Response of Moringa oleifera Seeds and Fixed Oil Production to Vermicompost and NPK Fertilizers under Calcareous Soil Conditions
Abstract
:1. Introduction
2. Results
2.1. Parameters of Mature Pods
2.2. Yield of Mature Pods
2.3. Parameters of Mature Seeds
2.4. Yield of Mature Seeds
2.5. Fixed Oil Percentage
2.6. Fixed Oil Yield
2.7. Fixed Oil Analysis
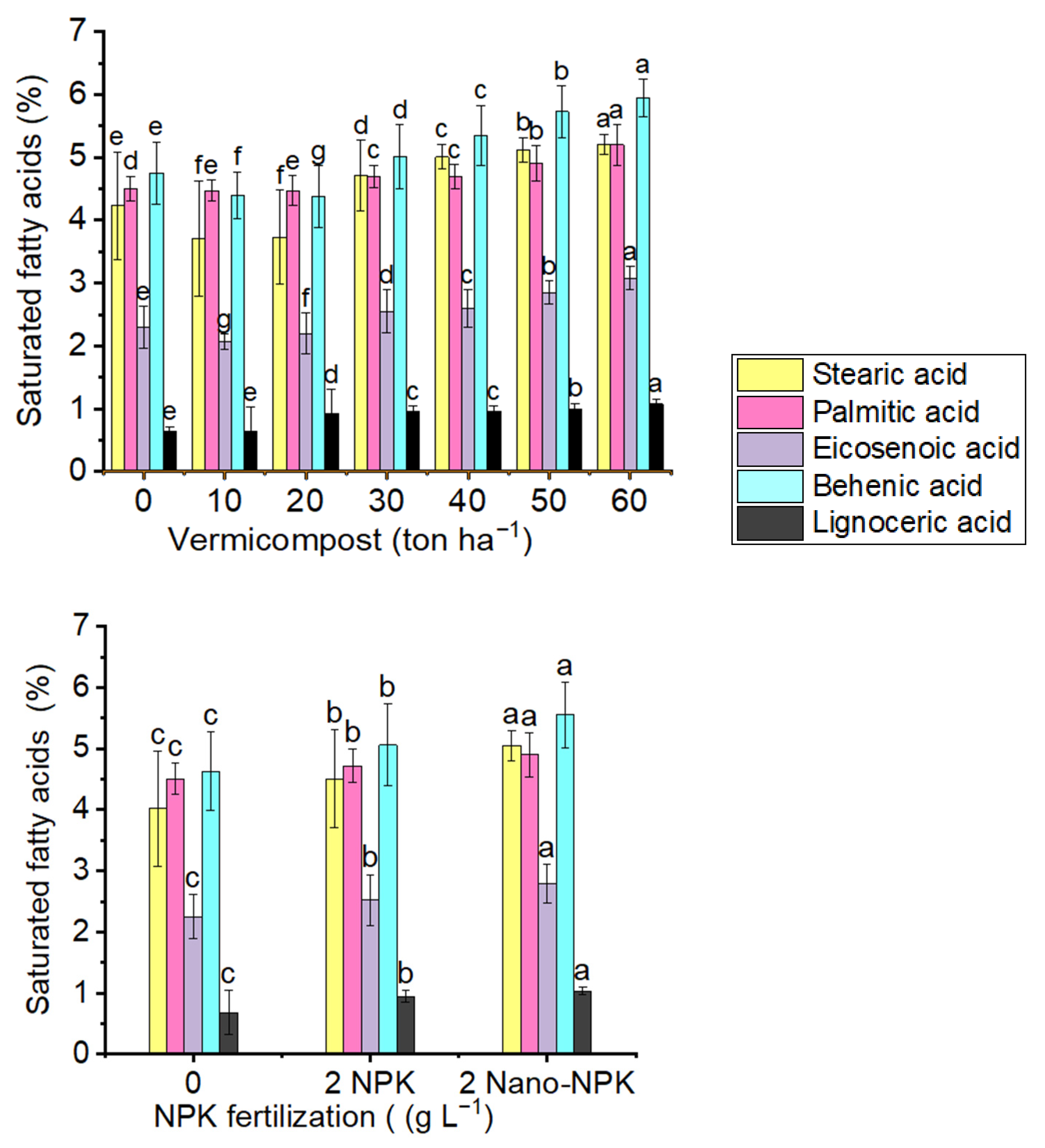
2.7.1. Saturated Fatty Acids
2.7.2. Unsaturated Fatty Acids
3. Discussion
3.1. Effects of Calcareous Soil
3.2. Effects of Vermicompost
3.3. Effects of NPK Fertilizer
3.4. Effects of Combination Treatments of Vermicompost and NPK
4. Materials and Methods
4.1. Plant Material
4.2. Treatment
4.3. Nano-NPK Preparation
4.4. Culture of Seeds
4.5. Parameters and Measurements
4.5.1. Pods and Seeds Parameters and Yield
4.5.2. Chemical Constituents of Seeds
Fixed Oil Content of Seeds
Fixed Oil Analysis (GC/MS Analysis)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramachandran, C.; Peter, K.V.; Gopalakrishnan, P.K. Drumstick (Moringa oleifera): A multipurpose. Indian vegetable. Econ. Bot. 1980, 34, 276–283. [Google Scholar] [CrossRef]
- Morton, J.F. The horseradish tree, Moringa pterygosperma (Moringaceae) a boon to arid lands. Econ. Bot. 1991, 45, 318–333. [Google Scholar] [CrossRef]
- Rockwood, J.L.; Anderson, B.G.; Casamatta, D.A. Potential uses of Moringa oleifera and an examination of antibiotic efficacy conferred by M. oleifera seed and leaf extracts using crude extraction techniques available to underserved indigenous populations. Int. J. Phytother. Res. 2013, 3, 61–71. [Google Scholar]
- Warra, A.A.A. Review of Moringa oleifera Lam. seed oil prospects in personal care formulations. Res. Rev. J. Pharm. Nanotechnol. 2014, 2, 31–34. [Google Scholar]
- Nadeem, M.; Imran, M. Promising features of Moringa oleifera oil: Recent updates and perspectives. Lipids Health Dis. 2016, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Aiyelaagbe, I.O.O. Nigerian Horticulture: Facing the challenges of human health and agricultural productivity. In Proceedings of the Keynote Address Presented at the 29th Annual National Conference of Horticultural Society of Nigeria, Makurdi, Nigeria, 24–29 July 2011; pp. 24–29. [Google Scholar]
- Fuglier, L.J. The Miracle Tree: Moringa oleifera, Natural Nutrition for the Tropics; Church World Service: Dakar, Senegal, 1999. [Google Scholar]
- Jones, P.D. Journal on the propagation and growing of multipurpose trees, Volume 19, 56, 60–78. In Vegetative and Reproductive tissue of the Multipurpose tree, Moringa oleifera. J. Agric. Food Chem. 1999, 51, 3546–3553. [Google Scholar]
- Elgabaly, M.M. Reclamation and management of the calcareous soils of Egypt. In FAO Soils Bulletin 21, Calcareous Soils: Report of the FAO/UNDP Regional Seminar on Reclamation and Management of Calcareous Soils; FAO Soils Bulletin: Cairo, Egypt, 1972; Volume 21, pp. 123–127. [Google Scholar]
- El-Hady, O.A.; Abo-Sedera, S.A. Conditioning effect of composts and acrylamide hydrogels on a sandy calcareous soil. II-Physico-bio-chemical properties of the soil. Int. J. Agric. Biol. 2006, 8, 876–884. [Google Scholar]
- FAO. FAO Soils Portal: Management of Calcareous Soils. 2016. Available online: http://www.fao.org/soils-portal/soil-management/managementof-some-problem-soils/calcareous-soils/ar/ (accessed on 1 April 2016).
- Aboukila, E.F.; Nassar, I.N.; Rashad, M.; Hafez, M.; Norton, J.B. Reclamation of calcareous soil and improvement of squash growth using brewers’ spent grain and compost. J. Saudi Soc. Agric. Sci. 2018, 17, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Wahba, M.M.; Labib, F.; Zaghloul, A. Management of Calcareous Soils in Arid Region. Int. J. Environ. Pollut. Environ. 2019, 2, 248–258. [Google Scholar]
- Parker, W.F. Influence of inorganic fertilizer on multipurpose trees in tropical regions. J. Propag. Grow Multipurp. Trees 1998, 15, 40–62. [Google Scholar]
- Sadat, M.S.I. Studies on the Effects of Different Levels of Nitrogen, Phosphorous and Potassium on the Growth Yield and Seed Production of Okra (Abelmoschus esculentus L.). Master’s Thesis, Deptartment of Horticulture, Bangladesh Agricultural University, Mymensingh, Bangladesh, 2000. [Google Scholar]
- Yadav, H.; Fatima, R.; Sharma, A.; Mathur, S. Enhancement of applicability of rock phosphate in alkaline soils by organic compost. Appl. Soil Ecol. 2017, 113, 80–85. [Google Scholar] [CrossRef]
- Mortvedt, J.J.; Murphy, L.S.; Follet, R.H. Fertilizer Technology and Application; Meister Publishing: Willoughby, OH, USA, 1999. [Google Scholar]
- DeRosa, M.R.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. J. 2010, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Gohari, A.A.; Noorhosseini Niyaki, S.A. Effects of iron and nitrogen fertilizers on yield and yield components of peanut (Arachis hypogaea L.) in staneh Ashrafiyeh, Iran. American-Eurasian. J. Agric. Environ. Sci. 2010, 9, 256–262. [Google Scholar]
- Sheykhbaglou, R.; Sedghi, M.; Shishevan, M.T.; Sharifi, R.S. Effects of Nano-Iron oxide particles on agronomic traits of soybean. Not. Sci. Biol. 2010, 2, 112–113. [Google Scholar] [CrossRef] [Green Version]
- Bozorgi, H.R. Effects of foliar spraying with marine plant ascophyll umnodosum extract and Nano-iron chelate fertilizer on fruit yield and several attributes of eggplant (Solanum melongena l.). ARPN J. Agric. Biol. Sci. 2012, 7, 357–362. [Google Scholar]
- Hagagg, L.F.; Mustafa, N.S.; Genaidy, E.A.E.; El-Hady, E.S. Effect of spraying Nano-NPK on growth performance and nutrients status for (Kalamat cv.) olive seedling. Biosci. Res. 2018, 15, 1297–1303. [Google Scholar]
- Bachmana, G.R.; Metzger, J.D. Growth of bedding plants in commercial potting substrate amended with vermicompost. Bioresour. Technol. 2008, 99, 3155–3161. [Google Scholar] [CrossRef]
- Rivera, M.C.; Wright, E.R. Research on vermicompost as plant growth promoter and disease suppressive substrate in latin America. Dyn. Soil Dyn. Plant. 2009, 3, 32–40. [Google Scholar]
- Brown, G.G. How do earthworms affect microfloral and faunal community diversity? Plant Soil. 1995, 170, 209–231. [Google Scholar] [CrossRef]
- Chaoui, H.; Edwards, C.A.; Brickner, M.; Lee, S.; Arancon, N. Suppression of the plant diseases, Pythium (damping off), Rhizoctonia (root rot) and Verticillum (wilt) by vermicomposts. In Proceedings of the Brighton Crop Protection Conference—Pests and Diseases, Brighton, UK, 18–21 November 2002; Volume II (8B-3), pp. 711–716. [Google Scholar]
- Singleton, D.R.; Hendrixb, P.F.; Colemanb, D.C.; Whitmana, W.B. Identification of uncultured bacteria tightly associated with the intestine of the earthworm Lumbricus rubellus (Lumbricidae; Oligochaeta). Soil Biol. Biochem. 2003, 35, 1547–1555. [Google Scholar] [CrossRef]
- Abd El Gayed, M.E.; Attia, E.A. Impact of growing media and compound fertilizer rates on growth and flowering of cocks comb (Celosia argentea) Plants. J. Plant Production. 2018, 9, 895–900. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, K.P. Enriching vermicompost by nitrogen fixing and phosphate solubilizing bacteria. Bioresour. Technol. 2001, 76, 173–175. [Google Scholar] [CrossRef]
- Goldstein, J. Compost suppresses diseases in the lab and fields. Biocycle 1998, 39, 62–64. [Google Scholar]
- Herencia, J.F.; Ruiz-Porras, J.C.; Melero, S.; Garcia-Galavis, P.A.; Morillo, E.; Maqueda, C. Comparison between organic and mineral fertilization for soil fertility levels, crop macronutrient concentrations, and yield. Agron. J. 2007, 99, 973–983. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Loeppert, R.H.; Hossner, L.R.; Amin, P.K. Formation of ferric oxyhydroxides from ferrous and ferric perchlorate in stirredcalcareous systems. Soil Sci. Soc. Am. J. 1984, 48, 677–683. [Google Scholar] [CrossRef]
- Tomati, U.; Grapppelli, A.; Galli, E. The hormone-like effect of earthworm casts on plant growth. Biol. Fertil Soils. 1988, 5, 288–294. [Google Scholar] [CrossRef]
- Nagavallemma, K.P.; Wani, S.P.; Stephane, L.; Padmaja, V.V.; Vineela, C.; Rao, M.B.; Sahrawat, K.L. Vermicomposting: Recycling wastes into valuable organic fertilizer. In Global Theme on Agrecosystems Report no.8. Patancheru 502324; International Crops Research Institute for the Semi-Arid Tropics: Patancheru, Andhra Pradesh, India, 2004; p. 20. [Google Scholar]
- Aguiar, N.O.; Olivares, F.L.; Novotny, E.H.; Dobbss, L.B.; Balmori, D.M.; Santos-Júnior, L.G.; Chagas, J.G.; Façanha, A.R.; Canellas, L.P. Bioactivity of humic acids isolated from vermicomposts at different maturation stages. Plant Soil. 2013, 362, 161–174. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Okorokova-Facanha, A.L.; Facanha, A.R. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane h+-atpase activity in maize roots. Plant Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, R.J.C.A.; Science, C.; Naidu, R. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A Review. Nutr. Cycl. Agroecosyst. 1998, 51, 123–137. [Google Scholar] [CrossRef]
- Johnston, A.E.; Poulton, P.R.; Coleman, K. Soil organic matter: Its importance in sustainable agriculture and carbon dioxide fluxs. Adv. Agron. 2009, 101, 1–57. [Google Scholar]
- Manlay, R.J.; Feller, C.; Swift, M.J. Historical evolution of soil organic matter concepts and their relationships with the fertility and sustainability of cropping systems. Agric. Ecosyst. Environ. 2007, 119, 217–233. [Google Scholar] [CrossRef]
- Burgin, A.J.; Groffman, P.M. Soil O2 controls denitrificationrates and N2O yield in a riparian wetland. J. Geophys. Res. Biogeosci. 2012, 117, G01010. [Google Scholar] [CrossRef] [Green Version]
- Zandonadi1, D.B.; Santos, M.P.; Busato, J.G.; Peres, L.E.P.; Façanha, A.R. Plant physiology as affected by humified organic matter. Theor. Exp. Plant Physiol. 2013, 25, 12–25. [Google Scholar] [CrossRef] [Green Version]
- Busato, J.G.; Lima, L.; Aguiar, N.O.; Canellas, L.P.; Olivares, F.L. Changes in labile phosphorus forms during maturation of vermicompost enriched with phosphorus-solubilizing and diazotrophic bacteria. Bioresour. Technol. 2012, 110, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Arancon, N.Q.; Edwards, C.A.; Babenko, A.; Cannon, J.; Galvis, P.; Metzger, J.D. Influences of vermicomposts, produced by earthworms and microorganisms from cattle manure, food waste and paper waste, on the germination, growth and flowering of petunias in the greenhouse. Appl. Soil Ecol. 2008, 39, 91–99. [Google Scholar] [CrossRef]
- Joshi, R.; Vig, A.P. Effect of vermicompost on growth, yield and quality of tomato (Lycopersicum esculentum L). AJBAS 2010, 2, 117–123. [Google Scholar]
- Salehi, A.; Ghalavand, A.; Sefidkan, F.; Asgharzadeh, A. Effect of zeolite bacterial inoculum of vermicomposting concentration NPK elements essential oil content and essential oil yield in organic farming chamomile Matricaria chamomilla. J. Med. Aromat. Plants Iran Res. 2011, 27, 188–201. [Google Scholar]
- Madahi, S. Investigating the Effect of Organic Biological and Chemical Fertilizers and Soil Organic Carbon on the Yield of Saffron (Crocus Sativus, L.). Master’s Thesis, Faculty of Agriculture, Ferdowsi University of Mashhad, Mashhad, Iran, 2015. [Google Scholar]
- Oftadeh, A.; Aminifard, M.H.; Behdani, M.A.; Moradineghad, F. The Effect of different levels of nitroxin and vermicompost on yield and photosynthetic pigmentation of saffron (Crocus sativus L.). J. Saffron Res. 2017, 5, 163–179. [Google Scholar]
- Vengadaramana, A.; Jashothan, P.T.J. Effect of organic fertilizers on the water holding capacity of soil in different terrains of Jaffna peninsula in Sri Lanka. J. Nat. Prod. Plant Resour. 2012, 2, 500–503. [Google Scholar]
- Sánchez, N.; Ledin, S.; Ledin, I. Biomass production and chemical composition of Moringa oleifera under different management regimes in nicaragua. Agrofor. Syst. 2006, 66, 231–242. [Google Scholar] [CrossRef]
- Isaiah, M.A. Effects of inorganic fertilizer on the growth and nutrient composition of Moringa (Moringa oleifera). J. Emerg. Trends Eng. Appl. Sci. 2013, 4, 341–343. [Google Scholar]
- Dania, S.O.; Akpansubi, P.; Eghagara, O.O. Comparative effects of different fertilizer sources on the growth and nutrient content of Moringa (Moringa oleifera) seedling in a greenhouse trial. Adv. Agric. 2014, 726313. [Google Scholar] [CrossRef]
- Von Uexküll, H.R.; Date, M.E.R.A.; Grundon, N.J.; Raymet, G.E.; Probert, M.E. Global Extent, Development and Economic Impact of Acid Soils, Plant–Soil Interactions at Low pH: Principles and Management; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 5–19. [Google Scholar]
- Bavaresco, L.; Poni, S. Effect of calcareous soil on photosynthesis rate, mineral nutrition, and source-sink ratio of table grape. J Plant Nutr. 2007, 26, 2123–2135. [Google Scholar] [CrossRef]
- Khan, M.J.; Qasimwheat, M. Integrated use of boiler ash as organic fertilizer and soil conditioner with NPK in calcareous soil. Songklanakarin. J. Sci. Technol. 2008, 30, 281–289. [Google Scholar]
- Semida, W.M.; Abd El-Mageed, T.A.; Howladar, S.M.; Mohamed, G.F.; Rady, M.M. Response of Solanum melongena l. seedlings grown under saline calcareous soil conditions to a new organo-mineral fertilizer. J. Plant Nutr. 2015, 25, 1018–7081. [Google Scholar]
- Haukioja, E.; Ossipov, V.; Koricheva, J.; Honkanen, T.; Larsson, S.; Lempa, K. Biosynthetic origin of carbon-based secondary compounds: Cause of variable responses of woody plants to fertilization. Chemoecology 1998, 8, 133–139. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Dominguez, J.; Subler, S.; Edwards, C.A. Changes in biochemical properties of cow manure during processing by earthworms (Eisenia andrei, Bouché) and the effects on seedling growth. Pedobiologia 2000, 44, 709–724. [Google Scholar] [CrossRef] [Green Version]
- Pant, A.P.; Radovich, T.J.K.; Hue, N.V.; Talcottb, S.T.; Krenek, K.A. Vermicompost extracts influence growth, mineral nutrients, phytonutrients and antioxidant activity in pakchoi (Brassica rapa cv. Bonsai, Chinensis group) grown under vermicompost and chemical fertilizer. J. Sci. Food Agric. 2009, 89, 2383–2392. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.V.; Ajranabhiah, S.N.V. Biological activity of earthworm Casts an assessment of plant growth promoter levels in casts. Proc. Indian Acad. Sci. 1986, 95, 341–351. [Google Scholar] [CrossRef]
- Rezaian, S.; Paseban, M. The effect of micronutrients and manure fertilizers on the quantity and quality of khorasan saffron. Acta Hortic. 2006, 739, 25–33. [Google Scholar] [CrossRef]
- Mohammad-Zadeh, A.R.; Paseban, M. The effect of the source and quantities of organic fertilizers on saffron crop yield. In Proceedings of the 10th Iranian Soil Science Conference, Karaj, Iran, 26–28 August 2007; Volume 26–28, pp. 813–814. [Google Scholar]
- Omidi, H.; Naghdibadi, H.A.; Golzad, A.H.; Torabi Fotoukian, M.H. The effect of hemical and bio-fertilizer source of nitrogen on qualitative and quantitative yield of saffron (Crocus sativus L.). J. Med. Plant. 2009, 8, 98–109. [Google Scholar]
- Ajami-Khals, M.R. Effects of the Weight of the Corms and Application of Organic and Chemical Fertilizers on Yield of Saffron. Master’s Thesis, Faculty of Agriculture, University of Torbath, Torbath, Iran, 2017. [Google Scholar]
- Gholizadeh, Z.; Aminifard, M.H.; Sayari, M.H. Investigating the effect of urban waste compost and corm’s weight on quality traits and secondary metabolites of saffron (Crocus sativus L.). Herb Prod. 2017, 40, 53–65. [Google Scholar]
- Ghanbari, J.; Khajoei-Negad, G.; Van Ruth, S.; Aghighi, S. The possibility for improvement of flowering, corm properties, bioactive compounds, and nutritional regimes. Ind. Crops Prod. 2019, 135, 301–310. [Google Scholar] [CrossRef]
- Jami, N.; Rahimia, A.; Naghizadehb, M.; Sedaghati, E. Investigating the use of different levels of Mycorrhiza and Vermicompost on quantitative and qualitative yield of saffron (Crocus sativus L.). Sci. Hortic. 2020, 262, 109027. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwars, C.A.; Bierman, P.; Wech, C.; Metzger, J.D. Influence of vermicompost on field strawberries. J. Bioresour. Technol. 2004, 93, 145–153. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Arancon, N.; Edwards, C.A.; Metzger, J.D. Incorporation of wastes in to greenhouse container media for production of marigolds. J. Bioresour. Technol. 2002, 81, 103–108. [Google Scholar] [CrossRef]
- Liuc, J.; Pank, B. Fertility levels on growth and oil yield of Roman Chamomile. J. Sci. Pharm. 2005, 46, 63–69. [Google Scholar]
- Muscolo, A.; Bovalo, F.; Gionfriddo, F.; Nardi, F. Earthworm humic matter produces auxin-like effects on Daucus Carota Cell growth and nitrate metabolism. J. Soil Biol. Biochem. 1999, 31, 1303–1311. [Google Scholar] [CrossRef]
- Brown, P.H.; Graham, R.B.; Nicholas, D.J.D. The effects of manganese and nitrate supply on the levels of phenolics and lignin in young wheat plants. Plant Soil. 1984, 81, 437–440. [Google Scholar] [CrossRef]
- Estiarte, M.; Filella, I.; Serra, J.; Pefiuelas, J. Effects of nutrient and water stress on leaf phenolic content of peppers and susceptibility to generalist herbivore Helicoverpa armigera (Hubner). Oecologia 1994, 99, 387–391. [Google Scholar] [CrossRef]
- Law-Ogbomo, K.E.; Ojeniyi, S.O.; OMazi, F.E. Combined and sole application of compost and NPK effect on Okra yield, soil and nutrient content. Nigeria. J. Soil Sci. 2013, 23, 130–135. [Google Scholar]
- Nieto, K.F.; Frankenberger, W.T. Biosynthesis of cytokinins in soil. Soil Sci. Soc. Am. J. 2011, 53, 735–740. [Google Scholar] [CrossRef]
- Aryal, J.; Tamrakar, A.S. Domestic organic waste composting in madhyapur thimi, bhaktapur. Nepal J. Sci. Technol. 2013, 14, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Vine, H. Experiments on the Maintenance of soil Fertility in Ibadan, Nigeria, Emp. J. Expt. Agric. 1953, 21, 65–71. [Google Scholar]
- Cooke, G.W. Fertilizer for Maximum Yield; Cambridge University Press: Cambridge, UK, 1982; p. 465. [Google Scholar]
- Solubo, R.A. Studies on white yam (Discorea rotundata) II. Changes in nutrient content with age. Exp. Agric. 1972, 8, 107–115. [Google Scholar]
- Johnson, C.D.; Decoteau, D.R. Nitrogen and Potassium FertilityAffects Jalapeño Pepper Plant Growth, Pod Yield and Pungency. HortScience 1996, 31, 1119–1123. [Google Scholar] [CrossRef]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Schneider, K.D.; Martens, J.R.T.; Zvomuya, F.; Reid, D.K.; Fraser, T.D.; Lynch, D.H.; O’Halloran, I.P.; Wilson, H.F. Options for Improved Phosphorus Cycling and Use in Agriculture at the Field and Regional Scales. Environ. Qual. 2019, 48, 1247–1264. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Mian, A.; Maathuis, F.J.M. Overexpression of the rice AKT1 potassium channel affects potassium nutrition and rice drought tolerance. J. Exp. Bot. 2016, 67, 2689–2698. [Google Scholar] [CrossRef] [Green Version]
- Zahoor, R.; Dong, H.; Abid, M.; Zhao, W.; Wang, Y.; Zhou, Z. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 2017, 137, 73–83. [Google Scholar] [CrossRef]
- Hu, W.; Lu, Z.; Meng, F.; Li, X.; Cong, R.; Ren, T.; Lu, J. Potassium fertilization reduces silique canopy temperature variation in Brassica napus to enhance seed yield. Ind. Crop. Prod. 2021, 168, 113604. [Google Scholar] [CrossRef]
- Ling, F.; Silberbush, M. Response of maize to foliar vs. soil application of nitrogen-phosphorus-potassium fertilizers. J. Plant Nutr. 2002, 25, 2333–2342. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.P.B.; Moreira, A.; Guimarães, C.M. Foliar fertilization of crop plants. J. Plant. Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Danish, S.; Kiran, S.; Fahad, S.; Ahmad, N.; Ali, M.A.; Tahir, F.A.; Rasheed, M.K.; Shahzad, K.; Li, X.; Wang, D.; et al. Alleviation of chromium toxicity in maize by Fe fortification and chromium tolerant ACC deaminase producing plant growth promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2019, 185, 109706. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tarafdar, J.C.; Biswas, P. Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. J. Nanopart. Res. 2013, 15, 1415–1417. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.M.M.; Hasaneen, M.N.A.; Omer, A.M. Nano-chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Spanish. J. Agric. Res. 2016, 14, 1–9. [Google Scholar]
- Elshamy, M.T.; ELKhallal, S.M.; Husseiny, S.M.; Farroh, K.Y. Application of Nano-chitosan NPK fertilizer on growth and productivity of potato plant. J. Sci. Res. Sci. 2019, 36, 424–441. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B. (Ed.) Springer Handbook of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in Nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Silberstein, O.; Wittwer, S.H. Foliar application of phosphatic nutrients to vegetable crops. In Proceedings of the American Society for Horticultural Science; American Society for Horticultural Science: College Park, MD, USA, 1951; Volume 58, pp. 179–190. [Google Scholar]
- Dixon, R.C. Foliar fertilization improves nutrient use efficiency. Fluids 2003, 11, 22–23. [Google Scholar]
- Liu, R.; Lal, R. Synthetic apatite Nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 6. [Google Scholar] [CrossRef]
- Fagbenro, J.A. Effect of inorganic and organic NPK fertilizers on the growth of three tropical hardwood seedlings grown in an Ultisol. In Nursery Production and Stand Establishment of Broad Leaves to Promote Sustainable Forest Management; Ciccarese, L., Finno, A., Eds.; International Union of Forest Research Organization (IUFRO): Vienna, Austria; Italian Environmental Protection Agency (ANPA): Rome, Italy; Dalarna University: Falun, Sweden, 2001; pp. 79–91. [Google Scholar]
- Ainika, J.N.; Amans, E.B. Growth and yield response of vegetable Amaranth to NPK fertilizer and farmyard manure at Samaru, Nigeria. Proceedings of 29th Annual National Conference of Horticultural Society of Nigeria, (Hortson’11), Makurdi, Nigeria, 24–29 July 2011. [Google Scholar]
- Ghafariyan, M.H.; Malakouti, M.J.; Dadpour, M.R.; Stroeve, P.; Mahmoudi, M. Effects of magnetite Nanoparticles on soybean chlorophyll. Environ. Sci.Technol. 2013, 47, 10645–10652. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodzadeh, H.; Aghili, R.; Nabavi, M. Physiological effects of TiO2 Nanoparticles on wheat (Triticum aestivum). Tech. J. Eng. Appl. Sci. Sci. Expl. Publi. 2013, 3, 1365–1370. [Google Scholar]
- Delfani, M.; Firouzabadi, M.B.; Farrokhi, N.; Makarian, H. Some physiological responses of black-eyed pea to iron and magnesium Nanofertilizers. Commun. Soil Sci. Plant Anal. 2014, 45, 11. [Google Scholar] [CrossRef]
- Farnia, A.; Ghorbani, A. Effect of K Nanofertilizer and N bio-fertilizer on yield and yield components of red bean (Phaseolus vulgaris L.). Int. J. Biosci. 2014, 5, 296–303. [Google Scholar]
- Oyedeji, S.; Animasaun, D.A.; Bello, A.A.; Agboola, O.O. Effect of NPK and poultry manure on growth, yield, and proximate composition of three Amaranths. J. Bot. 2014, 828750. [Google Scholar] [CrossRef]
- Bărăscu, N.M.I.; Duda, M.M.; Donescu, V. The effect of high NPK levels on potato yield size structure and tubers starch content. Sci. Pap. Ser. A. Agronomy 2015, 58, 136–142. [Google Scholar]
- Mokrani, K.; Hamdi, K.; Tarchoun, N. ‘Potato (Solanum Tuberosum, L.) Response to Nitrogen, Phosphorus and Potassium Fertilization Rates’, Communications in Soil Science and Plant Analysis. Commun. Soil Sci. Plant Anal. 2018, 49, 1314–1330. [Google Scholar] [CrossRef]
- Khalid, A.K.; Shedeed, M.R. Effect of NPK and foliar nutrition on growth, yield and chemical constituents in Nigella sativa L. J. Mater. Environ. Sci. 2015, 6, 1709–1714. [Google Scholar]
- Hasaneen, M.N.A.; Abdel-aziz, H.M. Effect of foliar application of engineered Nanomaterials: Carbon Nanotubes NPK and chitosan Nanoparticles NPK fertilizer on the growth of French bean plant. Biochem. Biotechnol. Res. 2016, 4, 68–76. [Google Scholar]
- Soylu, S.; Sade, B.; Topal, A.; Akgün, N.; Gezgin, S.; Hakki, E.E.; Babaoglu, M. Responses of irrigated durum and bread wheat cultivars to boron application in a low boron calcareous soil. Turk. J. Agric. For. 2005, 29, 275–286. [Google Scholar]
- Soleimani, R. The effects of integrated application of micronutrient on wheat in low organic carbon conditions of alkaline soils of western Iran. In Proceedings of the 18th World Congress of Soil Science, Philadelphia, PA, USA, 9–15 July 2006; p. 22. [Google Scholar]
- Arif, M.; Chohan, M.A.; Ali, S.; Gul, R.; Khan, S. Response of wheat to foliar application of nutrients. J. Agric. Biol. Sci. 2006, 1, 30–34. [Google Scholar]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Shinwari, Z.K.; Ahmad, N.; Kim, Y.H.; Lee, I.J. Effect of foliar and soil application of nitrogen, phosphorus and potassium on yield components of lentil. Pak. J. Bot. 2011, 43, 391–396. [Google Scholar]
- Jubeir, S.M.; Ahmed, W.A. Effect Of Nano-Fertilizers And Application Methods On Vegetative Growth And Yield Of Date Palm. Iraqi J. Agric. Sci. 2019, 50, 267–274. [Google Scholar]
- Alzreejawi, S.A.M.; Al-Juthery, H.W.A. Effect of spray with Nano-NPK, Complete Micro Fertilizers and Nano Amino Acids on Some Growth and Yield Indicators of Maize (Zea mays L.); Earth and Environmental Science 553 012010; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Rafiullah, M.; Khan, J.; Muhammad, D.; Fahad, S.; Adnan, M.; Wahid, F.; Alamri, S.; Khan, F.; Dawar, K.M.; Irshad, I.; et al. Phosphorus nutrient management through synchronization of application methods and rates in wheat and maize crops. Plants 2020, 9, 1389. [Google Scholar] [CrossRef]
- Toscano, P.; Godino, G.; Belfiore, T.; Briccoli-Bati, C. Foliar Fertilization: A valid alternative for olive cultivar. In Proceedings of the International Symposium on Foliar Nutrition of Perennial Fruit Plants, Meran, Italy, 11–15 September 200l.
- Goyal, S.; Chander, K.; Mundra, M.C.; Kapoor, K.K. Influence of inorganic fertilizers and organic amendments on soil organic matter and soil microbial properties under tropical conditions. Biol. Fertil. Soils 1999, 29, 196–200. [Google Scholar] [CrossRef]
- Kaur, K.; Kapoor, K.K.; Gupta, A.P. Impact of organic manures with and without mineral fertilizers on soil chemical and biological properties under tropical conditions. J. Plant Nutr. Soil Sci. 2005, 168, 117–122. [Google Scholar] [CrossRef]
- Valiki, S.R.H.; Ghanbari, S.; Golmohammadzadeh, S.; Tat, O.F. The effect of vermicompost and npk fertilizer on yield, growth parameters and essential oil of fennel (Foeniculum vulgare). Int. J. Life Sci. 2015, 9, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Bajracharya, S.K.; Sherchan, D.P.; Bhattarai, S. Effect of vermicompost in combination with bacterial and mineral fertilizers on the yield of vegetable soybean. Korean J. Crop. Sci. 2007, 52, 100–103. [Google Scholar]
- Bhattarai, B.P.; Tomar, C.S. Effect of integrated nutrient management on leaf nutrient status of Walnut (Juglans regia L.). Nepal J. Sci. Technol. 2009, 10, 63–67. [Google Scholar]
- Thakur, A.K.; Uphoff, N.; Antony, E. An assessment of physiological effects of system of rice intensification (SRI) practices compared with recommended rice cultivation practices in India. Exp. Agric. 2010, 46, 77–98. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wu, L.; Dong, C.; Li, Y. Rice yield, nitrogen utilization and ammonia volatilization as influenced by modified rice cultivation at varying nitrogen rates. Agric. Sci. 2010, 1, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Prativa, K.C.; Bhattarai, B.P. Effect of integrated nutrient management on the growth, yield and soil nutrient status in tomato. NJST 2011, 12, 23–28. [Google Scholar]
- Morshedi, A. An investigation into the effects of sowing time, N and P fertilizers on seed yield, oil and protein production in canola. Arch. Agron. Soil Sci. 2011, 57, 533–547. [Google Scholar] [CrossRef]
- Rogério, F.; Silva, T.R.B.; Santos, J.I.; Poletine, J.P. Phosphorus fertilization influences grain yield and oil content in crambe. Ind. Crops Prod. 2013, 41, 266–268. [Google Scholar] [CrossRef]
- Xie, Y.P.; Niu, X.X.; Niu, J.Y. Effect of phosphorus fertilizer on growth, phosphorus uptake, seed yield, yield components and phosphorus use efficiency of oilseed flax. Agron. J. 2016, 108, 1257–1266. [Google Scholar] [CrossRef]
- Anwar, F.; Ashraf, M.; Bhanger, M.I. Interprovenance variation in the composition of Moringa oleifera oilseeds from pakistan. J. Am. Oil Chem. Soc. 2005, 82, 45–51. [Google Scholar] [CrossRef]
- Xie, Y.; Yanb, Z.; Niuc, Z.; Coulterd, J.A.; Niuc, J.; Zhanga, J.; Wanga, B.; Yanc, B.; Zhaoa, W.; Wang, L. Yield, oil content, and fatty acid profile of flax (Linum usitatissimum L.) as affected by phosphorus rate and seeding rate. Ind. Crops Prod. 2020, 145, 112087. [Google Scholar] [CrossRef]
- Darakeh, S.A.S.S.; Weisany, W.; Diyanat, M.; Ebrahimi, R. Bio-organic fertilizers induce biochemical changes and affect seed oil fatty acids composition in black cumin (Nigella sativa Linn). Ind. Crops Prod. 2021, 164, 113383. [Google Scholar] [CrossRef]
- Climate-Data.org. Climate: Madinat Mubarak. 2021.
- Jackson, M.L. Methods of Chemical Analysis; Prentice Hall of India: New Delhi, India, 1973. [Google Scholar]
- Cottenie, A.; Verloo, M.; Kikens, L. Chemical Analysis of Plants and Soils; RUG Laboratory of Analytical and Agrochemistry: Gent, Belgium, 1982. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 12th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1980. [Google Scholar]
- Atteya, A.K.G.; Amer, H.M. Influence of Seaweed Extract and Amino Acids on Growth, Productivity and Chemical Constituents of Hibiscus sabdariffa L. Plants Biosci. Res. 2018, 15, 772–791. [Google Scholar]
- SAS Institute Inc. SAS/STAT User’s Guide, 6.03th ed.; SAS Institute: Cary, NC, USA, 1988. [Google Scholar]
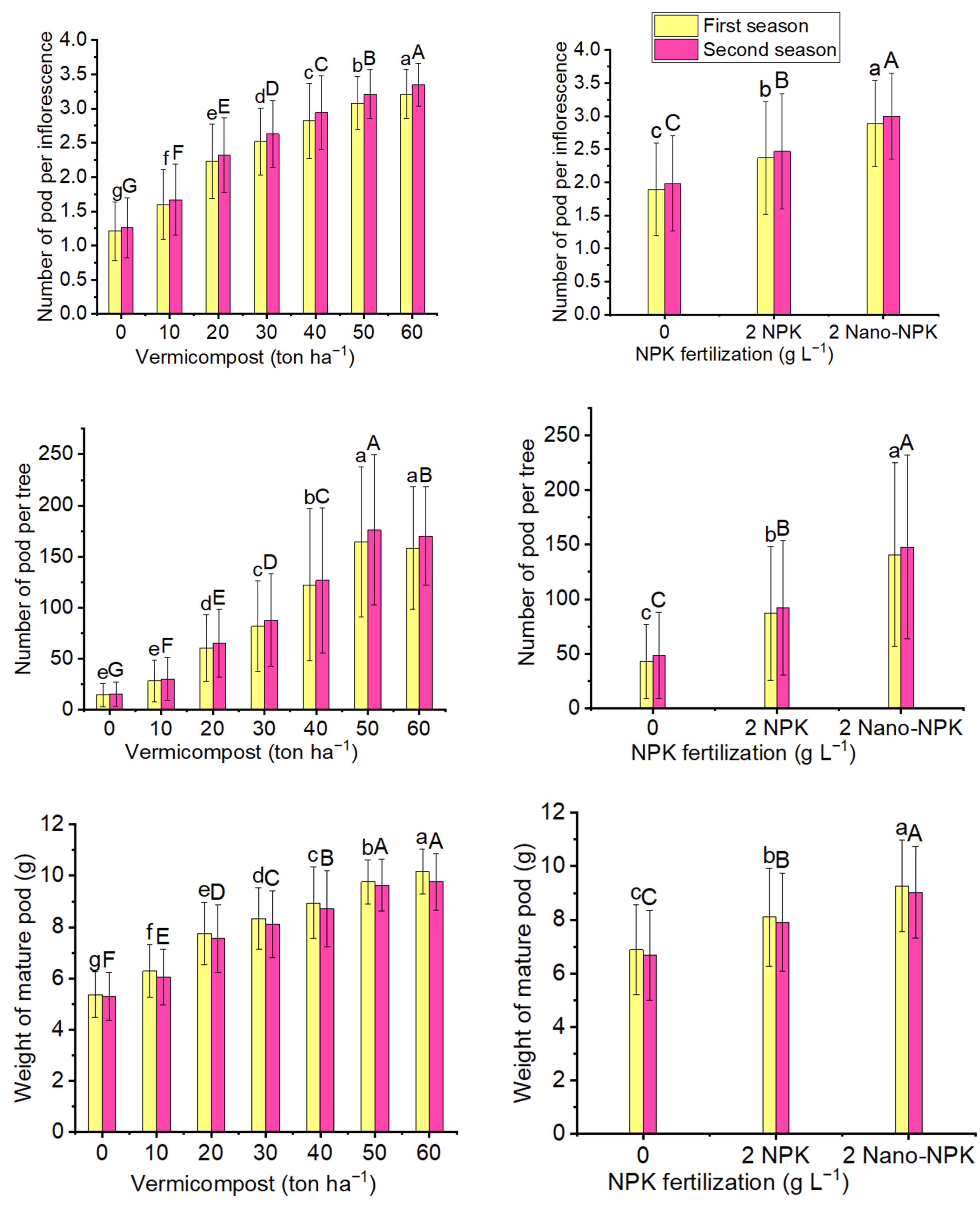
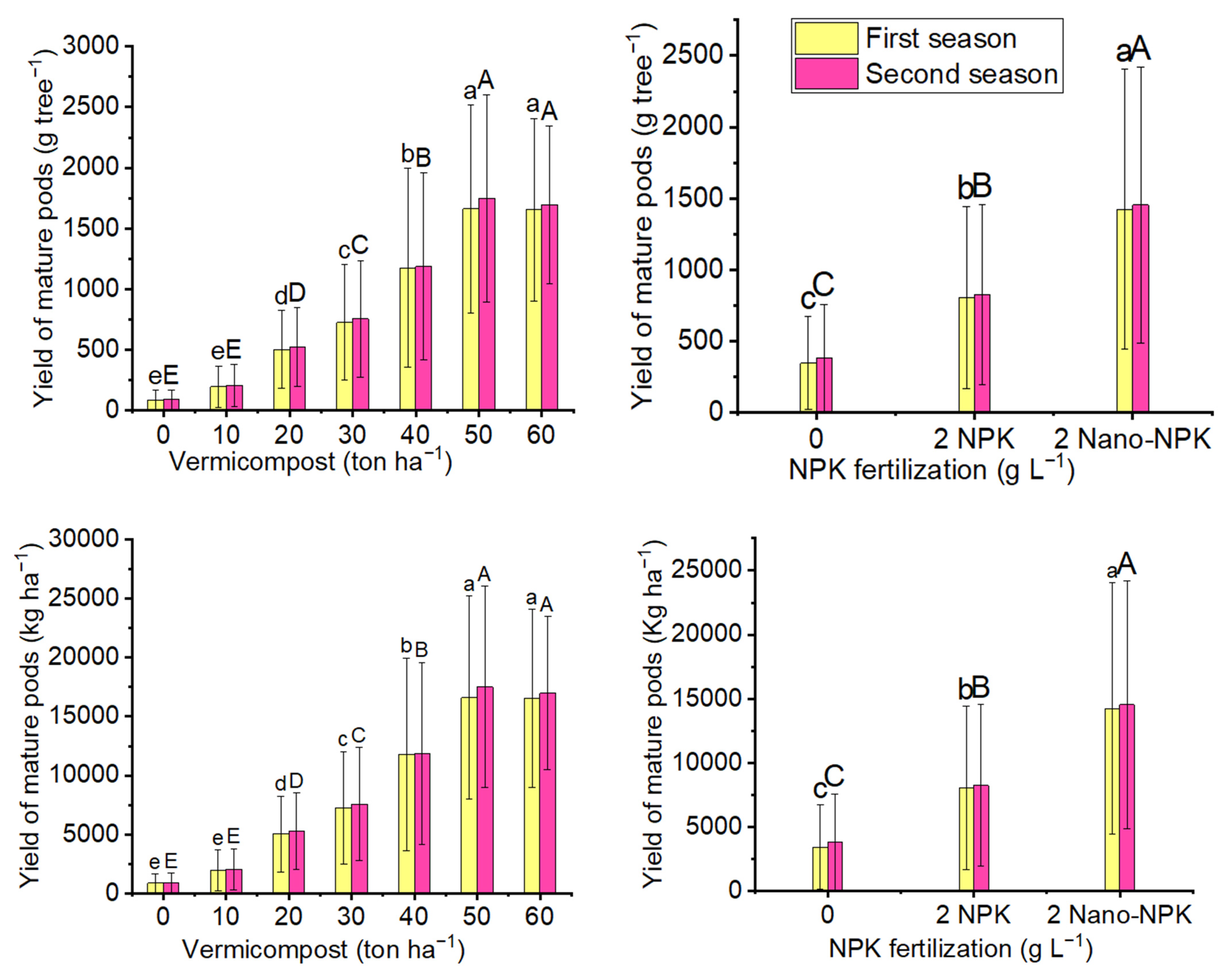
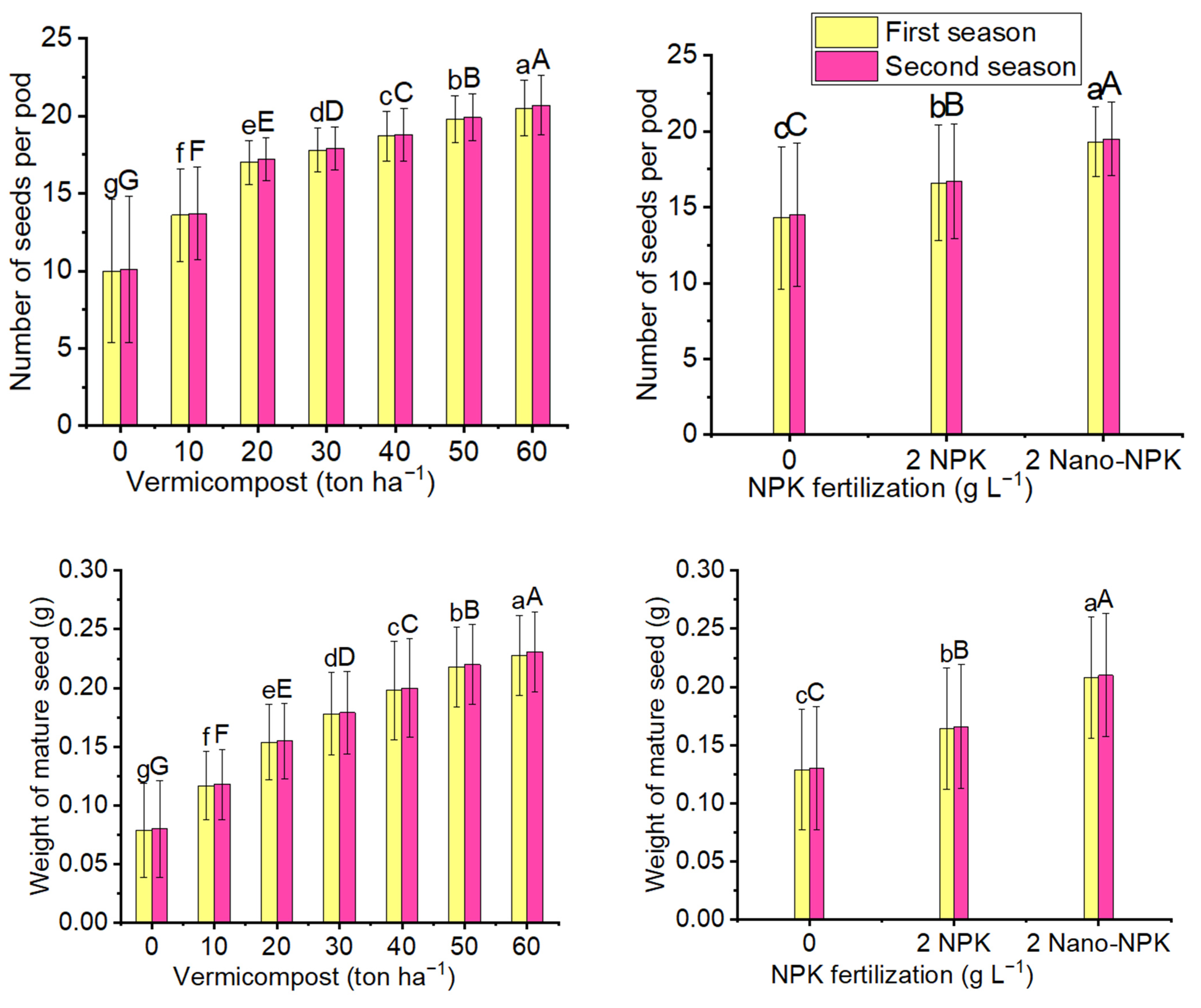
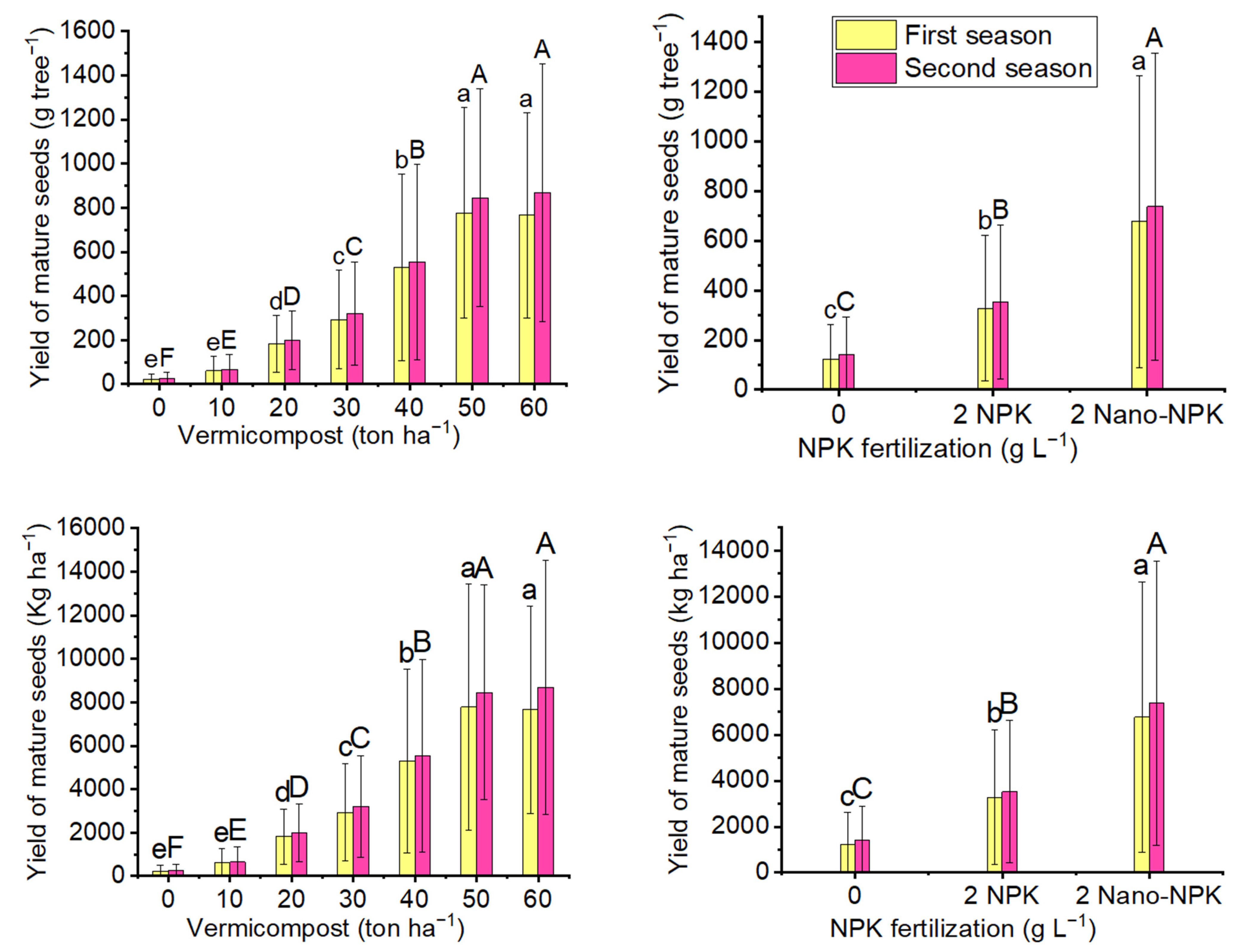


| Treatments | Number of Pods per Inflorescence | |
|---|---|---|
| 1st Season | 2nd Season | |
| T1 | 0.88 ± 0.06 r | 0.92 ± 0.01 u |
| T2 | 0.98 ± 0.07 r | 1.02 ± 0.01 t |
| T3 | 1.77 ± 0.13 n | 1.84 ± 0.02 p |
| T4 | 1.18 ± 0.08 q | 1.23 ± 0.02 s |
| T5 | 1.37 ± 0.10 p | 1.43 ± 0.02 r |
| T6 | 2.26 ± 0.16 kl | 2.36 ± 0.03 m |
| T7 | 1.57 ± 0.11 o | 1.64 ± 0.02 q |
| T8 | 2.36 ± 0.17 k | 2.46 ± 0.03 l |
| T9 | 2.75 ± 0.20 hi | 2.87 ± 0.04 i |
| T10 | 1.96 ± 0.14 m | 2.05 ± 0.03 o |
| T11 | 2.55 ± 0.18 j | 2.66 ± 0.03 k |
| T12 | 3.04 ± 0.22 ef | 3.18 ± 0.04 f |
| T13 | 2.16 ± 0.16 l | 2.25 ± 0.03 n |
| T14 | 2.95 ± 0.21 fg | 3.07 ± 0.04 g |
| T15 | 3.34 ± 0.24 bc | 3.48 ± 0.04 c |
| T16 | 2.65 ± 0.19 ij | 2.77 ± 0.03 j |
| T17 | 3.14 ± 0.23 de | 3.28 ± 0.04 e |
| T18 | 3.44 ± 0.25 ab | 3.59 ± 0.05 b |
| T19 | 2.85 ± 0.21 gh | 2.97 ± 0.04 h |
| T20 | 3.24 ± 0.23 cd | 3.38 ± 0.04 d |
| T21 | 3.53 ± 0.25 a | 3.69 ± 0.05 a |
| Treatments | Number of Pods per Tree | Weight of Mature Pods (g) | ||
|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | |
| T1 | 5.6 ± 0.9 l | 6.0 ± 0.1 s | 4.39 ± 0.09 u | 4.27 ± 0.35 m |
| T2 | 9.2 ± 1.5 kl | 9.8 ± 0.2 rs | 5.30 ± 0.10 t | 5.17 ± 0.42 l |
| T3 | 29.3 ± 5.0 ijk | 31.4 ± 0.8 o | 6.43 ± 0.13 p | 6.26 ± 0.51 k |
| T4 | 11.6 ± 1.9 kl | 12.4 ± 0.3 r | 5.51 ± 0.11 s | 5.36 ± 0.44 l |
| T5 | 19.0 ± 3.2 jkl | 20.4 ± 0.5 q | 5.71 ± 0.11 r | 5.56 ± 0.46 l |
| T6 | 54.5 ± 9.3 gh | 58.4 ± 1.4 l | 7.65 ± 0.15 m | 7.45 ± 0.61 i |
| T7 | 23.7 ± 4.0 ijkl | 25.4 ± 0.6 p | 6.22 ± 0.12 q | 6.06 ± 0.50 k |
| T8 | 63.3 ± 10.9 fg | 67.9 ± 1.7 k | 8.06 ± 0.16 l | 7.85 ± 0.64 hi |
| T9 | 95.2 ± 16.4 e | 102.0 ± 2.5 h | 8.98 ± 0.18 i | 8.74 ± 0.72 efj |
| T10 | 35.8 ± 6.1 hij | 38.4 ± 0.9 n | 6.94 ± 0.14 o | 6.75 ± 0.55 j |
| T11 | 76.6 ± 13.2 ef | 82.1 ± 2.0 j | 8.36 ± 0.16 k | 8.15 ± 0.67 gh |
| T12 | 132.8 ± 23.0 d | 142.4 ± 3.5 e | 9.69 ± 0.19 f | 9.44 ± 0.77 d |
| T13 | 42.6 ± 7.2 ghi | 45.6 ± 1.1 m | 7.14 ± 0.14 n | 6.95 ± 0.57 j |
| T14 | 117.3 ± 20.3 d | 125.8 ± 3.1 f | 9.49 ± 0.19 g | 9.24 ± 0.76 d |
| T15 | 207.0 ± 36.0 b | 209.1 ± 5.2 c | 10.20 ± 0.20 c | 9.93 ± 0.81 c |
| T16 | 85.3 ± 14.7 e | 94.1 ± 3.9 i | 8.77± 0.17 j | 8.54 ± 0.70 fg |
| T17 | 164.7 ± 28.6 c | 170.7 ± 4.2 d | 9.79 ± 0.19 e | 9.92 ± 0.15 c |
| T18 | 243.8 ± 41.1 a | 263.5 ± 6.6 a | 10.71± 0.21 b | 10.43 ± 0.86 b |
| T19 | 95.4 ± 9.4 e | 116.3 ± 4.4 g | 9.28 ± 0.18 h | 9.04 ± 0.74 de |
| T20 | 158.0 ± 27.4 c | 167.2 ± 4.1 d | 10.00 ± 0.20 d | 9.31 ± 0.69 d |
| T21 | 202.6 ± 38.6 b | 226.6 ± 8.1 b | 11.22 ± 0.22 a | 10.93 ± 0.90 a |
| Treatments | Yield of Mature Pods (g Tree−1) | Yield of Mature Pods (kg ha−1) | ||
|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | |
| T1 | 25 ± 4 l | 26 ± 2 o | 246 ± 43 l | 256 ± 24 o |
| T2 | 49 ± 9 l | 51 ± 5 no | 489 ± 88 kl | 509 ± 48 no |
| T3 | 189 ± 35 jkl | 197 ± 19 lmn | 1888 ± 349 jkl | 1966 ±186 lmn |
| T4 | 64 ±12 kl | 66 ± 6 no | 639 ± 116 kl | 665 ± 63 no |
| T5 | 109 ± 20 kl | 113 ± 11mno | 1088 ± 200 kl | 1133 ± 107 mno |
| T6 | 418 ± 78 hij | 436 ± 41 jk | 4180 ± 781 hij | 4356 ± 412 jk |
| T7 | 148 ± 27 kl | 154 ± 15 mno | 1478 ± 272 jkl | 1539 ± 145 mno |
| T8 | 511 ± 96 ghi | 533 ± 50 ij | 5112 ± 958 ghi | 5328 ± 503 ij |
| T9 | 856 ± 161 f | 892 ± 84 g | 8560 ± 1611 ef | 8922 ± 843 g |
| T10 | 249 ± 46 jkl | 260 ± 25 lm | 2491 ± 462 ijkl | 2595 ± 245 lm |
| T11 | 642 ± 121 fgh | 669 ± 63 hi | 6421 ± 1206 fgh | 6692 ± 632 hi |
| T12 | 1289 ± 243 d | 1344 ± 127 e | 12894 ± 2434 d | 13441 ± 1271 e |
| T13 | 305 ± 57 ijk | 317 ± 30 kl | 3046 ± 566 ijk | 3173 ± 300 kl |
| T14 | 1115 ± 210 de | 1162 ± 110 f | 11151 ± 2103 de | 11624 ± 1099 f |
| T15 | 2116 ± 401 b | 2078 ± 197 c | 21157 ± 4009 b | 20783 ± 1965 c |
| T16 | 750 ± 141 fg | 806 ± 97 gh | 7498 ± 1409 fg | 8058 ± 970 gh |
| T17 | 1616 ± 306 c | 1692 ± 46 d | 16160 ± 3057 c | 16924 ± 462 d |
| T18 | 2616 ± 482 a | 2750 ± 260 a | 26158 ± 4820 a | 27496 ± 2600 a |
| T19 | 887 ± 103 ef | 1054 ± 123 f | 8867 ± 1031 ef | 10536 ± 1231 f |
| T20 | 1583 ± 299 c | 1559 ± 155 d | 15826 ± 2992 c | 15586 ± 1546 d |
| T21 | 2276 ± 473 b | 2479 ± 271b | 22758 ± 4727 a | 24795 ± 2708 b |
| Treatments | Number of Seeds per Pod | Mature Seed Weight(g) | ||
|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | |
| T1 | 5.1 ± 0.1 u | 5.2 ± 0.1 u | 0.031 ± 0.001 u | 0.031 ± 0.001 u |
| T2 | 9.2 ± 0.2 t | 9.3 ± 0.2 t | 0.082 ± 0.002 t | 0.083 ± 0.002 t |
| T3 | 15.7 ± 0.3 p | 15.9 ± 0.3 p | 0.123 ± 0.003 p | 0.125 ± 0.002 p |
| T4 | 10.2 ± 0.2 s | 10.3 ± 0.2 s | 0.093 ± 0.002 s | 0.094 ± 0.002 s |
| T5 | 13.3 ± 0.2 r | 13.4 ± 0.3 r | 0.103 ± 0.002 r | 0.104 ± 0.002 r |
| T6 | 17.2 ± 0.3 m | 17.3 ± 0.3 m | 0.155 ± 0.003 m | 0.157 ± 0.003 m |
| T7 | 15.3 ± 0.3 q | 15.5 ± 0.3 q | 0.113 ± 0.002 q | 0.114 ± 0.002 q |
| T8 | 17.4 ± 0.3 l | 17.5 ± 0.3 l | 0.162 ± 0.004 l | 0.164 ± 0.003 l |
| T9 | 18.4 ± 0.3 i | 18.5 ± 0.4 i | 0.185 ± 0.004 i | 0.187 ± 0.004 i |
| T10 | 16.3 ± 0.3 o | 16.5 ± 0.3 o | 0.144 ± 0.003 o | 0.146 ± 0.003 o |
| T11 | 17.6 ± 0.3 k | 17.7 ± 0.3 k | 0.166 ± 0.004 k | 0.167 ± 0.003 k |
| T12 | 19.4 ± 0.3 f | 19.6 ± 0.4 f | 0.221 ± 0.005 f | 0.224 ± 0.004 f |
| T13 | 16.7 ± 0.3 n | 16.8 ± 0.3 n | 0.151 ± 0.003 n | 0.153 ± 0.003 n |
| T14 | 19.0 ± 0.3 g | 19.2 ± 0.4 g | 0.195 ± 0.004 g | 0.198 ± 0.004 g |
| T15 | 20.3 ± 0.3 c | 20.5 ± 0.4 c | 0.247 ± 0.005 c | 0.250 ± 0.005 c |
| T16 | 18.1 ± 0.3 j | 18.2 ± 0.4 i | 0.180 ± 0.004 i | 0.182 ± 0.004 i |
| T17 | 19.7 ± 0.3 e | 19.9 ± 0.4 e | 0.216 ± 0.005 e | 0.218 ± 0.004 e |
| T18 | 21.5 ± 0.4 b | 21.6 ± 0.4 b | 0.257 ± 0.006 b | 0.260 ± 0.005 b |
| T19 | 18.7 ± 0.3 h | 18.8 ± 0.4 h | 0.190 ± 0.004 h | 0.192 ± 0.004 h |
| T20 | 20.0 ± 0.3 d | 20.2 ± 0.4 d | 0.226 ± 0.005 d | 0.229 ± 0.004 d |
| T21 | 22.8 ± 0.4 a | 23.0 ± 0.4 a | 0.267 ± 0.006 a | 0.270 ± 0.005 a |
| Treatments | Yield of Mature Seeds (g Tree−1) | Yield of Mature Seeds (kg ha−1) | ||
|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | |
| T1 | 1 ± 0 l | 1 ± 0 p | 9 ± 2 l | 10 ± 0 p |
| T2 | 7 ± 1 l | 8 ± 0 op | 70 ± 13 l | 76 ± 4 op |
| T3 | 57 ± 11 jkl | 62 ± 3 mn | 572 ± 113 jkl | 622 ± 30 mn |
| T4 | 11 ± 2 kl | 12 ± 1 op | 110 ± 21 kl | 119 ± 6 op |
| T5 | 26 ± 5 kl | 28 ± 1 nop | 261 ± 51 kl | 284 ± 14 nop |
| T6 | 146 ± 29 hijk | 159 ± 8 jk | 1460 ± 290 hijk | 1588 ± 77 jk |
| T7 | 41 ± 8 kl | 45 ± 2 no | 413 ± 81 kl | 449 ± 22 no |
| T8 | 179 ± 36 hij | 195 ± 9 j | 1794 ± 358 hijk | 1953 ± 95 j |
| T9 | 325 ± 65 fg | 354 ± 17 h | 3253 ± 651 fg | 3541 ± 172 h |
| T10 | 85 ± 17 jkl | 92 ± 4 lm | 847 ± 167 jkl | 922 ± 45 lm |
| T11 | 224 ± 45 ghi | 244 ± 12 i | 2238 ± 447 ghi | 2436 ± 118 i |
| T12 | 572 ± 115 de | 623 ± 30 e | 5723 ± 1149 de | 6231 ± 302 e |
| T13 | 108 ± 21 ijkl | 117 ± 6 kl | 1076 ± 213 ijkl | 1171 ± 57 kl |
| T14 | 437 ± 88 ef | 476 ± 23 f | 4374 ± 877 ef | 4763 ± 231 f |
| T15 | 1043 ± 210 b | 1070 ± 55 c | 10431 ± 2100 b | 10701 ± 551 c |
| T16 | 279 ± 56 gh | 313 ± 15 h | 2788 ± 557 gh | 3127 ± 147 h |
| T17 | 704 ± 127 cd | 741 ± 33 d | 7043 ± 1273 cd | 7413 ± 335 d |
| T18 | 1350 ± 265 a | 1482 ± 70 a | 13501 ± 2654 a | 14821 ± 696 a |
| T19 | 340 ± 76 fg | 423 ± 20 g | 3403 ± 757 fg | 4226 ± 200 g |
| T20 | 719 ± 160 c | 772 ± 42 d | 7189 ± 1597 c | 7723 ± 419 d |
| T21 | 1237 ± 323 a | 1408 ± 85 b | 12372 ± 3232 a | 14083 ±8 47 b |
| Treatments | Fixed Oil Percentage (%) | Fixed Oil Content (mL Plant−1) | ||
|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | |
| T1 | 36.57 ± 3.07 a | 36.49 ± 2.40 ab | 0.3 ± 0.1 h | 0.4 ± 0.0 r |
| T2 | 38.69 ± 1.22 a | 37.48 ± 2.12 a | 4.2 ± 0.7 gh | 4.5 ± 0.3 r |
| T3 | 32.41 ± 6.29 b | 35.52 ± 1.84 bc | 17.8 ± 0.9 fgh | 22.6 ± 1.1 o |
| T4 | 37.49 ± 2.82 a | 36.78 ± 2.09 ab | 2.6 ± 0.4 h | 2.8 ± 0.1 r |
| T5 | 36.55 ± 0.71 a | 37.83 ± 1.50 a | 9.5 ± 2.0 gh | 10.7 ± 0.2 q |
| T6 | 29.17± 0.91 cd | 33.78 ± 1.10 c | 42.5 ± 8.1 ef | 53.6 ± 1.1 l |
| T7 | 37.22 ± 1.16 a | 36.54 ± 1.19 ab | 15.3 ± 2.9 fgh | 16.4 ± 0.3 p |
| T8 | 36.72 ± 1.15 a | 36.05 ± 1.18 ab | 65.7 ± 12.5 de | 70.3 ± 1.4 k |
| T9 | 28.17 ± 0.88 cd | 28.64 ± 0.93 ef | 91.4 ± 17.5 cd | 101.3 ± 2.0 h |
| T10 | 36.89 ± 1.71 a | 35.14 ± 1.14 bc | 32.2 ± 6.1 fg | 32.4 ± 1.0 n |
| T11 | 32.19 ± 1.01 b | 31.61 ± 1.03 d | 71.9 ± 13.7 d | 76.9 ± 1.5 J |
| T12 | 26.98 ± 0.40 de | 27.65 ± 0.90 fg | 154.4 ± 31.1 b | 172.2 ± 3.4 e |
| T13 | 36.22 ± 1.13 a | 35.56 ± 1.16 bc | 38.9 ± 7.4 ef | 41.6 ± 0.8 m |
| T14 | 23.14 ± 0.72 fg | 22.72 ± 0.74 ij | 101.0 ± 19.3 c | 108.1 ± 2.2 g |
| T15 | 25.15 ± 0.79 ef | 24.69 ± 0.80 h | 261.8 ± 50.4 a | 264.0 ± 5.3 c |
| T16 | 30.18 ± 0.94 bc | 29.63 ± 0.97 e | 83.9 ± 16.0 cd | 92.5 ± 4.4 i |
| T17 | 24.79 ± 0.69 ef | 24.07 ± 1.24 hi | 174.8 ± 37.4 b | 178.2 ± 2.0 d |
| T18 | 20.06 ± 0.31 h | 20.21 ± 0.89 k | 270.5 ± 52.2 a | 299.1 ± 2.3 a |
| T19 | 27.16 ± 0.85 de | 26.67 ± 0.87 g | 92.3 ± 10.8 cd | 112.5 ± 5.0 g |
| T20 | 21.44 ± 0.95 gh | 21.11 ± 0.73 jk | 153.3 ± 26.1 b | 163.0 ± 7.9 f |
| T21 | 20.01 ± 0.17 h | 20.09 ± 0.84 k | 247.4 ± 27.8 a | 282.4 ± 7.2 b |
| Treatments | Yield of Fixed Oil (L ha−1) | |
|---|---|---|
| 1st Season | 2nd Season | |
| T1 | 3 ± 1 h | 4 ± 0 r |
| T2 | 42 ± 7 gh | 45 ± 3 r |
| T3 | 178 ± 9 fgh | 226 ± 11 o |
| T4 | 26 ± 4 h | 28 ± 1 r |
| T5 | 95 ± 20 gh | 107 ± 2 q |
| T6 | 425 ± 81 ef | 536 ± 11 l |
| T7 | 153 ± 29 fgh | 164 ± 3 p |
| T8 | 657 ± 125 de | 703 ± 14 k |
| T9 | 914 ± 175 cd | 1013 ± 20 h |
| T10 | 322 ± 61 fg | 324 ± 10 n |
| T11 | 719 ± 137 d | 769 ± 15 j |
| T12 | 1544 ± 311 b | 1722 ± 34 e |
| T13 | 389 ± 74 ef | 416 ± 8 m |
| T14 | 1010 ± 193 c | 1081 ± 22 g |
| T15 | 2618 ± 504 a | 2640 ± 53 c |
| T16 | 839 ± 160 cd | 925 ± 44 i |
| T17 | 1748 ± 374 b | 1782 ± 20 d |
| T18 | 2705 ± 522 a | 2991 ± 23 a |
| T19 | 923 ± 108 cd | 1125 ± 50 g |
| T20 | 1533 ± 261 b | 1630 ± 79 f |
| T21 | 2474 ± 278 a | 2824 ± 72 b |
| Treatments | Stearic Acid | Palmitic Acid | Eicosenoic Acid | Behenic Acid | Lignoceric Acid |
|---|---|---|---|---|---|
| T1 | 3.12 ± 0.10 i | 4.31 ± 0.15 j | 2.01 ± 0.06 n | 4.26 ± 0.14 n | 0.84 ± 0.02 g |
| T2 | 4.61 ± 0.16 f | 4.56 ± 0.15 h | 2.16 ± 0.07 j | 4.64 ± 0.16 j | 0.92 ± 0.02 def |
| T3 | 4.96 ± 0.17 c | 4.64 ± 0.16 f | 2.72 ± 0.09 e | 5.35 ± 0.18 e | 1.00 ± 0.03 c |
| T4 | 3.03 ± 0.10 j | 4.32 ± 0.15 j | 1.97 ± 0.06 o | 4.02 ± 0.13 o | 0.15 ± 0.10 h |
| T5 | 3.16 ± 0.10 i | 4.51 ± 0.15 i | 2.04 ± 0.06 m | 4.36 ± 0.15 m | 0.88 ± 0.02 fg |
| T6 | 4.9 ± 0.17 cd | 4.57 ± 0.16 h | 2.20 ± 0.07 i | 4.83 ± 0.16 i | 0.94± 0.02 de |
| T7 | 3.00 ± 0.10 j | 4.20 ± 0.14 k | 1.91 ± 0.06 p | 3.81 ± 0.13 p | 0.14 ± 0.10 h |
| T8 | 3.51 ± 0.12 h | 4.59 ± 0.16 g | 2.10 ± 0.07 l | 4.41 ± 0.15 l | 0.86 ± 0.02 g |
| T9 | 4.67 ± 0.16 e | 4.63 ± 0.16 f | 2.61 ± 0.08 g | 4.9 ± 0.17 gh | 0.9 ± 0.02 cd |
| T10 | 4.01 ± 0.13 g | 4.60 ± 0.16 g | 2.14 ± 0.07 k | 4.53 ± 0.15 k | 0.85 ± 0.02 g |
| T11 | 4.94± 0.17 cd | 4.64 ± 0.16 f | 2.63 ± 0.08 fg | 4.87 ± 0.17 hi | 1.00 ± 0.03 c |
| T12 | 5.18± 0.18 ab | 4.85 ± 0.17 d | 2.88 ± 0.09 d | 5.63 ± 0.19 d | 1.05 ± 0.03 b |
| T13 | 4.90 ± 0.17 d | 4.59 ± 0.16 g | 2.23 ± 0.07 h | 4.92 ± 0.17 g | 0.91 ± 0.02 ef |
| T14 | 4.95± 0.17 cd | 4.64 ± 0.16 f | 2.65 ± 0.09 f | 5.20 ± 0.18 f | 0.88 ± 0.02 fg |
| T15 | 5.19± 0.18 ab | 4.88 ± 0.17 c | 2.90 ± 0.09 d | 5.94 ± 0.20 c | 1.07± 0.03 ab |
| T16 | 4.9 ± 0.17 cd | 4.66 ± 0.16 e | 2.64 ± 0.08 f | 5.23 ± 0.18 f | 0.86 ± 0.02 g |
| T17 | 5.20± 0.18 ab | 4.88 ± 0.17 c | 2.95 ± 0.10 c | 5.91 ± 0.20 c | 1.05 ± 0.03 b |
| T18 | 5.20± 0.18 ab | 5.20 ± 0.18 b | 2.97 ± 0.10 c | 6.04 ± 0.21 b | 1.08± 0.03 ab |
| T19 | 5.17 ± 0.18 b | 4.87 ±0.17 c | 2.89 ± 0.09 d | 5.62 ± 0.19 d | 1.06 ± 0.03 b |
| T20 | 5.22± 0.18 ab | 5.21 ± 0.18 b | 3.13 ± 0.10 b | 6.06 ± 0.21 b | 1.08± 0.03 ab |
| T21 | 5.23 ± 0.18 a | 5.52 ± 0.19 a | 3.23 ± 0.11 a | 6.15 ± 0.21 a | 1.11 ± 0.03 a |
| Treatments | Oleic Acid | Linoleic Acid | α-Linolenic Acid | Palmitoleic Acid | Paullinic Acid |
|---|---|---|---|---|---|
| T1 | 71.87 ± 1.80 b | 4.03 ± 0.09 c | 0.72 ± 0.01 c | 2.34 ± 0.05 f | 2.43 ± 0.05 h |
| T2 | 70.97 ± 1.78 d | 3.83 ± 0.09 g | 0.56 ± 0.01 g | 3.04 ± 0.07 b | 2.27 ± 0.05 j |
| T3 | 67.48 ± 1.69 j | 2.66 ± 0.06 n | 0.31 ± 0.01 l | 1.31 ± 0.02 k | 2.76 ± 0.06 b |
| T4 | 72.46 ± 1.82 a | 4.11 ± 0.09 b | 0.79 ± 0.01 b | 2.56 ± 0.05 d | 2.72 ± 0.06 c |
| T5 | 71.77 ± 1.80 b | 4.01 ± 0.09 d | 0.66 ± 0.01 d | 2.12 ± 0.04 g | 2.49 ± 0.05 g |
| T6 | 70.67 ± 1.77 e | 3.53 ± 0.08 h | 0.05 ± 0.05 q | 3.03 ± 0.07 b | 2.38 ± 0.05 i |
| T7 | 72.56 ± 1.82 a | 4.18 ± 0.09 a | 0.83 ± 0.01 a | 3.13 ± 0.07 a | 2.78 ± 0.06 a |
| T8 | 71.47 ± 1.79 c | 3.98 ± 0.09 e | 0.64 ± 0.01 e | 2.42 ± 0.05 e | 2.38 ± 0.05 i |
| T9 | 70.27 ± 1.76 f | 3.23 ± 0.07 j | 0.48 ± 0.00 i | 2.12 ± 0.04 g | 2.68 ± 0.06 d |
| T10 | 71.37 ± 1.79 c | 3.93 ± 0.09 f | 0.61 ± 0.01 f | 2.63 ± 0.06 c | 2.18 ± 0.04 k |
| T11 | 69.97 ± 1.76 g | 3.03 ± 0.07 k | 0.41 ± 0.00 j | 1.35 ± 0.02 j | 2.58 ± 0.05 f |
| T12 | 65.49 ± 1.64 k | 2.63 ± 0.06 o | 0.29 ± 0.01 m | 1.21 ± 0.02 l | 2.64 ± 0.06 e |
| T13 | 70.47 ± 1.77ef | 3.43 ± 0.08 i | 0.50 ± 0.00 h | 2.04 ± 0.04 h | 2.49 ± 0.05 g |
| T14 | 69.47 ± 1.74 h | 2.83 ± 0.06 l | 0.34 ± 0.01 k | 1.22 ± 0.02 l | 2.09 ± 0.04 m |
| T15 | 62.40± 1.56 m | 2.42 ± 0.05 q | 0.02 ± 0.00 r | 1.14 ± 0.02 m | 2.68 ± 0.06 d |
| T16 | 68.48 ± 1.72 i | 2.73 ± 0.06 m | 0.32 ± 0.01 l | 1.52 ± 0.03 i | 2.07 ± 0.04 n |
| T17 | 60.40 ± 1.51 n | 2.38 ± 0.05 r | 0.20 ± 0.01 o | 1.06 ± 0.02 n | 2.16 ± 0.04 l |
| T18 | 58.41 ± 1.46 o | 2.32 ± 0.05 s | 0.19 ± 0.01 o | 0.92 ± 0.01 o | 2.06 ± 0.04 n |
| T19 | 63.39 ± 1.59 l | 2.53 ± 0.05 p | 0.27 ± 0.01 n | 1.20 ± 0.02 l | 2.49 ± 0.05 g |
| T20 | 57.41 ± 1.44 p | 2.22 ± 0.05 t | 0.14 ± 0.01 p | 0.88 ± 0.01 p | 2.01 ± 0.04 o |
| T21 | 57.02 ± 1.43 q | 2.12 ± 0.04 u | 0.02 ± 0.00 r | 0.07 ± 0.05 q | 1.98 ± 0.04 p |
| Treatments | |
|---|---|
| T1 | Vermicompost control plus NPK control (Control) |
| T2 | Vermicompost control plus 2 gL−1 NPK |
| T3 | Vermicompost control plus 2 gL−1 Nano-NPK |
| T4 | 10 ton ha−1 vermicompost plus NPK control |
| T5 | 10 ton ha−1 vermicompost plus 2 gL−1 NPK |
| T6 | 10 ton ha−1 vermicompost plus 2 gL−1 Nano-NPK |
| T7 | 20 ton ha−1 vermicompost plus NPK control |
| T8 | 20 ton ha−1 vermicompost plus 2 gL−1 NPK |
| T9 | 20 ton ha−1 vermicompost plus 2 gL−1 Nano-NPK |
| T10 | 30 ton ha−1 vermicompost plus NPK control |
| T11 | 30 ton ha−1 vermicompost plus 2 gL−1 NPK |
| T12 | 30 ton ha−1 vermicompost plus 2 gL−1 Nano-NPK |
| T13 | 40 ton ha−1 vermicompost plus NPK control |
| T14 | 40 ton ha−1 vermicompost plus 2 gL−1 NPK |
| T15 | 40 ton ha−1 vermicompost plus 2 gL−1 Nano-NPK |
| T16 | 50 ton ha−1 vermicompost plus NPK control |
| T17 | 50 ton ha−1 vermicompost plus 2 gL−1 NPK |
| T18 | 50 ton ha−1 vermicompost plus 2 gL−1 Nano-NPK |
| T19 | 60 ton ha−1 vermicompost plus NPK control |
| T20 | 60 ton ha−1 vermicompost plus 2 gL−1 NPK |
| T21 | 60 ton ha−1 vermicompost plus 2 gL−1 Nano-NPK |
| Vermicompost Property | ||
|---|---|---|
| Organic matter | % | 44.57 |
| C | % | 17.02 |
| N | % | 1.82 |
| Mn | % | 0.03 |
| B | mg g−1 | 0.054 |
| Cu | mg g−1 | 0.25 |
| Fe | mg g−1 | 1.27 |
| Mg | mg g−1 | 6.01 |
| Na | mg g−1 | 1.48 |
| P2O5 | mg g−1 | 4.61 |
| K | mg g−1 | 1.93 |
| EC | ds m−1 | 1.78 |
| pH | 7.2 | |
| Soil Property | ||
|---|---|---|
| Organic matter | 0.75 | |
| CaCO3 | % | 28.62 |
| Sand | % | 65.3 |
| Silt | % | 15.8 |
| Clay | % | 18.9 |
| Texture class | Sandy clay loam | |
| pH | 8.51 | |
| EC | ds m−1 | 1.72 |
| N | % | 0.032 |
| HCO3- | mg g−1 | 0.099 |
| P2O4 | mg g−1 | 0.004 |
| K+ | mg g−1 | 0.287 |
| Fe | mg g−1 | 0.0038 |
| Zn | mg g−1 | 0.0014 |
| Mn | mg g−1 | 0.0035 |
| Cu | mg g−1 | 0.00059 |
| B | mg g−1 | 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atteya, A.K.G.; Albalawi, A.N.; El-Serafy, R.S.; Albalawi, K.N.; Bayomy, H.M.; Genaidy, E.A.E. Response of Moringa oleifera Seeds and Fixed Oil Production to Vermicompost and NPK Fertilizers under Calcareous Soil Conditions. Plants 2021, 10, 1998. https://doi.org/10.3390/plants10101998
Atteya AKG, Albalawi AN, El-Serafy RS, Albalawi KN, Bayomy HM, Genaidy EAE. Response of Moringa oleifera Seeds and Fixed Oil Production to Vermicompost and NPK Fertilizers under Calcareous Soil Conditions. Plants. 2021; 10(10):1998. https://doi.org/10.3390/plants10101998
Chicago/Turabian StyleAtteya, Amira K. G., Aishah N. Albalawi, Rasha S. El-Serafy, Khalil N. Albalawi, Hala M. Bayomy, and Esmail A. E. Genaidy. 2021. "Response of Moringa oleifera Seeds and Fixed Oil Production to Vermicompost and NPK Fertilizers under Calcareous Soil Conditions" Plants 10, no. 10: 1998. https://doi.org/10.3390/plants10101998






