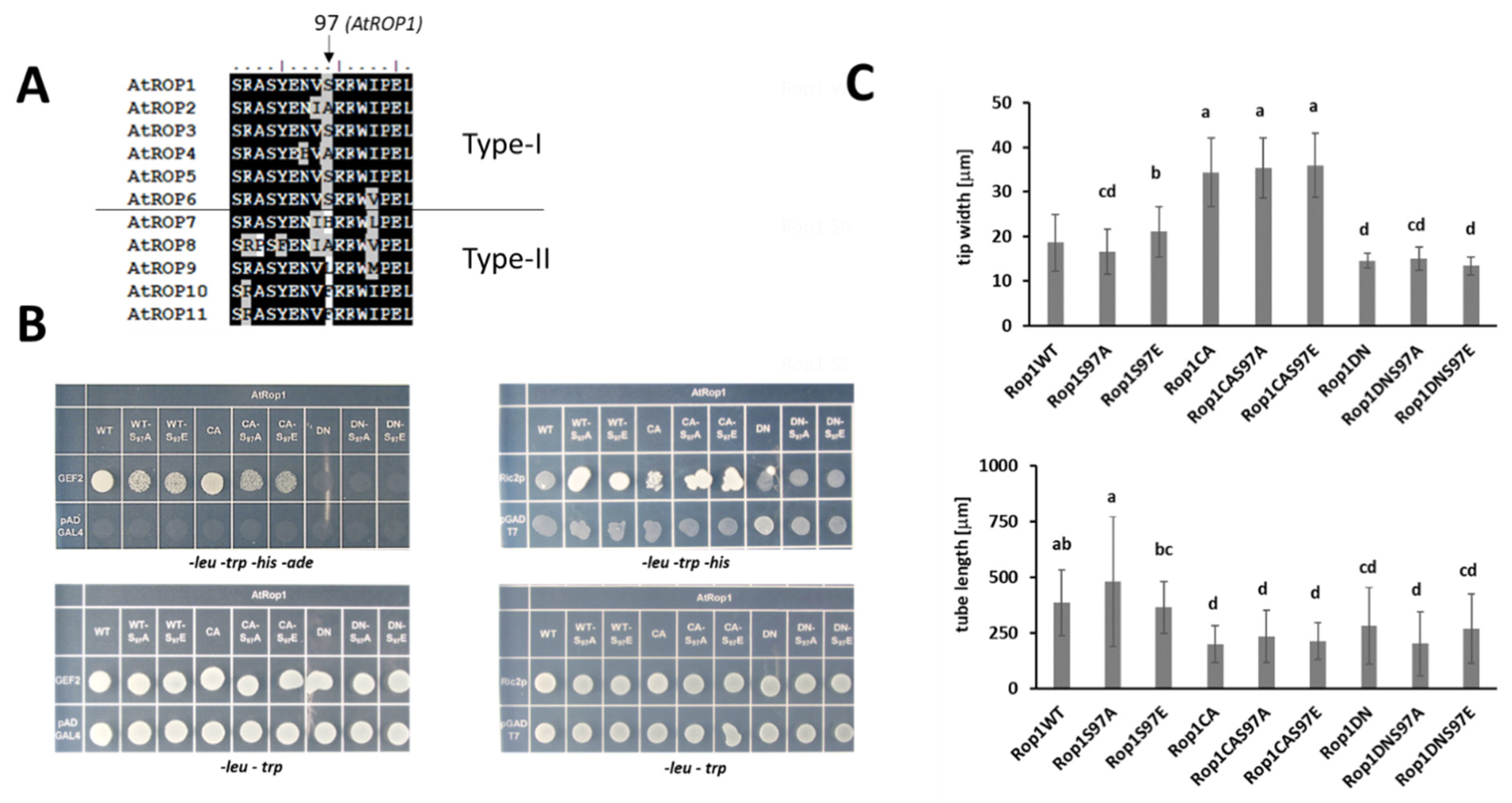
2.3. CPKs Phosphorylate the S97 Residue of AtROP1 but the S97E Phosphomimic Mutation Has No Significant Effect on the Function of It
2.4. The CPK17/34 Kinases Can Phosphorylate the ROP1 Protein at Several Sites In Vitro
3. Discussion
4. Materials and Methods
4.1. Molecular Cloning
4.2. Protein Purification, In Vitro Kinase Assay
4.3. Mass Spectrometry
4.4. Protein-Protein Interaction
4.5. Pollen Transformation and Microscopy
4.6. Accession Numbers
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and Biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Macara, I.G.; Lounsbury, K.M.; Richards, S.A.; McKiernan, C.; Bar-Sagi, D. The Ras Superfamily of GTPases. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1996, 10, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Bourne, H.R.; Sanders, D.A.; McCormick, F. The GTPase Superfamily: A Conserved Switch for Diverse Cell Functions. Nature 1990, 348, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.L.; Hall, A. Rho GTPases and Their Effector Proteins. Biochem. J. 2000, 348 Pt 2, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.; Wittinghofer, A. GEFs, GAPs, GDIs and Effectors: Taking a Closer (3D) Look at the Regulation of Ras-Related GTP-Binding Proteins. Curr. Opin. Struct. Biol. 1997, 7, 786–792. [Google Scholar] [CrossRef]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and Their Regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Boureux, A.; Vignal, E.; Faure, S.; Fort, P. Evolution of the Rho Family of Ras-like GTPases in Eukaryotes. Mol. Biol. Evol. 2007, 24, 203–216. [Google Scholar] [CrossRef]
- Wherlock, M.; Mellor, H. The Rho GTPase Family: A Racs to Wrchs Story. J. Cell Sci. 2002, 115, 239–240. [Google Scholar] [CrossRef]
- Wennerberg, K.; Der, C.J. Rho-Family GTPases: It’s Not Only Rac and Rho (and I like It). J. Cell Sci. 2004, 117, 1301–1312. [Google Scholar] [CrossRef]
- Brembu, T.; Winge, P.; Bones, A.M.; Yang, Z. A RHOse by Any Other Name: A Comparative Analysis of Animal and Plant Rho GTPases. Cell Res. 2006, 16, 435–445. [Google Scholar] [CrossRef]
- Berken, A.; Wittinghofer, A. Structure and Function of Rho-Type Molecular Switches in Plants. Plant Physiol. Biochem. PPB/Soc. Fr. Physiol. Vég. 2008, 46, 380–393. [Google Scholar] [CrossRef]
- Fehér, A.; Lajkó, D.B. Signals Fly When Kinases Meet Rho-of-Plants (ROP) Small G-Proteins. Plant Sci. 2015, 237, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Feiguelman, G.; Fu, Y.; Yalovsky, S. ROP GTPases Structure-Function and Signaling Pathways. Plant Physiol. 2018, 176, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Berken, A. ROPs in the Spotlight of Plant Signal Transduction. Cell. Mol. Life Sci. CMLS 2006, 63, 2446–2459. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, H.; Yang, Z. Arabidopsis RopGAPs Are a Novel Family of Rho GTPase-Activating Proteins That Require the Cdc42/Rac- Interactive Binding Motif for Rop-Specific GTPase Stimulation. Plant Physiol. 2000, 124, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Berken, A.; Thomas, C.; Wittinghofer, A. A New Family of RhoGEFs Activates the Rop Molecular Switch in Plants. Nature 2005, 436, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Fricke, I.; Berken, A. Molecular Basis for the Substrate Specificity of Plant Guanine Nucleotide Exchange Factors for ROP. FEBS Lett. 2009, 583, 75–80. [Google Scholar] [CrossRef]
- Stouten, P.F.W.; Sander, C.; Wittinghofer, A.; Valencia, A. How Does the Switch II Region of G-Domains Work? FEBS Lett. 1993, 320, 1–6. [Google Scholar] [CrossRef]
- Roberts, P.J.; Mitin, N.; Keller, P.J.; Chenette, E.J.; Madigan, J.P.; Currin, R.O.; Cox, A.D.; Wilson, O.; Kirschmeier, P.; Der, C.J. Rho Family GTPase Modification and Dependence on CAAX Motif-Signaled Posttranslational Modification. J. Biol. Chem. 2008, 283, 25150–25163. [Google Scholar] [CrossRef]
- Alan, J.K.; Berzat, A.C.; Dewar, B.J.; Graves, L.M.; Cox, A.D. Regulation of the Rho Family Small GTPase Wrch-1/RhoU by C-Terminal Tyrosine Phosphorylation Requires Src. Mol. Cell Biol. 2010, 30, 4324–4338. [Google Scholar] [CrossRef]
- Tong, J.; Li, L.; Ballermann, B.; Wang, Z. Phosphorylation of Rac1 T108 by Extracellular Signal-Regulated Kinase in Response to Epidermal Growth Factor: A Novel Mechanism To Regulate Rac1 Function. Mol. Cell Biol. 2013, 33, 4538–4551. [Google Scholar] [CrossRef]
- Chang, F.; Lemmon, C.; Lietha, D.; Eck, M.; Romer, L. Tyrosine Phosphorylation of Rac1: A Role in Regulation of Cell Spreading. PLoS ONE 2011, 6, e28587. [Google Scholar] [CrossRef]
- Kwon, T.; Kwon, D.Y.; Chun, J.; Kim, J.H.; Kang, S.S. Akt Protein Kinase Inhibits Rac1-GTP Binding through Phosphorylation at Serine 71 of Rac1. J. Biol. Chem. 2000, 275, 423–428. [Google Scholar] [CrossRef]
- Nusser, N.; Gosmanova, E.; Makarova, N.; Fujiwara, Y.; Yang, L.; Guo, F.; Luo, Y.; Zheng, Y.; Tigyi, G. Serine Phosphorylation Differentially Affects RhoA Binding to Effectors: Implications to NGF-Induced Neurite Outgrowth. Cell. Signal. 2006, 18, 704–714. [Google Scholar] [CrossRef]
- Forget, M.-A.; Desrosiers, R.R.; Gingras, D.; Béliveau, R. Phosphorylation States of Cdc42 and RhoA Regulate Their Interactions with Rho GDP Dissociation Inhibitor and Their Extraction from Biological Membranes. Biochem. J. 2002, 361, 243–254. [Google Scholar] [CrossRef]
- Zhao, J.; Mialki, R.K.; Wei, J.; Coon, T.A.; Zou, C.; Chen, B.B.; Mallampalli, R.K.; Zhao, Y. SCF E3 Ligase F-Box Protein Complex SCFFBXL19 Regulates Cell Migration by Mediating Rac1 Ubiquitination and Degradation. FASEB J. 2013, 27, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Abdrabou, A.; Brandwein, D.; Liu, C.; Wang, Z. Rac1 S71 Mediates the Interaction between Rac1 and 14-3-3 Proteins. Cells 2019, 8, 1006. [Google Scholar] [CrossRef] [PubMed]
- Fodor-Dunai, C.; Fricke, I.; Potocký, M.; Dorjgotov, D.; Domoki, M.; Jurca, M.E.; Ötvös, K.; Žárský, V.; Berken, A.; Fehér, A. The Phosphomimetic Mutation of an Evolutionarily Conserved Serine Residue Affects the Signaling Properties of Rho of Plants (ROPs): Signaling Properties of Phospho-ROPs. Plant J. 2011, 66, 669–679. [Google Scholar] [CrossRef]
- Weiß, L.; Reiner, T.; Mergner, J.; Kuster, B.; Fehér, A.; Hensel, G.; Gahrtz, M.; Kumlehn, J.; Engelhardt, S.; Hückelhoven, R. Posttranslational Modification of the RHO of Plants Protein RACB by Phosphorylation and Cross-Kingdom Conserved Ubiquitination. bioRxiv 2020. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Li, H.; Lin, Y.; Heath, R.M.; Zhu, M.X.; Yang, Z. Control of Pollen Tube Tip Growth by a Rop GTPase–Dependent Pathway That Leads to Tip-Localized Calcium Influx. Plant Cell 1999, 11, 1731–1742. [Google Scholar]
- Zhang, Y.; He, J.; McCormick, S. Two Arabidopsis AGC Kinases Are Critical for the Polarized Growth of Pollen Tubes. Plant J. 2009, 58, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Bögre, L.; Ökrész, L.; Henriques, R.; Anthony, R.G. Growth Signalling Pathways in Arabidopsis and the AGC Protein Kinases. Trends Plant Sci. 2003, 8, 424–431. [Google Scholar] [CrossRef]
- Devarenne, T.P.; Ekengren, S.K.; Pedley, K.F.; Martin, G.B. Adi3 Is a Pdk1-Interacting AGC Kinase That Negatively Regulates Plant Cell Death. EMBO J. 2006, 25, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, E.H.; Offringa, R. Evolutionary Adaptations of Plant AGC Kinases: From Light Signaling to Cell Polarity Regulation. Front. Plant Sci. 2012, 3, 250. [Google Scholar] [CrossRef]
- Zegzouti, H. Structural and Functional Insights into the Regulation of Arabidopsis AGC VIIIa Kinases. J. Biol. Chem. 2006, 281, 11. [Google Scholar] [CrossRef]
- Vlad, F.; Turk, B.E.; Peynot, P.; Leung, J.; Merlot, S. A Versatile Strategy to Define the Phosphorylation Preferences of Plant Protein Kinases and Screen for Putative Substrates. Plant J. 2008, 55, 104–117. [Google Scholar] [CrossRef]
- Myers, C.; Romanowsky, S.M.; Barron, Y.D.; Garg, S.; Azuse, C.L.; Curran, A.; Davis, R.M.; Hatton, J.; Harmon, A.C.; Harper, J.F. Calcium-Dependent Protein Kinases Regulate Polarized Tip Growth in Pollen Tubes. Plant J. 2009, 59, 528–539. [Google Scholar] [CrossRef]
- Yan, A.; Xu, G.; Yang, Z.-B. Calcium Participates in Feedback Regulation of the Oscillating ROP1 Rho GTPase in Pollen Tubes. Proc. Natl. Acad. Sci. USA 2009, 106, 22002–22007. [Google Scholar] [CrossRef]
- Fehér, A.; Jurca, M.E.; Fodor-Dunai, C.; Dorjgotov, D. Regulation of ROP GTPase Signalling at the Gene Expression Level: A Review. Open Plant Sci. J. 2008, 2, 37–46. [Google Scholar] [CrossRef][Green Version]
- Poraty-Gavra, L.; Zimmermann, P.; Haigis, S.; Bednarek, P.; Hazak, O.; Stelmakh, O.R.; Sadot, E.; Schulze-Lefert, P.; Gruissem, W.; Yalovsky, S. The Arabidopsis Rho of Plants GTPase AtROP6 Functions in Developmental and Pathogen Response Pathways1[C][W][OA]. Plant Physiol. 2013, 161, 1172–1188. [Google Scholar] [CrossRef] [PubMed]
- Van Aelst, L.; D’Souza-Schorey, C. Rho GTPases and Signaling Networks. Genes Dev. 1997, 11, 2295–2322. [Google Scholar] [CrossRef]
- Zhao, Z.; Manser, E. PAK and Other Rho-Associated Kinases–Effectors with Surprisingly Diverse Mechanisms of Regulation. Biochem. J. 2005, 386, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Dorjgotov, D.; Jurca, M.E.; Fodor-Dunai, C.; Szűcs, A.; Ötvös, K.; Klement, É.; Bíró, J.; Fehér, A. Plant Rho-Type (Rop) GTPase-Dependent Activation of Receptor-like Cytoplasmic Kinases in Vitro. FEBS Lett. 2009, 583, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Huesmann, C.; Reiner, T.; Hoefle, C.; Preuss, J.; Jurca, M.E.; Domoki, M.; Fehér, A.; Hückelhoven, R. Barley ROP Binding Kinase1 Is Involved in Microtubule Organization and in Basal Penetration Resistance to the Barley Powdery Mildew Fungus. Plant Physiol. 2012, 159, 311–320. [Google Scholar] [CrossRef]
- Lajkó, D.B.; Valkai, I.; Domoki, M.; Ménesi, D.; Ferenc, G.; Ayaydin, F.; Fehér, A. In Silico Identification and Experimental Validation of Amino Acid Motifs Required for the Rho-of-Plants GTPase-Mediated Activation of Receptor-like Cytoplasmic Kinases. Plant Cell Rep. 2018, 37, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Reiner, T.; Hoefle, C.; Huesmann, C.; Ménesi, D.; Fehér, A.; Hückelhoven, R. The Arabidopsis ROP-Activated Receptor-like Cytoplasmic Kinase RLCK VI_A3 Is Involved in Control of Basal Resistance to Powdery Mildew and Trichome Branching. Plant Cell Rep. 2015, 34, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Aggarwal, M.; Zheng, W.-G.; Wu, H.-M.; Cheung, A.Y. Receptor-like Kinases as Surface Regulators for RAC/ROP-Mediated Pollen Tube Growth and Interaction with the Pistil. AoB Plants 2011, 2011, plr017. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Cui, Y.; Ge, F.-R.; Chai, S.; Zhang, W.-T.; Feng, Q.-N.; Jiang, L.; Li, S.; Zhang, Y. AGC1. 5 Kinase Phosphorylates RopGEFs to Control Pollen Tube Growth. Mol. Plant 2018, 11, 1198–1209. [Google Scholar] [CrossRef]
- Jamin, A.; Yang, Z. Interactions between Calcium and ROP Signaling Regulate Pollen Tube Tip Growth. In Coding and Decoding of Calcium Signals in Plants; Luan, S., Ed.; Signaling and Communication in Plants; Springer: Berlin/Heidelberg, Germany, 2011; pp. 25–39. ISBN 978-3-642-20828-7. [Google Scholar]
- Yang, H.; You, C.; Yang, S.; Zhang, Y.; Yang, F.; Li, X.; Chen, N.; Luo, Y.; Hu, X. The Role of Calcium/Calcium-Dependent Protein Kinases Signal Pathway in Pollen Tube Growth. Front. Plant Sci. 2021, 23, 346. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, Y.; Yang, Z. A Genome-Wide Functional Characterization of Arabidopsis Regulatory Calcium Sensors in Pollen Tubes. J. Integr. Plant Biol. 2009, 51, 751–761. [Google Scholar] [CrossRef]
- Delormel, T.Y.; Boudsocq, M. Properties and Functions of Calcium-Dependent Protein Kinases and Their Relatives in Arabidopsis Thaliana. New Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef] [PubMed]
- Jurca, M.E.; Bottka, S.; Fehér, A. Characterization of a Family of Arabidopsis Receptor-like Cytoplasmic Kinases (RLCK Class VI). Plant Cell Rep. 2008, 27, 739–748. [Google Scholar] [CrossRef]
- Enders, T.A.; Frick, E.M.; Strader, L.C. An Arabidopsis Kinase Cascade Influences Auxin-Responsive Cell Expansion. Plant J. 2017, 92, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.F.; Harmon, A. Plants, Symbiosis and Parasites: A Calcium Signalling Connection. Nat. Rev. Mol. Cell Biol. 2005, 6, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Curran, A.; Chang, I.-F.; Chang, C.-L.; Garg, S.; Miguel, R.M.; Barron, Y.D.; Li, Y.; Romanowsky, S.; Cushman, J.C.; Gribskov, M.; et al. Calcium-Dependent Protein Kinases from Arabidopsis Show Substrate Specificity Differences in an Analysis of 103 Substrates. Front. Plant Sci. 2011, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Mergner, J.; Frejno, M.; List, M.; Papacek, M.; Chen, X.; Chaudhary, A.; Samaras, P.; Richter, S.; Shikata, H.; Messerer, M.; et al. Mass-Spectrometry-Based Draft of the Arabidopsis Proteome. Nature 2020, 579, 409–414. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Nomura, Y.; Wang, L.; Nakagami, H.; Somers, D.E. Quantitative Circadian Phosphoproteomic Analysis of Arabidopsis Reveals Extensive Clock Control of Key Components in Physiological, Metabolic, and Signaling Pathways. Mol. Cell. Proteom. 2015, 14, 2243–2260. [Google Scholar] [CrossRef] [PubMed]
- Potocký, M.; Pejchar, P.; Gutkowska, M.; Jiménez-Quesada, M.J.; Potocká, A.; Alché, J.D.D.; Kost, B.; Žárský, V. NADPH Oxidase Activity in Pollen Tubes Is Affected by Calcium Ions, Signaling Phospholipids and Rac/Rop GTPases. J. Plant Physiol. 2012, 169, 1654–1663. [Google Scholar] [CrossRef]
- Hwang, J.; Vernoud, V.; Szumlanski, A. A Tip-Localized RhoGAP Controls Cell Polarity by Globally Inhibiting Rho GTPase at the Cell Apex. Current Biol. 2008, 18, 1907–1916. [Google Scholar] [CrossRef]
- Li, Z.; Takahashi, Y.; Scavo, A.; Brandt, B.; Nguyen, D.; Rieu, P.; Schroeder, J.I. Abscisic Acid-Induced Degradation of Arabidopsis Guanine Nucleotide Exchange Factor Requires Calcium-Dependent Protein Kinases. Proc. Natl. Acad. Sci. USA 2018, 115, E4522–E4531. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, S.; Tian, H.; He, Y.; Xiong, W.; Guo, L.; Wu, Y. CPK3-Phosphorylated RhoGDI1 Is Essential in the Development of Arabidopsis Seedlings and Leaf Epidermal Cells. J. Exp. Bot. 2013, 64, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Klahre, U.; Becker, C.; Schmitt, A.C.; Kost, B. Nt-RhoGDI2 Regulates Rac/Rop Signaling and Polar Cell Growth in Tobacco Pollen Tubes. Plant J. 2006, 46, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Klement, E.; Gyula, P.; Viczián, A. Detection of Phytochrome Phosphorylation in Plants. In Phytochromes: Methods and Protocols; Hiltbrunner, A., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 41–67. ISBN 978-1-4939-9612-4. [Google Scholar]
- Benkő, P.; Jee, S.; Kaszler, N.; Fehér, A.; Gémes, K. Polyamines Treatment during Pollen Germination and Pollen Tube Elongation in Tobacco Modulate Reactive Oxygen Species and Nitric Oxide Homeostasis. J. Plant Physiol. 2020, 244, 153085. [Google Scholar] [CrossRef] [PubMed]
- Kost, B.; Spielhofer, P.; Chua, N.H. A GFP-Mouse Talin Fusion Protein Labels Plant Actin Filaments in Vivo and Visualizes the Actin Cytoskeleton in Growing Pollen Tubes. Plant J. 1998, 16, 393–401. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ménesi, D.; Klement, É.; Ferenc, G.; Fehér, A. The Arabidopsis Rho of Plants GTPase ROP1 Is a Potential Calcium-Dependent Protein Kinase (CDPK) Substrate. Plants 2021, 10, 2053. https://doi.org/10.3390/plants10102053
Ménesi D, Klement É, Ferenc G, Fehér A. The Arabidopsis Rho of Plants GTPase ROP1 Is a Potential Calcium-Dependent Protein Kinase (CDPK) Substrate. Plants. 2021; 10(10):2053. https://doi.org/10.3390/plants10102053
Chicago/Turabian StyleMénesi, Dalma, Éva Klement, Györgyi Ferenc, and Attila Fehér. 2021. "The Arabidopsis Rho of Plants GTPase ROP1 Is a Potential Calcium-Dependent Protein Kinase (CDPK) Substrate" Plants 10, no. 10: 2053. https://doi.org/10.3390/plants10102053
APA StyleMénesi, D., Klement, É., Ferenc, G., & Fehér, A. (2021). The Arabidopsis Rho of Plants GTPase ROP1 Is a Potential Calcium-Dependent Protein Kinase (CDPK) Substrate. Plants, 10(10), 2053. https://doi.org/10.3390/plants10102053






