Elemental Composition of Algae-Based Supplements by Energy Dispersive X-ray Fluorescence
Abstract
:1. Introduction
2. Results
2.1. Elemental Composition
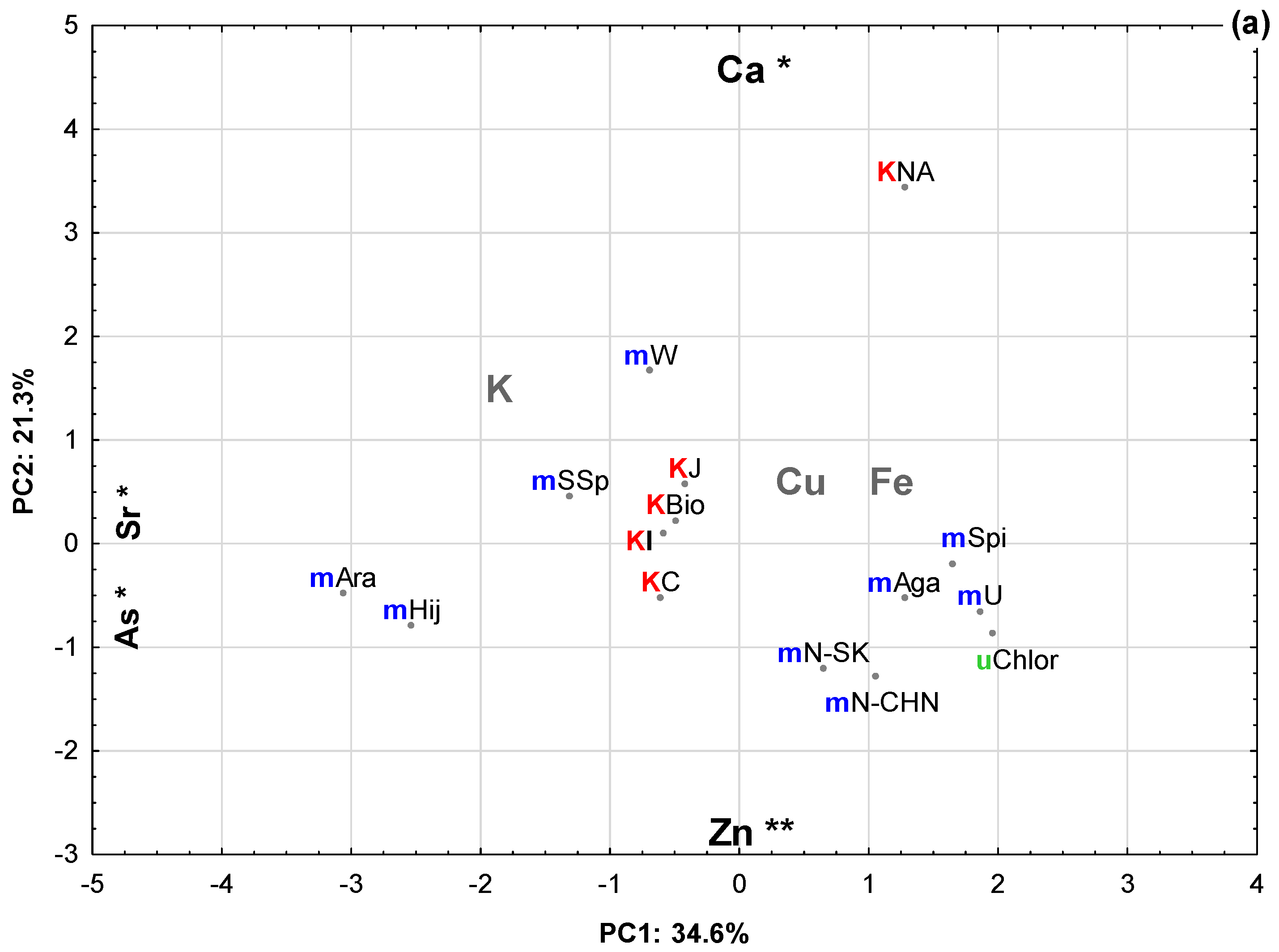
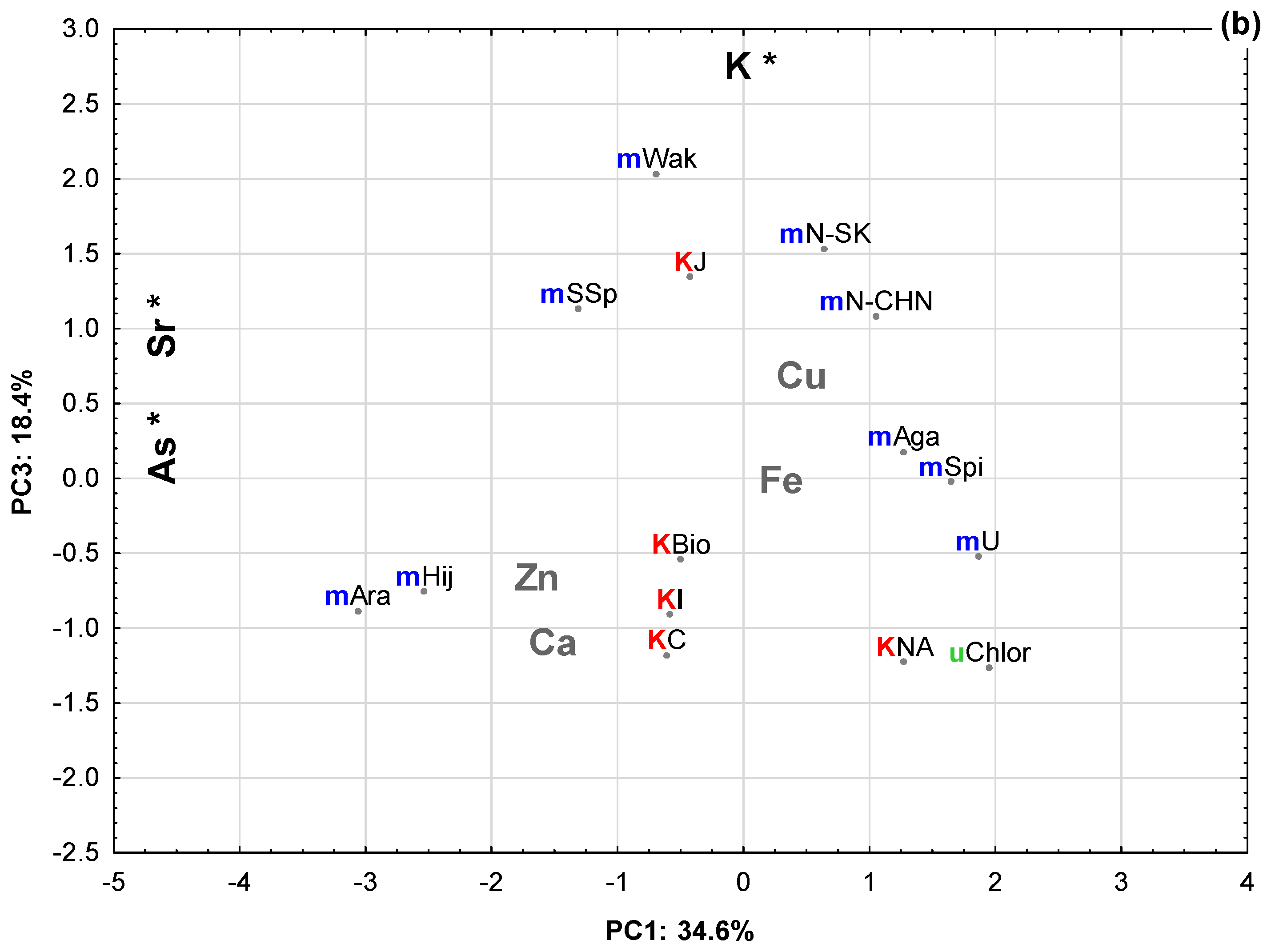
2.2. Principal Component Analysis (PCA)
2.3. Discussion
3. Materials and Methods
3.1. XRF Preparation and Analysis
3.2. Principal Component Analysis
3.3. Statistical Analysis and Control Assurance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arasaki, S.; Arasaki, T. Vegetables from the Sea; Japan Publishing Inc.: Tokyo, Japan, 1983; p. 196. [Google Scholar]
- FAO Fishery and Aquaculture Statistics. Global Aquaculture Production. In FAO Fisheries and Aquaculture Department; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/fishery/ (accessed on 28 January 2021).
- Dillehay, T.D.; Ramirez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pinot-Navarro, J.D. Monte Verde: Seaweed, food, medicine and the peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Falkenberg, M.; Nakano, E.; Zambotti-Villela, L.; Zatelli, G.A.; Philippus, A.C.; Imamura, K.B.; Velasquez, A.M.A.; Freitas, R.P.; Tallarico, L.F.; Colepicolo, P.; et al. Bioactive compounds against neglected diseases isolated from macroalgae: A review. J. Appl. Phycol. 2019, 31, 797–823. [Google Scholar] [CrossRef] [Green Version]
- Fields, F.J.; Lejzerowicz, F.; Schroeder, D.; Ngoi, S.M.; Tran, M.; McDonald, D.; Jiang, L.; Chang, J.T.; Knight, R.; Mayfield, S. Effects of the microalgae Chlamydomonas on gastrointestinal health. J. Funct. Foods 2020, 65, 103738. [Google Scholar] [CrossRef]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, C.; Cai, Y.; Wang, D.; Fang, Y.U. The study of antioxidant activities of fucoidan from Laminaria japonica. High. Technol. Lett. 2005, 11, 91–94. [Google Scholar]
- Boulho, R.; Marty, C.; Freile-Pelegrin, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Díaz, O.; Tapia, Y.; Muñoz, O.; Montoro, R.; Velez, D.; Almela, C. Total and inorganic arsenic concentrations in different species of economically important algae harvested from coastal zones of Chile. Food Chem. Toxicol. 2012, 50, 744–749. [Google Scholar] [CrossRef]
- Besada, V.; Andrade, J.M.; Schultze, F.; González, J.J. Heavy metals in edible seaweeds commercialised for human consumption. J. Mar. Syst. 2009, 75, 305–313. [Google Scholar] [CrossRef]
- Paz, S.; Rubio, C.; Frías, I.; Gutiérrez, A.J.; González-Weller, D.; Martín, V.; Revert, C.; Hardisson, A. Toxic metals (Al, Cd, Pb and Hg) in the most consumed edible seaweeds in Europe. Chemosphere 2019, 218, 879–884. [Google Scholar] [CrossRef]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed; Pomin, V.H., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2011; Chapter 2; pp. 15–47. [Google Scholar]
- Reboredo, F.H.; Barbosa, A.; Silva, M.; Carvalho, M.L.; Santos, J.P.; Pessoa, M.F.; Lidon, F.; Ramalho, J.C.; Guerra, M. Mineral content of food supplements of plant origin, by energy dispersive x-ray fluorescence: A risk assessment. Exp. Health 2020, 12, 917–927. [Google Scholar] [CrossRef]
- Pelica, J.; Barbosa, S.; Reboredo, F.; Lidon, F.; Pessoa, M.F.; Calvão, T. The paradigm of high concentration of metals of natural or antrophogenic origin in the soils—The case of Neves-Corvo mining area-South of Portugal. J. Geochem. Expl. 2018, 186, 12–23. [Google Scholar] [CrossRef]
- Reboredo, F.; Pelica, J.; Lidon, F.; Pessoa, M.F.; Ramalho, J.C.; Calvão, T.; Simões, M.; Guerra, M. Heavy metal content of edible plants collected close an area of intense mining activity (Southern Portugal). Environ. Monit. Assess. 2018, 190, 484. [Google Scholar] [CrossRef]
- Reboredo, F.; Simões, M.; Jorge, C.; Martinez, J.; Mancuso, M.; Guerra, M.; Ramalho, J.; Pessoa, M.F.; Lidon, F. Metal content in edible crops and agricultural soils due to intensive use of fertilizers and pesticides in Terras da Costa de Caparica (Portugal). Environ. Sci. Pollut. Res. 2019, 26, 2512–2522. [Google Scholar] [CrossRef]
- Reboredo, F.; Henriques, F. Some observations on the leaf ultrastructure of Halimione portulacoides (L.) Aellen grown in a medium containing copper. J. Plant. Physiol. 1991, 137, 717–722. [Google Scholar] [CrossRef]
- Reboredo, F. Cadmium accumulation by Halimione portulacoides (L.) Aellen. A seasonal study. Mar. Environ. Res. 1992, 33, 17–29. [Google Scholar] [CrossRef]
- Reboredo, F. Copper and zinc uptake by Halimione portulacoides (L.) Aellen. A long-term accumulation experiment. Bull. Environ. Contam. Toxicol. 1991, 46, 442–449. [Google Scholar] [CrossRef]
- Reboredo, F. The interaction between copper and zinc and their uptake by Halimione portulacoides (L.) Aellen. Bull. Environ. Contam. Toxicol. 1994, 52, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Hasegawa, H. High levels of inorganic arsenic in rice in areas where arsenic-contaminated water is used for irrigation and cooking. Sci. Total Environ. 2011, 409, 4645–4655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Dhabi, N.A. Heavy metal analysis in commercial Spirulina products for human consumption. Saudi J. Biol. Sci. 2013, 20, 383–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; et al. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO Fisheries and Aquaculture Circular No. 1229; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Bio-Based Industries Consortium (BIC). Mapping Portugal’s Bio-Based Potential Country Report; European Forestry House: Brussels, Belgium, 2021; 61p. [Google Scholar]
- FAO. The Global Status of Seaweed Production, Trade and Utilization; Globefish Research Programme: Rome, Italy, 2018; Volume 124, p. 120. [Google Scholar]
- Bolton, J.J. The biogeography of kelps (Laminariales, Phaeophyceae): A global analysis with new insights from recent advances in molecular phylogenetics. Helgol. Mar. Res. 2010, 64, 263–279. [Google Scholar] [CrossRef]
- Palma, P.; Alvarenga, P.; Palma, V.L.; Fernandes, R.M.; Soares, A.M.V.M.; Barbosa, I.R. Assessment of anthropogenic sources of water pollution using multivariate statistical techniques: A case study of the Alqueva’s reservoir, Portugal. Environ. Monit. Assess. 2010, 165, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, N.; Taipina, M.; Raposo, N.; Dias, J.; Carvalho, M.J.; Amaral, O.; Lidon, F.C. Development of biscuits with green banana flour irradiated by 60Co: Preservation in modified atmosphere packaging. Emir. J. Food Agric. 2018, 30, 498–502. [Google Scholar]
- Sá Monteiro, M.; Sloth, J.; Holdt, S.; Hansen, M. Analysis and Risk Assessment of Seaweed. EFSA J. 2019, 17, e170915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrea-Marín, M.T.; Pomares-Alfonso, M.S.; Gómez-Juaristi, M.; Sánchez-Muniz, F.J.; de la Rocha, S.R. Validation of an ICP-OES method for macro and trace element determination in Laminaria and Porphyra seaweeds from four different countries. J. Food Comp. Analy. 2010, 23, 814–820. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for Iodine. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA J. 2014, 12, 3660. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Ryu, K.Y.; Choi, J.Y.; Nho, E.Y.; Habte, G.; Choi, H.; Kim, M.H.; Park, K.S.; Kim, K.S. Determination of toxic heavy metals and speciation of arsenic in seaweeds from South Korea. Food Chem. 2015, 169, 464–470. [Google Scholar] [CrossRef]
- Rose, M.; Lewis, J.; Langford, N.; Baxter, M.; Origgi, S.; Barber, M.; MacBain, H.; Thomas, K. Arsenic in seaweed--forms, concentration and dietary exposure. Food Chem. Toxicol. 2007, 45, 1263–1267. [Google Scholar] [CrossRef]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef] [Green Version]
- Ronan, J.M.; Stengel, D.M.; Raab, A.; Feldmann, J.; O’Hea, L.; Bralatei, E.; McGovern, E. High proportions of inorganic arsenic in Laminaria digitata but not in Ascophyllum nodosum samples from Ireland. Chemosphere 2017, 186, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Australia New Zealand Food Standards Authority, Australia New Zealand Food Standards Code, Standard 1.4.1—Contaminants and Natural Toxicants. 2013. Available online: https://www.foodstandards.gov.au/code/Pages/default.aspx (accessed on 12 September 2021).
- Chiocchetti, G.M.; Vélez, D.; Devesa, V. Effect of subchronic exposure to inorganic arsenic on the structure and function of the intestinal epithelium. Toxicol. Lett. 2018, 286, 80–88. [Google Scholar] [CrossRef]
- Official Journal of the European Union Commission Regulation (EU) 2015/1006 of 25 June 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Inorganic Arsenic in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R1006&from=EN (accessed on 12 September 2021).
- Stamoulis, K.C.; Assimakopoulos, P.A.; Ioannides, K.G.; Johnson, E.; Soucacos, P.N. Strontium-90 concentration measurements in human bones and teeth in Greece. Sci. Total Environ. 1999, 229, 165–182. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrød, O. Strontium, calcium and magnesium in brown algae. Nature 1967, 215, 1167–1168. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a beneficial element for crop production. Front. Plant. Sci. 2017, 8, 597. [Google Scholar] [CrossRef] [Green Version]
- Dumon, J.C.; Ernst, W.H.O. Titanium in Plants. J. Plant. Physiol. 1988, 133, 203–209. [Google Scholar] [CrossRef]
- EFSA Lead dietary exposure in the European population. Scientific Report of European Food Safety Authority (EFSA). EFSA J. 2012, 10, 2831. [Google Scholar]
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef] [PubMed]
- WHO World Health Organization (WHO) Lead Poisoning and Health. Key Facts. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 18 February 2021).
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Rubio, C.; Napoleone, G.; Luis-González, G.; Gutiérrez, A.J.; González-Weller, D. Metals in edible seaweed. Chemosphere 2017, 173, 572–579. [Google Scholar] [CrossRef] [PubMed]
- de la Rocha, S.R.; Sánchez-Muniz, F.J.; Gómez-Juaristi, M.; Larrea-Marín, M.T. Trace elements determination in edible seaweeds by an optimized and validated ICP-MS method. J. Food Comp. Anal. 2009, 22, 330–336. [Google Scholar] [CrossRef]
- Rzymski, P.; Budzulak, J.; Niedzielski, P.; Klimaszyk, P.; Proch, J.; Kozak, L.; Poniedziałek, B. Essential and toxic elements in commercial microalgal food supplements. J. Appl. Phycol. 2019, 31, 3567–3579. [Google Scholar] [CrossRef] [Green Version]
- Apaydın, G.; Aylıkcı, V.; Cengiz, E.; Saydam, M.; Küp, N.; Tıraşoğlu, E. Analysis of metal contents of seaweed (Ulva lactuca) from Istanbul, Turkey by EDXRF. Turk. J. Fish. Aquat. Sci. 2010, 10, 215–220. [Google Scholar] [CrossRef]
- Nakamura, Y.; Narukawa, T.; Yoshinaga, J. Cancer risk to Japanese population from the consumption of inorganic arsenic in cooked Hijiki. J. Agric. Food Chem. 2008, 56, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Almela, C.; Algora, S.; Benito, V.; Clemente, M.J.; Devesa, V.; Súñer, M.A.; Vélez, D.; Montoro, R. Heavy Metal, Total Arsenic, and Inorganic Arsenic Contents of Algae Food Products. J. Agric. Food Chem. 2002, 50, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Kejžar, J.; Hudobivnik, M.J.; Nečemer, M.; Ogrinc, N.; Rutar, J.M.; Ulrih, N.P. Characterization of algae dietary supplements using antioxidative potential, elemental composition, and stable isotopes approach. Front. Nutr. 2021, 7, 618503. [Google Scholar] [CrossRef]
- Official Journal of the European Union, Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011, 46p. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R1169&from=PT (accessed on 12 September 2021).
- Pessanha, S.; Alves, M.; Sampaio, J.M.; Santos, J.P.; Carvalho, M.L.; Guerra, M. A novel portable energy dispersive X-ray fluorescence spectrometer with triaxial geometry. JINST 2017, 12, P01014. [Google Scholar] [CrossRef]
- Santos, S.C.P.-L.; Cruz, M.E.; Barroso, A.M.E.; Fonseca, C.P.S.; Guerra, M.; Carvalho, M.L.; Santos, J.P. Elemental characterization of plants and soils in Panasqueira tungsten mining region. J. Soils Sediments 2014, 14, 778–784. [Google Scholar] [CrossRef]
- Cardoso, P.; Mateus, T.; Velu, G.; Singh, R.P.; Santos, J.P.; Carvalho, M.L.; Lourenço, V.M.; Lidon, F.; Reboredo, F.; Guerra, M. Localization and distribution of Zn and Fe in grains of biofortified bread wheat lines through micro and triaxial-X-ray spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2018, 141, 70–79. [Google Scholar] [CrossRef]

| Element | KelpC | KelpBio | KelpI | KelpNA | KelpJ |
|---|---|---|---|---|---|
| Ca | 8220 ± 130b | 8700 ± 100b | 9970 ± 450b | 33,200 ± 3500a | 5190 ± 350c |
| K | 15,100 ± 230b | 14,760 ± 340b | 17,000 ± 400b | 2810 ± 270c | 22,600 ± 1900a |
| S | 10,500 ± 900bc | BDL | 14,400 ± 1800a | 3000 ± 1000c | BDL |
| I | 160 ± 50d | 550 ± 70c | 490 ± 50c | 860 ± 160b | 2270 ± 90a |
| Cu | 4.0 ± 1.0b | 4.0 ± 0.8b | 3.6 ± 0.4b | 3.0 ± 0.7b | 11.0 ± 0.6a |
| Fe | 404 ± 5a | 116 ± 16b | 381 ± 14a | 71 ± 5c | 29 ± 2d |
| Mn | 19.1 ± 1.7a | 16.9 ± 2.7a | 20.0 ± 1.8a | BDL | BDL |
| Ni | BDL | 6 ± 3a | BDL | BDL | BDL |
| Zn | 36 ± 2a | 28 ± 1b | 28 ± 1b | 7 ± 1c | 9 ± 1c |
| Sr | 640 ± 39a | 582 ± 31a | 690 ± 50a | 83 ± 7c | 362 ± 22b |
| Ti | BDL | 25 ± 7b | 34 ± 20b | 27 ± 6b | 86 ± 8a |
| As | 34.0 ± 0.6c | 29 ± 2d | 40 ± 1b | 8.7 ± 0.2e | 60 ± 6a |
| Element | Hijiki | Agar | Arame | Sea Spaghetti | Wakame |
|---|---|---|---|---|---|
| Ca | 3900 ± 2700c | 3830 ± 220c | 9900 ± 1200a | 7400 ± 400b | 6960± 190b |
| K | 6260 ± 430c | 120 ± 23d | 9800 ± 1000b | 57,000 ± 5000a | 62,000 ± 5000a |
| Cu | 5.6 ± 0.9c | 13.4 ± 2.0a | 9.0 ± 1.7ab | 5.0 ± 1.1c | 4.0 ± 0.8c |
| Fe | 70 ± 10a | 34 ± 3bc | 58 ± 22ab | 29 ± 3c | 43 ± 2b |
| Mn | 27.8 ± 3.5a | 11.8 ± 2.0b | BDL | 22.0 ± 2.4a | BDL |
| Ni | BDL | BDL | 3.5 ± 0.1 | 4.0 ± 0.2 | BDL |
| Zn | 36 ± 2a | 21 ± 3b | 35 ± 17ab | 30 ± 1a | 7 ± 1c |
| I | 250 ± 60b | BDL | 400 ± 29a | BDL | BDL |
| Sr | 1420 ± 100b | 9.7 ± 1.2d | 2220 ± 230a | 690 ± 80c | 560 ± 70c |
| As | 66 ± 17a | 3.9 ± 0.3d | 60 ± 18ab | 36 ± 2c | 47.0 ± 0.6b |
| Pb | 13 ± 1a | BDL | 19 ± 11a | 9.7± 0.5a | 10.0 ± 0.8a |
| Ulva | NoriSK | NoriCHN | Chlorella | Spirulina | |
|---|---|---|---|---|---|
| Ca | 5300 ± 500a | 3030 ± 240b | 4680 ± 200a | 1400 ± 80c | 970 ± 420c |
| K | 18,600 ± 1500b | 29,100 ± 1800a | 13,800 ± 440c | 7420 ± 340d | 11,220 ± 620c |
| S | 21,800 ± 2000a | 7000 ± 4800b | 17,700 ± 1000a | 5200 ± 900b | BDL |
| Cu | 10.0 ± 1.3b | 21 ± 3a | 23 ± 5a | 4.6 ± 0.4c | 7 ± 3b |
| Fe | 792 ± 24a | 153 ± 62c | 180 ± 80c | 826 ± 33a | 330 ± 41b |
| Mn | 151 ± 12a | 24.9 ± 2.6c | 24.4 ± 1.9c | 49 ± 3b | BDL |
| Zn | 18 ± 6ab | 26 ± 2a | 24 ± 3a | 20 ± 1b | 14 ± 6bc |
| Sr | 73 ± 8a | 52 ± 5b | 33 ± 3c | 11.0 ± 1.2d | 12.0 ± 0.9d |
| Ti | 24.8 ± 3.1a | BDL | BDL | BDL | 27.0 ± 2.1a |
| As | 10.8 ± 0.4b | 22.7 ± 1.1a | 19 ± 3a | 7.0 ± 0.8c | 4.0 ± 1.6cd |
| Eigenvalue | Total Variance (%) | Cumulative Eigenvalue | Cumulative (%) | |
|---|---|---|---|---|
| 1 | 2.42 | 34.57 | 2.42 | 34.57 |
| 2 | 1.49 | 21.31 | 3.91 | 55.88 |
| 3 | 1.29 | 18.40 | 5.20 | 74.27 |
| 4 | 1.01 | 14.44 | 6.21 | 88.71 |
| 5 | 0.40 | 5.75 | 6.61 | 94.47 |
| 6 | 0.27 | 3.89 | 6.89 | 98.36 |
| 7 | 0.11 | 1.64 | 7 | 100 |
| Components | |||
|---|---|---|---|
| Attribute | PC1 | PC2 | PC3 |
| Ca | −0.02 | 0.82 * | −0.33 |
| K | −0.24 | 0.22 | 0.71 * |
| Cu | 0.26 | −0.53 | 0.51 |
| Fe | 0.53 | −0.35 | −0.46 |
| Zn | −0.57 | −0.60 ** | −0.34 |
| Sr | −0.93 * | −0.07 | −0.24 |
| As | −0.91 * | 0.01 | 0.16 |
| Studied Species | Ca (800 mg) | Cu (1 mg) | Fe (14 mg) | I (150 μg) | K (2000 mg) | Mn (2 mg) | Zn (10 mg) |
|---|---|---|---|---|---|---|---|
| KelpC | 5.75 | 0.0028 | 0.283 | 112 | 10.6 | 0.013 | 0.025 |
| KelpBio | 6.09 | 0.0028 | 0.081 | 385 | 10.3 | 0.012 | 0.020 |
| KelpI | 6.98 | 0.0025 | 0.266 | 345 | 11.9 | 0.014 | 0.020 |
| KelpNA | 4.74 | 0.0004 | 0.010 | 123 | 0.40 | -- | 0.001 |
| Chlorella | 9.80 | 0.032 | 5.78 | -- | 51.9 | 0.34 | 0.14 |
| Spirulina | 6.79 | 0.049 | 2.31 | -- | 78.5 | -- | 0.098 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reboredo, F.H.; Junior, W.; Pessoa, M.F.; Lidon, F.C.; Ramalho, J.C.; Leitão, R.G.; Silva, M.M.; Alvarenga, N.; Guerra, M. Elemental Composition of Algae-Based Supplements by Energy Dispersive X-ray Fluorescence. Plants 2021, 10, 2041. https://doi.org/10.3390/plants10102041
Reboredo FH, Junior W, Pessoa MF, Lidon FC, Ramalho JC, Leitão RG, Silva MM, Alvarenga N, Guerra M. Elemental Composition of Algae-Based Supplements by Energy Dispersive X-ray Fluorescence. Plants. 2021; 10(10):2041. https://doi.org/10.3390/plants10102041
Chicago/Turabian StyleReboredo, Fernando H., Walter Junior, Maria F. Pessoa, Fernando C. Lidon, José C. Ramalho, Roberta G. Leitão, Maria Manuela Silva, Nuno Alvarenga, and Mauro Guerra. 2021. "Elemental Composition of Algae-Based Supplements by Energy Dispersive X-ray Fluorescence" Plants 10, no. 10: 2041. https://doi.org/10.3390/plants10102041
APA StyleReboredo, F. H., Junior, W., Pessoa, M. F., Lidon, F. C., Ramalho, J. C., Leitão, R. G., Silva, M. M., Alvarenga, N., & Guerra, M. (2021). Elemental Composition of Algae-Based Supplements by Energy Dispersive X-ray Fluorescence. Plants, 10(10), 2041. https://doi.org/10.3390/plants10102041













